Abstract
Gliomas are rich in extracellular nucleotides that modulate glioma cell production of multiple cytokines including interleukin (IL)-6, which strongly contributes to glioma cell proliferation. However, little is known about how nucleotide signaling modulates microglial/macrophage (MG/MP) cytokine production in the context of gliomas, nor how MG/MP purinergic P2 receptor expression changes in the tumor micro-environment. We hypothesized that: 1) expression of key P2Y receptors will be augmented in glioma-derived MG/MP, and 2) selective activation of these receptors in vitro will regulate microglial production of IL-6 and glioma cell proliferation. We tested these hypotheses using the murine GL261 glioma model. Compared to MG/MP isolated from the normal brain tissue, CD11b+ cells isolated from GL261 tumors expressed higher levels of several P2 receptors, including P2Y14 receptors. To evaluate microglial P2Y14 receptor function in the context of tumor cells, we first cultured N9 microglia in transwells with GL261 cells and found that microglial P2Y14 mRNA levels were similarly increased in transwell cultures. GL261 cells did not express detectable P2Y14 levels either when they were cultured alone or in transwell cultures with N9 cells. Selective P2Y14 receptor activation with UDP-glucose (UDPG) did not affect IL-6 levels in either cell type cultured alone, but in transwell cultures, UDPG decreased IL-6 protein levels in the medium. Application of conditioned medium from UDPG-treated microglia reduced GL261 cell proliferation. Together, these data suggest that P2Y14 receptors may be a key a receptor involved in glioma cell-MG/MP communication in the tumor environment.
Keywords: Microglia, purinergic receptor, cytokine, transwell
1. Introduction
Glioblastoma Multiforme (GBM) is the fastest growing and most invasive of all astrocytomas, accounting for over 28% of all primary brain tumors and almost 80% of all malignant CNS tumors [1]. Resident CNS immune cells, microglia, infiltrate the tumor and gather in number. Microglia are capable of phagocytosis, antigen presentation to T cells, and releasing pro-inflammatory cytokines [2]; however, within gliomas, microglia are unable to elicit an effective cell mediated anti-tumor immune response sufficient for tumor cell containment or ultimate regression [3, 4]. GBMs are characterized by a highly immunosuppressive microenvironment containing cytokines such as interleukin (IL) IL-6 [5], produced predominantly by glioma-associated microglia/macrophages in rodent glioma models [6]. Higher IL-6 mRNA levels correlate with poorer prognosis and survival in glioma patients, and are thought to contribute to glioma malignancy by promoting tumor cell proliferation and migration [7]. Indeed, GL261 tumor size is significantly reduced in IL-6 null mice [6].
Tumor microenvironments are characterized by chronically high levels of nucleotides and their metabolites [8] that have increased durations of action, in part, due to the strong suppression of ectonucleotidase expression and activity [9], the extracellular enzymes responsible for degrading nucleotides. One early study demonstrated that a single injection of the enzyme apyrase (which degrades the extracellular nucleotide ATP) at the time of intracranial implantation of C6 glioma cells decreased tumor size and mitotic index [10]. Although not yet clear, these data suggest an important role for nucleotides in glioma pathology via effects either on the tumor cells themselves or on tumor-associated microglia/macrophages, or both. Whereas nucleotides are potent mitogens for a number of human and rodent glioma cell lines [11, 12], nucleotide effects on microglial activities have been poorly studied in the tumor context.
Nucleotides exert their effects by activating purinergic P2X (ligand-gated ion channels) and P2Y (heterotrimeric G-protein coupled) receptors, whose signaling has emerged as an important component of glioma progression [13]. P2X receptors (P2X1–P2X7) and P2Y receptors are sensitive to adenine-containing (e.g. P2Y1, P2Y2, P2Y11, P2Y12, P2Y13) and uracil-containing (e.g. P2Y2, P2Y4, P2Y6, P2Y14) nucleotides and their metabolites [14], and are increasingly implicated in a number of tumor promoting activities. In particular, P2Y1, P2Y2 and P2Y12 activation promotes glioma cell proliferation contributing to glioma malignancy [15, 16]. Microglia express multiple purinergic receptors in the healthy CNS [17–19] and their activation can decrease microglial production of several pro-inflammatory mediators important for the immune response, including IL-6 [20–22]. However, P2Y receptor activation can also increase IL-6 production in microglia/macrophages [23, 24], suggesting that P2Y receptors in microglia may be especially important in the tumor environment. Interestingly, the sugar containing nucleotides, uridine 5′-diphosphoglucose (UDPG) and metabolites which are endogenous ligands for the P2Y14 receptor, are found in human astrocytomas and other tumor cell types at levels capable of activating P2Y14 receptors [25–27]. UDPG is hydrolyzed more slowly than ATP, suggesting that these uracil and sugar containing nucleotides may exert long-lasting effects.
Here we hypothesized that the expression and function of key P2Y nucleotide receptors would be altered in glioma-derived microglia. Our objectives were to: 1) identify which P2 receptors were altered in syngeneic mouse GL261 glioma-derived microglia/macrophages (MG/MP), and 2) study functional consequences of up-regulated P2 receptors in murine N9 microglia grown in transwells with GL261 tumor cells. We found that among other P2 receptors, P2Y14 receptor expression was up-regulated in MG/MP both from GL261 tumors, and in microglia grown in transwell co-cultures, suggesting that this receptor may be important for microglial function in tumors. Selective P2Y14 receptor activation in the transwell culture system reduced microglial IL-6 production, and decreased tumor cell proliferation, suggesting an important role for P2Y14 receptors in microglia-glioma cell communication.
