Abstract
Purpose
To examine DNA methylation as a mechanism linking diet, physical activity, weight status, and breast cancer risk.
Methods
Insufficiently active women of varying weight status, without a history of cancer, completed a maximal exercise test, clinical measurement of height and weight, and a dietary intake measure. They also provided blood samples, which were analyzed to ascertain average methylation of candidate genes related to breast cancer (BRCA1, RUNX3, GALNT9, and PAX6) and inflammation (TLR4 and TLR6).
Results
Elevated weight status (r=−.18, p<.05) and poorer aerobic fitness (r=.24, p<.01) were each associated with decreased methylation of inflammation genes. Methylation of inflammation genes statistically mediated the relationship between weight status and cancer gene methylation (standardized indirect effect =.12, p<.05) as well as between cardiorespiratory fitness and cancer gene methylation (standardized indirect effect = −.172, p<01). However, recent dietary behavior was not associated with methylation of either inflammation or cancer genes.
Conclusions
Both weight status and cardiovascular fitness are associated with methylation of genes associated with both inflammation and cancer. Methylation of inflammatory genes might serve as a mechanistic link between lifestyle factors and methylation changes in genes that increase risk for breast cancer.
Keywords: BMI, Diet, Exercise, Breast Cancer, DNA Methylation
Introduction
Obesity and breast cancer are high-priority public health issues in the United States. More than 1 in 3 adults in the US are obese, and about 1 in 8 women will be diagnosed with breast cancer in their lifetime [1, 2]. Importantly, these two public health issues are strongly interrelated. In a recent position statement, the American Society of Clinical Oncology named obesity as a major unrecognized risk factor for cancer; being overweight or obese increases risk for several types of cancer, including breast cancer, and is also associated with worse prognosis after cancer diagnosis and treatment [3]. Elevated weight status is associated with increased risk of death from cancer [4], with an estimated 90,000 annual cancer deaths in the United States that are due to excess weight. Despite the well-established link between increased body weight and cancer risk, the mechanisms underlying this relationship are largely unknown [5].
One important question regards whether increased body weight itself is most important in breast cancer etiology, or whether obesity-associated health behaviors, specifically, diet and exercise, also play an important role. This quandary exists partially because there are also well-established relationships in the literature between these two health behaviors and cancer risk. For example, increased fruit and vegetable consumption, which is associated with better weight management and reduced risk of obesity [6, 7], is thought to protect against several types of cancer[8, 9]. Across numerous meta-analyses and reviews, physical activity has been associated with reduced risk of developing various forms of cancer, including breast cancer (e.g., [10, 11]). Physical activity after cancer diagnosis also has implications for psychological and mortality outcomes (e.g., [12, 13]). For example, a recent meta-analysis of physical activity and breast cancer survival found that post-diagnosis physical activity reduced breast cancer deaths by 34% [13]. It is clear that both diet and physical activity influence cancer risk and outcomes, but the precise biological mechanisms underpinning these relationships are only beginning to be explored. Thus, we understand that body weight, diet, and exercise (which are clearly interrelated) all influence cancer risk. However, in this state of mechanistic uncertainty, the extent to which these are either unique or overlapping risks, as well as the biological processes by which they might independently or synergistically affect cancer risk, are largely unknown.
A number of mechanisms have been hypothesized as potential pathways through which obesity, diet, and physical activity may influence breast cancer risk, and one of the most promising is inflammation. Inflammation is a crucial biological mechanism that appears to underlie this relationship, as obesity is associated with chronic systemic inflammation, which can increase tumor-promoting activity [14] as well as risk of cancer incidence, as is the case with breast cancer [15]. Moreover, behaviors implicated in weight management and cancer prevention, namely physical activity and healthy diet, are inversely associated with inflammation [16, 17], which could indicate contributing or moderating effects on the relationship between weight and cancer.
