Abstract
Background
Basal-like breast cancers, originally recognized by gene expression profiling, can be clinically identified using immunohistochemical (IHC) definitions that require estrogen receptor (ER) negativity. However, some basal cases are ER-positive and are mistakenly considered to be luminal by standard IHC approaches, leading to suboptimal treatment choices. Nestin, an intermediate filament expressed in many stem cells, is a recently-identified positive marker of basal-like phenotype independent of ER status. In this study we evaluated its clinical associations and prognostic capacity in a large breast cancer cohort.
Methods
A tissue microarray series of clinically-annotated invasive breast cancers with 12.6 years median follow-up was assessed for nestin expression by IHC. Kaplan-Meier and Cox regression models were used to evaluate the prognostic significance of nestin status, for the primary endpoint of breast-cancer-specific-survival (BCSS).
Results
Among 3641 cases interpretable for nestin by IHC, positive staining was found in 371 cases (10%) and was significantly associated with poor prognostic factors including other markers of basal-like differentiation. Patients with nestin-positive tumors had a significantly lower 10 year BCSS (HR=1.97, 95%CI: 1.62–2.40; P<0.001). Importantly, within the large group of 2323 ER+ cases, nestin positivity identified a subgroup of 120 patients (5%) with a significantly inferior 10 years BCSS (HR=1.50, 95%CI: 1.10–2.13; P=0.02).
Conclusions
Nestin IHC positivity is associated with the poor clinical outcomes and reduced survival rates that characterize the gene expression basal-like subtype. This easily-applicable tool identifies ER+ poor prognosis basal phenotype patients that are currently being missed by “Triple-negative” or “Core basal” IHC definitions.
Keywords: Basal-like, Intrinsic subtyping, Immunohistochemistry, PAM50, Nestin, Prognostic capacity
Introduction
The identification of different intrinsic breast cancer subtypes by gene expression profiling [1, 2] has led to improvements in diagnosis, prognosis and prediction of patients’ outcomes [3–8]. However, gene expression assays are hard to perform in routine clinical settings (e.g. in community hospitals, or in jurisdictions lacking specialized molecular diagnostic laboratory facilities) due to their complexity and high costs, leading to the common preference to use immunohistochemical (IHC) surrogate markers or panels to assign breast cancer subtype as a more feasible and inexpensive approach. In clinical practice, such IHC markers guide decisions to treat luminal subtypes with endocrine therapy, and for human epidermal growth factor receptor-2 (HER2)-positive breast cancers with anti-HER2 targeted agents. However, very limited gains have been made in identifying and tailoring therapies for the aggressive basal-like subtype of breast cancer, which has the worst 10 year prognosis and is refractory to existing targeted treatments [9–11].
The basal-like subtype, as originally recognized by gene expression, [12, 13] is often clinically identified using a “Triple negative” IHC definition, characterized by combined negativity for estrogen receptor (ER), progesterone receptor (PR) and HER2. Another IHC definition commonly used in research studies and sometimes in clinical practice, termed “Core basal,” adds a requirement for triple negative cases to also be positive for epidermal growth factor receptor (EGFR) and/or cytokeratin 5/6 (CK5/6). This definition more specifically identifies basal-like cases and has a stronger association with poor prognosis than the “Triple negative” definition [9, 14]. However, both the “Triple negative” and “Core basal” definitions necessarily require ER negativity, and therefore miss the potentially important subset of basal-like cases that are ER positive by IHC [15, 16].
Work conducted by researchers at MD Anderson and independently by our own group has shown that a high fraction (50%) of weakly positive ER positive breast cancers (characterized by 1–10% positive nuclear staining) are classified as basal-like when using gene expression profile methods as a gold standard [15, 16]. These cases do not meet “Triple negative” or “Core basal” IHC definitions; instead, they are considered luminal cases by IHC [17], leading to suboptimal treatment choices. Patients in this subgroup are currently treated with endocrine therapy [18] that will not only likely be ineffective, risking side effects without benefit, but also lead to missed opportunities to initiate more effective chemotherapy-based treatments in the adjuvant setting. Any subsequent metastatic disease might well also end up suboptimally treated. Thus, an easily accessible IHC surrogate marker for basal-like breast cancer that can be applied on routine formalin-fixed, paraffin-embedded (FFPE) tissues of breast cancer and can be interpreted independently of ER status would be an important diagnostic tool, particularly to help guide therapy for ER weakly positive cases that constitute from 1 – 6.7% of breast cancers and represent a problem for clinicians making treatment decisions [19, 15, 20].
