Abstract
Background
Approximately 20% of patients with heartburn and normal endoscopic findings do not symptomatically improve on proton pump inhibitor (PPI) therapy making diagnosis and treatment uncertain. A biomarker distinguishing PPI-responsive from PPI-refractory heartburn is desirable.
Aims
We performed a pilot study assessing whether carboxy(C)-terminal fragments (CTFs) of e-cadherin in esophageal biopsies or amino(N)-terminal fragments (NTFs) of e-cadherin in serum could serve this purpose.
Methods
Twenty-nine patients with endoscopy-negative heartburn had esophageal biopsies for CTFs on Western blot and blood for serum NTFs on ELISA. All patients received dexlansoprazole 30 mg daily for 4 weeks and heartburn was assessed by daily diary entry. Post-treatment blood samples were obtained for serum NTFs. A control group without GERD symptoms (n=6) had biopsies for CTFs and a second control group (n=20) blood serum for serum NTFs.
Results
27 of 29 patients (93.1%) with endoscopy-negative heartburn, but 0 of 6 controls, were positive for CTFs. All patients and controls had measureable serum NTFs but mean NTFs were significantly higher in those with PPI-responsive heartburn compared to those with PPI-refractory heartburn and controls. Following treatment, 24 of 29 (82.8) patients had relief of heartburn, which associated with a decline in mean NTFs compared to controls. NTFs in PPI-refractory patients (n=5) were similar to controls before and after PPI therapy.
Conclusions
When heartburn responds to PPI, elevated serum NTFs decline to normal. These data suggest that cleaved products of e-cadherin may serve as biomarkers of NERD. Further data are needed to assess and confirm this concept.
Keywords: dysphagia, odynophagia, heartburn, functional heartburn, gastroesophageal reflux disease
Introduction
Estimates suggest that approximately one-quarter of American adults experience heartburn at least once per week [1–3]. However, the distinction between physiologic reflux and gastroesophageal reflux disease (GERD) is challenging, as this distinction is based in part on the subjective reporting of troublesome symptoms thought to arise from the reflux of gastric contents [4]. Furthermore, when evaluated by upper endoscopy, around 70% of patients with GERD symptoms exhibit no evidence of mucosal injury. This forces clinicians to stratify patients into etiologic categories defined by the presence or absence of abnormal acid exposure, symptom-reflux association, and subjective response to proton pump inhibitor (PPI) therapy. Many of these patients ultimately receive a diagnosis of functional heartburn, which constitutes the diagnosis in approximately 50% of PPI non-responders [5–7].
Given the ambiguity and complexity surrounding the etiology of heartburn symptoms, patients frequently continue PPI therapy for protracted periods of time despite the medication being ineffective. This subjects patients to excess cost and potential risk [8]. As such, a biomarker that distinguishes patients with non-erosive reflux disease (NERD) from functional heartburn and monitors the success or failure of PPI therapy in NERD patients is highly desirable.
Previously, our laboratory identified that acid-damaged esophageal epithelium developed an increase in paracellular permeability in an experimental rabbit model.
Subsequently, we reported this same change in endoscopic biopsies of esophageal epithelium in patients with both erosive- and non-erosive reflux disease. Moreover, the permeability change in esophageal epithelium in GERD patients was associated with and in part due to the cleavage of the zonula adherens transmembrane junctional protein, e-cadherin. As markers of this cleavage, in patients with GERD, carboxy(C)-terminal fragments (CTFs) of e-cadherin were found in biopsies of esophageal epithelium and elevated levels of the amino(N)-terminal fragments (NTFs) of e-cadherin were found in serum [9]. There findings suggested that identification of products of cleaved e-cadherin might be useful for making the diagnosis of GERD, especially in patients with NERD, and for monitoring the healing of acid-induced esophageal injury in NERD patients treated with PPI therapy. Here we describe the results of a proof of principle pilot study to assess whether CTFs of e-cadherin in esophageal biopsy or elevated NTFs of e-cadherin in the serum of patients are potentially useful as biomarkers for either the diagnosis of NERD or for monitoring its responsiveness to PPI therapy.