Another promising mechanism is DNA methylation, an epigenetic process that involves the addition of a methyl group to the 5′ position of the cytosine pyrimidine ring within cytosine-phosphate-guanine (CpG) dinucleotides. Together with other epigenetic mechanisms (e.g., histone modifications), DNA methylation functions as a switch that turns relevant genes on and off, a mechanism that is crucial for development, differentiation, and genomic stability. A small but growing body of evidence has demonstrated the importance of considering methylation as a mechanism by which weight status, diet, and/or exercise may influence breast cancer risk and outcomes. Xu and colleagues observed that differential variability in genome-wide methylation patterns are a feature of both cancer and obesity [18]. Another study assessing genome-wide levels of methylation observed relationships between body mass index (BMI) and methylation of candidate genes for cancer [19]. Separately, others have suggested changes in methylation patterns as a possible mechanism through which consumption of fruits and vegetables may reduce cancer occurrence [20–22]. Finally, physical activity levels [23] and cardiorespiratory fitness assessed by VO2 max [24] have been shown to be associated with differential methylation of genes specific to breast cancer, and one study found a positive association between physical activity levels and global genomic DNA methylation, though this association was attenuated when adjusted for covariates [25].
Notably, methylation may exert differential effects based upon the particular genes and sites that are affected. In some cases, increased methylation of genes is associated with positive health outcomes. One relevant example is methylation of toll-like receptor genes (e.g., TLR4 and TLR6) associated with inflammation and breast cancer cell survival/proliferation—greater methylation of TLRs can reduce gene expression and downstream signaling via competitive binding that blocks transcription factors and bacterial DNA binding, thus reducing the inflammatory signaling associated with chronic illness and tumor cell survival [26, 27]. However, in most cases, higher levels of methylation are associated with negative outcomes. For example, in cancer, the healthy state of low levels of methylation in the promoter regions of tumor suppressor genes (e.g., RUNX3 gene [28]) is disrupted such that higher levels of methylation may silence their action. In essence, the hypermethylation of these CpG islands turns off or silences the gene that prevents the proliferation of cells that characterize tumor development[29, 30]. Emerging evidence suggests that it is now possible to detect methylation patterns that predict the development of certain cancers among at-risk individuals, even several years before clinical diagnosis (e.g., [31]). Thus, there is great value in enhancing our understanding of the influence of DNA methylation on cancer-related processes, including inflammation, as well as on specific cancer genes.
In further investigating associations between BMI, diet, physical activity and DNA methylation patterns that may relate to breast cancer outcomes, it will be necessary to explore both moderators and mechanisms of these relationships. With regard to moderation, there is much debate in the literature regarding the interaction of BMI, diet, physical activity, and health outcomes. One example is the question of whether it is possible to be “fit and fat” [32]. That is, is there an interaction between BMI and physical activity such that BMI is only a risk factor for cancer when levels of physical activity are low? Some evidence has suggested that healthy diet and physical activity can be protective against cancer death regardless of weight status [33], but it is not clear whether the same factors can protect against cancer risk for individuals with obesity. Thus, the first goal of this analysis is to examine possible main effects and interactions of lifestyle behaviors and body weight in their relationship to methylation of genes associated with inflammation and breast cancer. With regard to mechanism, some have suggested that chronic inflammation plays a key role in the development of cancer [15] and it is well known that BMI, diet, and physical activity are associated with inflammatory processes. The second goal of this analysis is thus to determine whether the influence of lifestyle factors on methylation of cancer genes might be mediated by their association with methylation of inflammation genes.
Methods
Participants
276 women between the ages of 30 and 45 participated in the study as part of a larger randomized controlled trial (RCT) examining the effects of exercise intensity and duration on DNA methylation, particularly of genes related to breast cancer. Participants were recruited from the University of Colorado community and the Denver metro area. Due to the nature of the parent RCT, initial recruitment criteria required that participants engaged in 60 minutes or less of voluntary vigorous or moderate-level physical activity per week for at least the past six months. Participants were excluded if they reported that they smoked cigarettes, had a history of breast cancer, were receiving cancer treatment of any type, were on a restricted diet, were Type I or Type II diabetic, had any cardiovascular or respiratory diseases, were pregnant or planning to become pregnant in the next six months, or were otherwise unhealthy in a way that would prevent them from completing a sixteen-week exercise intervention. Inclusion criteria included a body mass index (BMI) less than 35 kg/m2, being physically capable of participating in moderate-level intensity physical activity, and having a regular menstrual cycle if not using a birth control method. All participants had to consent to random assignment to intervention condition. 271 of consented participants successfully completed all baseline measures prior to randomization. The Colorado Multiple Institutional Review Board approved the study and all study participants provided written informed consent.