Nestin, a type VI intermediate filament originally characterized because of its expression in neural progenitor cells [21, 22] has recently been identified as a useful positive IHC marker of the basal-like subtype [23–25]. In addition to its expression in neural tissues, nestin has been shown to be expressed in the endothelium of immature blood vessels [26, 27] and in the basal and myoepithelial layers of mammary cells [23]. Functionally, nestin is associated with tumor invasiveness, cancer progression and poor prognosis [28–30], including in triple negative breast cancers [31, 32]. Several studies have now associated nestin with basal-like differentiation, aggressive pathological characteristics and worse clinical behavior [24, 30, 33]. When our group evaluated 46 proposed basal IHC markers that had been published in the literature, we found nestin to have the best combination of sensitivity and specificity for any individual positive IHC marker of the basal-like subtype, when compared to an RNA-based PAM50 assay as a gold standard [25].
In the current study, we assess the prognostic capacity of nestin IHC expression on a large tissue microarray series corresponding to a population-based provincial cohort of early-stage breast cancer, annotated with detailed clinical data including long-term outcomes. We also specifically evaluate the capacity of nestin to identify ER+ patients that display a basal-like phenotype and prognosis.
Methods
This study was approved in accordance with the ethical standards of the institutional board of the University of British Columbia and British Columbia Cancer Agency (BCCA).
Study Population
The current study used a cohort which originally included 4543 samples from patients diagnosed with invasive breast cancer in the province of British Columbia, Canada and who were referred to the BCCA in the period January 1986 – September 1992 [34]. Cases are linked to well-annotated clinical data regarding age, staging, histology, pathological factors, treatment and a long-term follow up with a median of 12.6 years. Exclusion criteria included: in situ disease, recurrent or metastatic disease at diagnosis, and male breast cancer. Treatment decisions at the time of patients’ diagnosis were made in adherence to the provincial guidelines recommended by BCCA, based on different clinicopathological factors including ER levels as determined by dextran-coated charcoal (DCC) ligand binding assay. The DCC assay was the method used in clinical practice at the time of patients’ diagnosis and upon which treatment decisions were made. This technique quantitatively measures ER protein as a continuous variable. However, due to its high costs and technical challenges related to its requirement for fresh-frozen tissue, it was later replaced by IHC methods that are semi-quantitative but correlate directly with morphology, for which centrally-determined scores are also available on the BCCA cohort [34]. Accordingly, in this study we examined the nestin expression in the ER+ group as determined by both methods (DCC and IHC) to overcome any differences in methodology that might bias the results.
For each case in the current study, archival FFPE tissue blocks from the primary tumor excision were originally retained at Vancouver General Hospital for central ER testing.
Clinical outcomes were periodically updated by the BCCA Breast Cancer outcome unit and last updated in 2008.
Tissue microarrays (TMAs) and Immunohistochemistry
Hematoxylin and eosin slides corresponding to each patient’s block were reviewed and areas of invasive breast carcinoma were circled by pathologists and used for TMA construction. Tissue cores of 0.6mm diameter were extracted from circled areas in each donor block and transferred to a recipient block as previously described [35–37]. The study required a series of 17 TMA blocks, from which 4 μm serial sections were cut and stained for nestin using the Ventana Systems Discovery XT semi-automated immunostainer (Ventana medical Systems Inc. Tucson, AZ USA). The staining for nestin was applied as previously published by Parry et al. [31] and validated by our group [25, 38]. Slides underwent antigen retrieval with standard Cell Conditioning 1 (Ventana Medical Systems) followed by 60 minutes of primary antibody incubation with heat, and detected using a DAB Map Detection Kit (Ventana Medical Systems). The primary antibody applied was a commercial mouse monoclonal anti-nestin antibody at a dilution of 1:50 (Santa Cruz Biotechnology Inc., clone 10C2, Dallas, Texas USA), followed by an incubation of the slides with a secondary antibody (Ventana universal secondary antibody) for an additional 32 minutes. Staining and visual scoring for additional IHC markers of ER, PR, HER2, CK5 and EGFR were previously detailed in publications from the Genetic Pathology Evaluation Center [9, 34, 39].
