Abstract
Background
Current diagnosis of drug addiction like other mental disorders is based on clinical symptoms not on neural pathophysiology and consequently, does not provide useful information on the underlying pathophysiology and may impede the efforts to identify the underlying mechanisms. Identifying the functional deficits that are relevant to addiction and can be traced to the neural systems will greatly facility our understanding of the heterogeneity of the condition and improve the future diagnosis and treatment. Cocaine addiction is characterized by the continued use despite the dire consequences and the deficit in inhibitory control may play a key role in this process. This study aimed to develop a paradigm to measure the punishment-induced inhibitory regulation of reward-seeking behavior.
Methods
Rats were first trained to self-administer sucrose pellets under a chained schedule and then the breaking points (BPs) under the progressive-ratio schedule and the intensity-response effects of footshock punishment on sucrose SA were measured. Subsequently, the rats went on to self-administer intravenous cocaine and the BPs and the punishment intensity-response effects were similarly determined.
Results
The areas under the punishment intensity-response curves (AUCs) were calculated and used as an indicator of the sensitivity of the inhibitory system. The BPs for cocaine were not correlated with the AUCs. Furthermore, the change in the BPs for cocaine induced by changing cocaine dose did not predict the change in the AUCs.
Conclusion
The intensity-response effects of punishment can be used to measure the function or sensitivity of the inhibitory system independent of the motivational state.
Keywords: cocaine, motivation, punishment, self-administration, research domain criteria
Introduction
Drug addiction is a chronic, relapsing, mental disorder and characterized by the compulsive drug-seeking and drug-taking behaviors. Its diagnosis is largely based on the pathological pattern of drug use (American Psychiatric Association 2013). One prominent issue with such an approach is the heterogeneity of the diagnosis. Patients with the same diagnosis could present different combinations of the symptoms. Such a heterogeneity poses a significant problem for treatment because the heterogeneous patients unlikely respond to the same treatment equally well. Incorporation of the neuroscience-based criteria indicating the impairments of the neural systems or functional domains may significantly improve the diagnosis (Cuthbert and Insel 2010; Insel et al. 2010). The NIMH has proposed Research Domain Criteria (RDoC) that presently have identified five brain systems or functional domains germane to mental disorders: negative valence system, positive valence system, cognitive systems, systems for social processes, and arousal/regulatory systems (Insel et al. 2010). Heeding such a shift, the National Institute of Alcohol Abuse and Alcoholism (NIAAA) has proposed Alcohol Addiction RDoC (AARDoC) and temporarily proposed six domains: reward, stress, affect, incentive salience, executive function, and social processes relevant to alcohol addiction (Litten et al. 2015). Encouraged by these developments, a recent effort has been made to propose the Addictions Neuroclinical Assessment (ANA) that encourages assessment on three functional domains in drug addiction: incentive salience, negative emotionality and, executive function (Kwako et al. 2017; Kwako et al. 2016). To be successful, the functional constructs under each domain have to be identified and the paradigms for measuring these constructs need to be developed.
One of the most important constructs in drug addiction is the compulsive drug use, typically defined as the continued drug use despite the dire consequences. Identifying the functional domains important to development of the compulsive drug use is critical for guiding the research on the neural mechanisms underlying drug addiction. Over the years several theories, not mutually exclusive, have been proposed. The incentive-sensitization theory proposes that repeated drug exposures sensitize the neural circuit responsible for attributing the incentive salience to the drug and drug-associated stimuli and consequently, lead to the abnormally elevated motivation for the drug that drives the compulsive behavior (Robinson and Berridge 1993; 2008). The theory of the “dark side” of addiction emphasizes the role of the negative affect induced by a combination of reward deficit and stress surfeit (Koob and Mason 2016). Others have proposed that repeated drug uses strengthen stimulus-response association and ultimately, promote habit-like behaviors. Automation of the learned behavior is thought to contribute to the compulsive drug use (Everitt and Robbins 2016; Hyman 2005). Although these theories are well supported by the experimental data, they still fall short of explaining the fact that the dire consequences have no impact on the behavior of addicted patients. Such an observation suggests that the inhibitory control function may be impaired in drug addiction. Indeed, the deficit in inhibitory control has been demonstrated after exposure to drugs of abuse (Jentsch et al. 2002); (Calu et al. 2007; Krueger et al. 2009; Schoenbaum et al. 2004) and proposed to play a critical role in compulsive drug-seeking and drug-taking behaviors (Belin et al. 2013; Everitt 2014; Jentsch and Taylor 1999; Robinson and Berridge 2008).
Although compulsive drug use is typically defined as the continued drug use in the face of the negative consequences, the drug-seeking and drug-taking behaviors in the previous studies rarely result in negative consequences until recently (Belin et al. 2008; Chen et al. 2013; Deroche-Gamonet et al. 2004; Kasanetz et al. 2013; Pascoli et al. 2015; Xue et al. 2012). These studies provide evidence that development of compulsive drug use is influenced by both genetic/epigenetic factors and extent of drug use. Despite the progress, it is still unclear what functional changes drive the compulsive drug behavior. Either elevated motivation or impaired inhibitory control may explain the results. Thus, a paradigm is needed to measure them separately. We and others have demonstrated that cocaine SA is inhibited by footshock punishment in an intensity-dependent manner (Bentzley et al. 2014; Johanson and Schuster 1975; Xue et al. 2012). The rate of change in the punishment effects may indicate the function or sensitivity of the inhibitory system independent of the motivation. To test this idea, a modified paradigm based on our previous study (Xue et al. 2012) was used to determine the intensity-response effects of footshock punishment on cocaine SA. To determine whether the effects of punishment are related to the motivational state, the same rats were also tested under the progressive-ratio (PR) schedule of reinforcement to determine the breakpoints (BPs), an indicator of the motivational levels. If the sensitivity of the inhibitory system is independent of the motivation, then the two should not be correlated. We further reasoned that increasing the motivation for cocaine should not alter the sensitivity of the inhibitory system. To test this idea, we investigated whether increasing cocaine dose can alter the punishment sensitivity.