Methods
Men and non-pregnant/non-lactating women, 18–75 years old, with a history of heartburn at least three times per week for at least 3 months were recruited as cases for this study. All patients either had not taken PPI therapy or had been off PPI therapy for at least 1 month prior to enrollment. After a 1 week run-in period to quantify heartburn frequency and severity, those with at least moderate heartburn, using a scale of none, mild, moderate and severe, on at least 3 out of the last 7 days underwent upper endoscopy to establish the absence of esophageal mucosal damage (endoscopy negative). Eligible endoscopy-negative patients had esophageal biopsy performed 5 cm above the top of the gastric folds for Western blot analysis of CTFs of e-cadherin. At the time of this endoscopy, a peripheral venous blood sample was obtained for baseline levels of NTFs of e-cadherin by ELISA.
Two different control groups were utilized for this study. One control group was assembled to process CTFs of e-cadherin on Western blot from biopsies of esophageal epithelium. This first control group was recruited from individuals undergoing upper endoscopy for other indications in which there was no clinical or endoscopic evidence of esophageal pathology. These control subjects were investigated for ‘unexplained abdominal pain or heme positive stool’ and specifically had no esophageal symptoms including heartburn, dysphagia, chest pain, and regurgitation as well as no esophageal pathology on endoscopy including erosions, Barrett’s esophagus, stricture, or visible inflammation. The second control group was used to process serum levels of NTFs of e-cadherin by ELISA. Here, blood samples were purchased from Innovative Research (Novi, MI) from 20 healthy subjects whose demographics approximated that of the patient population.
The cases were then all provided 4 weeks of open label treatment with oral dexlansoprazole 30 mg once per day. During the 4-week period, heartburn response was quantified daily by questionnaire which scored the worst episode of heartburn for the day as 0 for none, 1 for mild (doesn’t interfere with activities), 2 for moderate (severe enough to interfere with activities), and 3 for severe (severe enough to prevent activities). At the end of the treatment period, the subject was classified as: a) ‘complete PPI responder’ if over the last 7 days of treatment, the patient experienced no heartburn for 6 of 7 days and only mild heartburn on one day; b) ‘partial PPI responder’ if over the last 7 days of treatment, the patient experienced no heartburn for 6 of 7 days and moderate or severe heartburn on one day; or c) ‘non-responder to PPI’ if over the last 7 days of treatment, the patient experienced heartburn of any degree on two or more days. At the end of treatment, subjects had a repeat blood sample for measurement of serum NTFs. Serum NTFs in the patient groups were compared to serum NTFs from the group of healthy control subjects. CTFs on Western blot and NTFs on ELISA were assessed by an investigator unaware of the patient’s treatment response. The reviewer of the Western blots was also blind to case versus control status.
To detect the CTFs of e-cadherin in biopsies of esophageal epithelium, we used antibodies against the intracellular carboxy(C)-terminus of human e-cadherin (Catalog#334000, Zymed Laboratories, San Franciso, CA). Signals were detected using an Odyssey Infrared Imaging System (LICOR, Inc., Lincoln, NE) and categorized as either present or absent based on visualization of the blot.
For quantification of NTFs, levels of soluble amino(N)-terminus of e-cadherin were measured in serum using a commercially available sandwich ELISA kit (Invitrogen, Carlsbad, CA). In brief, a monoclonal antibody to e-cadherin is coated onto microtitre plates. Samples containing an unknown amount of e-cadherin are incubated in the wells at 37°C for 2 hours. A second, detecting, monoclonal antibody (conjugated with peroxidase) is incubated in the wells at 37°C for 1 hour. Peroxidase substrate solution (H2O2 and tetramethylbenzidine) is added and results in a color change. The reaction is terminated by the addition of 1M H2SO4 and absorbance of the sample measured using a microtitre plate reader at 450 nm. Each sample is measured twice and the average value used for determination of e-cadherin concentration from a standard curve plotted using values obtained from standard solutions provided with the kit. Values are presented in units of absorbance.
Descriptive statistics were used to summarize the patient cohort. Bivariable analysis was conducted using Fisher’s exact test for the comparison between the presence or absence of CTFs and response to dexlansoprazole; Student’s T-test was used for comparing the levels of serum NTFs and response to dexlansoprazole. The University of North Carolina Institutional Review Board approved this study.