Nestin Scoring System and intrinsic subtyping
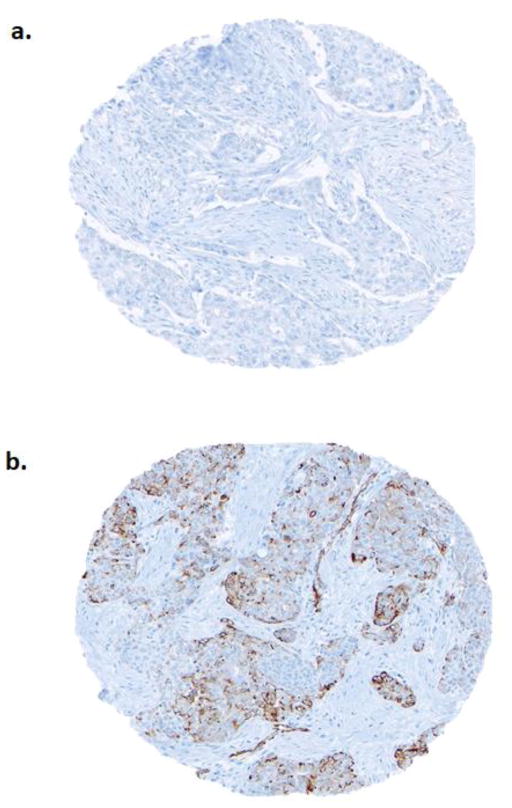
The scoring system used for nestin followed the prespecified, published criteria reported by Parry et al [31] which have been subsequently applied and validated by our group [25, 38]. Nestin expression was considered positive if cytoplasmic staining of any intensity above background was observed in≥1% of invasive breast carcinoma cells. For statistical analysis, nestin expression was binarized into negative (<1%) and positive (≥1%) categories. Scoring pathologists had no access to clinical data, and assessed staining without reference to any previous biomarker results. Representative negative and positive stains for nestin are displayed in Fig 1. Stained TMA slides were digitally scanned using a Bliss System (Bacus Laboratories/Olympus America, Lombard, IL USA). The primary image data are available for public access via the website of Genetic Pathology Evaluation Center (www.gpecimage.ubc.ca; username: nestin; password: abc123).
Fig. 1.
Nestin staining by immunohistochemistry. Tumors with <1% cytoplasmic staining for nestin were scored as negative (a) whereas those ≥1% were scored as positive (b)
The association between nestin expression and clinical outcomes was later tested in different subgroups of breast cancer, with a pre-planned focus on the ER positive subgroup. This ER positive cohort of cases was previously profiled using the quantitative reverse transcriptase (qRT)-PCR PAM50 gene expression method [40], and thus we used this method as a reference to validate the performance capacity of nestin in the ER positive setting. The methods involved in PAM50 intrinsic subtyping including FFPE macrodissection, RNA extraction, cDNA synthesis and qRT-PCR subtype predictions have been previously reported [40–41].
Statistical Analysis
The χ2 method was used to test associations between nestin status and standard clinicopathological variables. The primary outcome was breast cancer specific survival (BCSS), defined as time from diagnosis to either death caused by breast cancer or to last follow up. For univariate analyses, BCSS was quantified by Kaplan-Meier curves in the whole group and among different subgroups stratified according to ER status. Significance of differences in survival were assessed by log-rank test. For multivariate analyses, Cox proportional hazards models were used to assess the hazard ratio (HR) of different prognostic covariates with survival. P≤0.05 was considered as statistically significant. All statistical analyses were performed using SPSS version 23.
Results
Cohort Characteristics
Among 4543 cases included in the original cohort, 551 were excluded for various reasons (recurrent or metastatic disease at diagnosis, male breast cancer, insufficient material for TMA construction, and cases with missing cores in the TMA sections). Among the remaining cases, 3641 were interpretable for nestin IHC staining results and defined the study cohort. The clinicopathological characteristics of patients included in the study cohort are listed in Table 1. Adjuvant systemic therapy (chemotherapy, hormonal therapy or their combination) was prescribed in 58% of the overall patients, while 42% did not receive any adjuvant systemic therapy following local therapy.
Table 1.
