Abstract
Objective
To assess whether lipoprotein (a) [Lp(a)] levels and other lipid levels were predictive of progression of atherosclerosis burden as assessed by carotid MRI in subjects who have been treated with low density lipoprotein cholesterol (LDL-C) lowering therapy and participated in the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) Trial.
Approach
AIM-HIGH was a randomized, double-blind study of subjects with established vascular disease, elevated triglycerides, and low high-density lipoprotein cholesterol. One hundred fifty-two AIM-HIGH subjects underwent both baseline and 2-year follow-up carotid artery MRI. Plaque burden was measured by the percent wall volume (%WV) of the carotid artery. Associations between annualized change in %WV with baseline and on-study (1 year) lipid variables were evaluated using multivariate linear regression. P-values were adjusted for multiple comparisons.
Results
Average %WV at baseline was 41.6 ± 6.8% and annualized change in %WV over 2 years ranged from −3.2% to 3.7%/year (mean: 0.2 ± 1.1%/year, p=0.032). Increases in %WV was significantly associated with higher baseline Lp(a) [β = 0.34 per 1-SD increase of Lp(a), 95% CI: 0.15 to 0.52, p<0.001] after adjusting for clinical risk factors and other lipid levels. On-study Lp(a) had a similar positive association with %WV progression (β = 0.33, 95% CI: 0.15 to 0.52, p<0.001).
Conclusions
Despite intensive lipid therapy, aimed at aggressively lowering LDL-C to <70 mg/dL, carotid atherosclerosis continued to progress as assessed by carotid MRI and that elevated Lp(a) levels were independent predictors of increases in atherosclerosis burden.
Keywords: lipoprotein, lipids, atherosclerosis, MRI
Subject codes: Lipids and Cholesterol, MRI, Atherosclerosis
Introduction
Lipoprotein (a) [Lp(a)] has been identified to have a continuous and independent association with overall cardiovascular disease (CVD) risk [1,2]. Higher levels of Lp(a) are associated with risk of myocardial infarction [3,4]. In ischemic stroke patients, elevated Lp(a) levels are associated with the presence of carotid atherosclerosis [5]. Inaddition, the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) study found that Lp(a) levels at baseline and on-study were predictive of residual CVD risk [6]. Furthermore, Lp(a) was positively associated with high-risk plaque features including intraplaque hemorrhage, mural thrombus, or surface defects [7] and with plaque vascularity [8] in AIM-HIGH. The aim of our study was to assess whether Lp(a) and other lipid levels were predictive of progression of atherosclerosis burden as assessed by carotid MRI in subjects who have been treated with low density lipoprotein cholesterol (LDL-C) lowering therapy and participated in the AIM-HIGH Trial [9].
Material and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
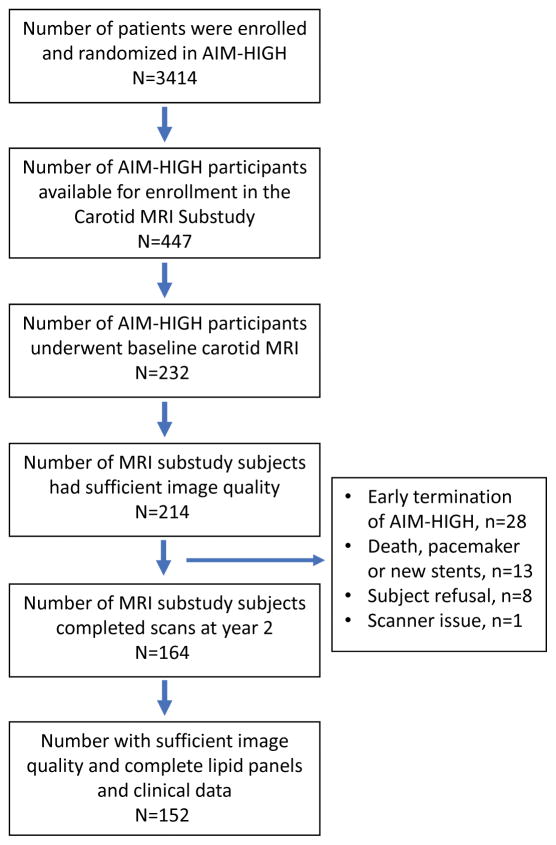
A total of 152 subjects from the AIM-HIGH MRI substudy were included in the analysis after excluding subjects with insufficient image quality or missing clinical measurements as shown by the flowchart of subject inclusion/exclusion in Figure 1. Subjects were 45 to 79 years old (median: 62), with 81% male, 12% non-white, 26% current smokers, 27% with a history of diabetes and 82% with a history of hypertension (Table 1). Median total-C, LDL-C, HDL-C, triglycerides, and Lp(a) at baseline were 145, 72, 35, 162, and 32 nmol/L, respectively. Baseline %WV ranged from 28% to 60% (median: 40%, SD: 7.1%). Most baseline variables were distributed similarly in the monotherapy and combination therapy arms (Table 1), though the monotherapy group had more white subjects (p=0.025) and lower Lp(a) (p=0.020) than the combination therapy group.