Results
Patient demographic information
There were 40 heartburn patients assessed for eligibility in this study. Of these 40 patients, 29 reported moderate heartburn on 3 or more of 7 days and had normal appearing esophageal mucosa on upper endoscopy. These 29 patients were thus eligible for inclusion in the study and initiation of dexlansoprazole. The mean age at study initiation was 41.5 years, and most patients were female (93%) and white (59%). Six control patients consented to provide upper endoscopy samples for CTF measurement, and 20 blood samples from healthy controls served as the comparator group for the NTF analysis. The mean age of controls was 43 years, and they were also predominantly female (65%) and white (81%). Prior to treatment, the mean absorbance values for serum NTFs of e-cadherin in complete, partial and non-responders were 0.277 ± 0.019, 0.239 ± 0.016, and 0.216 ± 0.031, respectively. For the control group, the mean absorbance value was 0.211 ± 0.014.
Clinical response to dexlansoprazole treatment
The majority of patients within the treatment cohort responded clinically when assessed at the end of the study. There were 24 patients (83%) who responded clinically, noting no heartburn symptoms in the final 6 of 7 days. Of these 24 responders, 15 were considered complete responders with no more than mild heartburn on 1 of the last 7 days; 9 were considered partial responders with moderate or severe heartburn on only 1 of the last 7 days. As such, 5 of the 29 patients (17%) were non-responders, as noting heartburn on two or more of the last 7 days of dexlansoprazole treatment.
CTF findings in esophageal biopsies
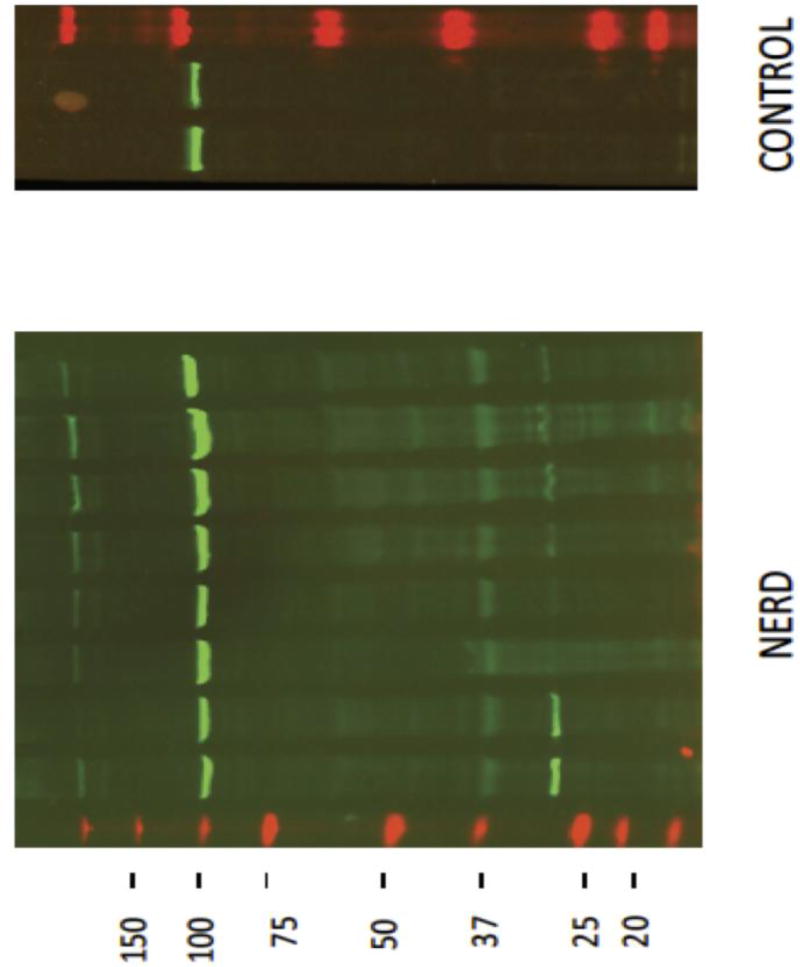
The esophageal biopsies from all 6 controls were found to have only a single 120 kilodalton (kD) CTF band on Western blot, representing intact e-cadherin. None of the 6 controls were positive for an additional CTF band. At baseline the esophageal biopsies from the intervention cohort similarly showed a 120 kD CTF band consistent with intact e-cadherin. However, 27 of the 29 (93%) patients within the treatment cohort also had one or more additional smaller-sized CTF bands, representing cleaved fragments of e-cadherin (Figure 1). The most prominent of the smaller-sized CTF bands had molecular weights of 30 and 35 kD.
Figure 1.