All participants were female and the average age was 37.48 years (SD = 4.56). The majority (57.2%) of the participants self-identified as White, while 13.3% identified as Black, 5.2% as Asian, 16.6% as Hispanic or Latina, 0.4% as Native Hawaiian or Pacific Islander, and 7.4% as more than one race.
Measures
BMI
Body Mass Index (BMI) was calculated in kg/m2 based on participants’ height and weight measured by a registered nurse practitioner during the participants’ baseline visit.
VO2max
As a measure of objective physical fitness, participants’ maximal oxygen capacity (VO2max) was obtained using an individualized maximal exercise test on a motorized treadmill. Participants were initially brought to a comfortable speed corresponding to 70% of their age-predicted maximal heart rate. While maintaining this speed, the treadmill grade was increased by 2.0% increments every two minutes until maximal VO2 was reached. VO2max, measured in milliliters of oxygen consumption per kilogram of body weight per minute (ml/kg/min), was assessed using an online computer-assisted open-circuit spirometry (PavoMedics, Sandy, UT), and confirmed by a respiratory quotient ≥1.1 and/or a detected plateau in VO2.
Fruit and vegetable consumption
Dietary data were collected using the Antioxidant Nutrient Questionnaire [34]. Fruit and vegetable consumption was selected as the dietary variable of interest due to prior links with both genetic methylation and cancer risk [20–22]. Estimated fruit and vegetable exposures across the last month were calculated as a sum of the times (ranging from 0 to 60) that the participant reported consuming a medium-sized serving, as defined by the questionnaire, of each of 23 fruits and 23 vegetables within the past month.
Gene methylation
To determine the methylation of CpG sites for the selected cancer/tumor suppressor genes (BRCA1, RUNX3, GALNT9, and PAX6) and inflammatory genes (TLR4 and TLR6), pyrosequencing was performed at EpigenDX (Worcester, MA) using previously published procedures [35]. The cancer gene assays covered a total of 27 CG dinucleotides located 5-upstream from the translational start site (TSS) and on the 5’ untranslated region (UTR) (−76 to +23, and −12 to +76 from TSS), Intron 1 (+41806 to +41854 from TSS), and Intron 4 (+12543 to +12609 from TSS) for BRCA1, RUNX3, GALNT9, and PAX6, respectively. The inflammatory gene assays covered a total of six CpG sites located on the 5’ UTR (+27 to +51 from TSS) and on the 5’ upstream region (−1291 to −1269 from TSS) for TLR4 and TLR6, respectively.
The selected genes and corresponding CpG locations were chosen based upon previously published findings implicating their increased methylation to be associated with breast cancer (BRCA1 and RUNX3), tumor suppression failure (BRCA1, PAX6, and RUNX3), tumor metastasis (GALNT9), and reduced inflammatory signaling (TLR4 and TLR6) [26, 28, 36–38]. All primers are owned by EpigenDx.
The analyzed DNA was presented as percent methylation at each of the CpGs. Similar to our prior work [24] and to reduce alpha inflation due to the number of tests conducted, the average percent methylation at each CpG was used to calculate the average methylation score for each gene. The average methylation scores for each cancer-associated gene were then combined and averaged to create one “cancer genes” methylation score used in analyses. Similarly, the average percent methylation scores for each of the sites on TLR4 and TLR6 were combined and averaged to create one “inflammatory genes” methylation score for percent methylation.
Procedures
Following recruitment and a phone screening for eligibility, participants were brought to the study location for two baseline sessions (orientation and fitness test). In addition to obtaining informed consent during the orientation appointment, approximately 6mL (two 3mL glass tubes) of blood was drawn by a trained phlebotomist, a physical examination was performed by a registered nurse to determine that each participant was healthy enough for exercise, and objective measures of height and weight were obtained. An online questionnaire was completed to determine fruit and vegetable consumption. Following the orientation visit, participants completed the VO2max test during a separate appointment.
