Abstract
Nicotine improves attention and processing speed in individuals with schizophrenia. Few studies have investigated the effects of nicotine on cognitive control. Prior functional magnetic resonance imaging (fMRI) research demonstrates blunted activation of dorsal anterior cingulate cortex (dACC) and rostral anterior cingulate cortex (rACC) in response to error and decreased post-error slowing in schizophrenia. Participants with schizophrenia (n=13) and healthy controls (n=12) participated in a randomized, placebo-controlled, crossover study of the effects of transdermal nicotine on cognitive control. For each drug condition, participants underwent fMRI while performing the Stop Signal Task where participants attempt to inhibit pre-potent responses to “Go (motor activation)” signals when an occasional “Stop (motor inhibition)” signal appears. Error processing was evaluated by comparing “Stop Error” trials (failed response inhibition) to “Go” trials. Resting state fMRI data were collected prior to the task. Participants with schizophrenia had increased nicotine-induced activation of right caudate in response to errors compared to controls (DRUG × GROUP effect: pcorrected<0.05). Both groups had significant nicotine-induced activation of dACC and rACC in response to errors. Using right caudate activation to errors as a seed for resting state functional connectivity analysis, relative to controls, participants with schizophrenia had significantly decreased connectivity between the right caudate and dACC/bilateral dorsolateral prefrontal cortices. In sum, we replicated prior findings of decreased post-error slowing in schizophrenia and found that nicotine was associated with more adaptive (i.e., increased) post-error reaction time (RT). This proof-of-concept pilot study suggests a role for nicotinic agents in targeting cognitive control deficits in schizophrenia.
Keywords: functional magnetic resonance imaging, schizophrenia, caudate, anterior cingulate, error
INTRODUCTION
The prevalence of smoking in individuals with schizophrenia is at least three-fold higher than smoking rates in the general population, contributing to higher rates of smoking-related morbidity and mortality in schizophrenia (Grant et al. 2004; Kelly et al. 2011). Higher rates of tobacco dependence in those with schizophrenia may be due to shared neurobiological abnormalities in schizophrenia and nicotine dependence (Lyons et al. 2002; Moran et al. 2013a). In addition, reduced negative affect, normalization of sensory gating, and improvements in cognitive performance with nicotine and nicotinic agonists have been described in individuals with schizophrenia (Smith et al. 2002; Leonard et al. 2007; Barr et al. 2008; Jubelt et al. 2008; Mann-Wrobel et al. 2011; Dutra et al. 2012). Cognitive deficits are a core feature of schizophrenia and predict poor functional outcome (Elvevåg and Goldberg 2000; Green et al. 2004). Accordingly, understanding the effects of nicotine on various domains of cognition in schizophrenia and its underlying neural circuitry may aid drug development of nicotinic agents targeted to improve cognitive function without the deleterious health effects of smoking.
Studies evaluating the effect of nicotine on cognitive function in schizophrenia have demonstrated faster processing speed (reaction time: RT) and increased accuracy in tasks of attention compared to placebo (Harris et al. 2004; Barr et al. 2008; AhnAllen et al. 2008). Intrusive saccades decrease after smoking and nicotine normalizes smooth pursuit abnormalities in individuals with schizophrenia (Olincy et al. 1998; Tregellas et al. 2005). Collectively, these studies show a range of beneficial effects of nicotine and warrant further study in other domains of cognition known to be impaired in schizophrenia.
In the current proof-of-concept study, we evaluated the effects of nicotine on the behavioral and neural correlates of cognitive control using the Stop Signal Task (SST). In this task, participants respond to a “Go (motor activation)” signal and withhold a pre-potent motor response when a “Stop (motor inhibition)” signal appears. Studies employing the SST have been used to probe distinct aspects of cognitive control including pre-potent response inhibition, error detection and dynamic adjustments of behavior in response to errors (Ray Li 2006; Aron and Poldrack 2006; Li et al. 2008a, b). The SST is based on a “horse race” model of response inhibition that assumes competition of excitatory (represented by speeded response to a Go signal) and inhibitory neural processes (represented by inhibition of motor response when a Stop signal is presented). If the speed of the excitatory process exceeds that of the inhibitory process, a Go motor response will be made. In contrast, if the speed of the inhibitory process is faster in response to a Stop signal, a response will be successfully inhibited. The task adapts to the participant’s performance by varying the delay between the onset of the Go stimulus and the Stop signal. As this delay becomes longer, it becomes more difficult to inhibit a pre-potent motor response. The Stop Signal Reaction Time (SSRT) is calculated based on individual participant’s performance (see Methods) and is used as an index of the efficiency of individual response inhibition, where shorter SSRT denotes better response inhibition. Functional magnetic resonance imaging (fMRI) studies using the SST in healthy individuals have found a consistent set of regions that subserve successful response inhibition, including right inferior frontal gyrus, anterior insula, and pre-supplementary motor area (Cai et al. 2014; Cieslik et al. 2015). Prior studies using the SST in schizophrenia have found impaired response inhibition (longer SSRT) associated with reduced inferior frontal gyrus activation (Hughes et al. 2012).
An important line of research using the SST is the investigation of the neural underpinnings of error detection and dynamic adjustments of performance in response to errors, evaluated by focusing on trials when participants incorrectly respond to a Stop signal (Li et al. 2008a, b). Consistent with research using other tasks (Carter et al. 1998), studies using the SST have identified the dorsal anterior cingulate cortex (dACC) as a key region in error processing. Moreover, among healthy controls, fMRI studies have shown activation of the dACC and the rostral ACC (rACC) in response to errors (Carter et al. 1998; Kerns et al. 2004; Polli et al. 2005). The dACC is engaged in cognitive processes through its strong connections with the dorsolateral prefrontal cortex (DLPFC) and is thought to mediate subsequent shifts in behavior in response to errors through its connections with premotor areas (Devinsky et al. 1995; Bush et al. 2000; Taylor et al. 2007). Activation of the dACC in response to errors correlates with post-error slowing and predicts subsequent DLPFC activation (Kerns et al. 2004). Post-error slowing, a phenomenon in which individuals slow down following an error, is thought to be associated with efforts to respond more cautiously to avoid subsequent mistakes, and is thus assumed to be an adaptive response (King et al. 2010; Dutilh et al. 2012). In contrast to the dACC and its associated “cognitive control” network, the rACC is part of an “affective” network that has strong connections to limbic areas such as the amygdala, anterior insula and orbitofrontal cortices (Devinsky et al. 1995; Bush et al. 2000; Cole and Schneider 2007). Activation of the rACC is thought to signal the affective or motivational response to errors (Polli et al. 2005). These fMRI findings have been corroborated and extended by electrophysiological studies of the error-related negativity, a negative potential that occurs 50–100 msec after error commission. In particular, source localization studies converge on the dACC and rACC as generators of the ERN (Van Veen and Carter 2002; Roger et al. 2010). The magnitude of the ERN is directly related to fMRI signal in the dACC and rACC (Mathalon et al. 2003).