An illustration of Western blots using a C-terminal antibody to e-cadherin on endoscopic esophageal biopsies for a group of 8 patients with non-erosive reflux disease (NERD) and that from 2 healthy control subjects. Note that all subjects have a band for intact e-cadherin at 120 kD while only those with NERD have one or more smaller bands (C-terminal fragments, CTFs). One prominent CTF in those with NERD is noted to migrate at 35 kD and another at 30 kD - see arrows. Only 2 of 6 controls are shown for illustrative purposes.
[Note: The band for non-erosive reflux disease patients rides above the 100 kD marker and hence is a 120 kD band for intact e-cadherin; the 35 kD band for NERD patients resides immediately below the 37 kD levels and is thus consistent with a 35 kD band.]
As noted previously, 24 of 29 (83%) of the treatment cohort were either full or partial responders and 5 of 29 of the treatment cohort were non-responders to dexlansoprazole. The 2 patients with biopsies negative for CTFs on Western blot were both responders to dexlansoprazole and all 5 patients who were non-responders to dexlansoprazole were positive for CTFs on Western blot.
NTF findings in serum
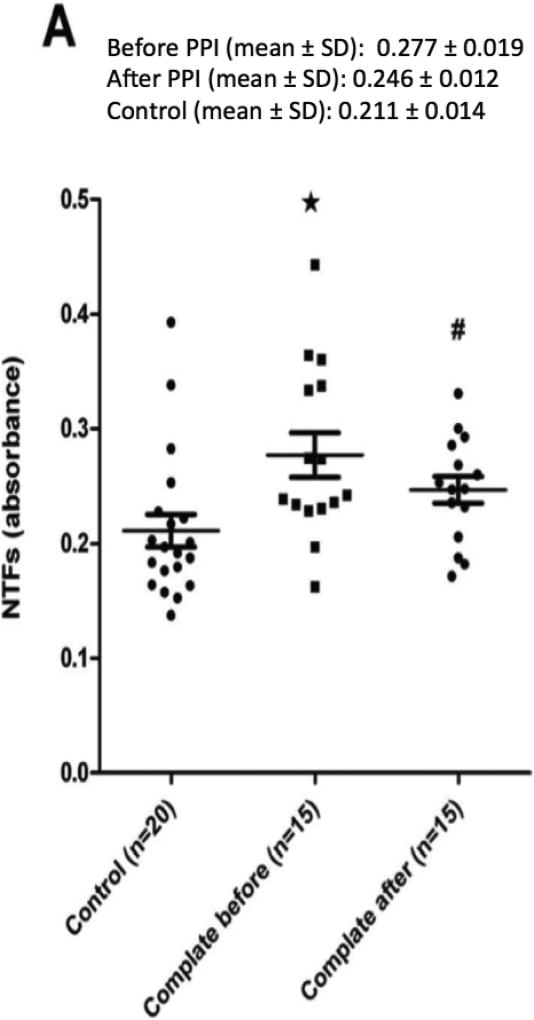
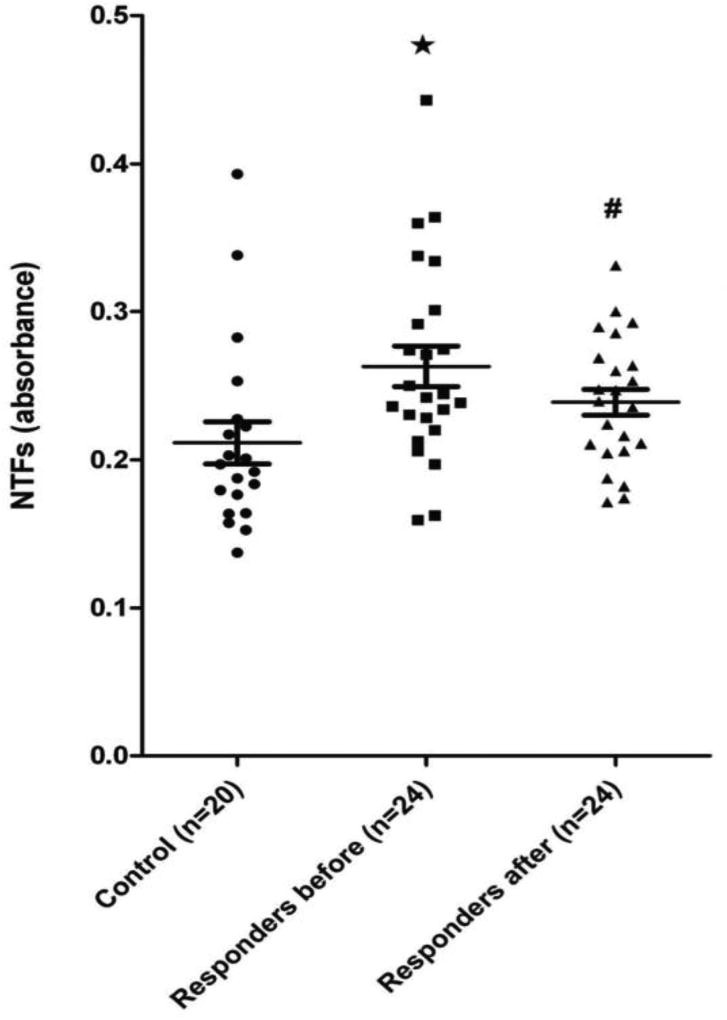
Baseline levels of serum NTFs were similar between responders to dexlansoprazole, non-responders, as well as the serum control group (Figure 2). Moreover, whether complete or partial responders were viewed independently (Figure 2) or combined together as responders (Figure 3), their NTF values were significantly higher than those of non-responders and controls.