Materials and Methods
Subjects and Drugs
Male outbred Wistar rats (300–390g, Charles River, n = 41) were housed individually in plastic home cages in a temperature- and humidity-controlled colony room on a 12-h reverse light-dark cycle (lights off at 08:00). Experiments were conducted during the dark phase (between 09:00 and 18:00). Cocaine hydrochloride (the National Institute on Drug Abuse, Bethesda, MD) was dissolved in physiological saline to prepare the solutions with concentrations of 2.5, and 5 mg/ml (salt), respectively. All procedures followed the Guidelines for the Care and Use of Laboratory Animals (National Research Council 2011) and were approved by University of Tennessee Health Science Center Animal Care and Use Committee.
Sucrose SA
The rats were placed on a restricted diet to reach ~85% of free feeding body weights and thereafter, fed daily with ~20 g regular rat chow. They were first trained to press a lever for a sucrose pellet (45 mg, Research Diet, New Brunswick, NJ) in a standard operant conditioning chamber equipped with two metal levers (Med Associates Inc., St. Albans, VT). Once they were able to obtained 100 reinforcements within two hours, the training with the chained schedule of reinforcement began. The schedule requires sequential responses on the two levers, designated as the seeking and taking levers, respectively. Only one lever is available at one time. Pressing the seeking lever leads to lever retraction and access to the taking lever after a delay. Pressing the taking lever results in reward delivery and lever retraction followed by a timeout period. Under such a schedule, reward-seeking and reward-taking behaviors can be investigated separately (Pelloux et al. 2007; Vanderschuren and Everitt 2004; Xue et al. 2012). In this study, 2-second delay and 5-second timeout were used in daily 30-minute sessions (Xue et al. 2012). The session ended when 30 minutes had passed or 100 reinforcements had been earned, whichever occurred first. Training continued until the rats reached the criteria: the rates of reinforcement varied < 10% for three consecutive sessions and a minimum of 10 training sessions.
Motivation for Sucrose
The BPs (Roberts et al. 1989a), defined as the last ratio finished under the PR schedule, were determined in a 4-hour test session. The schedule was only effective for the seeking lever while the schedule for the taking lever remained the same as during sucrose SA. The PR session ended if the rats had failed to respond within a half hour or 4-hour had passed, whichever occurred first. The BPs were measured for different values of sucrose (one and three pellets) in the same rats. The order of the two tests was counterbalanced among the rats and SA training continued between the tests to ensure the stable SA behavior before next test.
Effects of Punishment on Sucrose-seeking Behavior
Because compulsive cocaine use may be related to the basal sensitivity of the inhibitory system to punishment, the intensity-response effects of punishment on sucrose SA were measured before the rats were exposed to cocaine SA. The electric footshock was delivered to the grid floor of the operant conditioning chamber immediately after the seeking but not the taking responses. The four different levels of current intensity including 0.2, 0.3, 0.4, and 0.5 mA with a duration of 0.5 second were tested in a session consisting of four 15-min blocks. The first response on the seeking lever marked the onset of the session and the current intensity was increased in each subsequent block beginning from 0.2 mA. To determine whether an increase in reward value alters the effects of punishment, the intensity-response effects of punishment were determined for one and three sucrose pellets in the same rats, respectively. To minimize the potential impact of satiation on performance, the maximum number of reinforcements was set for each block: 25 or eight reinforcements for one or three pellets, respectively. The chained schedule was used to avoid simultaneous delivery of sucrose and footshock that facilitates counterconditioning between reward and footshock (Dickinson and Pearce 1976; Pearce and Dickinson 1975). The counterconditioning may turn footshock into part of the stimuli predicting reward and consequently, reduce the effect of punishment. A recent study showed that the effect of counterconditioning can be minimized by temporally separating reward and punishment (Pelloux et al. 2007).
Surgery
After the sucrose tests, a subpopulation of the rats (n = 30) randomly selected from the 41 rats were catheterized under the anesthesia with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg), intramuscularly. The other 11 rats were used for other purposes and their BPs and sensitivity to punishment were not significantly differ from those of the 30 rats except for the sensitivity at three pellets between the two groups (t = 2.10, p = 0.04). However, the significance disappeared after adjusting for the four multiple tests (2 value × 2 behavior). The catheter was made of a ~12-cm polyurethane tubing (MRE-037; Braintree Scientific, Inc. MA, USA) connected to the rat vascular access button (VAB95BS; Instech, PA, USA). The button was placed subcutaneously in the mid-scapular region and the tubing was tunneled under the skin and inserted into the right external jugular vein with a length of ~3.5 cm. Buprenorphine (0.03 mg/kg, subcutaneously) was given for the post-surgical analgesia. Catheter patency was evaluated by injecting 0.1 ml Brevital (1%) through the catheters as necessary and loss of muscle tone within five seconds indicates a patent catheter.
Cocaine SA
After 5- or 6-day recovery from surgery, the rats began to self-administer 0.125 mg/infusion of cocaine in daily 2-hour sessions under the same chained schedule as described for sucrose SA except that the timeout period was 20 seconds and a 10-second compound stimulus consisting of the two flashing cue lights and tone was presented after onset of cocaine infusions. The session ended when two hours had passed or 80 infusions had been self-administered, whichever occurred first. The training was conducted 6–7 days per week until they reached the criterion: the number of cocaine infusions varied by < 20% for three consecutive sessions. Following the PR and punishment tests for the dose of 0.125 mg (see below), the rats began to self-administer 0.25 mg/infusion of cocaine for another 10 sessions followed by the PR and punishment tests for the dose of 0.25 mg. A volume of 0.05 ml of 2.5 mg/ml or 5 mg/ml of cocaine was infused over a period of 0.55 second to deliver 0.125 or 0.25 mg, respectively.
Motivation for Cocaine
The BPs for cocaine were measured similarly as described for sucrose except that the timeout was 20 seconds and the 10-second stimulus associated with cocaine SA was presented. The order of the tests for two doses of cocaine was sequential from the lower to higher dose.
Effects of Punishment on Cocaine-seeking Behavior
The effects of punishment were determined similarly as described for sucrose except that no maximum number of cocaine infusions was set and each block lasted half hour. Because the rats regulated the number of cocaine infusions in daily 2-hr sessions, there was less concern over the satiation effects from the early blocks on the performance of the later blocks. To determine whether an increase in cocaine dose alters the effects of punishment, the intensity-response effects of punishment on cocaine SA were sequentially determined in the same rats, respectively.