There is ample evidence from fMRI studies that individuals with schizophrenia have impaired error processing with less dACC and rACC activation in response to errors and lower amplitude of the error-related negativity relative to controls (Carter et al. 2001; Mathalon et al. 2002; Laurens et al. 2003; Kerns et al. 2005; Polli et al. 2008; Becerril et al. 2011). In addition to abnormalities in ACC activation, abnormal connectivity in areas of the dACC-cognitive control network has been described. Voegler et al. found decreased dACC/mid-cingulate activation in response to errors and decreased connectivity between dACC/mid-cingulate and DLPFC in individuals with schizophrenia (Voegler et al. 2016). Consistent with decreased error-related dACC activation, multiple studies have described impaired post-error slowing in schizophrenia (Carter et al. 2001; Kerns et al. 2005). However, the literature on post-error slowing is mixed, as several studies have shown intact post-error slowing in schizophrenia (Mathalon et al. 2002; Polli et al. 2006). Collectively, prior findings in schizophrenia suggest dysfunctional activation of error-related ACC regions, decreased connectivity between dACC and associated areas of the cognitive control network, and aberrant adjustment of performance in response to errors.
Nicotine-induced activation of the dACC and rACC using fMRI has been observed in pre-clinical work and during tasks of attention in both healthy individuals and individuals with schizophrenia (Jacobsen et al. 2004; Hong et al. 2011; Bruijnzeel et al. 2014). To our knowledge, there are no studies examining the effects of acute nicotine on error processing and performance adjustments following errors in healthy individuals or those with schizophrenia. We hypothesized that nicotine-related activation of dACC, rACC and associated regions may be associated with enhanced error processing in schizophrenia. To test this hypothesis, we performed a randomized, double-blind, placebo-controlled trial in which participants received transdermal nicotine or placebo in two sessions approximately a week apart while performing a version of the SST adapted for fMRI. In addition, participants underwent resting state fMRI to evaluate functional connectivity within error-related networks.
We expected to replicate previous findings of decreased activation of dACC and rACC in response to errors and/or decreased connectivity between error-related network regions in schizophrenia. We further hypothesized that nicotine would improve abnormalities in error processing in schizophrenia by increasing activation of dACC and rACC in response to errors and/or increasing functional connectivity between dACC/rACC and other regions of cognitive control/affective networks. In association with these neural alterations, we also hypothesized that nicotine would lead to more adaptive post-error behavioral adjustments (i.e., increase post-error slowing). Although the primary focus of this study was on the effect of nicotine on error processing, we also evaluated whether nicotine improved response inhibition in schizophrenia (i.e., decrease SSRT) in conjunction with activation of regions associated with successful response inhibition (e.g., anterior insula, right inferior frontal gyrus).
METHODS
Participants and Study Design
Study Participants
Fifteen participants with schizophrenia and fourteen control participants (18–55 years old, all right-handed) were recruited from the community. Written, informed consent for a protocol approved by the Massachusetts General Hospital and McLean Hospital Institutional Review Boards was obtained from each participant prior to study procedures. Diagnosis of schizophrenia in patients and the absence of any axis I condition for controls were confirmed by the Structured Clinical Interview for DSM-IV (First et al. 2002). Control participants were excluded if they reported a history of a first-degree relative with a psychotic disorder. All participants were required to have negative urine toxicology screens at all study visits. Individuals with a history of neurological disorders or head injury with neurological sequelae were excluded. Structural MRI for all participants were reviewed by a radiologist and no structural abnormalities were identified. Expired carbon monoxide measurement (CO) was used to confirmed smoking status (CO<5 ppm for non-smokers; CO>10 ppm for smokers).
Four participants (2 controls and 2 individuals with schizophrenia) did not complete the study due to nausea/vomiting after nicotine administration and were excluded from analyses. The final sample included 13 adults with schizophrenia and 12 controls (flow chart in Figure S1). Twelve of the 13 participants with schizophrenia were taking antipsychotic medications (n=2 clozapine, n=10 other atypical antipsychotic). Chlorpromazine equivalent dose (CED: mg/day) and D2 receptor occupancy for antipsychotic medications were calculated (Woods 2003; Lako et al. 2013). Please see Table 1 for details on demographics and clinical characteristics. Although group difference in smoking status was not significant, there were more smokers in the schizophrenia group than the control group (46.2% vs. 16.7%; p=0.13). Nicotine dependence severity was measured by the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al. 1991). To evaluate putative effects of nicotine withdrawal on fMRI results, smokers completed the Minnesota Nicotine Withdrawal Scale (MNWS; 0 – 24 score range) immediately after smoking a cigarette (before patch administration) and after the scan (Toll et al. 2007).
Table 1.
Demographics and Clinical Characteristics
| Control (n=12) |
Schizophrenia (n=13) |
t | p | |
|---|---|---|---|---|
| Age (mean ± SD) | 38.8 ± 10.7 | 37.3 ± 8.1 | 0.4 | 0.69 |
| Gender (Female/Male) | 6/6 | 4/9 | χ2=1.0 | 0.33 |
| Ethnicity (White/Non-white) | 7/5 | 8/5 | χ2=0.03 | 0.87 |
| Education (yrs) | 15.1 ± 2.2 | 14.0 ± 2.1 | 1.3 | 0.21 |
| IQ | 104.4 ± 11.0 | 102.1 ± 8.2 | 0.6 | 0.55 |
| Nicotine dose administered (mg) | 14.0 ± 6.0 | 16.2 ± 6.0 | −0.9 | 0.38 |
|
| ||||
| Symptom scales and antipsychotic medication | ||||
|
| ||||
| SANS total score | — | 32.0 ± 15.6 | — | — |
|
| ||||
| BPRS total score | — | 37.7 ± 10.3 | — | —— |
|
| ||||
| Chlorpromazine Equivalents (mg/day) | — | 389.7 ± 308.0 | — | — |
|
| ||||
| D2 receptor occupancy (%) | — | 68.2 ± 26.0 | — | — |
|
| ||||
| Smoking characteristics | ||||
|
| ||||
| Current smokers: n (%) | 2 (16.7%) | 6 (46.2%) | — | 0.13a |
|
| ||||
| FTND | 5.0 ± 1.4 | 5.0 ± 1.6 | <0.001 | 1.00 |
|
| ||||
| Cigarettes per day | 14.0 ± 5.7 | 11.2 ± 4.8 | 0.7 | 0.51 |
Fisher’s exact test
SANS: Scale for the Assessment of Negative Symptoms; BPRS: Brief Psychiatric Rating Scale; Fagerstrom Test for Nicotine Dependence (FTND)
Study Design
A randomized, double-blind, placebo-controlled, cross-over design was utilized in which each participant was administered either transdermal nicotine or identical placebo patch on two separate study sessions performed at least 7 days apart. Order of drug was counterbalanced within each group. Participants received different doses of nicotine depending on the experience of adverse events and smoking status (range 7 – 28 mg); please see Supplement and Table S1 for details on nicotine patch administration and dosing. Three hours after patch application, participants underwent fMRI while performing resting state followed by the SST. We chose three hours to coincide with maximal nicotine concentrations (Gupta et al. 1995) and this time frame also avoids peak withdrawal symptoms, which typically occur 6 – 12 hours after smoking cessation (Hughes et al. 1994).
MRI Data Acquisition
Participants were scanned on a 3T Siemens Trio scanner with a 12-channel head coil at the McLean Imaging Center. High resolution (1×1×1 mm3) T1-weighted MPRAGE images were acquired. Resting state data were obtained prior to SST to avoid task-related effects. During resting state acquisition, participants were instructed to rest with eyes open for 6.2 minutes. Functional MR images were acquired with interleaved acquisition tilted −30° from the AC-PC line using a gradient-echo echoplanar imaging (EPI) sequence. EPI parameters for resting state were: 124 volumes; echo time/repetition time (TE/TR)=30/3000 msec; flip angle 85°; field of view 504×504 mm2; voxel dimensions 3.0 mm isotropic; 47 axial slices. EPI parameters for SST were: 161 volumes, TE/TR=30/2000 msec; flip angle 90°; FOV 384×384 mm2; voxel dimensions 3.125 × 3.125 × 3.0 mm; 32 axial slices.
