Abstract
Background
Increased esophagogastric junction (EGJ) distensibility is thought to contribute to gastroesophageal reflux disease (GERD). Using the functional lumen imaging probe (FLIP), we aimed to assess the esophageal response to distension among patients undergoing esophageal pH monitoring.
Methods
25 patients (ages 22 – 73; 13 females) that underwent ambulatory wireless esophageal pH testing while off proton-pump inhibitors were evaluated with FLIP during sedated upper endoscopy. Esophageal reflux was quantified by total percent acid exposure time (AET; < 6% was considered normal). FLIP studies were analyzed using a customized program generate FLIP topography plots to identify esophageal contractility patterns and to calculate the EGJ-distensibility index (DI). Reflux symptoms were assessed with the GERDQ. Values reflect median (interquartile range).
Results
Among all patients, the AET was 7.2% (3.7 – 11.1) and EGJ-DI was 4.2 (2.5 – 7.6) mm2/mmHg. Repetitive antegrade contractions (RACs) were induced in 19/25 (76%) of patients; AET was lower among patients with (6.1%, 3 – 7.8) than without (14.9, 8.5 – 22.3) RACs (p = 0.009). Correlation was weak and insignificant between AET and EGJ-DI, GERDQ and AET, and GERDQ and EGJ-DI. Patients with abnormal AET (n = 16) and normal AET (n = 9) had similar EGJ-DI, 4.6 mm2/mmHg (2.9 – 9.2) vs 3.2 (2.2 – 5.1), p = 0.207 and GERDQ, p = 0.138.
Conclusions
Abnormal esophageal acid exposure was associated with an impaired contractile response to volume distention of the esophagus. This supports that acid exposure is dependent on acid clearance mechanisms.
Keywords: functional lumen imaging probe, GERD, impedance, peristalsis, reflux
Abbreviated abstract
Among patients evaluated for reflux with wireless esophageal pH testing and the functional lumen imaging probe, a response to volumetric distension comprising repetitive, antegrade contractions was associated with a reduced degree of esophageal acid exposure. The esophagogastric-junction distensibility index, however, was poorly correlated with esophageal acid exposure.
Introduction
Gastroesophageal reflux disease (GERD) is a commonly encountered condition that often involves one or more contributing pathophysiologic abnormalities.1 Esophagogastric junction (EGJ) incompetence is a primary factor related to the development of GERD, thus measurement of alterations in EGJ-distensibility may help characterize patients. Studies utilizing barostat distension of the EGJ demonstrated increased EGJ-distensibility (i.e. greater EGJ diameters at lower distending-pressures) among GERD patients with and without hiatal hernia.2, 3 However, use of the barostat is limited by its cumbersome nature and accurate assessments of esophageal distensibility are somewhat limited with traditional methods of esophageal disease evaluation (i.e. upper endoscopy, manometry, and barium-radiography).
The functional lumen imaging probe (FLIP) utilizes high-resolution impedance planimetry to measure the relationship of luminal dimensions and distensive pressure (i.e. distensibility) during controlled, volumetric distension. The FLIP is commercially available and can be easily applied during a sedated upper endoscopy, thus it offers the potential to aid clinical characterization of patients presenting with GERD symptoms. Although EGJ-distensibility was assessed with FLIP in several previous studies, results regarding the association between EGJ-distensibility and GERD have been inconsistent.4–8 Distension-induced contractility can also be assessed using the FLIP, though this evaluation has not been previously described among patients evaluated for GERD.9, 10
The role of FLIP for diagnostic evaluation and targeted management in GERD remains appealing, however inconsistent results among prior studies applying FLIP measurement of EGJ-distensibility in GERD limits support of this application. Thus, we aimed to assess the association of esophageal acid exposure with the esophageal response to distension utilizing FLIP.