DNA was isolated from whole blood for methylation analyses. Blood samples were stored at +4°C until DNA could be extracted and incubated. The DNA was extracted per manufacturer’s instructions using the Gentra Puregene Blood Kit (Cat No. 158389). The DNA was quantified using Invitrogen’s Qubit™ dsDNA BR Assay Kit (Cat. No. Q-32853) and cryogenically stored at −80°C.
Due to failed blood draws, low sample concentration, and amplification failure, we were able to extract viable methylation data for 186–188 participants depending on the candidate gene. Because some participants were excluded from the study after blood samples were collected (due to complications discovered during the physical examination, failure to return for a second appointment, or an inability to complete the VO2max test), BMI data were available for 141 of these participants and 130 of these participants had a valid VO2max test.
Analytic Plan
The first goal of the current study was to examine associations between BMI, diet, physical activity, and DNA methylation patterns that may relate to breast cancer outcomes. We were interested in both main effects and interactions. Thus, we first examined bivariate correlations between the lifestyle behaviors and DNA methylation of both breast cancer-related genes and inflammation-related genes. These correlations are presented in the Results section below.
From these bivariate correlations, to parse the associations we observed further, we ran multivariate regression models predicting inflammatory gene methylation from BMI, VO2max, and fruit and vegetable consumption. As we did not observe any significant bivariate associations between our lifestyle variables of interest and cancer gene methylation, we do not present regression models for that outcome. Additionally, since we observed a high correlation between BMI and VO2max in these data, we expected suppression effects in a model that included both variables; thus, we first examined their relationships separately (but together with fruit and vegetable consumption), and later together. In these regression models, we first examined main effects only and later included predicted interactions between lifestyle behavior markers (e.g., BMI and VO2max, in order to examine the “fit and fat” hypothesis).
Finally, in order to examine whether inflammation may mediate relationships between lifestyle factors and cancer-related gene methylation, we estimated mediational models via path analysis (c.f., [39]) using Mplus software.
Results
Descriptive Statistics
The average VO2max was 27.11 ml/kg/minute (SD=5.11, Range=17.9–41.7), indicating on average “fair” cardiorespiratory fitness at baseline for women in this age range, consistent with the fact that these women were all suboptimally active. Participants were, on average, on the border between overweight and obese, with a mean BMI of 29.88 (SD=5.25, Range=17.8–40.2). In terms of typical BMI cutoffs of clinical significance, 21.8% of participants were considered “normal” weight (BMI between 18.5 and 25), 36.1% were considered “overweight” (BMI between 25 and 30), and 41.2% were considered “obese” (BMI above 30), and .9% participant was considered “underweight”. Participants reported eating an average of 140.94 servings of fruits and vegetables per month (SD=111.65, Range=7.5–598), or approximately 4.7 servings per day on average. On average, cancer genes (for which higher levels of methylation are typically associated with worse outcomes, i.e., reduced action of tumor suppressor genes) were 32.06% methylated (SD=2.58, Range=24.44–39.63), while inflammatory genes (for which higher levels of methylation are typically associated with better outcomes, i.e., reduced inflammatory signaling) were 9.77% methylated (SD=3.24, Range=4.37–21.63). These descriptive statistics as well as methylation proportions for the individual candidate genes are presented in Table 1.
Table 1.