Stop Signal Task
We utilized a variant of the SST (Bonnelle et al. 2012), a two-choice reaction time task in which participants are instructed to press a button indicating the direction of a Go signal (green arrow pointing to right or left). During an initial screening visit, participants performed a continuous reaction time (CRT) version outside of the scanner where they responded as fast as possible to Go stimuli without any stop signals to determine each individual’s initial stop signal delay (SSD), the delay between the onset of Go signal and Stop signal. The initial SSD was calculated as the mean Go RT for the CRT – 200 msec.
During the fMRI sessions, while performing the SST, a Stop signal (red dot above arrow) was presented on occasional trials, which required participants to inhibit their response to the Go signal. A staircase adaptation procedure was employed in which the SSD was dynamically adjusted every two Stop trials. If the cumulative accuracy on Stop trials was > 50%, SSD was increased by 50 msec. In contrast, if cumulative accuracy on Stop trials was < 50%, SSD was decreased by 50 msec. Using this procedure, performance for each participant was adjusted such that they correctly inhibited the response on ~ 50% of the Stop trials. In addition, a critical SSD was computed as the mean RT where the probability of responding equals the probability of inhibiting (i.e., time delay between Go and Stop signal where participants successfully inhibited their response to a Stop signal). The SSRT for each participant was estimated by subtracting median Go RT from the critical SSD. The SSRT is thought to reflect the latency between presentation of the stop signal and onset of participant’s inhibitory response process.
A total of 184 trials were presented over one run that lasted approximately 5.5 minutes. In addition to Go trials and Stop trials, which composed approximately 70% and 20% of trials, respectively, there were 16 rest trials. In order to mitigate strategic slowing down, during which participants respond more slowly to Go trials to avoid missing a Stop signal, negative feedback (“Speed up!”) was presented in place of a trial if the participant’s RT exceeded the 95th percentile of their current RT distribution. Each Go signal was presented for 1250 msec followed by a fixation cross presented for 350 msec. The Stop signal was presented according to the adaptive procedure described above. Stimuli were presented in a randomized fashion with an equal number of left and right responses for both Go and Stop trials.
When participants make errors on tasks requiring execution or inhibition of motor responses, post-error slowing may occur, in which participants slow down in the subsequent Go trial after an error. We used correct Go trials that followed correct Stop responses (post-stop correct: pSC) and correct Go trials that followed Stop errors (post-stop error: pSE) to calculate post-error RT (King et al. 2010):
For analysis of SSRT, outliers were removed according to commonly employed lenient criteria for the SST (Congdon et al. 2012): 1) successful inhibition on Stop trials < 25% or > 75%; 2) correct response on Go trials < 60%; and 3) SSRT estimates that are negative or < 50 msec. Negative SSRT values are thought to reflect strategic slowing of responses to Go trials. These values are routinely excluded in SSRT estimates as they violate the horse-race model (Congdon et al. 2012). Generalized strategic slowing to Go trials may also decrease likelihood of dynamic increases in RT after errors. Therefore, for post-error RT, the same criteria were used to exclude outliers except we restricted successful inhibition criterion (#1) to Stop trials used for post-error RT estimation (i.e., Stop trials preceding a correct Go trial) in order to ensure we had sufficient number of trials of each type. This resulted in removal of 5 participants: 2 controls (both non-smokers) and 3 individuals with schizophrenia (1 non-smoker and 2 smokers) for post-error RT and SSRT calculation (Figure S1).
Statistical Analyses
SST – Behavioral Data
The pre-specified primary behavioral variable was post-error RT. Other secondary variables of interest included SSRT, median correct Go RT, mean correct Go RT, standard deviation for mean correct Go RT, and the number of negative feedbacks. For each variable, we used a repeated measures mixed model with DRUG (Nicotine vs. Placebo), GROUP (Control vs. Schizophrenia), and SESSION (first vs. second MRI session) as factors. Because of the difference in smoking rates between the two groups, SMOKING STATUS was included in all models. DRUG × GROUP was included in initial models and removed in the absence of a significant interaction. Since prior research demonstrates faster RT with nicotine, a global increase in RT may attenuate any increase in post-error RT associated with nicotine. We therefore included mean correct Go RT as a covariate in models examining post-error RT.
SST – fMRI Data: Whole Brain Voxelwise Analysis
Analyses for the SST were conducted using FSL version 5.0.6. To allow for signal stabilization, the first 4 volumes were removed. Pre-processing steps included motion correction using the middle image as a reference (MCFLIRT), skull-stripping and brain extraction, slice time correction, spatial smoothing with a Gaussian kernel full-width half maximum 6 mm and high-pass temporal filtering. An in-house program was used to detect artifacts due to motion and intensity spiking (spike defined as ≥ 100 voxels exhibiting ≥ 2% difference of time point from mean intensity). Functional MRI data were co-registered to corresponding anatomical images, which were spatially normalized to the MNI template.
Individual participant’s data were analyzed using the general linear model with regressors for Go Correct, Stop Correct, Stop Error, negative feedback and regressors for six motion parameters and artefactual time points detected by in-house software. Stimulus waveforms were convolved with gamma variate hemodynamic response functions (normalization of probability density function of single gamma function). The contrasts of interest were for errors (Stop Error – Go contrast) and for successful response inhibition (Stop Correct – Go Correct, Stop Correct – Stop Error). Each contrast has advantages and drawbacks. The Stop Error – Go contrast isolates error-related activations due to failure to inhibit pre-potent response without confound of response versus no response. The Stop Correct – Go Correct contrast is considered more sensitive to detecting regions associated with successful response inhibition; however, Stop Correct and Go trials differ on visual stimulus and motor response. The Stop Correct – Stop Error contrast is considered a conservative and specific strategy to isolate regions required to successfully inhibit responses (Boehler et al. 2010). Stop Correct and Stop Error trials are matched on task requirement to inhibit response and visual stimulus, but differ on execution of a motor response. Whole-brain group analyses for each contrast were performed with FMRIB’s Local Analysis of Mixed Effects (FLAME) with DRUG as a within-subject factor, GROUP as a between-subjects factor, and DRUG × GROUP interaction. Control for multiple comparisons was performed using a z-statistic of 3.1 (p=0.001) to define contiguous clusters (minimum cluster size of 116 voxels) for pcorrected=0.05 threshold.
For any contrast where there were no significant DRUG, GROUP or DRUG × GROUP effects, we used one-sample t-tests to present significant fMRI activations (pcorrected<0.05) for each group separately using a design matrix that modeled between-session variance. For any cluster identified with a significant DRUG effect, to evaluate whether the DRUG effect was significant within each group, we performed repeated measures mixed models using mean contrast parameter estimate averaged over each region within each group separately.
SST – fMRI Data: ROI Analysis
In addition to whole brain analysis, ROI analyses were planned a priori for the Stop Error – Go contrast in dACC and rACC due to their critical role in error processing (see Introduction). Since rACC activation was observed with whole-brain analysis, we did not proceed with ROI analysis. For the dACC, we created a 10-mm sphere around MNI coordinates of X=4, Y=28, Z=26 obtained from a previous study in an independent cohort of individuals with schizophrenia and controls matched on age, gender and smoking status (Moran et al. 2013b). Repeated measure mixed models were performed with mean contrast parameter from Stop Error – Go contrast (averaged over ROI) as dependent variable, and DRUG, GROUP, and DRUG × GROUP as independent variables.