Methods
Subjects
Patients presenting to the Esophageal Center of Northwestern for evaluation of suspected GERD and scheduled for upper endoscopy with wireless pH monitoring between May 2016 and December 2016 were prospectively included. Indication for reflux monitoring was classified as typical reflux symptoms (heartburn or regurgitation), chest pain, extraesophageal reflux symptoms (cough, laryngitis, globus), or other. Upper endoscopy was completed using sedation with midazolam (2 – 15 mg) and fentanyl (0 – 300 mcg); propofol (in addition to midazolam and fentanyl) was used with anesthesiologist assistance at the discretion of the performing endoscopist in one case. Patients with previous upper gastrointestinal surgery, significant medical co-morbidities, eosinophilic esophagitis, severe reflux esophagitis (LA-classification C or D), or hiatal hernia > 3 cm were excluded.
Reflux symptoms were assessed and quantified using the GERDQ, a widely used, validated, 6-item self-report measure with questions evaluating common reflux symptoms (heartburn, regurgitation, chest pain), sleep disturbance, and antacid use.11 A GERDQ score > 8 was considered abnormal. Informed consent was obtained from each subject and the study protocol was approved by the Northwestern University Institutional Review Board.
Functional lumen imaging probe
The FLIP assembly consisted of a 240-cm long, 3-mm outer diameter catheter with an 18-CM, infinitely compliant balloon (up to a distension volume of 60 mL) mounted near the distal end of the catheter (EndoFLIP®; Crospon, Inc, Galway, Ireland). The balloon tapered at both ends to assume a 16-cm long cylindrical shape in the center that housed 17 impedance planimetry ring electrodes spaced at 1-cm intervals and a solid-state pressure transducer positioned at the distal end to provide simultaneous measurement of 16 channels of cross-sectional area (CSA) converted to diameter based on the assumption of circular geometry and intra-balloon pressure.
FLIP was completed immediately following sedated upper endoscopy while the patient was in the left lateral decubitus position. The FLIP probe was pressure-zeroed to atmospheric pressure and was placed trans-orally and positioned with the distal 2–3 impedance sensors beyond the EGJ as confirmed by demonstration of a waist in the impedance planimetry segment at a balloon distension volume of 20–30 ml. The FLIP assembly position was adjusted by the endoscopist during the study to maintain placement relative to the EGJ as visualized on real-time output. Simultaneous CSAs and intra-balloon pressures were measured during 10 ml stepwise distensions beginning with 20 ml and increasing to target volume of 70 ml with each incremental distension volume maintained for 20 – 30 seconds.
FLIP Data analysis
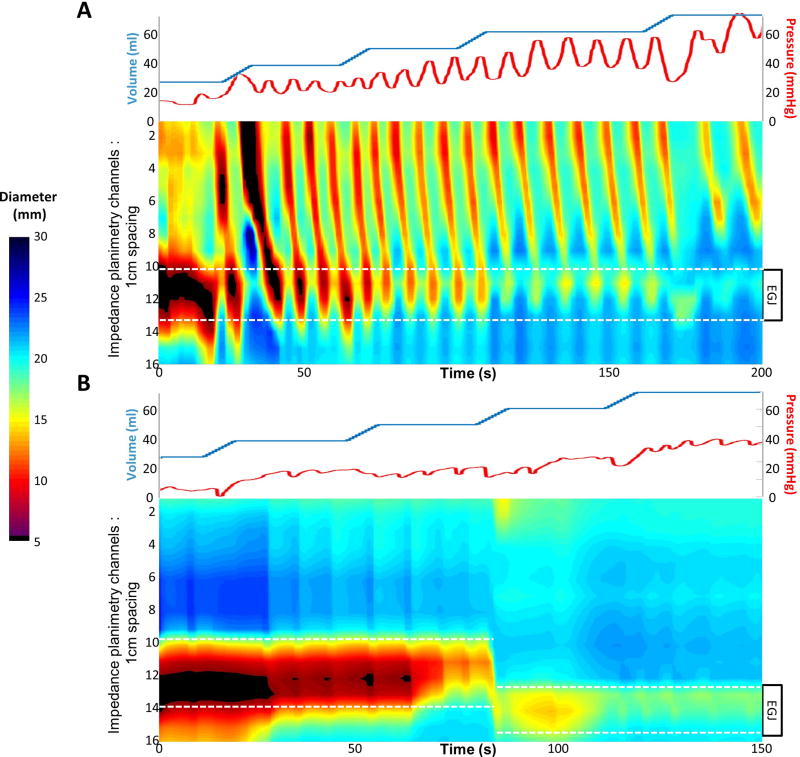
FLIP data including distension volume, intra-balloon pressure, and 16 channels of luminal diameter for each subject were exported to MATLAB (The Math Works, Natick, MA, USA) for analysis using a customized MATLAB program.12 This program applied a filter to minimize vascular and respiratory artifact and then generated tracings of each channel’s luminal diameter. Interpolation between channels was applied to generate color-coded topography plots by time with corresponding plots of volume distension and intra-balloon pressure; Figure 1. The program identified the EGJ-midline by searching for the minimal diameter of the distal impedance planimetry channels. The EGJ-distensibility index (EGJ-DI) was calculated by measuring the narrowest EGJ CSA and intra-balloon pressure at each data sample obtained during the time course at the 60-ml distension volume.13 The median values for narrowest EGJ CSA and intra-balloon pressure were then divided to calculate the EGJ-DI (CSA/pressure; mm2/mmHg).