Statistics
The rates of reinforcement were calculated as the number of reinforcements divided by the session durations. Because the durations of the SA and punishment sessions were different for cocaine and sucrose, the rates were scaled to half hour to facilitate comparisons. The rates from the SA training sessions before the punishment tests were used as the baseline controls. After normalizing the rates from each block of the punishment session to the baseline controls, the intensity-response curves were constructed and the area under the curves (AUCs) were calculated for each rat with the trapezoid method.
The Pearson or Spearman correlation coefficients were calculated depending on the data distribution. Either the D'Agostino & Pearson omnibus or Shapiro-Wilk normality test was used to determine whether the distribution deviates from the normal distribution (the latter test was used if the sample size was too small for the former test). The AUCs at different doses of cocaine or sucrose values were compared with either a paired t test or Wilcoxon signed-rank test. In addition, a two-way repeated measures analysis of variance (ANOVAs) was used to analyze the interaction between reward value and punishment intensity. All the analyses were conducted with the GraphPad Prism version 7.03 (GraphPad Software, La Jolla California). The significance level was set at 0.05.
Results
Effects of Punishment on Sucrose SA Is Independent of the Motivation for Sucrose
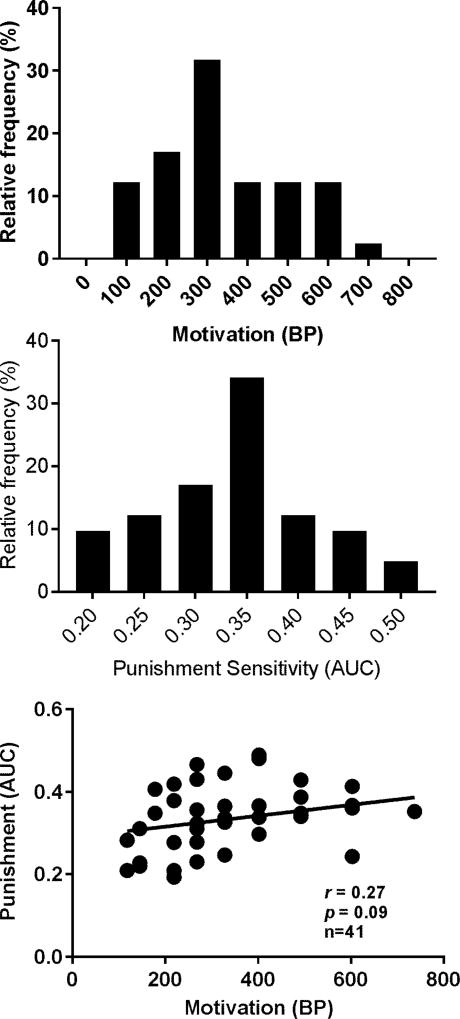
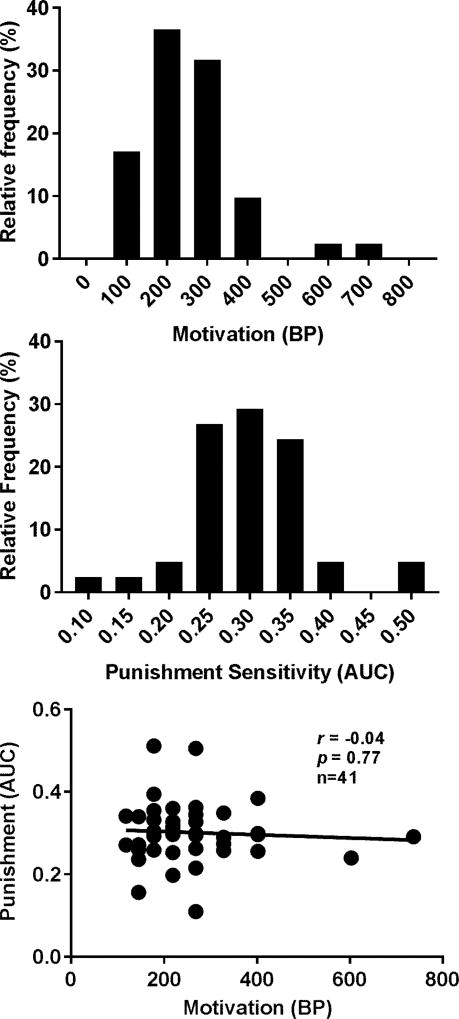
The BPs and AUCs for one sucrose pellet significantly deviated from the normal distribution (p < 0.001 and p = 0.03 for BPs and AUCs, respectively) and there was no significant correlation between the two. Interestingly, the distributions of BPs and AUCs for three sucrose pellets passed the normality test as shown in upper two panels of Figure 2 and no significant correlation was found between the two.
Figure 2.
Distributions of and correlation between the BPs and AUCs with sucrose SA reinforced by three sucrose pellet. Upper panel: distribution of the BPs. Middle panel: distribution of the AUCs. Lower Panel: Pearson correlation between the BPs and AUCs. No significant correlation was found between the two.