2. Materials and Methods
Materials
All chemicals were purchased from Sigma Chemical Company (St. Louis, MO) unless otherwise indicated. Tissue culture transwell inserts (Falcon® 0.4 μm pore 12-well) were purchased from Becton Dickinson (Franklin Lakes, NJ). Synthetic UDP glucose was purchased from MP Biomedicals (Solon, OH) as previously described [28].
Cell Culture
Murine N9 microglial cells were provided by Dr. Paula Ricciardi Castagnoli (University of Milan, Italy) [29], and GL261 tumor cells were obtained from the NIH National Cancer Institute, Frederick, MD. Both cell lines were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM; Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 100 U/ml penicillin/streptomycin (Cellgro) in 100 mm Sarstedt plates. Cells were grown to ~90% confluency and passaged every 2 days. GL261 or N9 cells were plated at 200,000 cells per well in 12 well plates. For transwell experiments, 60,000 N9 cells were plated in inserts and placed in 12 well plates containing adherent GL261 cells for 24 hours. N9 microglia were also grown on inserts in the presence of N9 microglia in the wells to account for possible alterations in transwell microglial gene expression resulting from differences in total cell numbers in the well. Comparisons were also made to N9 cells grown alone in wells with empty inserts, and no differences between either setup were noted. The 24 hour time point was chosen because differences in P2 receptor expression apparent at 24 hours were not different from later time points. The next day, cells were treated with 300 μM uracil 5′ diphosphoglucose (UDPG) or vehicle (250 mM HEPES); cells and culture media were harvested 24 hours later. For tumor cell implantation studies, GL261 cells were harvested by trypsinization and resuspended in 1XPBS to a concentration of 5×105 cells per 3 μl.
Animals and Tumor Cell Implantation
Eight week old male C57BL/6 mice were purchased from Harlan (Indianapolis, IN). All animals were used and maintained according to protocols approved by the University of Wisconsin Institutional Animal Care and Use Committee. Mice were anesthetized using isoflurane and immobilized using a stereotactic frame. Small bilateral holes were made 1mm lateral and rostral of bregma and cells were injected 2–3mm below the cranial surface. Three microliters of GL261 tumor cell suspension (n=5, 3 mice pooled per n) or 3 μl 1XPBS (n=4, 3 mice pooled per n) was delivered using a Hamilton syringe (Fischer Scientific, Tustin, CA). At the first clinical signs of tumor presence (approximately 14 days following tumor cell implantation), both sham and GL261 cell-injected mice were euthanized with an isoflurane overdose and the tumor was removed. For comparison, microglia were harvested from tissue from the corresponding tumor cell injection site of PBS-injected healthy (non-tumor) mice.
CD11b+ Cell Isolation from GL261 Tumors and Sham (PBS-Injected) Animals
Tumor and brain tissue was dissociated using the Neural Tissue Dissociation Kit (Miltenyi Biotec, Auburn, CA). CD11b+ cells were isolated using immunomagnetic cell separation as we have described previously [17].
Flow Cytometry
An aliquot of the CD11b+ cell fraction and the neuronal/tumor tissue digest prior to magnetic bead separation was taken for flow cytometry analysis to ascertain the purity of the isolated cells. These samples were incubated with and without antibodies to CD11b for proper instrument setup. After staining, all samples were filtered through 40μm filters and resuspended in PBS with 0.5% BSA; propidium iodide (PI) was added to identify live cells, and PI+ cells were not included in the analysis. A FACSCaliber fluorescence cell sorter equipped with a 15 mW, 488 nm air cooled argon ion laser and a 635 red diode (BD Scientific, Franklin Lakes, NJ) was used for analysis.
Real-time RT-PCR
Total RNA was isolated using TRI-reagent (Sigma) according to the manufacturer’s instructions. Purified RNA was digested with DNase I (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. 1μg of total RNA was converted to cDNA and real-time PCR was performed for P2X and P2Y receptors (primer sequences listed in Table 1) as we have described previously [17]. The relative amounts of each gene between samples were determined using the comparative CT method [30], and the values were normalized to the relative levels of 18s rRNA.
Table 1.
Primer sequences used in qRT-PCR
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| P2X1 | CAGAAAGGAAAGCCCAAGGTATT | CACGTCTTCACAGTGCCATTG |
| P2X2 | CAACATTGCAAGCCAGAAGA | CCTTGTGTGCCAGTTCTGTG |
| P2X3 | AAGGCTTCGGACGCTATGC | GATGACAAAGACAGAAGTGCCCT |
| P2X4 | AGACGGACCAGTGATGCCTAAC | TGGAGTGGAGACCGAGTGAGA |
| P2X5 | GATGTGGCAGACTTTGTCATTCC | CCTTCACGCTCAGCACAGATG |
| P2X6 | ACGTGTTCTTCCTGGTAACCAACT | TGGACATCTGCCCTGGACTT |
| P2X7 | ACAATGTGGAAAAGCGGACG | TCAATGCACACAGTGGCCA |
| P2Y1 | AGC AGA ATG GAG ACA CGA GTT TG | GGG ATG TCT TGT GAC CAT GTT ACA |
| P2Y2 | GAA GAA CTG GAG CAG GCG CT | CCA TTG CCC TGG ACC TGA TC |
| P2Y4 | CTG CAA GTT CGT CCG CTT TC | GTA TTG CCC GCA GTG GAT G |
| P2Y6 | TGA AAA CAA CGA GGA ACA CCA A | CAG CCT TTC CTA TGC TCG GA |
| P2Y12 | CAC AGA GGG CTT TGG GAA CTT A | TGG TCC TGC TTC TGC TGA ATC |
| P2Y13 | CAG CTG AGT CTC TTC CAA AAC AAA | TGC ATC CCA GTG GTG TTG AT |
| P2Y14 | CCA CCA CAG ACC CTC CAA AC | CAA CAC GGG AAT GAT CTG CTT T |
| 18s rRNA | CGC CGC TAG AGG TGA AAT TCT | CGA ACC TCC GAC TTT CGT TCT |
Cytokine Quantification
ELISA assays were used to detect murine IL-6 concentrations in the tissue culture medium, and levels were interpolated from standard curves, according to the manufacturer’s protocol (R&D Systems, Rockford, IL).