Figure 1. FLIP topography plots.
Distension volume (top, blue line), and intra-balloon pressure (top, red line), and FLIP topography (bottom) from two patients are displayed. The position of the esophagogastric junction (EGJ) is noted by the white dashed lines. A) Repetitive, antegrade contractions are present and the EGJ-distensibility index (DI) at the 60-ml fill volume was 6.7 mm2/mmHg. The worst day of total acid exposure time (AET) was 4.9%. B) No esophageal contractility was induced and the EGJ-DI at the 60-ml fill volume was 4.7 mm2/mmHg. The worst day AET was 29%. Figure used with permission from the Esophageal Center at Northwestern.
Esophageal body contractions were identified by a transient decrease of ≥ 5 mm in the measured luminal diameter detected in ≥2 adjacent axial impedance planimetry channels using the FLIP topography plots and 16 channel diameter tracing output.9, 14 Contractions were described in terms of propagation direction (antegrade or retrograde) based on the tangent line placed on the onset of contraction. Contractions were considered repetitive (repetitive, antegrade contractions, RACs, or repetitive, retrograde contractions, based on propagation direction) when ≥3 occurred consecutively. The presence of RACs and repetitive, retrograde contractions was not mutually exclusive, thus both could be present in a single patient over the course of the FLIP study.
Esophageal pH monitoring
All patients stopped proton-pump inhibitor therapy for at least 7 days prior to esophageal pH monitoring. The Bravo wireless pH capsule (Medtronic Inc, Shoreview, MN) was placed following endoscopy and FLIP. The delivery system was placed trans-orally into the esophagus and positioned so that the pH electrode was 6 cm proximal to the squamocolumnar junction, where the pH capsule was secured to the adjacent esophageal mucosa. The recording period extended for 96 hours with each test day analyzed separately. Patient position (supine, upright) and meal times were based on patient recording and diary; meal times were excluded from the analysis. Using esophageal pH < 4 as indicative of reflux, the measures outcomes included the % time pH <4 (acid exposure time, AET), number of reflux events, and longest reflux event. The worst day, i.e. most extreme measure, for each variable was included in analysis to maintain independence of variables. Abnormal AET was consider if any day had a total AET > 6%.15 Additionally, the number of abnormal days (0–4), i.e. days with total AET > 6%, was tabulated
Statistical analysis
Descriptive statistics for all continuous and ordinal measures were presented as median (IQR), unless otherwise stated. Correlations were assessed using Spearman’s rho. Groups were compared using the Kruskal-Wallis or Mann-Whitney U test for continuous variables and Χ2 or Fischer’s Exact tests for dichotomous and categorical variables. Analyses assumed a 5% level of statistical significance.