Descriptive Statistics
| Average/Frequency | SD/Range | |
|---|---|---|
| Age (years) | 37.48 | 30–45 |
|
| ||
| Race/Ethnicity | ||
|
| ||
| White/Caucasian American | 155 (57.2%) | |
|
| ||
| Black/African American | 36 (13.3%) | |
|
| ||
| Asian | 14 (5.2%) | |
|
| ||
| Hispanic/Latina | 45 (16.6%) | |
|
| ||
| Native Hawaiian/Pacific Islander | 1 (.4%) | |
|
| ||
| More than one race | 20 (7.4%) | |
| VO2max (ml/kg/min) | 27.11 | 5.11 |
| BMI (kg/m2) | 29.88 | 5.25 |
| Underweight | 2 (.9%) | |
| Normal | 47 (21.8%) | |
| Overweight | 78 (36.1%) | |
| Obese | 89 (41.2%) | |
| Monthly Fruit/Vegetable Exposures | 140.94 | 111.65 |
| PAX6 Methylation % | 6.58 | 1.07 |
| GALNT9 Methylation % | 75.12 | 6.28 |
| BRCA1 Methylation % | 0.29 | 0.84 |
| RUNX3 Methylation % | 46.3 | 8.42 |
| TLR6 Methylation % | 17.02 | 5.57 |
| TLR4 Methylation % | 2.53 | 1.22 |
| Cancer Gene Mean % Methylation | 32.06 | 2.58 |
| Inflammatory Gene Mean % Methylation | 9.77 | 3.24 |
Correlations between BMI, VO2max, Diet and Methylation
Bivariate correlations between our measures of interest are presented in Table 2. Of note, methylation of inflammatory genes was strongly negatively related to methylation of cancer genes. We also observed a significant negative correlation between BMI and methylation of inflammatory genes, and a significant positive correlation between VO2max and inflammatory gene methylation. Neither BMI nor VO2max were significantly associated with methylation of cancer genes. Fruit and vegetable consumption was not associated with methylation of either inflammatory genes or cancer genes. We also observed a strong negative relationship between BMI and VO2max. Finally, age was not related to methylation of either cancer genes or inflammatory genes in this dataset; thus, it was not included as a covariate in subsequent analyses.
Table 2.
Bivariate Correlation Table
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. BMI | |||||
| 2. VO2 | −0.57* | ||||
| 3. Fruit/Veg consump. | −0.15* | 0.12 | |||
| 4. Cancer gene methyl. | 0.12 | −0.08 | 0.06 | ||
| 5. Inflammatory gene methyl. | −0.18* | 0.24* | −0.02 | −0.66* | |
| 6. Age | 0.16* | −0.32* | 0.02 | −0.03 | −0.01 |
Note.
indicates p<.05.
Regression models
We first examined the relationship between inflammatory gene methylation, BMI, and fruit and vegetable consumption. This model is presented in Table 3. Controlling for fruit and vegetable consumption, BMI was significantly negatively associated with inflammatory gene methylation. Controlling for BMI, fruit and vegetable consumption was not significantly associated with inflammatory gene methylation. We also ran a model that included the interaction between BMI and fruit and vegetable consumption, in order to examine whether the relationship between BMI and inflammatory gene methylation might depend on the quality of ones’ diet; however, we did not observe a significant interaction between these variables (p=.79).
Table 3.
Inflammatory Genes by Fruit and Vegetable Consumption and BMI
| Coefficient | B | St. Error | t | p-value |
|---|---|---|---|---|
| Intercept | 13.25 | 1.82 | 7.27 | .000 |
| BMI | −0.12 | 0.06 | −2.11 | .037 |
| Fruit and Vegetable Consumption | −0.001 | 0.003 | −0.28 | .780 |
Notes. Multiple R2=.03
Next, we examined the relationship between inflammatory gene methylation, VO2max, and fruit and vegetable consumption. This model is presented in Table 4. Controlling for fruit and vegetable consumption, VO2max was significantly positively associated with inflammatory gene methylation. Controlling for VO2max, fruit and vegetable consumption was not significantly associated with inflammatory gene methylation. We also tested the interaction between VO2max and fruit and vegetable consumption, which was not significant (p=.78).
Table 4.
Inflammatory Genes by Fruit and Vegetable Consumption and VO2max
| Coefficient | B | St. Error | t | p-value |
|---|---|---|---|---|
| Intercept | 5.15 | 1.66 | 3.11 | .002 |
| VO2max | 0.18 | 0.06 | 2.86 | .005 |
| Fruit and Vegetable Consumption | −0.002 | 0.003 | −0.72 | .476 |
Notes. Multiple R2=.06
Lastly, we tested a model that included all three lifestyle predictors. In this model, we observed a trend such that VO2max was positively associated with methylation of inflammatory genes, controlling for BMI and fruit and vegetable consumption, though, consistent with suppression, this trend did not reach conventional levels of statistical significance (b=.14, p=.077). Neither BMI (b=−.056, p=.46) nor fruit and vegetable consumption (b=−.002, p=.46) were significantly related to methylation of inflammatory genes in the context of this regression model. Finally, we examined a regression model that included the interaction between BMI and VO2max, in order to test the hypothesis that the effect of BMI on methylation may differ for individuals with greater levels of physical fitness. We did not observe a significant interaction between these variables (p=.60).