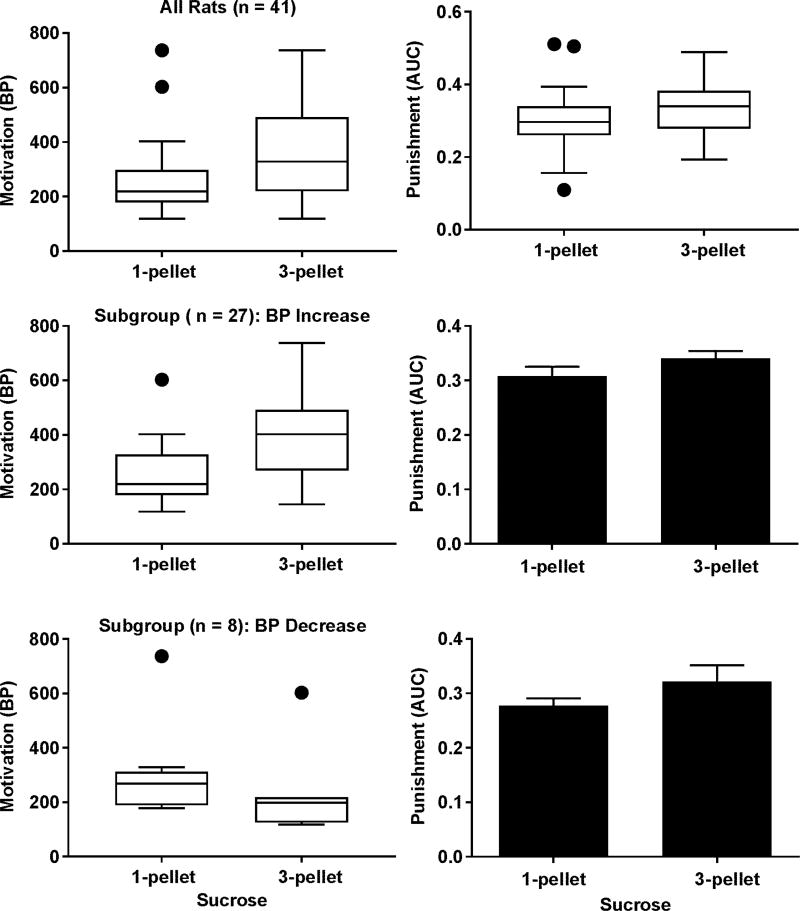
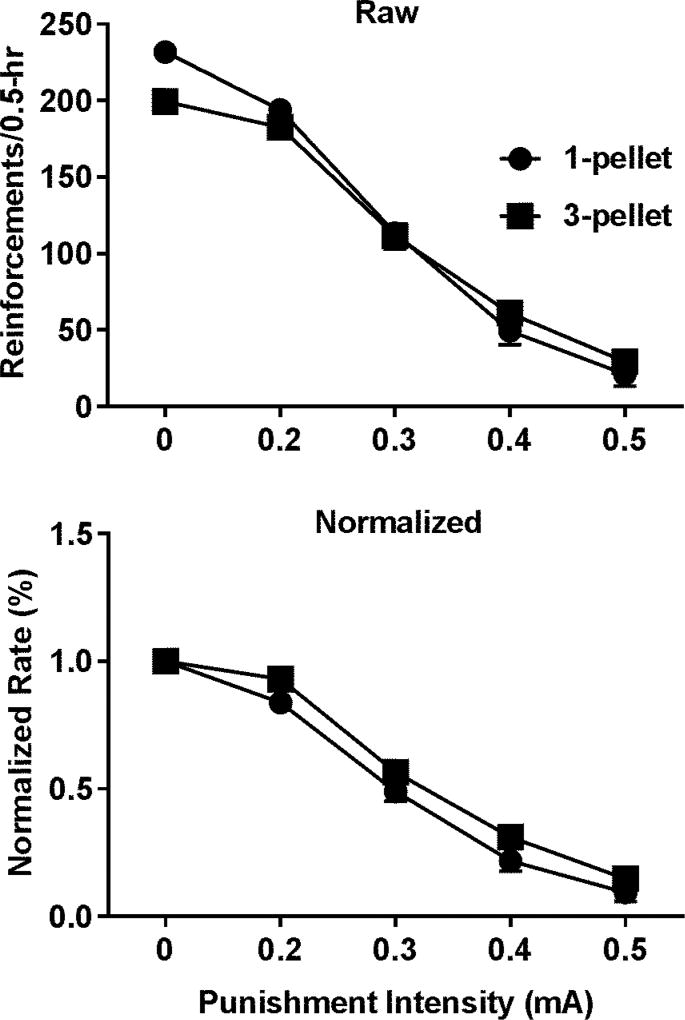
The BPs for three pellets were significantly higher than that for one pellet (the upper left panel of Figure 3, p < 0.001) but no significance was found for the differences in the AUCs (the upper right panel of Figure 3). We noticed that increasing the sucrose value caused either an increase or decrease or no change in the BPs among the rats. Thus, we were concerned that the failure to find the increase in the AUCs in response to the increased sucrose value may result from the mixture of the two subgroups of rats showing the increased and decreased BPs. To address this issue, a paired t test was used to test whether the AUCs differ in the two subgroups and no significant difference was found. In addition, if the motivation and punishment sensitivity are independent, then the sucrose value and punishment intensity should not interact with each other. To this end, the normalized data from the intensity-response effects of punishment on sucrose SA reinforced by one and three sucrose pellets were analyzed with the two-way repeated measures ANOVA. As shown in Figure 4, no significant interaction was found between the two. Note that the data were presented as either box and whisker plots or bar graphs indicating whether the parametric or non-parametric statistic tests were used.
Figure 3.
Impact of different motivational levels for sucrose on the punishment sensitivity. Upper left panel: box and whisker plot showing an increase in the BPs at the group level (Wilcoxon signed-rank test, p < 0.001). Upper right panel: box and whisker plot showing no significant change in the AUCs at the group level (Wilcoxon signed-rank test, p = 0.06). Middle left panel: box and whisker plot showing an increase in the BPs in a subgroup in response to increased sucrose value (Wilcoxon signed-rank test, p < 0.001). Middle right panel: bar graph showing no significant change in the AUCs within the BP-increasing subgroup (paired t test, p = 0.12). Lower left panel: box and whisker plot showing a decrease in the BPs in a subgroup in response to increased sucrose value (Wilcoxon signed-rank test, p < 0.01). Lower right panel: bar graph showing no significant change in the AUCs within the BP-decreasing subgroup (paired t test, p = 0.18). Data are represented as mean ± SEM in the bar graph. The box extends from 25th to 75th percentile. The upper whisker represents the largest value smaller than the sum of the 75th percentile and 1.5 times the inter-quartile distance or the maximum of the data if the maximum is smaller than the sum. The lower whisker represents the smallest value greater than the difference between the 25th percentile and 1.5 times the inter-quartile distance or the minimum of the data if the minimum greater than the difference. The filled circles represent data beyond the whiskers.
Figure 4.
Intensity-response effects of punishment on sucrose SA. Upper panel: effects on the raw rates of sucrose SA reinforced by different sucrose values. Lower panel: effects on the normalized rates of sucrose SA. No significant interaction between punishment intensity and sucrose value was found (two-way repeated measures ANOVA, p = 0.34). Data are represented as mean ± SEM.