Results
Subjects
25 patients completed the study protocol (ages 22 – 73, 13 females) and entail the included study cohort; 3 other patients consented but were excluded at the time of endoscopy for LA-C esophagitis (1), hiatal hernia > 3 cm (1), and a technical malfunction of the FLIP pressure sensor (1). Indication for reflux monitoring was for typical reflux symptoms in 13 (52%), chest pain in 4 (16%), extraesophageal symptoms in 6 (24), and other symptoms in 2 (8%: 1 for abdominal pain, 1 for nausea). Three patients underwent testing for both typical and extraesophageal symptoms. Endoscopy demonstrated a hiatal hernia ≤ 3cm in 7 (28%), LA-B esophagitis in one patient (also with hiatal hernia), and LA-A esophagitis in 2 patients (neither with hiatal hernia); endoscopies were otherwise normal. One patient did not complete the GERDQ, among the remaining 24 patients, median (IQR) GERDQ scores were 9 (6 – 10); 12 (50%) were abnormal.
Esophageal pH monitoring
Among the total cohort, the worst day of AET was 7.2% (3.7 – 11.1); there were 16 (64%) with abnormal esophageal acid exposure. All seven patients with hiatal hernia also had abnormal AET, as did all three with low-grade esophagitis; there otherwise were no significant differences in clinical characteristics between patients with and without abnormal AET (Table 1). Among those with abnormal AET, 8 (50%) had 1 day positive, 5 (31%) had 2 days positive, 2 (13%) had 3 days positive, and 1 (6%) had all 4 days positive.
Table 1.
Clinical characteristics by esophageal acid exposure.
| Abnormal AET | Normal AET | |
|---|---|---|
|
| ||
| n | 16 | 9 |
|
| ||
| Age, years, mean (range) | 50 (22 – 73) | 45 (27 – 67) |
|
| ||
| Gender (% female) | 50 | 56 |
|
| ||
| Indication for pH testing, n (%) | ||
| Typical symptoms | 9 (53) | 4 (44) |
| Chest pain | 1 (6) | 3 (33) |
| Extraesophageal symptoms | 6 (38) | 0 |
| Other | 0 | 2 (22) |
|
| ||
| Endoscopy findings | ||
| Esophagitis, n LA-A/B | 2/1 | 0 |
| Hiatal hernia, n (%) | 7 (44) | 01 |
|
| ||
| GERDQ, median (IQR) | 10 (6 – 11) | 7 (6 – 9) |
|
| ||
| GERDQ > 8, n (%) | 6 (40) | 6 (67) |
Abnormal acid exposure time (AET) was considered at a worst day of > 6%.
p-value < 0.05 when compared with abnormal AET.
Correlation (Spearman’s rho) of GERDQ with reflux parameters included 0.265 with total AET, 0.342 with upright AET, −0.227 with supine AET, 0.276 with number of reflux episodes, and −0.095 with longest reflux episode. None of these correlations were statistically significant.