Resting State Data
Analyses for resting state data were conducted using AFNI version 17.0.03 (1/26/2017) and MATLAB. After removal of the first four volumes, pre-processing steps included motion correction, brain extraction, and slice time correction. Resting state scans were co-registered to anatomical images, which were spatially normalized to the MNI template. In order to remove confounds that contribute to non-neural noise, five principal components of a combined white matter and cerebrospinal fluid mask (eroded by one voxel to avoid contamination by gray matter) and six motion parameters were detrended from resting state data by regression. Compared to regressing out global mean signal, this noise reduction method has the advantage of avoiding artefactual anticorrelations (Behzadi et al. 2007; Chai et al. 2012). In addition, global signal regression may distort between-group comparisons of inter-regional correlation (Saad et al. 2012.) Because spurious differences in functional connectivity could be related to differences in head motion between groups, additional control for motion was conducted (Jones et al. 2010; Van Dijk et al. 2012). Specifically, we calculated time points with significant change in motion from preceding TR as defined by framewise displacement of the sum of absolute values of temporal derivatives of 6 motion parameters of ≥ 0.5 mm and replaced their values by interpolating neighboring time points (Power et al. 2012). We found no significant difference in head motion between the two groups as defined by the percent of time points with significant change in motion (nicotine: t(23)=−0.1, p=0.88; placebo: t(23)=−0.6, p=0.55). Next, a bandpass filter (0.009 to 0.08 Hz) was applied and then data were spatially smoothed (full-width half maximum 6 mm). The primary analyses of interest were to examine effects of DRUG and GROUP on network connectivity relevant to error monitoring. We therefore defined seeds as areas of activation from the Stop Error – Go contrast with significant DRUG, GROUP or DRUG × GROUP effects. For each participant, cross-correlation coefficients were calculated between the average time course of each seed region and the time course of each voxel in the brain, and coefficients were Fisher z-transformed as a measure of intrinsic functional connectivity. A mixed effects model was used to detect areas with significant within-subject DRUG and between-subjects GROUP effects or DRUG × GROUP interaction. Data were corrected for multiple comparisons (pcorrected < 0.05; see Supplement for details).
fMRI Correlations
We calculated Pearson correlation coefficients between activation in significant brain areas with significant DRUG × GROUP or DRUG differences for the Stop Error – Go contrast (using mean contrast parameter averaged across cluster) and post-error RT. In order to determine if network connectivity dynamics influenced nicotine-induced activations to errors, we performed additional exploratory correlational analyses. Specifically, Pearson correlation coefficients were calculated between intrinsic functional connectivity measures using areas identified as having significant DRUG or GROUP differences in connectivity and nicotine-induced activations (i.e., clusters identified from whole-brain analysis of Stop Error – Go contrast). Correlations were performed for each drug condition separately. Comparisons of correlations for nicotine versus placebo were performed using methods that take into account non-independence of correlations (Steiger 1980). Comparison of correlations for control vs. schizophrenia was performed using Fischer’s r to z-transformation. All tests of hypotheses were two-sided with significance level of p<0.05 (uncorrected for multiple comparisons).
RESULTS
SST – Behavioral Data
For post-error RT, between-group comparisons using a repeated measures mixed effects model revealed a significant GROUP effect (β=−43.0, p=0.046) in which control participants had significantly longer post-error RT than the schizophrenia group. In addition, a significant DRUG effect emerged (β=40.0, p=0.03) such that nicotine was associated with increased post-error RT relative to the placebo condition (Figure 1, Table 2). There was no significant DRUG × GROUP interaction.
Figure 1.
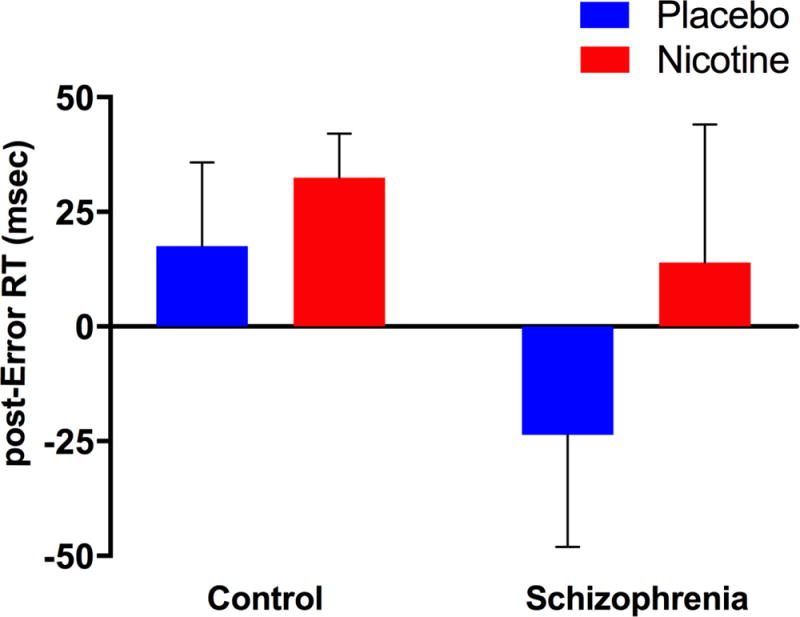
Post-error reaction time (RT): Participants with schizophrenia had significantly decreased post-error RT compared to control participants (GROUP effect: p=0.046). Nicotine was associated with increased post-error RT (DRUG effect: p=0.03).
Table 2.
SST Behavioral Data
| Controla | Schizophreniaa | |||
|---|---|---|---|---|
| Nicotine | Placebo | Nicotine | Placebo | |
| Post-error RT* (msec) (mean ± SD) | 32 ± 30 | 18 ± 58 | 14 ± 70 | −24 ± 77 |
| SSRT | 243 ± 39 | 236 ± 51 | 222 ± 36 | 208 ± 59 |
| Median Correct Go RT | 520 ± 196 | 528 ± 167 | 558 ± 146 | 578 ± 173 |
| Mean Correct Go RT | 522 ± 185 | 541 ± 162 | 581 ± 140 | 593 ± 156 |
| SD Correct Go RT* | 79 ± 33 | 99 ± 43 | 134 ± 38 | 143 ± 42 |
| Smiddle Accuracy (%) | 56 ± 13 | 53 ± 7 | 53 ± 7 | 51 ± 8 |
| Negative Feedback (n) | 11 ± 8 | 14 ± 7 | 16 ± 9 | 16 ± 10 |
Significant GROUP and DRUG effects p<0.05
RT=reaction time; SD=standard deviation for individual subject RT distribution; SSRT=smiddle signal reaction time
Data for post-error RT and SSRT were derived from 10 control participants (2 smokers, 8 nonsmokers) and 10 participants with schizophrenia (4 smokers, 6 non-smokers). See Methods for criteria used to exclude outliers from post-error RT/SSRT analyses. All other variables were derived from the full sample of participants included in the imaging analyses (12 controls and 13 participants with schizophrenia).