GL261 Proliferation assay
N9 microglia conditioned medium (N9 CM) was prepared by treating microglia in 6 well plates (2×105 cells/ml) with vehicle (250 mM Hepes) or 300 μM UDPG for 24 hours. The CM was removed, passed through 0.2 μm filters to remove cell debris, and then applied in triplicate to GL261 cells (1×105 cells/ml) plated in 96 well plates at a ratio of 1:1 for 24 hrs. As a control, GL261 cells were also treated directly with UDPG (150 μM) to account for the presence of UDPG in the N9 CM. The next day, GL261 cell numbers were quantified using CellTiter96 Aqueous Non-radioactive Cell Proliferation Assay (Promega, Madison, WI), according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using a Student’s T-test or a one-way RM-ANOVA pre hoc test followed by the Bonferroni Multiple Comparisons or Fisher’s LSD post hoc tests, using the InStat3 program (GraphPad Software Inc., LaJolla, CA). Statistical significance was set at the 95% confidence limit (p < 0.05). Quantitative data are expressed as the mean + SEM of 3–7 independent experiments.
3. Results
P2 nucleotide receptors are differentially expressed in GL261 glioma-derived CD11b+ cells
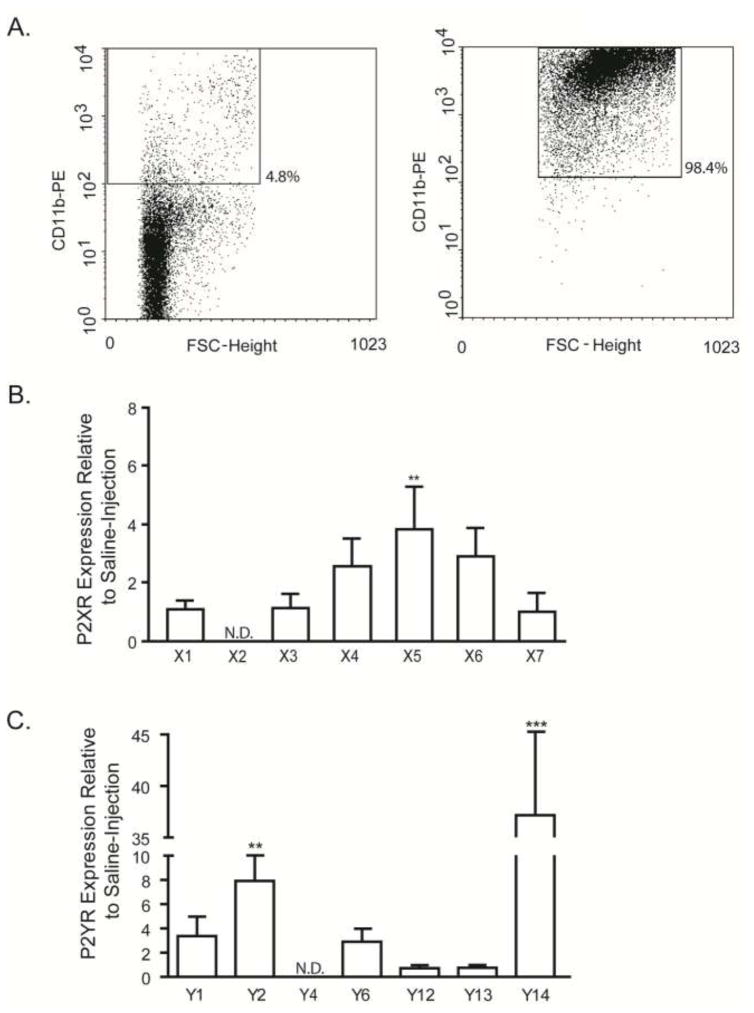
At this early stage of tumor progression, approximately 5% of total cells in GL261 tumor tissue labeled positive with CD11b-PE antibodies as determined by flow cytometry (Fig. 1A, left panel), similar to the normal 3–6% frequency of CD11b+ cells in control brain tissue (data not shown). After immunomagnetic purification, the cell population was >98% pure CD11b+ cells (Fig. 1A, right panel) which was used for qRT-PCR analyses of all P2X (Fig. 1B) and P2Y (Fig. 1C) receptors. Data are expressed as fold change relative to expression in CD11b+ cells from PBS-injected control brain tissue. While the expression of most P2X and P2Y receptors was unchanged in glioma-derived CD11b+ cells, P2X5, P2Y2 and P2Y14 were significantly upregulated by ~4, ~8 and ~37 fold, respectively. Neither P2X2 nor P2Y4 mRNA was detected in glioma-derived or PBS-injected CD11b+ cells, consistent with our previous report in microglia from the healthy CNS of male mice from this age [18].