Association of distension-induced contractility with reflux parameters
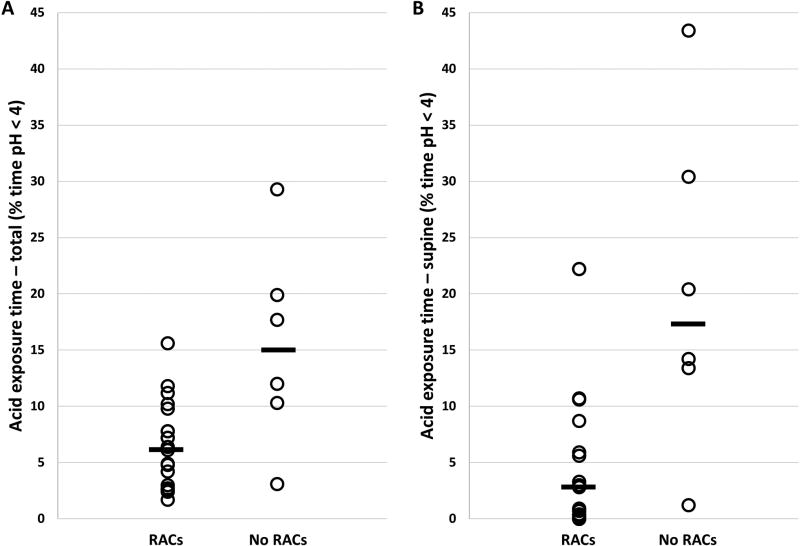
Esophageal contractility was observed on FLIP topography in all patients but one (96%); Figure 1. RACs were present in 19 (76%). Repetitive, retrograde contractions were present in 3 (12%), 2 of which also had RACs. Total AET was lower in patients exhibiting RACs, 6.1% (3.0 – 7.8), than those that did not generate RACs, 14.9% (8.5 – 22.3), p = 0.009; Figure 2. Among the six patients without RACs, five (83%) RACs had an abnormal AET while EGJ-DIs ranged from 0.4 – 9.0 mm2/mmHg (median 2.2 mm2/mmHg). The dosages of sedation agents were similar between patients with and without RACs: midazolam (median, IQR 7, 5–10 mg vs 5, 4–8 mg; p = 0.120); fentanyl (125, 100–175 mcg vs 100, 88 – 163 mcg; p = 0.265).
Figure 2. Acid exposure time (A-total; B-supine) among patients with and without distension-induced repetitive, antegrade contractions (RACs).
Group median values are displayed as black bars.
Supine AET also differed between patients with RACs, 2.8% (0.1 – 8.7), and without RACs, 17.3 (10.4 – 33.7), p = 0.007; Figure 2. Upright AET (1.7%, 0.9 – 2.3 vs 2%, 1.8 – 2.6; p = 0.198), number of reflux episodes (35, 20 – 48 vs 42, 30 – 55; p = 0.303), and longest reflux episode (21 minutes, 10 – 41 vs 40 minutes, 22 – 93; p = 0.106) were similar between patients with and without RACs. GERDQ was also similar between patients with and without RACs, 9 (7 – 10) vs 7 (6 – 10); p = 0.581.
Association of EGJ-distensibility with reflux parameters
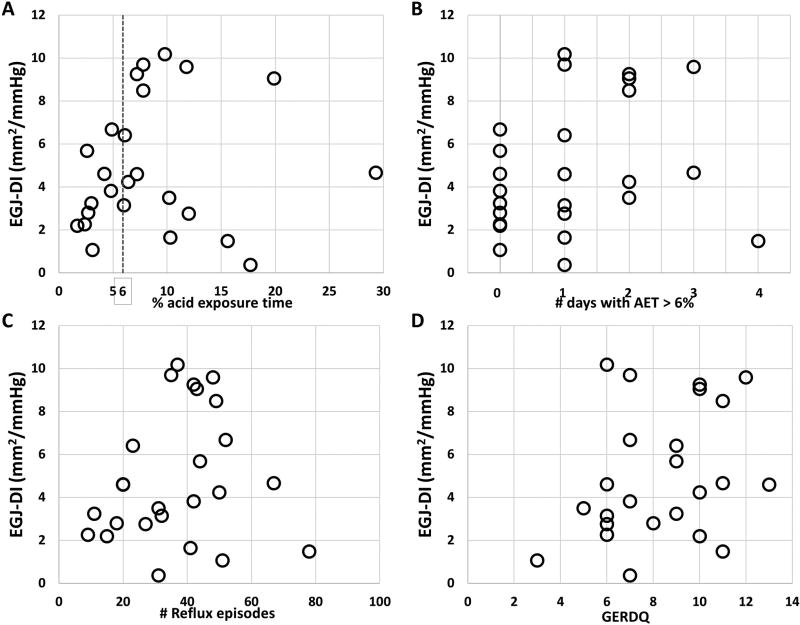
Among the total cohort, the EGJ-DI was 4.2 mm2/mmHg (2.5 – 7.6), with the median diameter at the 60-ml fill volume was 14.8 mm (11.8 – 18.9) and the median pressure was 40.9 mmHg (33.9 – 53). EGJ-DI and total AET were poorly correlated (rho = 0.169); Figure 3A. EGJ-DI did not differ between patients with abnormal AET, 4.6 mm2/mmHg (2.8 – 9.2), and normal AET, 3.2 (2.2 – 5.1); p = 0.207. EGJ-DI did not differ by number of days with abnormal AET, p = 0.179, Figure 3B.