The distribution of clinicopathological characteristics and their associations with nestin status in the whole series (n=3641)
| Clinico-pathological characteristic | Nestin negative (<1%) (n=3270) | Nestin positive (≥ 1%) (n=371) | Total (n=3641) | P-value |
|---|---|---|---|---|
| Age, years* | <0.001 | |||
| <60 | 1444 (50%) | 215 (63%) | 1659 (51%) | |
| ≥ 60 | 1451 (50%) | 124 (37%) | 1575 (49%) | |
| Tumor Size, cm | 0.2 | |||
| ≤2 | 1521 (53%) | 166 (49%) | 1687 (52%) | |
| >2 | 1361 (47%) | 172 (51%) | 1533 (48%) | |
| Tumor grade* | ||||
| 1–2 | 1347 (49%) | 78 (24%) | 1425 (46%) | <0.001 |
| 3 | 1428 (51%) | 251 (76%) | 1679 (54%) | |
| Lymph nodes* | 0.005 | |||
| Negative | 1797 (55%) | 232 (63%) | 2029 (56%) | |
| Positive | 1459 (45%) | 137 (37%) | 1596 (44%) | |
| Lymphovascular Invasion | 0.06 | |||
| Negative | 1492 (54%) | 196 (59%) | 1412 (46%) | |
| Positive | 1278 (46%) | 134 (41%) | 1688 (54%) | |
| ER status (by IHC)* | <0.001 | |||
| Negative | 678 (23%) | 218 (64%) | 896 (28%) | |
| Positive | 2207 (77%) | 120 (36%) | 2327 (72%) | |
| ER clinical status (by DCC)* | <0.001 | |||
| Negative (0–9 fmol/mg) | 448 (16%) | 184 (56%) | 632 (20%) | |
| Positive (>10 fmol/mg) | 2381 (84%) | 142 (44%) | 2523 (80%) | |
| ER positivity score (by IHC)* | <0.001 | |||
| 0 | 731 (27%) | 229 (69%) | 960 (31%) | |
| 1%–10% | 502 (18%) | 35 (11%) | 537 (18%) | |
| >10% | 1496 (55%) | 65 (20%) | 1561 (51%) | |
| HER-2 Status | ||||
| Negative | 2455 (87%) | 293 (88%) | 2748 (87%) | 0.6 |
| Positive | 383 (13%) | 42 (12%) | 425 (13%) | |
| KI67* | <0.001 | |||
| <14% | 1511 (57%) | 94 (30%) | 1605 (54%) | |
| ≥14% | 1141 (43%) | 216 (70%) | 1357 (46%) | |
| Systemic treatment* | <0.001 | |||
| No systemic therapy | 1351 (41%) | 190 (51%) | 1541 (42%) | |
| Tamoxifen only; no chemotherapy | 1132 (35%) | 56 (15%) | 1188 (33%) | |
| Chemotherapy only; no hormonal | 539 (17%) | 105 (28%) | 644 (18%) | |
| Chemotherapy + hormonal | 234 (7%) | 19 (5%) | 253 (7%) | |
| CK5* | <0.001 | |||
| Negative | 2418 (94%) | 209 (69%) | 2627 (91%) | |
| Positive | 156 (6%) | 93 (31%) | 249 (9%) | |
| EGFR* | <0.001 | |||
| Negative | 2363 (90%) | 173 (58%) | 2536 (87%) | |
| Positive | 259 (10%) | 127 (42%) | 386 (13%) | |
| Basal Subtype Groups* | ||||
| Core basal | 164 (6%) | 132 (40%) | 296 (10%) | <0.001 |
| Non-Core basal | 2632 (94%) | 200 (60%) | 2832 (90%) | |
| Triple Negative | 340 (12%) | 181 (55%) | 521 (17%) | <0.001 |
| Non-Triple negative | 2456 (88%) | 151 (45%) | 2607 (83%) |
Indicates significant difference, P≤0.05
ER: Estrogen receptor; IHC: Immunohistochemical; DCC: Dextran-coated charcoal of ligand binding assay; HER2: Human epidermal growth factor receptor-2; CK5: Cytokeratin 5; EGFR: Epidermal growth factor receptor.
Association of nestin IHC expression with clinicopathological characteristics
Among the 3641 cases interpretable for nestin by IHC, positive staining was found in 371 (10%) and was significantly associated with poor prognostic factors including younger age, higher grade, ER negativity and high proliferation index (P<0.001) (Table 1). Moreover, nestin positive expression was significantly associated with other markers of the basal-like phenotype: CK5, EGFR, “Core basal” and “Triple negative” IHC subtypes (P<0.001) (Table 1).
Univariate Breast Cancer Specific Survival (BCSS)
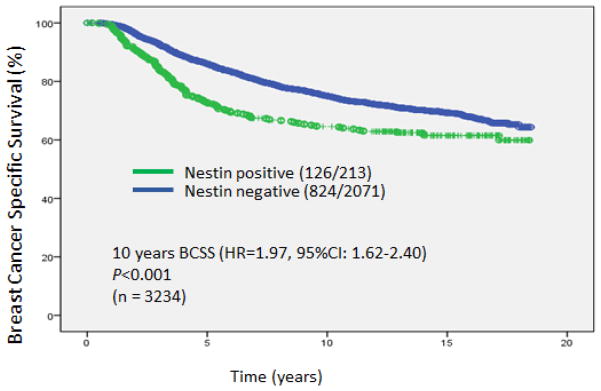
In the current study, median follow up was 12.6 years and BCSS was the primary endpoint. Within 10 years follow-up, patients with positive expression for nestin had a significantly lower BCSS (HR=1.97, 95%CI: 1.62–2.40; P<0.001) (Fig 2).