For Go RT variables, there were no significant effects of DRUG for median Go RT or mean Go RT. For variability of Go RT (standard deviation), there were significant effects of DRUG (β=−15.1, p=0.002) and GROUP (β=55.7, p<0.001), where nicotine was associated with decreased variability and patients had greater variability than controls. For median, mean and variability of Go RT, there were no significant DRUG × GROUP interactions. There were no significant effects of DRUG, GROUP or DRUG × GROUP interactions for SSRT or the number of negative feedbacks.
In summary, nicotine was associated with decreased variability of RT for Go trials. Although post-error RT was reduced in individuals with schizophrenia in comparison to controls, nicotine was associated with improved adaptive responses to errors as indicated by increased post-error RT. We did not detect a significant effect of nicotine on response inhibition efficiency, as measured by SSRT.
SST – fMRI Data
Stop Correct – Go
There were no significant DRUG or GROUP effects for the Stop Correct – Go contrast. Participants with schizophrenia and controls recruited a similar network of regions associated with successful response inhibition consistent with areas reported in the literature (Cai et al. 2014; Cieslik et al. 2015) that included inferior frontal gyrus and the anterior insula (Figure S2, Table S2).
Stop Correct – Stop Error
There were no significant DRUG, GROUP, or DRUG × GROUP effects. Using one-sample t-tests within each group, we found a significant increase in bilateral striatum (caudate/putamen) and precentral gyrus for controls, but no significant activations for schizophrenia (Figure S3, Table S3).
Stop Error – Go
Whole-brain analysis revealed a significant DRUG × GROUP interaction in the right caudate (anterior to the anterior commissure); relative to controls, participants with schizophrenia had greater nicotine-induced activation of the right caudate in response to errors (Figure 2, Table 3). Within-group analyses revealed a significant DRUG effect for the schizophrenia group (β=62.7, p<0.001) but not controls (β =17.0, p=0.23). Furthermore, 12 of the 13 participants with schizophrenia had greater activation of the right caudate for the nicotine than placebo condition (binomial P=0.003).
Figure 2.
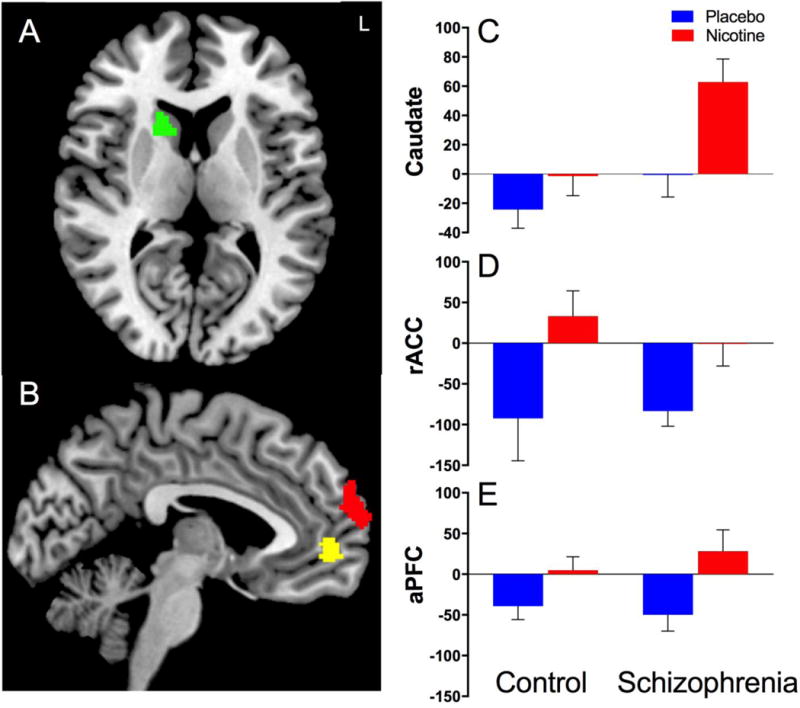
Stop Error – Go contrast from Stop Signal Task using whole-brain voxelwise fMRI analysis corrected for multiple comparisons (pcorrected<0.05). A. Significant DRUG × GROUP interaction where individuals with schizophrenia had increased nicotine-induced activation of the right caudate in response to errors compared to placebo. In contrast, there was no significant DRUG effect for control participants; individuals with schizophrenia had significantly greater right caudate activation than controls under the nicotine condition (C). B. Significant DRUG effects in the rostral anterior cingulate (rACC: yellow, D) and the dorsomedial anterior prefrontal cortex (aPFC: red, E) with increased nicotine-induced activation in both regions. Data in C-E represent mean contrast parameter extracted from clusters identified using whole-brain analysis for the Stop Error – Go contrast (averaged over each region).
Table 3.
Stop Error – Go Contrast: Whole-Brain Analysis
| Brain region | Side | Volume (# voxels) | Z value | Peak activity: MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| DRUG × GROUP | ||||||
| Caudate | R | 125 | 5.03 | 16 | 14 | 10 |
| DRUG (Nicotine > Placebo) | ||||||
| Dorsomedial superior frontal gyrus (anterior prefrontal cortex: aPFC) | Bilateral (L>R) | 631 | 4.86 | −8 | 64 | 26 |
| Rostral anterior cingulate cortex/Medial prefrontal cortex | Bilateral (L>R) | 142 | 4.92 | −6 | 52 | 0 |
Using whole-brain analysis, we also found significant DRUG effects in which nicotine was associated with increased activation of rACC/medial prefrontal cortex (MPFC) and bilateral anterior prefrontal cortex (aPFC) in the dorsomedial aspect of the superior frontal gyrus (BA10) in response to errors. Within-group analyses revealed significant DRUG effects for both the schizophrenia and control groups for rACC/MPFC and aPFC regions. Using an ROI for the dACC, we found a significant DRUG effect (β=35.8, p=0.02) with increased nicotine-induced dACC activation across all participants (Figure 3). There was no significant GROUP effect or DRUG × GROUP interaction.
Figure 3.
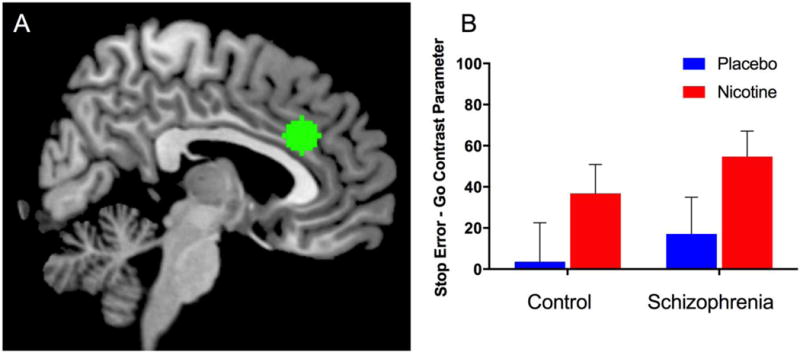
A. Dorsal anterior cingulate (dACC) region of interest (ROI). B. Significant DRUG effect for the Stop Error – Go contrast associated with increased nicotine-induced activation of dACC (p=0.02). There was no significant GROUP effect or DRUG × GROUP interactions. Data shown represent mean contrast parameter from Stop Error – Go contrast averaged over each ROI.