Effects of Punishment on Cocaine SA Is Independent of the Motivation for Cocaine
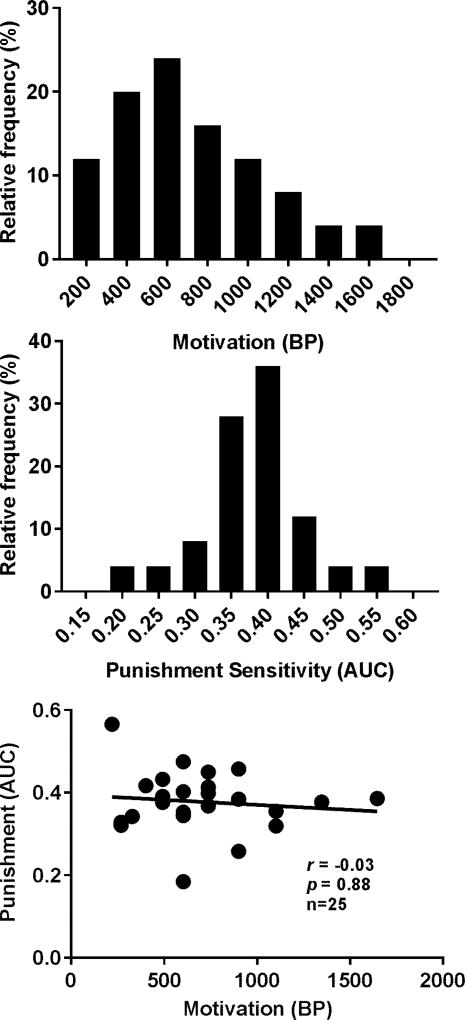
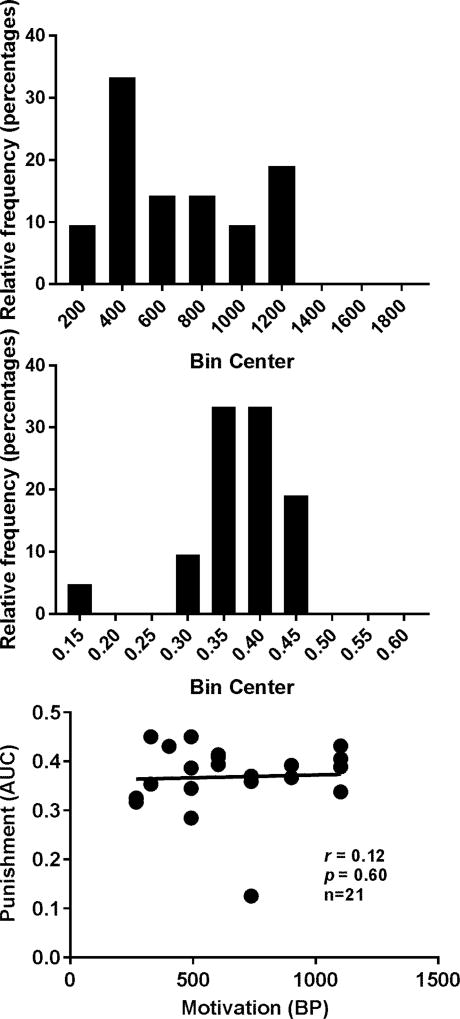
Five out of the 30 rats had catheter failures during cocaine SA of 0.125 mg/infusion. The remaining rats reached the SA training criterion on an average of 7.8 ± 0.6 days (mean ± SEM). The total cocaine intake before the tests was on the average of 175.9 ± 11.4 mg/kg (mean ±SEM). The distributions of the AUCs but not BPs passed the normality test as shown in Figure 5 (p = 0.14 and p = 0.03 for AUCs and BPs, respectively). There was no significant correlation between the two. During cocaine SA of 0.25 mg/infusion, four more rats had catheter failures. Thus the data analysis was conducted for the remaining 21 rats. At 0.25 mg/infusion, the distribution of the BPs but not AUCs passed the normality test as shown in Figure 6 (p = 0.25 and p < 0.0001 for BPs and AUCs, respectively). No significant correlation was found between the two.
Figure 5.
Distributions of and correlation between the BPs and AUCs with cocaine SA reinforced by the dose of 0.125 mg/infusion. Upper panel: distribution of the BPs. Middle panel: distribution of the AUCs. Lower Panel: Spearman correlation between the BPs and AUCs. No significant correlation was found.
Figure 6.
Distributions of the BPs and AUCs and their relationship with cocaine SA reinforced by the dose of 0.25 mg/infusion. Upper panel: distribution of the BPs. Middle panel: distribution of the AUCs. Lower Panel: Spearman correlation between the BPs and AUCs. No significant correlation was found.