Fig. 2.
Kaplan-Meier curves for Breast Cancer Specific Survival based on nestin status for the whole series
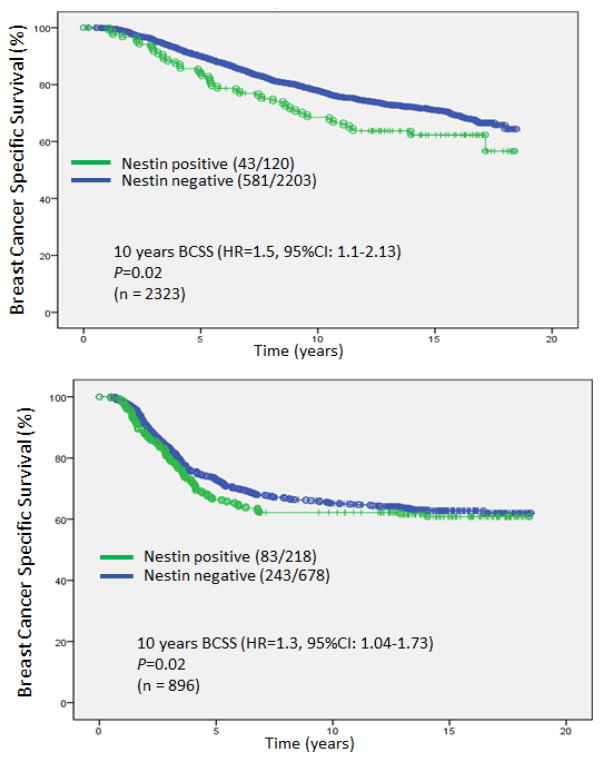
We further evaluated the prognostic significance of nestin expression within different IHC subgroups classified based on ER status. Importantly, within the large group of 2323 ER+ cases, nestin was positive in 120 patients (5%), and these patients had significantly inferior 10 year BCSS (HR=1.5, 95%CI: 1.1–2.13; P=0.02) (Fig 3a). Although the magnitude of hazard difference was not as pronounced, nestin was also significantly prognostic for 10 years BCSS among ER negative cases (HR=1.3, 95%CI: 1.04–1.73; P=0.02) (Fig 3b).
Fig. 3.
Kaplan-Meier curves for Breast Cancer Specific Survival based on nestin status for the estrogen (ER) positive subgroup (a) and the ER negative subgroup (b)
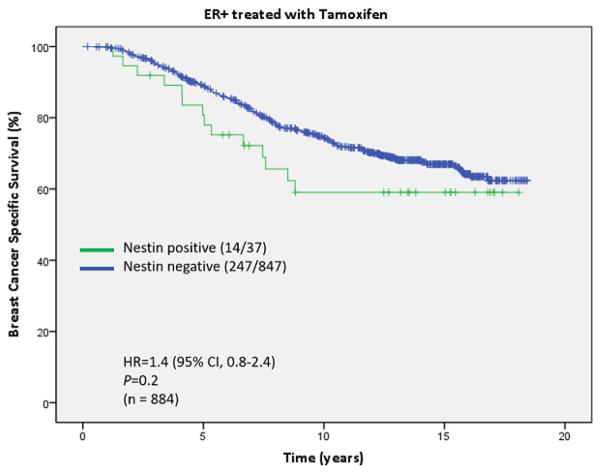
We also analyzed the prognostic significance of nestin in the patient subgroup that was ER positive and received adjuvant tamoxifen only. A trend for better BCSS was observed in nestin negative cases, but did not reach significance due to a small number of cases in the nestin positive subgroup (HR 1.4, P=0.2) (Fig 4).
Fig. 4.
Kaplan-Meier curves for Breast Cancer Specific Survival based on nestin status for the estrogen (ER) positive subgroup treated with tamoxifen only
Multivariate BCSS Analysis
To assess nestin expression as an independent prognostic marker for BCSS outcome, Cox proportional hazards model were built including different prognostic factors. Positive expression of nestin remained an independent poor prognostic factor at P= 0.05 for BCSS in the whole series (HR=1.24, 95%CI: 1.00–1.54) and within in the ER+ subgroup (HR=1.39, 95%CI: 1.00–1.93) (Table 2).
Table 2.