Statistical mediation of cancer gene methylation by lifestyle factors through inflammatory gene methylation
While we did not observe significant bivariate relationships between our lifestyle factors and cancer gene methylation, we did observe a strong negative correlation between inflammatory gene methylation and cancer gene methylation, and consistent relationships between BMI and inflammatory gene methylation and VO2max and inflammatory gene methylation. Thus, we were interested in testing whether VO2max and BMI may be indirectly associated with methylation of cancer genes through association with inflammatory gene methylation.
We estimated two path models to examine the indirect relationships between BMI and cancer gene methylation and VO2 and cancer gene methylation, controlling for fruit and vegetable consumption. These models are presented in Figure 1 and Figure 2. In both models we found evidence for significant indirect effects between BMI and cancer gene methylation and VO2 and cancer gene methylation through inflammatory gene methylation.
Figure 1.
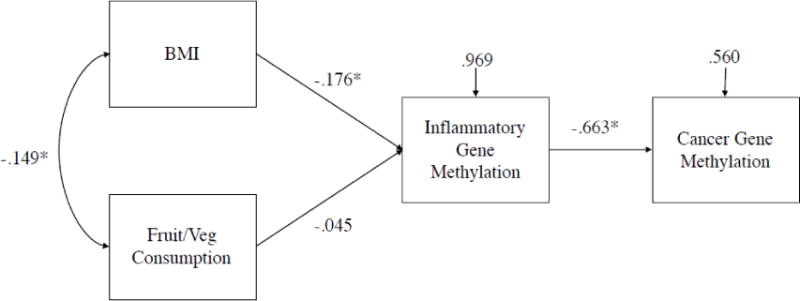
Note. Standardized path estimates and residual variances presented. * denotes p<.05. χ2(2)=.690, p=.71, CFI=1.00, RMSEA=.000. Standardized estimate of indirect effect from BMI to Cancer Gene Methylation = −0.117, p=.03.
Figure 2.
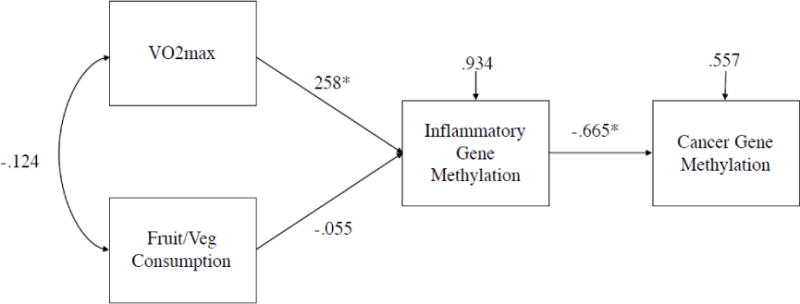
Note. Standardized path estimates and residual variances presented. * denotes p<.05. χ2(2)=1.96, p=.37, CFI=1.00, RMSEA=.000. Standardized estimate of indirect effect from VO2max to Cancer Gene Methylation = −0.172, p=.004.
Discussion
The present study sought to test the relationships between lifestyle factors of weight status, physical activity, and diet, and methylation of inflammatory and cancer-related candidate genes. Across a sample of adult women without a history of cancer, increased methylation of the inflammatory genes was associated with reduced methylation of the cancer candidate genes. Further, elevated maximal cardiorespiratory capacity and reduced body mass index were each associated with increased methylation of the inflammatory genes. Methylation of the inflammatory genes statistically mediated the relationships between weight status and cancer gene methylation as well as between cardiorespiratory fitness and cancer gene methylation. However, self-reported dietary behavior—measured here as fruit and vegetable consumption— was not associated with methylation of either inflammatory or cancer genes. Moreover, there were no moderating effects of diet or cardiorespiratory fitness on the relationships between weight status and methylation, suggesting that exercise and healthy diet may not protect individuals who are overweight or obese from the deleterious effects of their weight status on epigenetic modifications on inflammatory and cancer-related genes.