Figure 1. Purinergic P2X and P2Y receptor expression differs in CD11b+ cells derived from GL261 tumors and non-tumor mouse brain.
Staining with anti-CD11b antibodies was performed in neural tissue derived from GL261 cell-injected and PBS-injected mice (A). Approximately 4.8% of all cells were positive for CD11b as determined by flow cytometry (A; left). After immunomagnetic sorting to enrich for CD11b+ cells, this fraction consisted of an average of ~98% purity (A; right). Total RNA was harvested from CD11b+ cells isolated from both tumor and PBS-injected normal brain tissue, reverse transcribed and subjected to quantitative RT-PCR for P2X (B) and P2Y (C) receptor mRNA quantification. Data were normalized to 18s rRNA and expressed relative to the expression of each receptor in CD11b+ cells derived from PBS-injected normal brain tissue. P2X5, P2Y2 and P2Y14 expression levels were up-regulated in GL261 glioma-derived CD11b+ cells. ** p < 0.01, *** p < 0.001.
P2 receptor profiles of murine GL261 glioma and N9 microglial cells
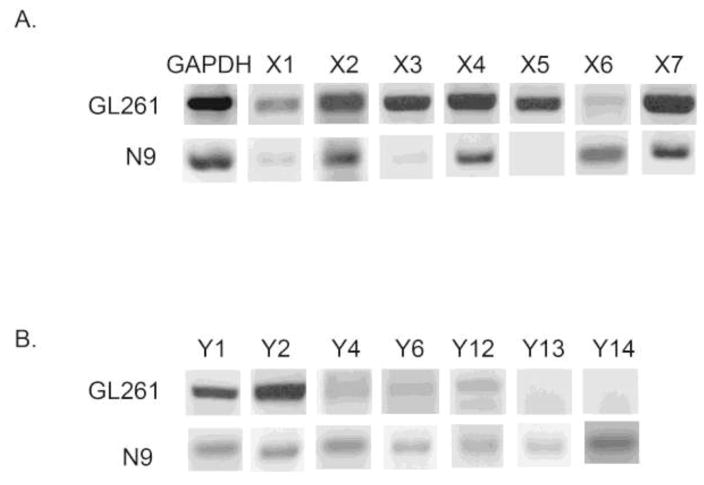
Although the GL261 cell line is studied frequently, to our knowledge, a comprehensive P2 receptor expression profile in these cells has not been performed. We found that GL261 glioma cells robustly express P2X2, P2X3, P2X4, P2X5, and P2X7; P2X1 and P2X6 were lowly detected (Fig. 2A). In contrast, only P2Y1 and P2Y2 were robustly detected; P2Y12 was lowly expressed. (Fig. 2B). We found that the N9 microglial cell line utilized for these experiments strongly expressed P2X2, P2X4, P2X6 and P2X7, whereas P2X5 was not detected and P2X1 and P2X3 expression was very low (Fig. 2A), in accordance with a previous report in N9 microglia where all P2X receptors were expressed, although P2X1, P2X3 and P2X5 mRNA levels were very low [31]. N9 cells also modestly expressed all known murine P2Y nucleotide receptors (Fig. 2B); consistent with the previous report [31].
Figure 2. P2X and P2Y receptor mRNA levels in N9 microglia and GL261 glioma cells.
Total RNA was harvested from N9 and GL261 cells, and semi-quantitative RT-PCR analysis was done for all known P2X (A) and P2Y (B) nucleotide receptors. GAPDH is shown as a loading control. Whole C57/BL6 mouse brain was used as a positive control for the primers (data not shown). GL261 express all P2X receptors whereas N9 microglia do not; N9 microglia express all P2Y receptors but GL261 cells do not. Experiments were independently repeated three times.