Figure 2.
A plot of the serum levels of N-terminal fragments (NTFs) of e-cadherin by ELISA using an N-terminal antibody in patients with non-erosive reflux disease (NERD) that are (A) completely, (B) partially, or (C) non-responsive to 4 weeks of treatment with dexlansoprazole 30 mg once per day. Serum from a group of healthy adult subjects served as a control. Values for NTFs are in absorbance. Note that prior to treatment NTFs for complete and partial responders are significantly higher than controls while for non-responders NTFs are similar to controls. Note also that after treatment the elevated NTF values for complete and partial responders decline to values that are no different than controls. Values are the mean ± SE, * p < 0.05 pre-treatment NERD values versus controls; # p < 0.05 post-treatment NERD values versus pre-treatment NERD values.
Figure 3.
A plot of the serum levels of N-terminal fragments (NTFs) of e-cadherin by ELISA using an N-terminal antibody in patients with non-erosive reflux disease (NERD) that combines both complete and partial responders to treatment for 4 weeks with dexlansoprazole 30 mg once per day. Serum from a group of healthy adult subjects served as a control. Values for NTFs are in absorbance. Note that before treatment the NTFs for this combined group of responders were significantly higher than controls and that after treatment these elevated values of NTFs decline, so that they are no longer significantly different than controls. Values are the mean ± SE, * p < 0.05 NERD versus controls.
Following treatment with dexlansoprazole, serum NTFs of complete and partial responders, either independently (Figure 2A and 2B) or combined as responders (Figure 3), declined significantly from baseline levels and were then similar to that of controls. In addition, baseline serum NTFs of non-responders to dexlansoprazole were both similar to controls at baseline but also remained unchanged following treatment with dexlansoprazole (Figure 2C). The mean absorbance for complete responders decreased to 0.26 ± 0.012 after PPI therapy. The absorbance values for partial and non-responders were 0.223 ± 0.015 and 0.217 ± 0.027, respectively.
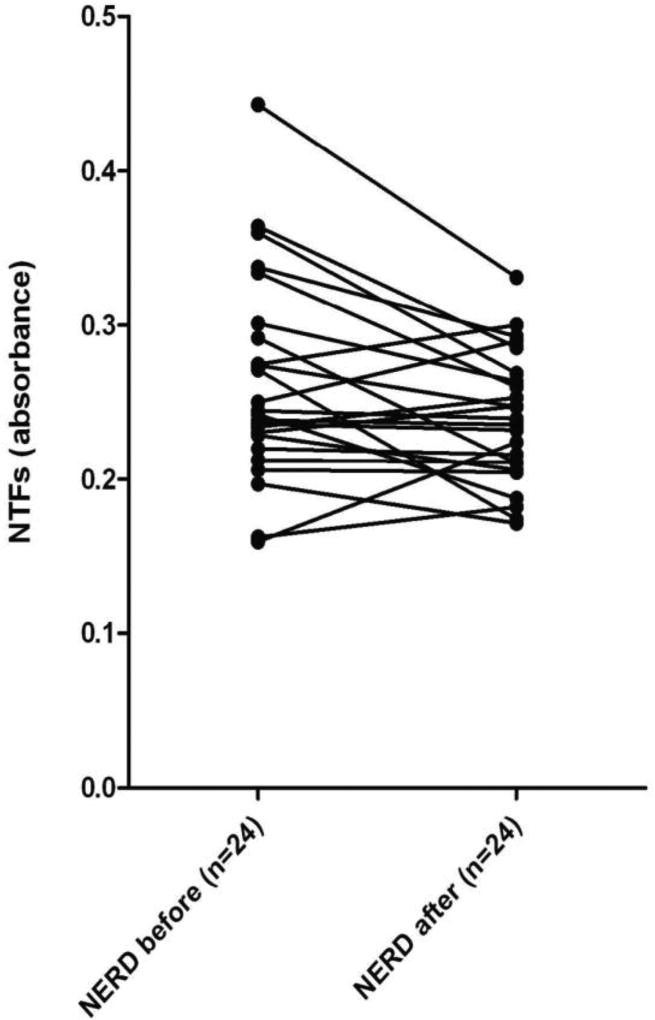
We also found that the majority of complete and partial responders (75%) exhibited a post-treatment decline in NTF values (Figure 4). When applying a receiver operator characteristics curve to the patient and control data (Figure 3), the area under the operator curve was 0.7615 ± 0.078 (95% CI 0.608 - 0.915). The calibration plot values on the ROC curve are 0.145 and 0.418, representing the range of the data. A cutoff absorbance value of ≥ 0.2280 maximized sensitivity and specificity, which were were 75% and 80%, respectively.