Limitations
While this study has numerous strengths, including its inclusion of dietary, physical activity, anthropomorphic, and genetic data in the same sample, it is not without limitations. First, the sample only includes women in midlife who have not been diagnosed with cancer, so it remains unknown whether these same patterns hold among men, individuals in other age ranges, and those who have already been diagnosed with cancer. Additionally, the present data are cross-sectional, and thus future examinations will be necessary to confirm the temporal ordering and causal mechanisms underlying these relationships. Finally, while VO2 max and body mass index were recorded using traditional clinical metrics and procedures, fruit and vegetable consumption was measured using exposures indicated in a self-report measure. The limitations of the self-report measure may underlie the lack of relationship between diet and the methylation measures in these data. However, fruit and vegetable consumption was related to BMI in this sample (r = −.15, p < .05), demonstrating at least some validity of this self-report exposure measure and an objective anthropomorphic outcome variable.
Epigenetic modifications, including methylation, present a mechanism by which behavioral and environmental factors influence gene expression and other biological processes relevant to health and disease states. The present study represents a first demonstration of the links between weight status, cardiorespiratory fitness, and the methylation of inflammatory and cancer genes in a single sample. The findings demonstrate that methylation of inflammatory genes, which is associated with lower weight status and better cardiorespiratory fitness, is negatively correlated with methylation of candidate genes associated with cancer. While prior research has identified inflammation as a key pathway linking obesity to increased cancer incidence and mortality (e.g., [14, 40]), the present findings go beyond the role of circulating inflammatory markers to highlight the relationships between weight status and methylation of inflammatory and cancer genes. Further, the findings demonstrate the importance of measuring methylation of specific genes, rather than whole-genome methylation approaches, in understanding the nuanced relationships between biobehavioral factors and changes in DNA methylation that have implications for ultimate health outcomes [41]. In sum, these findings demonstrate that both weight status and cardiovascular fitness are associated with methylation of genes associated with both inflammation and cancer, and that methylation of inflammatory genes might serve as a mechanistic link between lifestyle factors and methylation changes in genes that increase risk for breast cancer.
Acknowledgments
Funding: This research was funded by a grant from NIH/NCI (Grant number R01CA179963-04) to Angela Bryan.
Footnotes
Disclosure: The authors declared that they have no conflicts of interest.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009. 2011 (Vintage 2009 Populations) [Google Scholar]
- 2.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 3.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–74. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Morley TS, Kim M, et al. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aljadani HM, Patterson A, Sibbritt D, et al. Diet quality, measured by fruit and vegetable intake, predicts weight change in young women. J Obes. 2013 doi: 10.1155/2013/525161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He K, Hu FB, Colditz GA, et al. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes. 2004;28:1569–1574. doi: 10.1038/sj.ijo.0802795. [DOI] [PubMed] [Google Scholar]
- 8.Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360:861–868. doi: 10.1016/S0140-6736(02)09958-0. [DOI] [PubMed] [Google Scholar]
- 9.STEINMETZ KA, POTTER JD. Vegetables, Fruit, and Cancer Prevention. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 10.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 11.Lynch BM, Neilson HK, Friedenreich CM. Phys Act cancer. Springer; 2010. Physical activity and breast cancer prevention; pp. 13–42. [DOI] [PubMed] [Google Scholar]
- 12.Dimeo FC, Stieglitz R, Novelli-Fischer U, et al. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273–2277. [PubMed] [Google Scholar]