Co-culturing of N9 microglia with GL261 cells upregulates microglial P2Y receptor expression
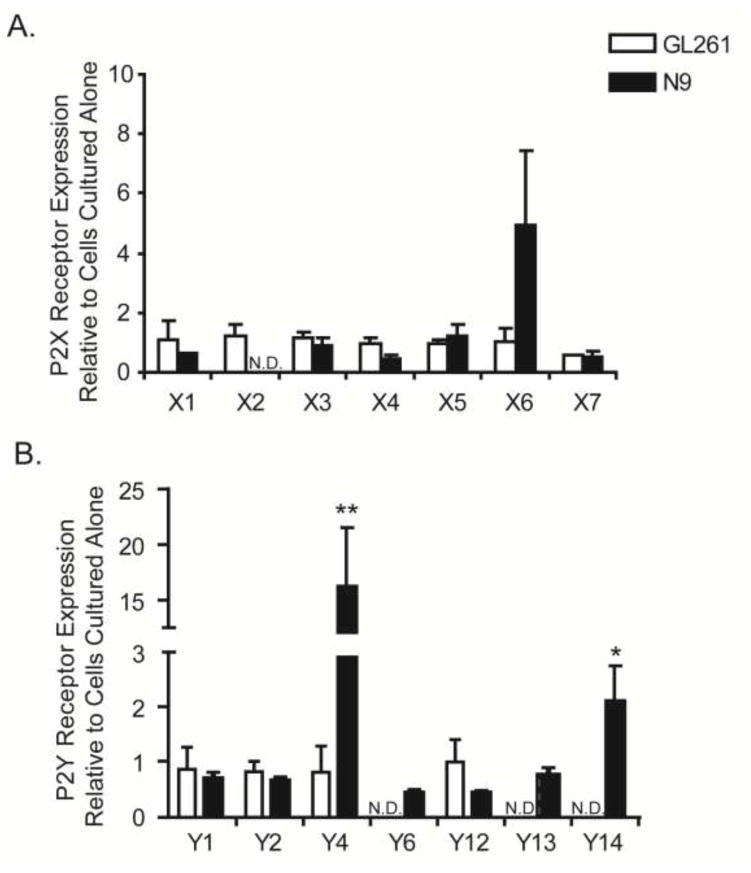
N9 microglia were co-cultured in inserts with GL261 glioma cells. While no statistically significant changes in basal microglial P2X receptor expression levels were observed in transwell cultures (Fig. 3A), P2X2 trended towards down-regulation, P2X5 became detectibly expressed and P2X6 trended towards increasing. Interestingly, P2Y4 and P2Y14 receptors were significantly up-regulated (Fig. 3B) in transwell cultures with glioma cells, the latter being consistent with changes also detected in glioma-derived CD11b+ cells (Fig. 1C). Although P2Y2 mRNA was significantly upregulated in CD11b+ cells derived from gliomas (Figure 2B), this was not recapitulated in the transwell system.
Figure 3. N9 microglia cultured in transwells with GL261 glioma cells upregulate P2Y4 and P2Y14 nucleotide receptor expression.
Transwell cultures containing N9 microglia in the insert and GL261 glioma cells in the well were co-cultured for 24 hours. Total RNA was harvested form each cell type individually, reverse transcribed and subjected to quantitative RT-PCR for all known rodent (A) P2X and (B) P2Y nucleotide receptors. Data were normalized to 18s rRNA. Fold changes are displayed relative to the expression of each gene in each cell line cultured alone: N9 cells cultured on inserts with N9 cells on the well, and GL261 cells in the well cultured with GL261 cells in the insert, to account for alterations due to differences in cell numbers in the co-cultures. * p<0.05, ** p < 0.01.
Selective activation of P2Y14 receptors decreases GL261 cell IL-6 release in GL261/N9 transwell cultures
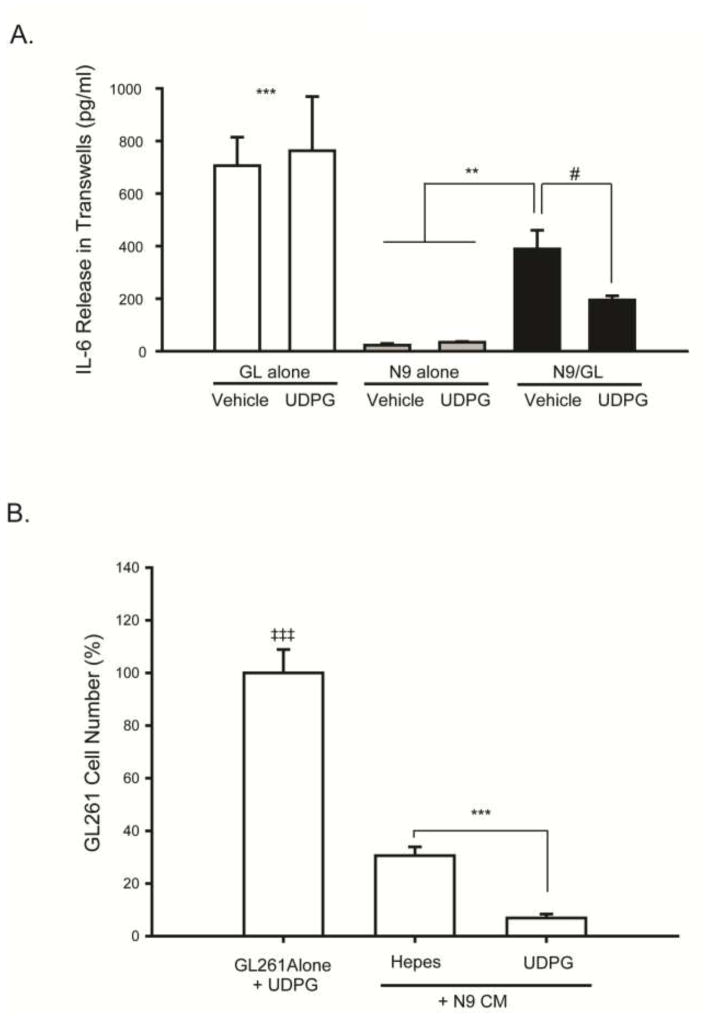
Since IL-6 has potent effects on glioma cell proliferation, we evaluated IL-6 levels in the medium of GL261 and N9 cells grown alone. While N9 cells alone produced very little IL-6, the GL261 cells produced high amounts (Fig. 4A). However, adding microglia to the transwell system reduced the amount of IL-6 in the wells by ~50% (Figure 4A), suggesting that microglia reduce glioma cell production of this cytokine. To begin to address the potential functional significance of microglial P2Y14 receptor upregulation in glioma transwell cultures, we next treated the transwell cultures with UDP glucose (UDPG), a selective P2Y14 receptor agonist. Since GL261 cells do not express detectible levels of P2Y14 either alone (Fig. 2B) or in co-culture (Fig. 3B), the effects of UDPG are likely mediated by the microglia. We observed an even further reduction in IL-6 levels in the transwell supernatants (Figure 4A) upon UDPG treatment, suggesting that P2Y14 receptor activation amplifies the inhibitory effect of microglia on GL261 cell IL-6 release.