Figure 4.
A plot of the pre- and post-treatment serum levels of N-terminal fragments (NTFs) of e-cadherin by ELISA using an N-terminal antibody in patients with non-erosive reflux disease (NERD). The lines connect the pairs of values for individual patients that either completely or partially responded to treatment with 4 weeks of dexlansoprazole 30 mg once per day. Values for NTFs are in absorbance.
Discussion
NERD is a clinical condition resulting from reflux damage to the esophageal epithelium. However, the injury is microscopic and subtle with the earliest and most sensitive pathologic feature being the presence on transmission electron microscopy of dilated intracellular spaces (DIS) within the esophageal stratified squamous epithelium [10–12]. DIS indicates that patients with NERD have an increase in paracellular permeability, and this increase in paracellular permeability is associated with acid damage to the intercellular junctional complex [13]. Not surprisingly, given that the damage is thought to be acid mediated, the majority of patients with NERD respond to PPI therapy with control of symptoms, which correlates with resolution of the DIS [14].
When investigating the cause for the increase in paracellular permeability in patients with non-erosive acid-damaged esophageal epithelium, we found that the junctional protein e-cadherin was cleaved into CTFs and NTFs [9]. Furthermore, we found that the loss of e-cadherin in esophageal epithelium results from its proteolytic cleavage by a membrane metalloproteinase, ADAM10 [9]. Cleavage of e-cadherin by ADAM10 can be identified in GERD patients by the presence of one or more CTFs of e-cadherin in biopsies of esophageal epithelium as well as by the presence of increased levels of jettisoned NTFs in serum. The NTFs are increased in serum owing to their solubility and small size, which allows jettisoned NTF fragments to diffuse into blood. E-cadherin is important as an intercellular adhesive bridge of the zonula adherens [15,16]. Moreover, since the zonula adherens plays an integral role in maintaining the integrity of tight junction between cells, the presence of cleaved e-cadherin likely contributes to the increase in paracellular permeability in PPI-responsive NERD [17,18]. Since CTFs on Western blot of endoscopic biopsies and elevated NTFs of e-cadherin by ELISA in serum are a reflection of acid injured esophageal epithelium, their measurement may be useful as a biomarker of NERD. Measurement of NTFs is particularly attractive, as it is measured in blood serum, which is minimally invasive.