Resting State fMRI Data
In order to investigate error-related network dynamics associated with nicotine use, we used the three clusters from the Stop Error – Go contrast associated with greater activation in response to nicotine as seed regions (right caudate, rACC and aPFC). Using the right caudate as seed region, voxelwise whole-brain analysis revealed clusters with significant GROUP differences in connectivity between the right caudate and dACC, and right caudate and bilateral DLPFC, key nodes of the cognitive control network (Cole and Schneider 2007), in which patients with schizophrenia had reduced connectivity (Figure 4; Table 4). Rostral ACC/MPFC and aPFC clusters identified overlapping regions in the occipital cortex (right cuneus) with significantly decreased connectivity in schizophrenia compared to controls. There were no significant DRUG effects or DRUG × GROUP interactions for connectivity measures.
Figure 4.
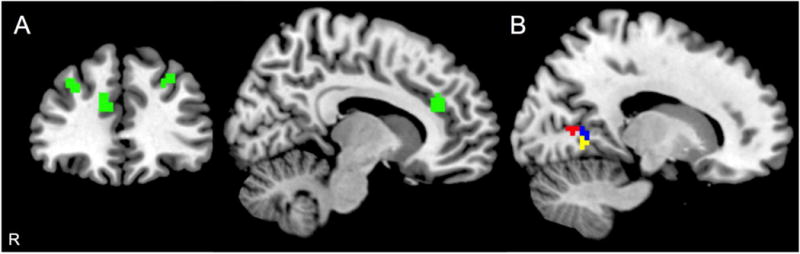
Resting state functional connectivity analysis. A. Right caudate activation from the Stop Error - Go contrast associated with a significant DRUG × GROUP effect (increased nicotine-induced activation in schizophrenia) was used as a seed region: decreased connectivity between the right caudate seed and dorsal anterior cingulate (dACC) and bilateral dorsolateral prefrontal cortices was found in participants with schizophrenia (green). B. Rostral anterior cingulate cortex (rACC) and anterior prefrontal cortex (aPFC) activations from the Stop Error – Go contrast with significant DRUG effect (increased nicotine-induced activation) were used as seed regions (rACC: yellow; aPFC: red): decreased connectivity between these seed regions and right cuneus was found in participants with schizophrenia. Areas of overlap shown in blue.
Table 4.
Resting State Data: Whole-Brain Analysis
| Seed Regiona | Areas of Decreased Connectivityb | Side | Volume (# voxels) | tc | Peak activity: MNI coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Right Caudate | Dorsolateral prefrontal cortex | R | 63 | 5.4 | 32 | 37 | 32 |
| Dorsal anterior cingulate | R | 26 | 5.6 | 11 | 34 | 30 | |
| Dorsolateral prefrontal cortex | L | 24 | 4.3 | −28 | 28 | 38 | |
| Anterior prefrontal cortex (dorsomedial superior frontal gyrus) | Cuneus | R | 24 | 3.8 | 17 | −56 | 5 |
| Rostral anterior cingulate cortex/Medial prefrontal cortex | Cuneus | R | 36 | 4.2 | 23 | −53 | 2 |
Clusters with significant DRUG effect or DRUG × GROUP effect from Stop Error – Go contrast were used as seed regions.
Areas with significant decreases in connectivity in schizophrenia compared to controls (GROUP effect: pcorrected < 0.05)
t-statistic for Control – Schizophrenia group comparison (peak voxel)
fMRI Correlations
To evaluate whether error-related activations influence nicotine-induced post-error slowing, we performed correlations between activations identified in the Stop Error – Go contrast and post-error RT. We found a significant positive correlation between post-error RT and dACC ROI activation for nicotine (r=0.45, p=0.04), replicating previous research that greater dACC activation is associated with increased post-error RT (Kerns et al. 2004). In contrast, there was a trend for a negative correlation between dACC and post-error RT in the placebo condition (r=−0.42, p=0.07). The correlation between dACC and post-error RT was significantly greater for the nicotine condition than for the placebo condition (z=2.57, p=0.01). While the positive correlation was significant for controls (r=0.88, p<0.001), it was not significant for the schizophrenia group (r=0.53, p=0.12). However, there was no significant difference in correlation for the nicotine condition between the two groups (z=−1.44, p=0.15). We also noted trend-level positive correlations between right caudate activation and post-error RT for the nicotine condition only in both control participants (r=0.60, p=0.07) and individuals with schizophrenia (r=0.58, p=0.08).
Because of the finding of greater nicotine-induced activation of the right caudate in response to errors in the presence of decreased resting state functional connectivity between the right caudate and dACC/bilateral DLPFC in individuals with schizophrenia, we examined correlations between right caudate activation and network connectivity. We found significant negative correlations between right caudate activation to errors and resting state connectivity between the right caudate and both right (r=−0.50, p=0.01) and left DLPFC (r=−0.45, p=0.03) and a trend for connectivity between right caudate and dACC (r=−0.34, p=0.09) for the nicotine condition but no significant correlations for the placebo condition (right DLPFC: r=−0.26, p=0.21; left DLPFC: r=−0.07, p=0.73; dACC: r=−0.11, p=0.61). These results suggest that nicotinic activation in response to errors was greater in those with lower network connectivity.
Control Analyses
Additional analyses were conducted to confirm that higher rates of smoking and nicotine dose in the schizophrenia group did not influence our findings. It is therefore possible that nicotine withdrawal during the placebo condition may have influenced group differences. We found that subjective nicotine withdrawal measured with the MNWS was negligible (see Supplement for details). To evaluate whether antipsychotic medication usage influenced our findings, we computed Pearson correlation coefficients for each drug condition between (1) CED/D2 receptor occupancy and (2) post-error RT and significant task-based (SST) and resting state fMRI measures. There were no significant correlations between CED/D2 receptor occupancy and post-error RT. We found a significant negative correlation between CED and activation in the right caudate for the placebo condition (r=−0.64, p=0.02) and a trend for the nicotine condition (r=−0.52, p=0.08). To establish that CED did not contribute to increased nicotine-induced activation in the right caudate, we used a repeated measures mixed model restricted to participants with schizophrenia with and without inclusion of CED as a covariate; DRUG effect in the schizophrenia group was significant regardless of including CED (DRUG effect p<0.001 in both models). Greater CED dose was associated with decreased activation; since we found increased activation in schizophrenia group, CED did not explain the increased activation and may have biased this finding towards the null. No other significant correlations were observed between CED/D2 receptor occupancy for the SST or resting state data.
DISCUSSION
In this study, we report the novel findings that nicotine administration is associated with a) increased activation of the right caudate in response to errors in participants with schizophrenia relative to controls; and b) increased activation of both rostral and dorsal ACC in response to errors in participants with and without schizophrenia. We also replicated prior research demonstrating decreased post-error RT in schizophrenia and demonstrate the novel finding that nicotine administration is associated with increased post-error RT.
Nicotine-related activation of the right caudate in response to errors was specific to participants with schizophrenia. Using this area as a seed for resting state functional connectivity analysis, we isolated areas of the cognitive control network with decreased connectivity with the right caudate in schizophrenia: dACC and bilateral DLPFC. We found positive correlations between error-related dACC activation and post-error RT, consistent with previous reports that dACC activation predicts post-error slowing in healthy controls and individuals with schizophrenia (Kerns et al. 2004; Becerril et al. 2011). There was also a trend for a positive correlation between right caudate activation to errors and post-error RT in the nicotine condition. We additionally found negative correlations between right caudate activation in response to errors and right caudate-dACC and right caudate-bilateral DLPFC connectivity specific to the nicotine condition. This constellation of findings suggests that nicotine-induced activation of the right caudate in response to errors may overcome deficits in functional connectivity within the cognitive control network during the baseline condition; greater nicotine-induced caudate activation was found in patients with the greatest decrements in connectivity associated with nicotine-induced increases in post-error RT.