Figure 4. Pharmacologic activation of P2Y14 receptors with UDPG decreases IL-6 release in N9 microglia and GL261 glioma transwell cultures, and conditioned medium from microglia treated with UDPG decreases GL261 tumor cell proliferation.
(A) ELISA assays were performed to measure IL-6 concentrations in the tissue culture medium from GL261 tumor cells alone, N9 microglia alone, or from N9/GL261 transwell cultures grown together for 24 hours treated with either vehicle (250 mM HEPES) or UDPG (300 μM). GL261 cells produced the most IL-6, but the presence of microglia in transwell culture reduced this, an effect that was further reduced by treatment with UDPG. # p=0.09, ** p<0.015, and *** p<0.001 GL alone vs. all others. (B) N9 microglia were cultured alone and treated with either vehicle (250 mM HEPES) or UDPG (300 μM) for 24 hours. The N9 cell conditioned medium (N9 CM) was filtered and then applied to GL261 cell cultures at a ratio of 1:1 for 24 hrs after which time, cell numbers were quantified using the MTS assay. N9 CM data are expressed relative to GL261 cells treated directly with UDPG to account for the presence of UDPG in the microglia conditioned medium. CM from UDPG-treated N9 cells reduced GL261 glioma cell proliferation. *** p < 0.001 vs. GL261 cells alone.
Conditioned medium from microglia treated with UDPG decreases GL261 glioma cell proliferation
Since soluble factors produced by microglia reduce transwell glioma cell production of IL-6, we next evaluated the effects of microglial UDPG treatment on glioma cell proliferation. Conditioned medium (CM) from vehicle- or UDPG-treated microglia was added to GL261 cells, and GL261 cell number was evaluated 24 hrs later. CM from UDPG-treated microglia reduced glioma cell proliferation by ~75%, suggesting that microglial P2Y14 receptor activation causes their release of a soluble molecule that interferes with glioma cell proliferation.
4. Discussion
Purinergic receptor signaling has functional consequences on both the microglial inflammatory response, and glioma progression. However, the role of P2Y receptors specifically, remains poorly understood in this context. Here we report that P2Y14 receptors are upregulated in CD11b+ cells in the tumor microenvironment both in vivo and in transwell cultures, and that P2Y14 activation in vitro decreases IL-6 production and glioma cell proliferation.
In the C57/B6 GL261 glioma model, tumor-associated CD11b+ cells significantly upregulated P2Y2 and P2Y14 mRNA levels, but whether these alterations reflect an anti-tumor response, or a modification that benefits the glioma cells is not yet clear. Microglial P2Y4 and P2Y14 receptor expression was significantly increased in transwell cultures, partially mirroring the effects observed in GL261 tumor-associated CD11b+ cells. However, the in vitro transwell culture system does not model the complex physiologic tumor environment, and the length of time over which cell-cell communication can be studied in transwells is limited by the highly proliferative nature of both cell types. Moreover, other cell types and molecules within the glioma and/or CNS environment are absent, including for example, neurons and non-transformed astrocytes. Also, since the tumor-associated CD11b+ cell population isolated here contains both microglia and tumor infiltrating macrophages, some of the differences observed in P2 receptor expression in tumor-associated CD11b+ cells vs. microglia in transwell cultures, may reflect alterations in P2 receptors in infiltrating macrophages that were absent in transwells.
It is interesting to note that although CD11b+ cells accumulate within and around gliomas [4], the expression of P2Y12 receptors, which are activated by ADP and are necessary for microglial chemotaxis and process extension [32, 33] are not up-regulated in glioma-derived CD11b+ cells, or in microglia grown in transwell cultures. Thus, P2Y12 purinergic receptors probably play a minor role in microglial chemotaxis in the tumor microenvironment. However, the absence of P2Y12 receptor mRNA upregulation is in line with known downregulation of P2Y12 receptor expression by ADP [34], an abundant nucleotide in the tumor microenvironment [8].
Up to 40% of GBMs can consist of tumor-associated macrophages and microglia [35]. Indeed, higher infiltration of macrophages within gliomas is associated with a poorer prognosis in patients, as they are thought to exacerbate immunosuppression [36]. We found that P2Y2 mRNA was increased in tumor-associated CD11b+ cells. Activation of P2Y2 receptors by UTP upregulates production of the monocyte recruiting chemokine CCL2, by several antigen presenting cell types including rat alveolar and peritoneal macrophages, human THP-1 monocytic cells, and primary human monocytes and macrophages [37, 38]. These observations suggest that P2Y2 upregulation in glioma-derived CD11b+ cells could aid tumor infiltration by microglia and macrophages, and may therefore represent a potential therapeutic target worthy of future study.