- 13.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 14.Font-Burgada J, Sun B, Karin M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016;23:48–62. doi: 10.1016/j.cmet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Tobias DK, Akinkuolie AO, Chandler PD, et al. Markers of Inflammation and Incident Breast Cancer Risk in the Women’s Health Study. Am J Epidemiol. 2017 doi: 10.1093/aje/kwx250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods JA, Vieira VJ, Keylock KT. Exercise, Inflammation, and Innate Immunity. Immunol Allergy Clin North Am. 2009;29:381–393. doi: 10.1016/j.iac.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106:S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Su S, Barnes VA, et al. A genome-wide methylation study on obesity. Epigenetics. 2013;8:522–533. doi: 10.4161/epi.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronn T, Volkov P, Gillberg L, et al. Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Hum Mol Genet. 2015;100:9440–9445. doi: 10.1093/hmg/ddv124. [DOI] [PubMed] [Google Scholar]
- 20.HIGDON J, DELAGE B, WILLIAMS D, DASHWOOD R. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Tollefsbol TO. Impact on DNA Methylation in Cancer Prevention and Therapy by Bioactive Dietary Components. Curr Med Chem. 2010;17:2141–2151. doi: 10.2174/092986710791299966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang FF, Morabia A, Carroll J, et al. Dietary Patterns Are Associated with Levels of Global Genomic DNA Methylation in a Cancer-Free Population. J Nutr. 2011;141:1165–1171. doi: 10.3945/jn.110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coyle YM, Xie X-J, Lewis CM, et al. Role of Physical Activity in Modulating Breast Cancer Risk as Defined by APC and RASSF1A Promoter Hypermethylation in Nonmalignant Breast Tissue. Cancer Epidemiol Prev Biomarkers. 2007;16 doi: 10.1158/1055-9965.EPI-06-0700. [DOI] [PubMed] [Google Scholar]
- 24.Bryan AD, Magnan RE, Hooper AEC, et al. Physical Activity and Differential Methylation of Breast Cancer Genes Assayed from Saliva: A Preliminary Investigation. Ann Behav Med. 2013;45:89–98. doi: 10.1007/s12160-012-9411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang FF, Cardarelli R, Carroll J, et al. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics. 2011;6:293–299. doi: 10.4161/epi.6.3.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Zhou H, Feng P, et al. Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. J Exp Clin Cancer Res. 2010;29:92. doi: 10.1186/1756-9966-29-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau QC, Raja E, Salto-Tellez M, et al. RUNX3 is frequently inactivated by dual mechanisms of protein mislocalization and promoter hypermethylation in breast cancer. Cancer Res. 2006;66:6512–6520. doi: 10.1158/0008-5472.CAN-06-0369. [DOI] [PubMed] [Google Scholar]
- 29.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 30.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- 31.Belinsky SA, Palmisano WA, Gilliland FD, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002;62:2370–2377. [PubMed] [Google Scholar]
- 32.Ortega FB, Ruiz JR, Labayen I, et al. The Fat but Fit paradox: what we know and don?t know about it. Br J Sports Med. 2017 doi: 10.1136/bjsports-2016-097400. bjsports-2016-097400. [DOI] [PubMed] [Google Scholar]
- 33.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–51. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satia JA, Watters JL, Galanko JA. Validation of an antioxidant nutrient questionnaire in whites and African Americans. J Am Diet Assoc. 2009;109:502–508.e6. doi: 10.1016/j.jada.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed K, Poulin ML, Yan L, Parissenti AM. Comparison of bisulfite sequencing PCR with pyrosequencing for measuring differences in DNA methylation. Anal Biochem. 2010;397:96–106. doi: 10.1016/j.ab.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Esteller M, Silva JM, Dominguez G, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. JNCI J Natl Cancer Inst. 2000;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 37.Pangeni RP, Channathodiyil P, Huen DS, et al. The GALNT9, BNC1 and CCDC8 genes are frequently epigenetically dysregulated in breast tumours that metastasise to the brain. Clin Epigenetics. 2015;7:57. doi: 10.1186/s13148-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyota M, Ho C, Ahuja N, et al. Identification of differentially methylated sequences in colorectal cancer by methylated CpG island amplification. Cancer Res. 1999;59:2307–2312. [PubMed] [Google Scholar]
- 39.Bryan A, Schmiege SJ, Broaddus MR. Mediational Analysis in HIV/AIDS Research: Estimating Multivariate Path Analytic Models in a Structural Equation Modeling Framework. AIDS Behav. 2007;11:365–383. doi: 10.1007/s10461-006-9150-2. [DOI] [PubMed] [Google Scholar]
- 40.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 41.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]