Figure 3. Correlation of esophageal distensibility with reflux parameters (A–C) and symptom score (D).
Correlation (Spearman’s rho) of EGJ-DI with upright AET was 0.196, supine AET was 0.074, number of reflux episodes (Figure 3C) was −.240, and longest reflux episode was 0.237. The correlation of GERDQ with EGJ-DI was 0.286 (Figure 3D). None of these correlations were statistically significant.
Discussion
We aimed to assess the association of esophageal acid exposure with the esophageal response to distension among patients undergoing GERD evaluation with wireless esophageal pH monitoring and FLIP and demonstrated that although EGJ-distensibility was not consistently associated with esophageal acid exposure, impairment in distension-induced contractility carried an association with greater esophageal acid exposure.
The pathophysiology related to GERD is multifactorial and involves factors associated with the anti-reflux barrier as well as those related to esophageal acid clearance.1 A study of healthy controls recognized that during acid reflux episodes, primary peristalsis is largely responsible for acid clearance in the awake state while secondary peristalsis is more integral in the sleep or supine state.16 In another study, after analyzing 595 episodes of documented GERD, nearly 82% of motor events during this period were due to primary peristaltic activity while the remaining 18% were due to secondary peristalsis or ineffective motility.17 Further, triggering of secondary peristalsis was shown to occur less frequently among patients with erosive and non-erosive GERD than asymptomatic controls.18, 19
The volumetric filling of the FLIP and associated distension of the esophageal induces secondary peristalsis. Because the esophageal distension is maintained over the course of the FLIP study, the normal esophageal response to this sustained esophageal distension is the manifestation of RACs.9 In our initial evaluation of FLIP-associated motility, we observed RACs in 80% of healthy, asymptomatic controls.9 Although this cohort of controls was not evaluated with manometry, we also frequently observed RACs on FLIP among dysphagia patients with normal esophageal peristalsis.10 Unfortunately, manometry was not routinely obtained among the GERD cohort evaluated for the present study to better assess the relationships between primary and secondary peristaltic function with esophageal acid exposure. However, our results support that an impaired contractile response to esophageal distension is associated with greater esophageal acid exposure. Secondary peristalsis likely plays a larger role in esophageal clearance during sleep when swallowing is suppressed, thus it seems fitting that we observed a more exaggerate increase in acid exposure during the supine periods among patients without induction of RACs.
Previous studies utilizing a barostat demonstrated that increased EGJ-distensibility was associated with GERD, however feasibility of barostat use limits its application to clinical practice.2, 3 The FLIP provides a clinically-available method to objectively assess esophageal distensibility EGJ however conflicting results on the association between EGJ-distensibility and GERD were observed among studies utilizing FLIP. An initial study applying the FLIP to patients with typical GERD symptoms demonstrated increased EGJ-DI (mm2/mmHg) compared with healthy controls.4 Additionally, EGJ- DI was positively correlated with reflux symptom scores among patients with Barrett’s esophagus and hiatal hernia.5 However, a subsequent study assessing patients with FLIP and wireless esophageal pH monitoring found that patients with GERD symptoms actually had lower EGJ-DI than controls, and EGJ-DI did not differ between patients with normal (n = 9) or abnormal (n = 9) esophageal acid exposure.6 Another study that evaluated patients treated with trans-oral incisionless fundoplication (TIF) demonstrated that pre-operative EGJ-DI was not correlated with pre-operative AET, nor was EGJ-distensibility at 6-month post-TIF follow-up associated with normalization of AET.7 Similarly, we did not observe a significant correlation of EGJ-DI with AET, nor did we observe a difference in EGJ-DI associated with categorized AET.