While the function of P2Y14 in microglia is still unclear, its expression is upregulated in microglia during neuropathic pain, and P2Y14 knockdown with RNAi improves pain behavior [39]. However, despite its reported expression in microglia more than a decade ago [39], few studies since have studied the role of this receptor in microglia. Interestingly, P2Y14 is also highly expressed by hematopoietic cells such as neutrophils [40] and may be important in injury-induced chemotaxis of both neutrophils [41] and bone marrow hematopoietic stem cells [42]. Additional studies are needed to determine if P2Y14 receptors play a similar role in gliomas for recruiting MG/MP to the growing tumor.
IL-6 contributes to glioma growth and progression, and increased levels within tumors correlate with poorer patient prognosis [43, 44]. Thus, reduced IL-6 protein levels by P2Y14 activation may be clinically important. We found that glioma cells, not N9 microglia, were the major producers of IL-6 in the GL261-N9 transwell cultures. This is in contrast to a recent report indicating that human primary microglia in culture produce high levels of IL-6 [45], but in accordance with other reports [46, 47]. Treatment of the transwell cultures with UDPG reduced IL-6 levels even further, suggesting that pharmacologic activation of P2Y14 receptors in the tumor microenvironment may be beneficial. We next evaluated glioma cell proliferation in the presence of conditioned medium from microglia treated with UDPG and found that tumor cell proliferation was also inhibited. While it is not yet clear that reductions in IL-6 by UDPG are responsible for the decrease in glioma cell proliferation, reducing IL-6 levels within gliomas via UDPG treatment may decrease tumor invasiveness and reduce tumor associated angiogenesis [48, 49]. Delivering this nucleotide to gliomas, and studying its effects on microglial immune activities will shed light on how modulation of purinergic receptors like P2Y14 in tumor-associated MG/MP may ultimately play a supportive role in glioma regression.
Immunotherapies to activate resident MG/MP populations within the glioma hold great promise; and though they are currently the subject of much research, no clinically useful therapies have been developed. Based on these data, we propose that manipulating endogenously expressed P2 receptors on microglia whose activation may result in the suppression of tumor promoting factors may be therapeutically useful as an adjuvant or in combination with standard glioma chemotherapies.
Acknowledgments
This work was supported by NIH R01NS049033 (JJW) and the University of Wisconsin Alumni Research Foundation. We would like to thank Dr. Victoria Richards for helpful discussions and contributions to this manuscript.
Footnotes
Compliance with ethical standards:
MAC and JJW declare that they have no conflicts of interest.
All research animal studies were conducted on protocols approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee, and complied with standards set forth by the Guide for the Care and Use of Laboratory Animals.
This article does not contain any studies using human participants performed by any of the authors.
References
- 1.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G for the German Glioma N. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 3.Badie B, Schartner J. Role of microglia in glioma biology. Microscopy Research & Technique. 2001;54:106–113. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- 4.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. Journal of Neuroscience Research. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 5.Yeung YT, McDonald KL, Grewal T, Munoz L. Interleukins in glioblastoma pathophysiology: implications for therapy. Br J Pharmacol. 2013;168:591–606. doi: 10.1111/bph.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a Dzaye OD, Hu F, Derkow K, Haage V, Euskirchen P, Harms C, Lehnardt S, Synowitz M, Wolf SA, Kettenmann H. Glioma Stem Cells but Not Bulk Glioma Cells Upregulate IL-6 Secretion in Microglia/Brain Macrophages via Toll-like Receptor 4 Signaling. J Neuropathol Exp Neurol. 2016;75:429–440. doi: 10.1093/jnen/nlw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CY, Li MC, Liao SL, Huang YL, Shen CC, Pan HC. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J Clin Neurosci. 2005;12:930–933. doi: 10.1016/j.jocn.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wink MR, Lenz G, Braganhol E, Tamajusuku ASK, Schwartsmann G, Sarkis JJF, Battastini AMO. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Letters. 2003;198:211. doi: 10.1016/s0304-3835(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 10.Morrone FB, Oliveira DL, Gammermann P, Stella J, Wofchuk S, Wink MR, Meurer L, Edelweiss MI, Lenz G, Battastini AM. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:226. doi: 10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claes P, Grobben B, Van Kolen K, Roymans D, Slegers H. P2Y(AC)(−)-receptor agonists enhance the proliferation of rat C6 glioma cells through activation of the p42/44 mitogen-activated protein kinase. Br J Pharmacol. 2001;134:402–408. doi: 10.1038/sj.bjp.0704271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrone FB, Jacques-Silva MC, Horn AP, Bernardi A, Schwartsmann G, Rodnight R, Lenz G. Extracellular Nucleotides and Nucleosides Induce Proliferation and Increase Nucleoside Transport in Human Glioma Cell Lines. Journal of Neuro-Oncology. 2003;64:211. doi: 10.1023/a:1025699932270. [DOI] [PubMed] [Google Scholar]