Figure 1.
Flowchart of the study population.
Table 1.
Clinical, lipid, and carotid plaque characteristics by treatment assignment.
| Variable | Treatment Assignment*
|
P-value† | |
|---|---|---|---|
| Monotherapy (N=86) | Combination Therapy (N=66) | ||
| Baseline Clinical Characteristics | |||
| Male sex | 67 (77.9) | 56 (84.8) | 0.31 |
| Age, years | 62 (46 – 78) | 60 (45 – 79) | 0.55 |
| White race | 80 (93.0) | 53 (80.3) | 0.025 |
| Body mass index, kg/m2 | 29 (17 – 44) | 30 (21 – 41) | 0.46 |
| Current smoker | 16 (18.6) | 10 (15.2) | 0.67 |
| History of diabetes | 25 (29.1) | 16 (24.2) | 0.58 |
| History of hypertension | 71 (82.6) | 54 (81.8) | >0.99 |
| Baseline Lipid Values | |||
| Total-C, mg/dl | 145 (88 – 257) | 144 (100 – 245) | 0.71 |
| LDL-C, mg/dl | 74 (29 – 178) | 72 (32 – 167) | 0.92 |
| HDL-C, mg/dl | 35 (20 – 51) | 34 (18 – 52) | 0.42 |
| Triglycerides, mg/dl | 166 (100 – 333) | 154 (101 – 388) | 0.34 |
| ApoB, mg/dl | 82 (41 – 159) | 86 (53 – 175) | 0.11 |
| ApoA1, mg/dl | 124 (85 – 167) | 119 (59 – 168) | 0.18 |
| Lp(a), nmol/L | 29 (0.5 – 324) | 47 (0.3 – 339) | 0.020 |
| On-study Lipid Values | |||
| Total-C, mg/dl | 140 (97 – 289) | 134 (87 – 223) | 0.63 |
| LDL-C, mg/dl | 69 (28 – 203) | 67 (26 – 126) | 0.48 |
| HDL-C, mg/dl | 39 (21 – 67) | 43 (21 – 73) | 0.007 |
| Triglycerides, mg/dl | 154 (74 – 477) | 131 (44 – 464) | 0.012 |
| ApoB, mg/dl | 77 (51 – 153) | 74 (30 – 144) | 0.10 |
| ApoA1, mg/dl | 128 (88 – 171) | 136 (72 – 210) | 0.041 |
| Lp(a), nmol/L | 24 (0.4 – 321) | 33 (0.4 – 353) | 0.15 |
Values are no. (%) or median (range);
Fisher’s exact test or Wilcoxon rank-sum test comparing treatment groups.
Supplemental Table S1 shows a comparison between subjects included in the present analysis and the remainder of the AIM-HIGH cohort (n=3262). Subjects in the MRI substudy were younger (median: 62 vs. 64 years, p=0.003), were less likely to be white (88% vs. 92%, p=0.042), had lower body mass index (median: 29 vs 30, p=0.002), less likely to be diabetic (27% vs. 34%, p=0.066) and more likely to have a history of hypertension (82% vs. 71%, p=0.002). There were no significant differences in baseline lipids or on-study lipids between the two cohorts. The MRI substudy cohort had a significantly lower rate of cardiovascular events at year 2 than the remainder of the AIM-HIGH cohort (6% vs. 11%, p=0.009).
Over one year, all lipid values except total-C significantly improved on average across both treatment groups (Table 2). However, HDL-C (p<0.001), triglycerides (p=0.033), ApoB (p=0.004), ApoA1 (p=0.001) and Lp(a) (p<0.001) improved more in the combination therapy arm than the monotherapy arm. On average, annualized change in %WV ranged from −3.2 to 3.7% per year (mean: 0.2 ± 1.1% per year, p=0.032) with no significant difference in the amount of change between treatment groups (p=0.67).