However, the current and previous studies evaluating GERD patients with FLIP assume the EGJ-DI is synonymous with EGJ-distensibility. The EGJ-DI, i.e. the narrowest single CSA within the EGJ divided by distensive pressure, was demonstrated as an effective metric to identify esophageal outflow obstruction among patients with achalasia and dysphagia.10, 13, 20 Thus, while the EGJ-DI appears well suited for the evaluation of dysphagia and its simplicity makes it appealing to clinical application, it may not be the ideal measure to assess GERD physiology.21 Gastroesophageal reflux is primarily an episodic event that occurs when the gastro-esophageal pressure gradient overcomes the anti-reflux barrier, which can occur at relatively small EGJ opening diameters induced by small pressure increments. While this GERD-related EGJ opening was able to be assess using the pressure-controlled barostat method, the volume-controlled distension of the typical FLIP study protocol and use of the EGJ-DI may not reflect the appropriate paradigm to assess reflux susceptibility.2, 3 Thus modifications to the FLIP study protocol, such as utilizing small volume or pressure (possibly zeroed to gastric pressure to reflect a reflux-generating pressure)-directed distension, and analytic approach, such as incorporation of other measures of EGJ biomechanics (e.g. yield pressure or elastic modulus), are likely needed to optimize FLIP use for GERD assessment.
Limitations of our study also include the fairly heterogeneous clinical cohort resulting from inclusion of both patients with typical and atypical reflux symptoms. While this was intended to aid providing a greater number of patients with normal AET (i.e. somewhat of a ‘patient-control’ group), studying a more homogenous GERD population, could potentially yield more consistent results related to EGJ-distensibility in GERD. Additionally, although we further attempted to supplement the GERD evaluation by utilizing a validated GERD questionnaire, we found poor correlations between the symptom scores with both FLIP and esophageal pH parameters, which speaks to the further complexity of symptom-generation related to reflux.
Ultimately, GERD pathogenesis and symptom generation is complex and multifactorial. Our results support the previous literature that FLIP-assessed EGJ-distensibility (using the EGJ-DI) is inconsistently associated with esophageal acid exposure, though our novel FLIP-motility paradigm observed that the esophageal contractile response to distension was associated with esophageal acid exposure. Competence of the EGJ remains an important factor in GERD, thus further study to optimize its evaluation is needed. While the role of FLIP in the GERD evaluation remains an evolving question, the esophageal response to distension appears to be an important component of esophageal function, thus its assessment holds promise to aid characterizing patients evaluated for reflux.
Key points.
The esophageal response to distension includes esophagogastric junction competence and secondary peristalsis, which are relevant to reflux disease and can be evaluated using the functional lumen imaging probe (FLIP).
A response to volumetric distension comprising repetitive, antegrade contractions was associated with a reduced degree of esophageal acid exposure among patients evaluated for reflux.
Esophageal acid exposure is dependent on mechanisms associated with acid clearance.
Acknowledgments
We would like to thank Katherine Ritter and Sophia Falmagne for their assistance with data acquisition.
Funding: This work was supported by a Northwestern Digestive Health Foundation award (DAC) and R01 DK079902 (JEP) from the Public Health service.
John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking)
Abbreviations
- AET
acid exposure time
- CSA
cross-sectional area
- DI
distensibility index
- EGJ
esophagogastric junction
- FLIP
functional lumen imaging probe
- GERD
gastroesophageal reflux disease
- RACs
repetitive antegrade contractions
Footnotes
Disclosures:
Dustin A. Carlson, Priya Kathpalia, Jenna Craft, Michael Tye, Zhiyue Lin, Peter J. Kahrilas: none
Author contributions: DAC contributed to study concept and design, obtaining funding, data analysis, data interpretation, drafting of the manuscript, and approval of the final version. PK contributed to data analysis, drafting of the manuscript, and approval of the final version. JC, MT, and ZL contributed to data analysis and approval of the final version. PJK contributed revising the manuscript critically, and approval of the final version. JEP contributed to study concept and design, revising the manuscript critically, and approval of the final version.