Multivariate analysis with hazard model for nestin in the whole series and in ER+ subgroup
| Multivariate Analysis for BCSS in the whole series (n=3234) | Multivariate Analysis for BCSS in the ER+ subgroup (n=2323) | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Nestin+ vs. Nestin− | 1.24 (1.00–1.54) | 0.05 | 1.39 (1.00–1.93) | 0.05 |
| Age ≥ 60 vs. < 60, years | 1.13 (0.98–1.31) | 0.09 | 1.12 (0.95–1.33) | 0.18 |
| Tumor size >2 vs. ≤2, cm | 1.59 (1.37–1.84) | <0.001 | 1.60 (1.34–1.92) | <0.001 |
| Grade {3} vs. {1,2} | 1.50 (1.28–1.77) | <0.001 | 1.50 (1.26–1.81) | <0.001 |
| Nodal status positive vs. negative | 1.90 (1.61–2.23) | <0.001 | 1.88 (1.55–2.30) | <0.001 |
| Lymphovascular Invasion positive vs. negative | 1.39 (1.18–1.63) | <0.001 | 1.28 (1.05–1.56) | 0.02 |
| ER positive vs. negative | 0.82 (0.70–0.97) | 0.02 | ---- | ---- |
| Her2 positive vs. negative | 1.37 (1.14–1.64) | 0.001 | 1.27 (0.97–1.65) | 0.08 |
| ki67 {≥14%} vs. {<14%} | 1.42 (1.22–1.64) | <0.001 | 1.60 (1.33–1.9) | <0.001 |
BCSS: Breast cancer specific survival; ER: Estrogen receptor; Her2: Human epidermal growth factor receptor-2.
The association of PAM50 intrinsic subtype with nestin IHC expression in the ER+ breast cancer subgroup
To establish the value of nestin to identify basal breast cancer cases within the ER+ subgroup, we tested the association of IHC nestin expression against a qRT-PCR PAM50 gene expression-determined subtype as a gold standard within the set of tamoxifen-treated, clinically ER+ patients. The gene expression subtypes of this cohort using the qRT-PCR PAM50 were available from a previously published study [33]. From a set of 842 ER+ cases originally subjected to PAM50 intrinsic subtyping by qRT-PCR, 672 were also evaluable for nestin expression by IHC. Nestin positive expression was significantly associated with basal breast cancer PAM50 subtype, while negative staining for nestin was associated with the non-basal intrinsic subtypes in the ER+ subgroup whether determined by biochemical (DCC) or by immunohistochemical (IHC) methods (Table 3).
Table 3.
A. PAM50 intrinsic subtype distribution within Estrogen Receptor Positive group A. ER assessment as determined by immunohistochemistry (IHC) B. ER assessment as determined by Dextran-Coated Charcoal of ligand binding assay (DCC)
| A. Estrogen Receptor Positive Subgroup (by IHC) | ||||
|---|---|---|---|---|
| PAM50 intrinsic subtype | Nestin negative (<1%) (n=640) | Nestin positive (≥ 1%) (n=32) | Total | P-value |
| 0.03 | ||||
| Luminal A | 314 (49%) | 10 (31%) | 324 | |
| Luminal B | 273 (42.7%) | 17 (53%) | 290 | |
| Her2-Enriched | 51 (8%) | 4 (13%) | 55 | |
| Basal-like | 2 (0.3%) | 1 (3%) | 3 | |
| Total | 640 | 32 | 672 | |
| B. Estrogen Receptor Positive Subgroup (by DCC) | ||||
| PAM50 intrinsic subtype | Nestin negative (<1%) (n=634) | Nestin positive (≥ 1%) (n=34) | Total | P-value |
| <0.001 | ||||
| Luminal A | 309 (49%) | 10 (29%) | 319 | |
| Luminal B | 269 (42%) | 17 (50%) | 286 | |
| Her2-Enriched | 51 (8%) | 4 (12%) | 55 | |
| Basal-like | 5 (1%) | 3 (9%) | 8 | |
| Total | 634 | 34 | 666 | |
Discussion
Despite the capacity of gene profiling methods to identify the basal-like subtype with high precision, the cost and complexity of molecular technologies has to date made their application in routine community clinical practice impractical. As such, inexpensive and easily applicable surrogate IHC markers are being developed to identify basal-like breast cancers. Nestin is an IHC basal marker that has been optimized and previously reported to be the best single positive IHC marker that identifies basal-like subtype regardless of ER status [25].
Using a large cohort of clinically-annotated invasive breast cancer cases, we provide evidence confirming this simple IHC marker, nestin, is significantly associated with basal-like differentiation, high risk clinicopathological factors and poor prognosis. These findings match the expected biology and aggressive clinical behavior that characterize the basal-like subtype as determined by gene expression [9–13]. Furthermore, the findings of the current study are consistent with studies reporting that nestin is associated with triple negative and basal-like differentiation [31, 32], poor clinicopatholgical features and reduced survival [28–30].