In this pilot study, we tested the hypothesis that the presence of cleaved fragments of e-cadherin (serum or esophageal tissue based) could be of value for the diagnosis of NERD and functional heartburn. We additionally questioned the utility of cleaved serum e-cadherin fragments in the monitoring of response to PPI therapy. This was explored by determining the proportion of subjects with heartburn and controls with CTFs of e-cadherin at baseline; we also assessed the level of NTFs from e-cadherin prior to and after treatment with 4 weeks of dexlansoprazole 30 mg once per day. In summation, 27 of 29 (93%) of the treatment cohort and 0 of 6 of the control group were positive for smaller-sized CTF bands on esophageal biopsy. There were also 25 of 29 patients (86%) in the treatment cohort who were PPI-responsive (complete or partial responders). After 4 weeks of PPI therapy, serum NTFs of e-cadherin of the 25 PPI-responsive patients were significantly lower than at baseline; similar trends were not seen in either the PPI-unresponsive patients or controls. This suggests that the biomarker may have utility in differentiating between these patient groups. Specifically, using an absorbance value for serum NTFs of ≥ 0.228 provided a sensitivity and specificity of 75% and 80%, respectively, for discriminating between the cohorts. Supporting the role of cleaved of NTFs of e-cadherin in determining PPI treatment response, the levels of serum NTFs in PPI-responsive patients were not stable and declined to levels exhibited by healthy controls after treatment. For the 5 PPI non-responsive subjects, serum NTF values were the same as controls both before and after 4 weeks of PPI therapy. From a mechanistic standpoint, these observations support the supposition that elevated serum NTFs found in heartburn patients derive from acid reflux-induced esophageal epithelial damage. The findings also suggest that the decline in serum NTFs following PPI therapy correlates with PPI-induced esophageal epithelial healing. Clinically, the biomarker may help determine when a PPI may be tapered or discontinued without immediate risk of relapse. For those with heartburn not responsive to empiric PPIs, particularly double/high dose, normal CTFs and/or normal NTFs of e-cadherin would support the conclusion that the symptoms prompting PPI use were unrelated to reflux disease and add confidence that PPI therapy could be safely discontinued
For this study, 2 different control groups were used. The first was to process serum levels of NTF of e-cadherin by ELISA and the second to process CTFs of e-cadherin on Western blot from biopsies of healthy esophageal epithelium. Two groups were chosen for economic and scientific reasons. First, for sera studies, it is cheaper and easier to obtain sera from a large number of healthy subjects using a commercial supplier. Second, for tissue studies, the controls were not truly ‘healthy subjects’ in that they were being evaluated for clinical reasons. However, there was no clinical or endoscopic reason to indicate a concurrent disease of the esophagus. In this way, we were able to avoid loss of time, effort and risks of endoscopy associated with recruiting healthy subjects to obtain biopsies of healthy esophageal epithelium.
Limitations exist for this paper. First, this study did not utilize 24-hour pH monitoring on or off therapy to correlate esophageal acid exposure with NTF levels. While this additional testing would be desirable, patient participation would be decreased. Future work, however, will provide this information. We also only obtained pre-treatment CTF levels. While post-treatment measurement of CTF may corroborate the changes documented in NTF levels, the additional cost, risk, and inconvenience of repeat upper endoscopy precluded this assessment in a proof of principle study. This study also utilized small sample sizes, which may have resulted in a chance difference in the treatment and control populations. As this was a pilot and thus hypothesis generating study, we did not control for potential confounding variables. It is important to confirm these results in a larger sample size, as we have documented dissimilar results in a cohort of Barrett’s esophagus patients post-radiofrequency ablation. Unlike in the NERD cohort documented in this paper, the post-radiofrequency ablation patients did not demonstrate elevated serum NTFs of e-cadherin. However, radiofrequency ablation damaged capillaries within the esophageal wall, which likely entrapped NTF’s within the ablated segments of Barrett’s esophagus. Nonetheless, as NERD and Barrett’s esophagus share similar pathogeneses, and these results are divergent, it is reasonable to confirm the current findings in a larger study [19]. Lastly, the treatment cohort and control group differed by baseline demographic features, which we could not control for in this study given the sample size.
The results of this pilot proof of principle study support the previously published concept that cleavage of e-cadherin plays an integral role in the pathogenesis of heartburn in NERD. Furthermore, fragments of e-cadherin are clinically useful as biomarkers of reflux disease. This resides in their use as an objective marker of reflux disease that informs about the integrity and state of healing of reflux-damaged esophageal epithelium. This information, if validated, could be applied to determining when PPI therapy could be tapered or stopped in responders to therapy and help predict the degree of risk of symptom relapse. Furthermore, this biomarker may help determine those patients who would benefit from centrally acting medications targeting visceral hyperalgesia (i.e. patients with functional heartburn). Ultimately, such a test would provide a population-level benefit by averting prolonged, expensive, potentially risky and ultimately futile therapeutic trials with acid suppression.
Acknowledgments
Grant supportThis study was supported by an investigator-initiated grant from Takeda Pharmaceuticals U.S.A. Inc. and in part by NIH award T32DK007634 (CCR).