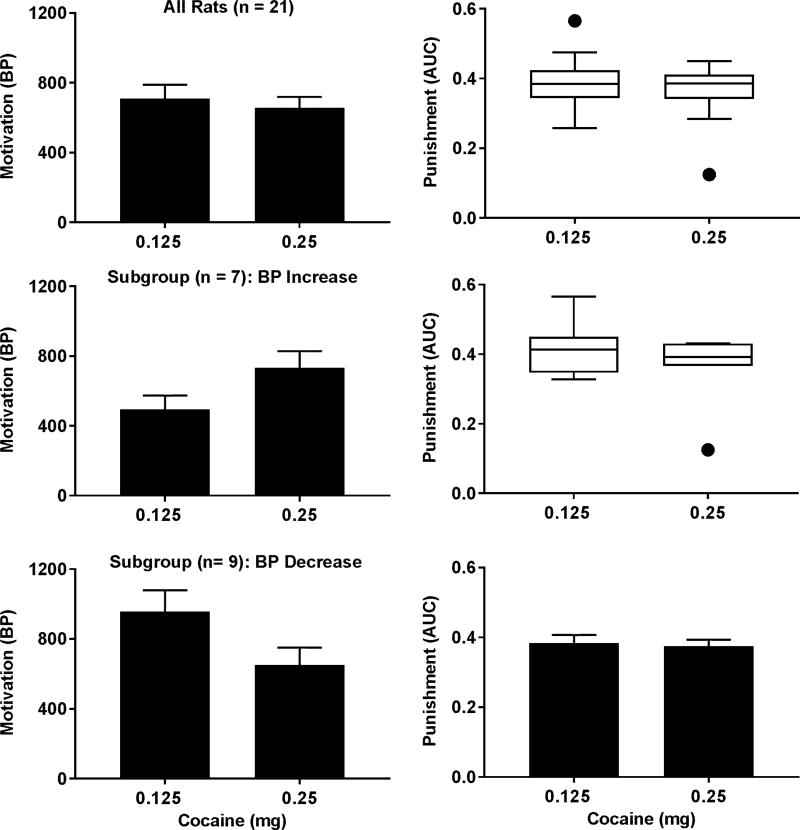
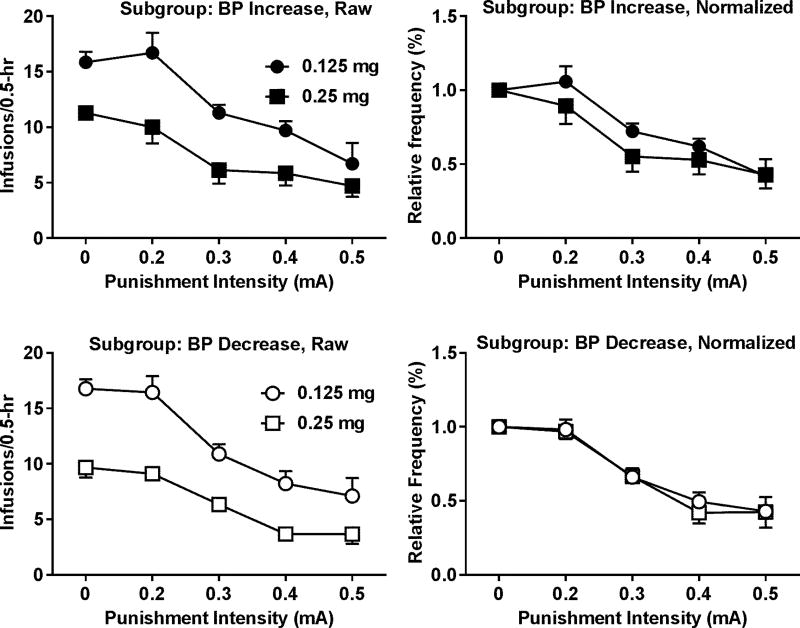
The Wilcoxon signed-rank test was used to determine the difference in the AUCs between the two doses of cocaine and revealed no significant difference (Figure 7). Like the observation for sucrose, we also noticed that increasing the dose did not increase the BPs for all the rats. Thus, the impact of motivation on the punishment was further analyzed in the two subgroups. The distributions of the BPs and AUCs passed the normality test except the BP-increasing subgroup at the dose of 0.25 mg/infusion (p = 0.0012). The Wilcoxon signed-rank test and the paired t test revealed no significant difference between the two doses within each subgroup. To further determine whether the punishment sensitivity is altered by the motivation for cocaine, the interaction between punishment intensity and cocaine dose was analyzed with the two-way repeated measures ANOVA on the normalized data for the BP-increasing and BP-decreasing subgroups, respectively. As shown in Figure 8, no significant interaction was found in either group.
Figure 7.
Impact of different motivational levels for cocaine on the punishment sensitivity. Upper left panel: bar graph showing no change in the BPs at the group level (paired t test, p = 0.44). Upper right panel: box and whisker plot showing no significant change in the AUCs at the group level (Wilcoxon signed-rank test, p = 0.51). Middle left panel: bar graph showing an increase in the BPs of the BP-increasing subgroup (paired t test, p < 0.01). Middle right panel: box and whisker plot showing no significant change in the AUCs within the BP-increase subgroup (Wilcoxon signed-rank test, p = 0.38). Lower left panel: bar graph showing a decrease in the BPs of the BP-decreasing subgroup (paired t test, p < 0.01). Lower right panel: bar graph showing no significant change in the AUCs within the BP-decrease subgroup (paired t test, p = 0.72). See Figure 3 for other details.
Figure 8.
Intensity-response effects of punishment on cocaine SA. Upper left panel: effects of punishment on the raw rates of cocaine SA reinforced by different doses of cocaine in the BP-increasing subgroup. Upper right panel: intensity-response effects of punishment on the normalized rates of cocaine SA in the BP-increasing subgroup. No significant interaction between punishment intensity and cocaine dose was found (F4, 24 = 1.54, p = 0.22). Lower left panel: effects of punishment on the raw rates of cocaine SA reinforced by the different doses of cocaine in the BP-decreasing subgroup. Lower right panel: effects of punishment on the normalized rates of cocaine SA in the BP-decreasing subgroup. No significant interaction between punishment intensity and cocaine dose was found (F4, 32 = 0.27, p = 0.89). Data are represented as mean ± SEM.
Discussion
Deficit in the inhibitory control over cocaine use may play a key role in development of the compulsive cocaine use. One challenge facing the preclinical studies is the lack of the animal models that can untangle the role of the inhibitory deficit from the elevated motivation for cocaine in the compulsive behavior. The model developed in the current study can be used to study the punishment-mediated inhibitory control over cocaine SA independent of the motivation measured with the PR schedule. Our data provide three lines of evidence supporting the idea. First, taking advantage of natural variations in the BPs and AUCs among the rats, we found no significant correlation between them at either dose of cocaine. Second, the rats showing dose-dependent increases or decreases in the BPs do not show corresponding changes in the AUCs. Third, cocaine dose does not interact with punishment intensity suggesting that the effects of punishment at the different levels of intensity remain the same regardless of the dose. These results demonstrate that the intensity-response effects of punishment can be used as a measurement of the inhibitory function independent of the motivation. The similar findings for sucrose further support this idea.