In addition to confirming previous findings, by using a much larger series, our cohort was sizeable enough to analyze the clinically-problematic issue of ER positive cases with a basal phenotype. We showed that nestin positivity identified a fraction (5%) of ER+ cases as associated with basal-like characteristics and reduced BCSS when compared to ER+ cases with negative nestin staining. These findings are in line with results from a previous study conducted by our group, showing that some ER+ cases that are currently being classified as luminal by IHC were actually basal-like by PAM50 gene expression assay and displayed an aggressive clinical behavior, suggesting that it was the PAM50 rather than the clinical IHC result that correctly predicted disease course [16]. Though the number of weakly ER positive cases in that series was underpowered to evaluate the performance of nestin in this specific subgroup, the evidence we present in the current study on the prognostic capacity of nestin using a much larger set including 2323 ER+ cases highlights an immediate clinical implication of these findings for the adjuvant treatment of ER+ cases overall. Currently, ER+ cases are all being assigned as luminal subtype by IHC, and those with an underlying basal phenotype are missed by “Triple negative” and “Core basal” definitions (and thus are offered unfavorable endocrine therapies instead of more effective early chemotherapies).
We recently suggested nestin to be one of two markers that can be used as an optimized basal IHC panel, consisting of either “nestin positive” or “inositol polyphosphate-4-phosphatase type IIB (INPP4B) negative” to accurately capture cases with the biology of basal-like breast cancer in the setting of ER positivity [38]. INPP4B, a signalling pathway enzyme that serves as a negative marker for the basal subtype, could not be reliably assessed on the current series due to the older age of the blocks and pre-analytical handling factors of the original source specimens. However, nestin, being a stable and relatively abundant intermediate filament protein, was robust to these issues, yielding a fraction of positive and negative cases very similar to values observed in previous reported cohorts [31, 32].
Current practices that approximate the basal breast cancer phenotype as “Triple negative” are convenient, can be easily extracted from patients’ reports, and are often used to guide contemporary treatment decisions. However, evidence for a potentially superior predictive effect for nestin than “Triple Negative” would require its application in the context of a randomized clinical trial. The retrospective data we present here regarding nestin and its relation to clinical outcomes does provide data supporting its application as an exploratory stratification factor on clinical trials materials so as to achieve a higher level of evidence for its prognostic and predictive capacity.
In conclusion, the current study presents data from a large TMA series including both ER+ and ER− cases, finding nestin expression to be an independent poor prognostic factor even in multivariate analyses. This study supports the potential clinical use of an easily-applicable IHC tool, particularly to identify clinically ER+ patients with a basal-like phenotype and poor prognosis that cannot be identified using “Triple-negative” or “Core basal” IHC definitions, and who could potentially be spared endocrine therapies that may be ineffective and/or receive adjuvant chemotherapy. Further studies, including tumor specimens from clinical trials datasets, are needed to validate the predictive capacity of nestin to improve treatment choices.
Acknowledgments
Funding Information
This work was supported by NIH SPECS grant (U01 CA114722-01). We thank Samuel Leung for his advice in statistical analysis.
Footnotes
Conflict of Interest:
Torsten O. Nielsen played a role in the development of the PAM50 gene expression classifier, which has been licensed to NanoString technologies (who did not participate or fund this work). None of the remaining authors have any financial or non-financial conflict of interest to declare for this work.