Caudate activation using the SST is commonly associated with successful response inhibition (i.e., not with errors) consistent with our finding of bilateral caudate activation in controls for the Stop Correct – Stop Error contrast (Chevrier et al. 2007; Li et al. 2008c; Padmala and Pessoa 2010; Boehler et al. 2010). Interestingly, fMRI investigations of post-error behavioral adjustments suggest that errors may facilitate processing of task-relevant activation. Using a task where participants were asked to classify faces as male or female, King et al. found error-related activation of dACC in response to errors and subsequent activation of bilateral fusiform face area (King et al. 2010). Although we identified bilateral caudate activation associated with successful response inhibition in controls, there were no significant activations for the Stop Correct – Stop Error contrast in schizophrenia. Thus, nicotinic actions on error processing in schizophrenia may have redirected neural resources towards task-relevant caudate activity in preparation for subsequent Stop trials.
The compensatory activation of the caudate in patients was specific to the nicotine condition. We speculate that nicotine-related activation of the caudate in response to errors may have been paradoxically facilitated by disease-related abnormalities in caudate neurotransmission. The caudate, along with the thalamus and substantia nigra, has the highest density of nicotinic acetylcholine receptors in the human brain (Paterson and Nordberg 2000). The caudate region with increased nicotine-induced activation in the patient group in the current study was located in the pre-commissural caudate head, corresponding to the associative striatum (Martinez et al. 2003). Molecular imaging studies have implicated the associative striatum as key to schizophrenia pathology; specifically, increased dopaminergic transmission in the associative striatum has been found in unmedicated patients with schizophrenia (Kegeles et al. 2010). Moreover, individuals at risk for psychosis have elevated presynaptic dopaminergic capacity in the associative striatum that predicts conversion to psychosis (Howes et al. 2009). In addition, increased glutamate levels in the associative striatum have been found in individuals at risk for psychosis and drug-naive patients experiencing a first episode of psychosis (Fuente-Sandoval et al. 2011; Plitman et al. 2016). Functional MRI activation in the caudate for the SST has been positively correlated with both glutamate levels using magnetic spectroscopy and presynaptic dopaminergic capacity using positron emission tomography in the associative striatum (Lorenz et al. 2015). Nicotine activates presynaptic nicotinic acetylcholine receptors on dopaminergic neurons in the mesoaccumbens system. In addition, nicotinic effects on the glutamatergic system potentiates excitation of dopaminergic transmission (Mansvelder et al. 2002). Thus, a parsimonious explanation of our findings is that disease-related abnormalities of glutamatergic and dopaminergic neurotransmission in the associative striatum in schizophrenia may underlie exaggerated nicotine-related activation of the caudate in response to errors, which served to compensate for error processing deficits in schizophrenia.
In response to errors, we identified significant nicotine-induced activation of dACC and rACC, key hubs of the cognitive control and affective networks, respectively (Polli et al. 2005; Cole and Schneider 2007). In contrast to multiple previous studies showing decreased dACC activation in response to errors in schizophrenia (Carter et al. 2001; Kerns et al. 2005; Polli et al. 2008; Becerril et al. 2011), there was no significant group difference in dACC activation in the placebo condition. Nonetheless, our finding of decreased connectivity between the caudate and dACC and DLPFC is consistent with prior studies demonstrating decreased connectivity between error-related dACC/middle cingulate region and DLPFC (Voegler et al. 2016). We did not find any significant effects of nicotine on resting state functional connectivity measures, consistent with our prior research in smokers with and without schizophrenia in which there was no effect of nicotine on the strength of functional connectivity between dACC and striatal regions (Moran et al. 2012). However, a recent study using graph theoretical methods found enhanced nicotine-induced connectedness of the ACC in individuals with schizophrenia but the opposite effect of nicotine in healthy individuals (Smucny et al. 2017). Our finding of negative correlations between right caudate activation and connectivity between right caudate and dACC/DLPFC suggests that enhanced nicotine-induced caudate activation in response to errors served as a compensatory mechanism to overcome functional dysconnectivity in the cognitive control network in schizophrenia.
Limitations
The major limitation of this paper is the small sample size, which increases the risk of Type I and Type II error. The crossover study design allows for the detection of within-group drug effects, but we likely did not have sufficient power to detect small between-group differences. We failed to replicate prior studies using the SST of impaired response inhibition efficiency (group differences in SSRT) or group differences in activation of regions associated with response inhibition in schizophrenia (Hughes et al. 2012). However, we found that both patient and control groups fundamentally recruit similar brain regions commonly reported for successful response inhibition, such as the anterior insula and inferior frontal gyrus (Cai et al. 2014). Because of the small sample size, we did not correct for multiple comparisons in analysis of behavioral data; accordingly, the current findings await replication in independent samples. Replication of our findings in groups matched on smoking status is required, as there was a higher rate of smoking in participants with schizophrenia compared with controls, and an associated increase in dose of nicotine administered to the patient group. Post-error RT was notably associated with large variance in the schizophrenia group in both drug conditions; this finding in particular should be interpreted with caution and requires replication in a larger cohort of non-smokers or groups balanced on smoking status. Even in the absence of subjective withdrawal symptoms, nicotine administration may have reversed nicotine withdrawal-related impairments in error monitoring. Another limitation was the short duration of the resting state scan (6.2 minutes with TR of 3 seconds). Critically, our fMRI findings of increased error-related activation of dACC and rACC (in the nicotine condition) are consistent with previous reports of the key roles of these regions in error processing (Polli et al. 2005). Decreased connectivity between the caudate and areas of the cognitive control network in schizophrenia are consistent with prior studies demonstrating dACC dysfunction in schizophrenia, increasing confidence in our findings (Carter et al. 2001; Laurens et al. 2003; Kerns et al. 2005; Polli et al. 2008; Becerril et al. 2011; Voegler et al. 2016). Finally, most participants with schizophrenia were prescribed antipsychotic medications. Post-error RT and fMRI data measurements were not correlated with CED or D2 receptor occupancy, with the exception of a negative correlation between CED and right caudate activation for the Stop Error – Go contrast. While participants with schizophrenia had increased nicotine-induced caudate activation, CED predicted decreased activation, suggesting that antipsychotic medications may have attenuated increased right caudate activation.
Conclusions
In summary, individuals with schizophrenia were characterized by deficits in error processing manifested by decreased post-error slowing compared to controls in association with decreased connectivity of the right caudate with other regions of the cognitive control network (dACC and bilateral DLPFC). The key novel finding of the current study is that nicotine improves various aspects of error processing in schizophrenia. Nicotine activated regions involved in cognitive control (dACC) and affective/motivational (rACC) responses to errors. Nicotine also enhanced dynamic adjustment of behavior (increased post-error RT) and activated task-relevant regions associated with successful response inhibition (caudate activation) in response to errors. This proof-of-concept work highlights the need for development of treatments that target nicotinic systems to improve cognitive control deficits in schizophrenia.
Supplementary Material
Acknowledgments
Financial support was received from NARSAD Young Investigator Awards, Brain and Behavior Research Foundation (to LES and LVM), the Pope-Hintz Fellowship Award, McLean Hospital (to LVM), and the Dupont-Warren Fellowship Award, Harvard Medical School (to LVM). This research was also supported by grants from National Institute of Mental Health (R01 MH068376 to DAP and K23 MH110564 to LVM) and NIDA K24 DA030443 to AEE. DAP was also partially supported by R37 MH068376. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health.