Recent preclinical studies demonstrate that the extent of cocaine use and genetic/epigenetic factors may interact to facilitate development of compulsive cocaine use. For example, cocaine SA became compulsive in a subgroup of rats after a prolonged period (more than 70 days) of cocaine SA (Deroche-Gamonet et al. 2004). In addition, an extended (6 hr) but not limited (1 hr) daily access to cocaine for approximately two weeks also facilitate development of the compulsive cocaine-seeking behavior (Pelloux et al. 2012; Pelloux et al. 2015). One unsolved issue is whether development of the compulsive behavior is related to the increased motivation for cocaine given that the motivation for cocaine increases after a period of cocaine SA (Ducret et al. 2016; Hao et al. 2010; Morgan et al. 2005; Smith et al. 2008). There are conflicting results in this regard (Deroche-Gamonet et al. 2004; Pelloux et al. 2012; Pelloux et al. 2007) and the reasons for the different results are not entirely clear. One possibility is that these studies arbitrarily chose different intensity levels of footshock. The effects of punishment on cocaine SA depend on the footshock intensity (Grove and Schuster 1974; Johanson 1977). If the intensity is too high, no animals would continue to respond. On the other hand, if the intensity is too low, the behavior might not be altered. Thus, choosing the intensity is a key factor in determining the effects of punishment. Thus, the difference in the intensity could contribute to the conflicting results. Another possibility is that these studies used different PR schedules. The BP is affected by how fast the response requirement is increased under the PR schedules (Bradshaw and Killeen 2012). More importantly, the results from these studies do not provide information on how the sensitivity or responsiveness of the inhibitory system is affected by the extent of cocaine exposure. To answer this question, the responses to different intensity levels of punishment need to be determined. The paradigm developed in this study avoids arbitrarily to choose the punishment intensity and may be used to measure the sensitivity of drug SA to punishment. This paradigm will be instrumental in facilitating the research on the neurobiological mechanisms underlying the compulsive drug use.
The importance of individual differences in the vulnerability to develop addiction are increasingly realized (Belin et al. 2009; Belin et al. 2008; Bentzley et al. 2014; Deroche-Gamonet et al. 2004). Analyzing data at the group or population level often misses such differences. Thus, paying attention to the behavioral changes at individual or subpopulation levels will be the key to identify the vulnerable ones and may provide important information on the mechanisms of drug addiction. Our study showed individual differences in response to the increase in cocaine dose. It has long been known that there are individual differences in the optimum dose that maintains the highest BPs (Bedford et al. 1978; Roberts et al. 1989b). Our data demonstrate that the AUCs do not change in the subgroups showing either a decrease or increase in the BPs. These analyses at the subgroups provide the evidence that the BPs and AUCs are independent of each other.
Note that the punishment was delivered immediately after the cocaine-seeking but not cocaine-taking responses in the current study. This approach avoids to deliver the reward and punishment simultaneously because such an arrangement facilitates counterconditioning between reward and footshock (Dickinson and Pearce 1976; Pearce and Dickinson 1975). The counterconditioning reduce the effectiveness of punishment and thus, make it difficult to draw the conclusion on the reduced effects of punishment. In addition, cocaine-seeking and cocaine-taking behaviors are differentially regulated (Roberts et al. 2013) and the chained schedule can separate the two processes to a degree. Thus, the AUCs in the current study should be thought as an indicator of the compulsive cocaine-seeking behavior. One concern is that the punishment was delivered immediately whereas the negative consequences rarely occur immediately in patients. Although it would be logical to simulate the real situation, we were concerned about what drives the drug-related behaviors under these conditions. It is likely that animals may underestimate or discount the probability of punishment in the delayed punishment situation. If animals do not believe that the negative consequences will occur, they will likely continue their drug-related behaviors. Under such a scenario, it is difficult to conclude that their behaviors are compulsive. We specifically want to know whether animals will continue drug use despite the fact that they know for sure that their behavior will get punished. Moreover, although the negative consequences usually do not occur immediately in patients, they are fully aware of the fact that it is just a matter of time that the dire consequences will occur. We believe that the animal model should be targeted at this core feature of drug addiction. This is why we did not use the procedures by others (Deroche-Gamonet et al. 2004; Vanderschuren and Everitt 2004) and used the FR1 schedule to avoid any uncertainty about punishment.
The BPs and AUCs for each dose of cocaine or value of sucrose were measured only once in the current study. There is a concern about the stability of these measures. There is evidence that the BPs obtained in the first PR session is a good predictor of the BPs obtained from the subsequent consecutive PR sessions (Roberts et al. 1989b). We also found that the BPs were significantly correlated for different values of sucrose (Pearson: r = 0.64, p < 0.0001) and different doses of cocaine (Pearson: r = 0.61, p = 0.003). These results provide evidence that our data cannot be explained by random performance. In addition, these approaches have been used before and significantly contributed to the current knowledge of drug addiction (Belin et al. 2009; Belin et al. 2008; Bentzley et al. 2014; Deroche-Gamonet et al. 2004). We would like to point out that the intervals between the measurements of the BPs or AUCs at two doses of cocaine were at least two weeks during which cocaine SA training continued in the current study. Depending on the extent of the drug exposure and genetic/epigenetic factors, the brain functions involved in regulation of drug-related behaviors may change. The initial response to cocaine does not necessarily predict how vulnerable individuals are to these changes (Deroche-Gamonet et al. 2004; Vanderschuren and Everitt 2004). Thus, there is no guarantee that the measurement of the same behavior will correlate after a period of cocaine SA. In addition, the performance repeated under the same schedule of reinforcement also reflects the impact of learning on the behavior. Animals may develop either tolerance or sensitization to footshock after repeated punishment sessions depending on the footshock intensity and individuals. Because the BPs and AUCs were measured only once for each reward value or dose in the current study, we are less concerned with the learning-related interpretation of our results. Lack of the correlation between the BPs and AUCs is consistent with the idea that the neural circuits involved in the motivation and punishment are different and disruption of one circuit may not necessarily correlate with disruption of the other after repeated cocaine uses. Of course, this conclusion needs to be further validated given the known limits of the BP as an indicator of the motivational state. For example, the BP is influenced by the step size of the PR schedule and the duration of the interval defining the last ratio (Bradshaw and Killeen 2012; Hursh and Silberberg 2008). Thus, other procedures such as those of the behavioral economics should be used to validate our results.