References
- 1.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Z, Fan C, Oh DS, Marron JS, He X, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 7.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–85. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 8.Cheang MC, Voduc KD, Tu D, Jiang S, Leung S, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res. 2012;18:2402–12. doi: 10.1158/1078-0432.CCR-11-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prat A, Adamo B, Fan C, Peg V, Vidal M, et al. Genomic analyses across six cancer types identify basal-like breast cancer as a unique molecular entity. Sci Rep. 2013;3:3544. doi: 10.1038/srep03544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18:123–33. doi: 10.1634/theoncologist.2012-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30:729–34. doi: 10.1200/JCO.2011.36.2574. [DOI] [PubMed] [Google Scholar]
- 16.Sheffield BS, Kos Z, Asleh-Aburaya K, Wang XQ, Leung S, et al. Molecular subtype profiling of invasive breast cancers weakly positive for estrogen receptor. Breast Cancer Res Treat. 2016;155:483–90. doi: 10.1007/s10549-016-3689-z. [DOI] [PubMed] [Google Scholar]
- 17.Deyarmin B, Kane JL, Valente AL, van Laar R, Gallagher C, et al. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;20:87–93. doi: 10.1245/s10434-012-2588-8. [DOI] [PubMed] [Google Scholar]
- 18.(EBCTCG) EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 19.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123:21–7. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 20.Khoshnoud MR, Löfdahl B, Fohlin H, Fornander T, Stål O, et al. Immunohistochemistry compared to cytosol assays for determination of estrogen receptor and prediction of the long-term effect of adjuvant tamoxifen. Breast Cancer Res Treat. 2011;126:421–30. doi: 10.1007/s10549-010-1202-7. [DOI] [PubMed] [Google Scholar]
- 21.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 22.Guérette D, Khan PA, Savard PE, Vincent M. Molecular evolution of type VI intermediate filament proteins. BMC Evol Biol. 2007;7:164. doi: 10.1186/1471-2148-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Cherukuri P, Li N, Cowling V, Spinella M, et al. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501–10. doi: 10.1158/0008-5472.CAN-05-4571. [DOI] [PubMed] [Google Scholar]
- 24.Krüger K, Wik E, Knutsvik G, Nalwoga H, Klingen TA, et al. Expression of Nestin associates with BRCA1 mutations, a basal-like phenotype and aggressive breast cancer. Sci Rep. 2017;7:1089. doi: 10.1038/s41598-017-00862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Won JR, Gao D, Chow C, Cheng J, Lau SY, et al. A survey of immunohistochemical biomarkers for basal-like breast cancer against a gene expression profile gold standard. Mod Pathol. 2013;26:1438–50. doi: 10.1038/modpathol.2013.97. [DOI] [PubMed] [Google Scholar]
- 26.Onisim A, Achimas-Cadariu A, Vlad C, Kubelac P, Achimas-Cadariu P. Current insights into the association of Nestin with tumor angiogenesis. J BUON. 2015;20:699–706. [PubMed] [Google Scholar]
- 27.Nowak A, Grzegrzolka J, Paprocka M, Piotrowska A, Rys J, et al. Nestin-positive microvessel density is an independent prognostic factor in breast cancer. Int J Oncol. 2017;51:668–76. doi: 10.3892/ijo.2017.4057. [DOI] [PubMed] [Google Scholar]
- 28.Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol. 2011;17:409–18. doi: 10.3748/wjg.v17.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neradil J, Veselska R. Nestin as a marker of cancer stem cells. Cancer Sci. 2015;106:803–11. doi: 10.1111/cas.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Chen B, Zhu J, Zhang R, Yao F, et al. Clinical implications for nestin protein expression in breast cancer. Cancer Sci. 2010;101:815–9. doi: 10.1111/j.1349-7006.2009.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parry S, Savage K, Marchiò C, Reis-Filho JS. Nestin is expressed in basal-like and triple negative breast cancers. J Clin Pathol. 2008;61:1045–50. doi: 10.1136/jcp.2008.058750. [DOI] [PubMed] [Google Scholar]
- 32.Piras F, Ionta MT, Lai S, Perra MT, Atzori F, et al. Nestin expression associates with poor prognosis and triple negative phenotype in locally advanced (T4) breast cancer. Eur J Histochem. 2011;55:e39. doi: 10.4081/ejh.2011.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Lu P, Zhang H, Xu H, Gao N, et al. Nestin positively regulates the Wnt/β-catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Res. 2014;16:408. doi: 10.1186/s13058-014-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheang MC, Treaba DO, Speers CH, Olivotto IA, Bajdik CD, et al. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol. 2006;24:5637–44. doi: 10.1200/JCO.2005.05.4155. [DOI] [PubMed] [Google Scholar]
- 35.Voduc D, Kenney C, Nielsen TO. Tissue microarrays in clinical oncology. Semin Radiat Oncol. 2008;18:89–97. doi: 10.1016/j.semradonc.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 37.Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, et al. Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol. 2002;117:723–8. doi: 10.1309/PEF8-GL6F-YWMC-AG56. [DOI] [PubMed] [Google Scholar]
- 38.Asleh-Aburaya K, Sheffield BS, Kos Z, Won JR, Wang XQ, et al. Basal biomarkers Nestin and INPP4b accurately identify intrinsic subtype in breast cancers that are weakly positive for estrogen receptor. Histopathology. 2016 doi: 10.1111/his.13038. [DOI] [PubMed] [Google Scholar]
- 39.Chia S, Norris B, Speers C, Cheang M, Gilks B, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–32. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastien RR, Rodríguez-Lescure Á, Ebbert MT, Prat A, Munárriz B, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]