References
- 1.Bredenoord AJ, Pandolfino JE, Smout AJ. Gastro-oesophageal reflux disease. Lancet. 2013;381(9881):1933–42. doi: 10.1016/S0140-6736(12)62171-0. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Shi G, Curry J, et al. Esophagogastric junction distensibility: a factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G1052–8. doi: 10.1152/ajpgi.00279.2001. [DOI] [PubMed] [Google Scholar]
- 3.Pandolfino JE, Shi G, Trueworthy B, et al. Esophagogastric junction opening during relaxation distinguishes nonhernia reflux patients, hernia patients, and normal subjects. Gastroenterology. 2003;125(4):1018–24. doi: 10.1016/s0016-5085(03)01210-1. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatek MA, Pandolfino JE, Hirano I, et al. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP) Gastrointest Endosc. 2010;72(2):272–8. doi: 10.1016/j.gie.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lottrup C, McMahon BP, Ejstrud P, et al. Esophagogastric junction distensibility in hiatus hernia. Dis Esophagus. 2016;29(5):463–71. doi: 10.1111/dote.12344. [DOI] [PubMed] [Google Scholar]
- 6.Tucker E, Sweis R, Anggiansah A, et al. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil. 2013;25(11):904–10. doi: 10.1111/nmo.12218. [DOI] [PubMed] [Google Scholar]
- 7.Smeets FG, Keszthelyi D, Bouvy ND, et al. Does Measurement of Esophagogastric Junction Distensibility by EndoFLIP Predict Therapy- responsiveness to Endoluminal Fundoplication in Patients With Gastroesophageal Reflux Disease? J Neurogastroenterol Motil. 2015;21(2):255–64. doi: 10.5056/jnm14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15(3):325–34. doi: 10.1016/j.cgh.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson DA, Lin Z, Rogers MC, et al. Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterol Motil. 2015;27(7):981–9. doi: 10.1111/nmo.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol. 2016;111(12):1726–35. doi: 10.1038/ajg.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonasson C, Wernersson B, Hoff DA, et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37(5):564–72. doi: 10.1111/apt.12204. [DOI] [PubMed] [Google Scholar]
- 12.Lin Z, Nicodeme F, Boris L, et al. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP) Neurogastroenterol Motil. 2013;25(11):e765–71. doi: 10.1111/nmo.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil. 2013;25(6):496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology. 2015;149(7):1742–51. doi: 10.1053/j.gastro.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13067. [DOI] [PubMed] [Google Scholar]
- 16.Barham CP, Gotley DC, Miller R, et al. Pressure events surrounding oesophageal acid reflux episodes and acid clearance in ambulant healthy volunteers. Gut. 1993;34(4):444–9. doi: 10.1136/gut.34.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anggiansah A, Taylor G, Bright N, et al. Primary peristalsis is the major acid clearance mechanism in reflux patients. Gut. 1994;35(11):1536–42. doi: 10.1136/gut.35.11.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoeman MN, Holloway RH. Integrity and characteristics of secondary oesophageal peristalsis in patients with gastro-oesophageal reflux disease. Gut. 1995;36(4):499–504. doi: 10.1136/gut.36.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwakiri K, Hayashi Y, Kotoyori M, et al. Defective triggering of secondary peristalsis in patients with non-erosive reflux disease. J Gastroenterol Hepatol. 2007;22(12):2208–11. doi: 10.1111/j.1440-1746.2006.04817.x. [DOI] [PubMed] [Google Scholar]
- 20.Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143(2):328–35. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 21.Gregersen H. Analysis of Functional Luminal Imaging Probe Data. Clin Gastroenterol Hepatol. 2017;15(8):1313–4. doi: 10.1016/j.cgh.2017.04.010. [DOI] [PubMed] [Google Scholar]