- 13.Braganhol E, Wink M, Lenz G, Battastini A. Purinergic Signaling in Glioma Progression. In: Barańska J, editor. Glioma Signaling. Springer; Netherlands: 2013. pp. 81–102. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- 15.Tu MT, Luo SF, Wang CC, Chien CS, Chiu CT, Lin CC, Yang CM. P2Y(2) receptor-mediated proliferation of C(6) glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br J Pharmacol. 2000;129:1481–1489. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krzeminski P, Suplat D, Czajkowski R, Pomorski P, Baranska J. Expression and functional characterization of P2Y1 and P2Y12 nucleotide receptors in long-term serum-deprived glioma C6 cells. Febs j. 2007;274:1970–1982. doi: 10.1111/j.1742-4658.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- 17.Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. Journal of Neuroscience Research. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crain J, Nikodemova M, Watters J. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. Journal of Neuroinflammation. 2009;6:24. doi: 10.1186/1742-2094-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crain JM, Watters JJ. Microglial P2 Purinergic Receptor and Immunomodulatory Gene Transcripts Vary By Region, Sex, and Age in the Healthy Mouse CNS. Transcriptomics: open access. 2015:3. doi: 10.4172/2329-8936.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- 21.Brautigam VM, Frasier C, Nikodemova M, Watters JJ. Purinergic receptor modulation of BV-2 microglial cell activity: Potential involvement of p38 MAP kinase and CREB. Journal of Neuroimmunology. 2005;166:113. doi: 10.1016/j.jneuroim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Ogata T, Chuai M, Morino T, Yamamoto H, Nakamura Y, Schubert P. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Research. 2003;981:174. doi: 10.1016/s0006-8993(03)03028-2. [DOI] [PubMed] [Google Scholar]
- 23.Straub RH, Pongratz G, Gunzler C, Michna A, Baier S, Kees F, Falk W, Scholmerich J. Immunoregulation of IL-6 secretion by endogenous and exogenous adenosine and by exogenous purinergic agonists in splenic tissue slices. J Neuroimmunol. 2002;125:73–81. doi: 10.1016/s0165-5728(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 24.Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. Journal of Neurochemistry. 2001;78:1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- 25.Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol. 2008;153:1528–1537. doi: 10.1038/sj.bjp.0707692. 0707692 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of Cellular UDP-Glucose as a Potential Extracellular Signaling Molecule. Mol Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- 27.Eigenbrodt E, Reinacher M, Scheefers-Borchel U, Scheefers H, Friis R. Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Crit Rev Oncog. 1992;3:91–115. [PubMed] [Google Scholar]
- 28.Brautigam VM, Dubyak GR, Crain JM, Watters JJ. The inflammatory effects of UDP-glucose in N9 microglia are not mediated by P2Y14 receptor activation. Purinergic Signal. 2008;4:73–78. doi: 10.1007/s11302-008-9095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. European Journal of Immunology. 1989;19:1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48:144–156. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 33.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham Margaret R, Nisar Shaista P, Mundell Stuart J. Molecular mechanisms of platelet P2Y12 receptor regulation. Biochemical Society Transactions. 2013;41:225–230. doi: 10.1042/bst20120295. [DOI] [PubMed] [Google Scholar]
- 35.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 36.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59:472–485. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stokes L, Surprenant A. Purinergic P2Y2 Receptors Induce Increased MCP-1/CCL2 Synthesis and Release from Rat Alveolar and Peritoneal Macrophages. J Immunol. 2007;179:6016–6023. doi: 10.4049/jimmunol.179.9.6016. [DOI] [PubMed] [Google Scholar]
- 38.Higgins KR, Kovacevic W, Stokes L. Nucleotides regulate secretion of the inflammatory chemokine CCL2 from human macrophages and monocytes. Mediators of inflammation. 2014;2014:293925. doi: 10.1155/2014/293925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi K, Fukuoka T, Iyamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- 40.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in human neutrophils. European Journal of Pharmacology. 2006;543:166. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 41.Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER, Harden TK. A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol Pharmacol. 2013;84:41–49. doi: 10.1124/mol.113.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BC, Cheng T, Adams GB, Attar EC, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, Hancock J, Choi PS, Haber DA, Luster AD, Scadden DT. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003;17:1592–1604. doi: 10.1101/gad.1071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shan Y, He X, Song W, Han D, Niu J, Wang J. Role of IL-6 in the invasiveness and prognosis of glioma. International journal of clinical and experimental medicine. 2015;8:9114–9120. [PMC free article] [PubMed] [Google Scholar]
- 44.McFarland BC, Hong SW, Rajbhandari R, Twitty GB, Jr, Gray GK, Yu H, Benveniste EN, Nozell SE. NF-kappaB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS One. 2013;8:e78728. doi: 10.1371/journal.pone.0078728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Sarkar S, Cua R, Zhou Y, Hader W, Yong VW. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012;33:312–319. doi: 10.1093/carcin/bgr289. [DOI] [PubMed] [Google Scholar]
- 46.Van Meir E, Sawamura Y, Diserens AC, Hamou MF, de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990;50:6683–6688. [PubMed] [Google Scholar]
- 47.Dubost JJ, Rolhion C, Tchirkov A, Bertrand S, Chassagne J, Dosgilbert A, Verrelle P. Interleukin-6-producing cells in a human glioblastoma cell line are not affected by ionizing radiation. J Neurooncol. 2002;56:29–34. doi: 10.1023/a:1014467804488. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Gao Q, Han S, Pan F, Fan W. The CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal transition by cooperatively activating STAT3-Twist signaling. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:973–981. doi: 10.1007/s13277-014-2717-z. [DOI] [PubMed] [Google Scholar]
- 49.Piperi C, Samaras V, Levidou G, Kavantzas N, Boviatsis E, Petraki K, Grivas A, Barbatis C, Varsos V, Patsouris E, Korkolopoulou P. Prognostic significance of IL-8-STAT-3 pathway in astrocytomas: correlation with IL-6, VEGF and microvessel morphometry. Cytokine. 2011;55:387–395. doi: 10.1016/j.cyto.2011.05.012. [DOI] [PubMed] [Google Scholar]