Abbreviations
- NERD
non-erosive reflux disease
- PPI
proton pump inhibitor
- CTFs
carboxy(C)-terminal fragments of e-cadherin
- NTFs
amino(N)-terminal fragments of e-cadherin
- DIS
dilated intercellular spaces
Footnotes
Disclosures: B Jovov, RC Orlando, Z Djukic and GS Orlando are co-inventers on a patent on the use of fragments of e-cadherin for the diagnosis and management of gastroesophageal reflux disease. RC Orlando has held in the past an investigator-initiated grant from Takeda Pharmaceuticals and from Astra Zeneca.
Author contributions
Biljana Jovov, Roy C. Orlando and Nicholas J. Shaheen were involved in all aspects of this study. Kathleen Ferrell, Amy Pruitt, Zorka Djukic and Geraldine S. Orlando provided technical expertise and assisted in data collection and analysis. Craig C. Reed critically appraised and revised the manuscript.
References
- 1.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–80. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locke GR, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126(7):1692–9. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 4.Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Medical Position Statement on the Management of Gastroesophageal Reflux Disease. Gastroenterology. 2008;135(4) doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 5.Patel A, Sayuk GS, Gyawali CP. Parameters on esophageal pH-impedance monitoring that predict outcomes of patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2015;13(5):884–91. doi: 10.1016/j.cgh.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bortoli N, Martinucci I, Savarino E, Bellini M, Bredenoord AJ, Franchi R, et al. Proton pump inhibitor responders who are not confirmed as GERD patients with impedance and pH monitoring: Who are they? Neurogastroenterol Motil. 2014;26(1):28–35. doi: 10.1111/nmo.12221. [DOI] [PubMed] [Google Scholar]
- 7.Penagini R, Sweis R, Mauro A, et al. Inconsistency in the diagnosis of functional heartburn: Usefulness of prolonged wireless ph monitoring in patients with proton pump inhibitor refractory gastroesophageal reflux disease. J Neurogastroenterol Motil. 2015;21(2):265–72. doi: 10.5056/jnm14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaezi MF, Yang Y-X, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterol Vaezi MF Complicat Prot Pump Inhib Ther Gastroenterol. 2017;(17):35623–8. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 9.Jovov B, Que J, Tobey NA, Djukic Z, Hogan BLM, Orlando RC. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol [Internet] 2011;106(6):1039–47. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese C, Fabbri A, Bortolotti M, et al. Dilated intercellular spaces as a marker of oesophageal damage: Comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18(5):525–32. doi: 10.1046/j.1365-2036.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 11.Caviglia R, Ribolsi M, Maggiano N, et al. Dilated intercellular spaces of esophageal epithelium in nonerosive reflux disease patients with physiological esophageal acid exposure. Am J Gastroenterol. 2005;100(3):543–8. doi: 10.1111/j.1572-0241.2005.40978.x. [DOI] [PubMed] [Google Scholar]
- 12.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: A morphological feature of acid reflux- damaged human esophageal epithelium. Gastroenterology. 1996;111(5):1200–5. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 13.Tobey NA, Hosseini SS, Argote CM, et al. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. 2004;99(1):13–22. doi: 10.1046/j.1572-0241.2003.04018.x. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese C, Bortolotti M, Fabbri A, et al. Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol. 2005;100(3):537–42. doi: 10.1111/j.1572-0241.2005.40476.x. [DOI] [PubMed] [Google Scholar]
- 15.Akhmanova A, Yap AS. Organizing Junctions at the Cell-Cell Interface. Cell. 2008;135:791–3. doi: 10.1016/j.cell.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Hartsock A, Nelson WJ. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochimica et Biophysica Acta - Biomembranes. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow WJ, Orlando RC. The pathogenesis of heartburn in nonerosive reflux disease: A unifying hypothesis. Gastroenterology. 2005;128:771–8. doi: 10.1053/j.gastro.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Tobey N, Argote C, Hosseini S, Orlando R. Calcium-switch technique and junctional permeability in native rabbit esophageal epithelium. AJP Gastrointest Liver Physiol. 2004;286(6):G1042–9. doi: 10.1152/ajpgi.00387.2003. [DOI] [PubMed] [Google Scholar]
- 19.Runge TM, Shaheen NJ, Djukic Z, et al. Cleavage of E-Cadherin Contributes to Defective Barrier Function in Neosquamous Epithelium. Dig Dis Sci. 2016;61(11):3169–75. doi: 10.1007/s10620-016-4315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]