Given the footshock was delivered while cocaine was being self-administered, there is a concern that cocaine may interfere with the effects of punishment by reducing sensation or perception of the pain induced by footshock. We are not aware of any studies on this issue. There are, however, studies of the effects of cocaine on the punished responding maintained by natural rewards. For example, cocaine inhibits rather than enhances the punished responding at the doses that increase or have no effects on the unpunished responding (Dworkin et al. 1989; McMillan 1975; Spealman 1979). If cocaine interferes with the perception of the pain induced by footshock, we would expect an increase in the punished responding. These studies reach a general conclusion that cocaine does not have anti-punishment effects suggesting that no analgesic effects of cocaine are induced in these studies. We acknowledge that there are differences in the extent of cocaine exposure between these studies and the punished cocaine SA. Nevertheless, there is strong evidence that development of the resistance to punishment reflects the deficit in the inhibitory function rather than the cocaine-related interference with punishment (Belin et al. 2009; Belin et al. 2008; Chen et al. 2013; Deroche-Gamonet et al. 2004; Kasanetz et al. 2013; Pascoli et al. 2015; Pelloux et al. 2007).
Realizing the drawbacks of the current diagnosis of mental disorders, the NIMH launched a research program to guide the research field to focus on the domain-based deficits underlying the behavioral symptoms of mental disorders (Insel et al. 2010). Identifying biology-based deficits in mental disorders will be critical to development of the future diagnosis system that will improve treatment selection and outcome. The same drawbacks also exist for the diagnosis of drug addiction and the efforts are being made to emphasize the neuroscience-based research on drug addiction (Kwako et al. 2017; Kwako et al. 2016; Litten et al. 2015). Following the example of the NIMH RDoC, the research domains and constructs that reflect the functions of brain systems relevant to drug addiction have to be identified. Modifying and refining these domains and constructs will be ongoing efforts based on the new information provided by the research field. This study is an example of our efforts in developing a paradigm to measure the functional deficits relevant to cocaine addiction.
Figure 1.
Distributions of and correlation between the BPs and AUCs with sucrose SA reinforced by one sucrose pellet. Upper panel: distribution of the BPs. Middle panel: distribution of the AUCs. Lower Panel: Spearman correlation between the BPs and AUCs. No significant correlation was found between the two.
Acknowledgments
The project was supported by Grant Number DA034776 (WLS) from the National Institute on Drug Abuse and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or NIH. All procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Footnotes
There is no conflict of interest in relation to this article.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing, Incorporated; Washington, DC: 2013. [Google Scholar]
- Bedford JA, Bailey LP, Wilson MC. Cocaine reinforced progressive ratio performance in the rhesus monkey. Pharmacol Biochem Behav. 1978;9:631–8. doi: 10.1016/0091-3057(78)90214-9. [DOI] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–8. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 2013;23:564–572. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw CM, Killeen PR. A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology (Berl) 2012;222:549–64. doi: 10.1007/s00213-012-2771-4. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–2. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Pearce JM. Preference and response suppression under different correlations between shock and a positive reinforcer in rats. Learn Motiv. 1976;7:66–85. [Google Scholar]
- Ducret E, Puaud M, Lacoste J, Belin-Rauscent A, Fouyssac M, Dugast E, Murray JE, Everitt BJ, Houeto JL, Belin D. N-acetylcysteine Facilitates Self-Imposed Abstinence After Escalation of Cocaine Intake. Biol Psychiatry. 2016;80:226–34. doi: 10.1016/j.biopsych.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Bimle C, Miyauchi T. Differential effects of pentobarbital and cocaine on punished and nonpunished responding. J Exp Anal Behav. 1989;51:173–184. doi: 10.1901/jeab.1989.51-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories--indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–82. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Grove RN, Schuster CR. Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav. 1974;2:199–208. doi: 10.1016/0091-3057(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–8. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–90. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johanson CE. The effects of electric shock on responding maintained by cocaine injections in a choice procedure in the rhesus monkey. Psychopharmacology (Berl) 1977;53:277–82. doi: 10.1007/BF00492364. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–88. [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Fiancette JF, Renault P, Piazza PV, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18:729–37. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Koob GF, Mason BJ. Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu Rev Pharmacol Toxicol. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Oo H, Olausson P, Taylor JR, Nairn AC. Prior chronic cocaine exposure in mice induces persistent alterations in cognitive function. Behav Pharmacol. 2009;20:695–704. doi: 10.1097/FBP.0b013e328333a2bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Grodin EN, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A reverse translational approach. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol Psychiatry. 2016;80:179–189. doi: 10.1016/j.biopsych.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of Alcohol Use Disorder: Understanding Mechanisms to Advance Personalized Treatment. Alcoholism-Clinical and Experimental Research. 2015;39:579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- McMillan DE. Determinants of drug effects on punished responding. Fed Proc. 1975;34:1870–9. [PubMed] [Google Scholar]
- Morgan D, Smith MA, Roberts DC. Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl) 2005;178:309–16. doi: 10.1007/s00213-004-1992-6. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Eighth. The National Academies; Washington, DC: 2011. [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Luscher C. Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron. 2015;88:1054–66. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Dickinson A. Pavlovian counterconditioning: changing the suppressive properties of shock by association with food. J Exp Psychol Anim Behav Process. 1975;1:170–7. doi: 10.1037//0097-7403.1.2.170. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ. Reduced Forebrain Serotonin Transmission is Causally Involved in the Development of Compulsive Cocaine Seeking in Rats. Neuropsychopharmacology. 2012;37:2505–14. doi: 10.1038/npp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 2007;194:127–37. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ. Differential vulnerability to the punishment of cocaine related behaviours: effects of locus of punishment, cocaine taking history and alternative reinforcer availability. Psychopharmacology (Berl) 2015;232:125–34. doi: 10.1007/s00213-014-3648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989a;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Gabriele A, Zimmer BA. Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neurosci Biobehav Rev. 2013;37:2026–36. doi: 10.1016/j.neubiorev.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989b;97:535–8. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ward SJ, Roberts DC. Lesions of the dorsomedial frontal cortex block sensitization to the positive-reinforcing effects of cocaine. Pharmacol Biochem Behav. 2008;88:238–46. doi: 10.1016/j.pbb.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Comparison of drug effects on responding punished by pressurized air or electric shock delivery in squirrel monkeys: pentobarbital, chlordiazepoxide, d-amphetamine and cocaine. J Pharmacol Exp Ther. 1979;209:309–15. [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Sun W. Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. Eur J Neurosci. 2012;35:775–783. doi: 10.1111/j.1460-9568.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]