Footnotes
Conflict of Interest: Over the past 3 years, Dr. Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Pfizer and Posit Science for activities unrelated to the current study. Dr. Evins has received research grant awards to her institution from Pfizer, Forum Pharmaceuticals, and GlaxoSmithKline and has received consulting fees from Pfizer and Reckitt Benckiser. All other authors declare no conflicts of interest.
References
- AhnAllen CG, Nestor PG, Shenton ME, et al. Early nicotine withdrawal and transdermal nicotine effects on neurocognitive performance in schizophrenia. Schizophr Res. 2008;100:261–269. doi: 10.1016/j.schres.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacol. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Becerril KE, Repovs G, Barch DM. Error processing network dynamics in schizophrenia. NeuroImage. 2011;54:1495–1505. doi: 10.1016/j.neuroimage.2010.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, et al. Pinning down response inhibition in the brain — Conjunction analyses of the Stop-signal task. NeuroImage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Alexander JC, Perez PD, et al. Acute nicotine administration increases BOLD fMRI signal in brain regions involved in reward signaling and compulsive drug intake in rats. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, et al. Dissociable Roles of Right Inferior Frontal Cortex and Anterior Insula in Inhibitory Control: Evidence from Intrinsic and Task-Related Functional Parcellation, Connectivity, and Response Profile Analyses across Multiple Datasets. J Neurosci. 2014;34:14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Mueller VI, Eickhoff CR, et al. Three key regions for supervisory attentional control: Evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, et al. Measurement and Reliability of Response Inhibition. Front Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain J Neurol. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Forstmann BU, et al. Testing theories of post-error slowing. Atten Percept Psychophys. 2012;74:454–465. doi: 10.3758/s13414-011-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra SJ, Stoeckel LE, Carlini SV, et al. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology (Berl) 2012;219:25–34. doi: 10.1007/s00213-011-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version (SCID) Biometric Research Department: New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- de la Fuente-Sandoval C, León-Ortiz P, Favila R, et al. Higher Levels of Glutamate in the Associative-Striatum of Subjects with Prodromal Symptoms of Schizophrenia and Patients with First-Episode Psychosis. Neuropsychopharmacology. 2011;36:1781–1791. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Okerholm RA, Eller M, et al. Comparison of the pharmacokinetics of two nicotine transdermal systems: nicoderm and habitrol. J Clin Pharmacol. 1995;35:493–498. doi: 10.1002/j.1552-4604.1995.tb04093.x. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacol. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Schroeder M, Ross TJ, et al. Nicotine Enhances but Does Not Normalize Visual Sustained Attention and the Associated Brain Network in Schizophrenia. Schizophr Bull. 2011;37:416–425. doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin M-C, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addict Abingdon Engl. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol Psychol. 2012;89:220–231. doi: 10.1016/j.biopsycho.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, et al. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. NeuroImage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelt LE, Barr RS, Goff DC, et al. Effects of transdermal nicotine on episodic memory in nonsmokers with and without schizophrenia. Psychopharmacology (Berl) 2008;199:89–98. doi: 10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kelly DL, McMahon RP, Wehring HJ, et al. Cigarette smoking and mortality risk in people with schizophrenia. Schizophr Bull. 2011;37:832–838. doi: 10.1093/schbul/sbp152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- King JA, Korb FM, von Cramon DY, Ullsperger M. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. J Neurosci. 2010;30:12759–12769. doi: 10.1523/JNEUROSCI.3274-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D₂ receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33:675–681. doi: 10.1097/JCP.0b013e3182983ffa. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Ngan ETC, Bates AT, et al. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain J Neurol. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Leonard S, Mexal S, Freedman R. Smoking, Genetics and Schizophrenia: Evidence for Self Medication. J Dual Diagn. 2007;3:43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Huang C, Yan P, et al. Neural Correlates of Post-error Slowing during a Stop Signal Task: A Functional Magnetic Resonance Imaging Study. J Cogn Neurosci. 2008a;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Chao HH-A, et al. Error-specific medial cortical and subcortical activity during the stop signal task: A functional magnetic resonance imaging study. Neuroscience. 2008b;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Sinha R, Lee T-W. Subcortical processes of motor response inhibition during a stop signal task. NeuroImage. 2008c;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RC, Gleich T, Buchert R, et al. Interactions between glutamate, dopamine, and the neuronal signature of response inhibition in the human striatum: Neurochemical Basis of Response Inhibition. Hum Brain Mapp. 2015;36:4031–4040. doi: 10.1002/hbm.22895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Kremen WS, et al. Nicotine and familial vulnerability to schizophrenia: a discordant twin study. J Abnorm Psychol. 2002;111:687–693. doi: 10.1037//0021-843x.111.4.687. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Bennett ME, Weiner EE, et al. Smoking history and motivation to quit in smokers with schizophrenia in a smoking cessation program. Schizophr Res. 2011;126:277–283. doi: 10.1016/j.schres.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, et al. Imaging Human Mesolimbic Dopamine Transmission with Positron Emission Tomography. Part II: Amphetamine-Induced Dopamine Release in the Functional Subdivisions of the Striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, et al. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002;111:22–41. [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Kochunov P, Hong LE. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophr Bull. 2013a;39:1373–1381. doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142:223–229. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LV, Tagamets MA, Sampath H, et al. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol Psychiatry. 2013b;74:467–474. doi: 10.1016/j.biopsych.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, et al. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacol. 1998;18:175–185. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Moment-to-moment fluctuations in fMRI amplitude and interregion coupling are predictive of inhibitory performance. Cogn Affect Behav Neurosci. 2010;10:279–297. doi: 10.3758/CABN.10.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, et al. Elevated Myo-Inositol, Choline, and Glutamate Levels in the Associative Striatum of Antipsychotic-Naive Patients With First-Episode Psychosis: A Proton Magnetic Resonance Spectroscopy Study With Implications for Glial Dysfunction. Schizophr Bull. 2016;42:415–424. doi: 10.1093/schbul/sbv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJS, Cain MS, et al. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci U S A. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJS, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain J Neurol. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJS, Vangel M, et al. Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophr Res. 2006;82:191–201. doi: 10.1016/j.schres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Li C-s. Imaging Response Inhibition in a Stop-Signal Task: Neural Correlates Independent of Signal Monitoring and Post-Response Processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger C, Bénar CG, Vidal F, et al. Rostral Cingulate Zone and correct response monitoring: ICA and source localization evidences for the unicity of correct- and error-negativities. NeuroImage. 2010;51:391–403. doi: 10.1016/j.neuroimage.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, et al. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacol. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smucny J, Wylie KP, Kronberg E, et al. Nicotinic modulation of salience network connectivity and centrality in schizophrenia. J Psychiatr Res. 2017;89:85–96. doi: 10.1016/j.jpsychires.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. doi: 10.1037/0033-2909.87.2.245. [DOI] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural Systems for Error Monitoring: Recent Findings and Theoretical Perspectives. The Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Toll BA, O’Malley SS, McKee SA, et al. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav J Soc Psychol Addict Behav. 2007;21:216–225. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Martin LF, Freedman R. FMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. Am J Psychiatry. 2005;162:391–393. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Voegler R, Becker MPI, Nitsch A, et al. Aberrant network connectivity during error processing in patients with schizophrenia. J Psychiatry Neurosci. 2016;41(2):E3–12. doi: 10.1503/jpn.150092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