Table 2.
Changes in lipids from baseline to 1-year of the study.
| Variable | All (N=152) | Treatment Assignment*
|
P-value† | |
|---|---|---|---|---|
| Monotherapy (N=86) | Combination Therapy (N=66) | |||
| Change in total-C, mg/dl | −2.2 ± 38.0 | −2.0 ± 39.6 | −2.5 ± 36.0 | 0.55 |
| Change in LDL-C, mg/dl | −5.3 ± 31.3‡ | −4.4 ± 34.1 | −6.6 ± 27.4‡ | 0.38 |
| Change in HDL-C, mg/dl | 6.4 ± 7.2‡ | 4.1 ± 6.2‡ | 9.5 ± 7.4‡ | <0.001 |
| Change in triglycerides, mg/dl | −12.5 ± 80.2‡ | −6.8 ± 69.7 | −19.9 ± 92.2‡ | 0.033 |
| Change in ApoB, mg/dl | −8.7 ± 25.7‡ | −3.8 ± 24.1 | −15.0 ± 26.6‡ | 0.004 |
| Change in ApoA1, mg/dl | 9.9 ± 16.9‡ | 5.2 ± 13.3‡ | 16.0 ± 19.1‡ | 0.001 |
| Change in Lp(a), nmol/L | −9.3 ± 35.6‡ | −0.5 ± 27.6 | −20.8 ± 41.5‡ | <0.001 |
Values are mean ± SD;
Wilcoxon rank-sum test comparing change in lipids between treatment groups;
Significant change in lipid values between baseline and on-study by Wilcoxon signed-rank test (p<0.05).
Age (p=0.043) and history of hypertension (p=0.061) had marginal associations with annualized change in %WV (Supplemental Table S2). None of the other non-lipid risk factors or treatment assignment were significantly associated with plaque progression. Associations between plaque progression and lipid variables with adjustments for treatment assignment, baseline %WV, and clinical risk factors are summarized in Table 3. Only Lp(a) at baseline (β = 0.33 per 1-SD increase, 95% CI: 0.15 to 0.51, p=0.001) and on-study (β = 0.31, 95% CI: 0.13 to 0.49, p=0.001) were significantly associated with plaque progression at the Bonferroni corrected level α = 0.0036. Baseline (β = 0.34 per 1-SD increase, 95% CI: 0.15 to 0.52, p<0.001) and on-study Lp(a) (β = 0.33, 95% CI: 0.15 to 0.52, p<0.001) remained independently associated with plaque progression after further adjustment for LDL-C, HDL-C, and triglycerides. The difference between baseline and on-study Lp(a) was not significantly associated with plaque progression (p=0.75).
Table 3.
Associations between individual baseline and on-study lipid variables and carotid plaque progression.
| Variable | Model 1
|
||
|---|---|---|---|
| β* | (95% CI) | P-value | |
| Baseline Lipid Values | |||
| Total-C | 0.09 | (−0.10, 0.28) | 0.34 |
| LDL-C | 0.05 | (−0.13, 0.24) | 0.59 |
| HDL-C | 0.06 | (−0.15, 0.26) | 0.58 |
| Triglycerides† | 0.08 | (−0.11, 0.27) | 0.39 |
| ApoB | 0.15 | (−0.04, 0.33) | 0.12 |
| ApoA1 | 0.07 | (−0.13, 0.27) | 0.47 |
| Lp(a)† | 0.33 | (0.15, 0.51) | 0.001 |
| On-study Lipid Values | |||
| Total-C | 0.04 | (−0.15, 0.23) | 0.66 |
| LDL-C | −0.01 | (−0.20, 0.18) | 0.94 |
| HDL-C | 0.01 | (−0.19, 0.22) | 0.88 |
| Triglycerides† | 0.07 | (−0.12, 0.26) | 0.47 |
| ApoB | 0.16 | (−0.04, 0.35) | 0.11 |
| ApoA1 | −0.03 | (−0.23, 0.17) | 0.76 |
| Lp(a)† | 0.31 | (0.13, 0.49) | 0.001 |
Model 1 = one lipid variable + adjustments for random assignment, baseline %WV, adjustment for sex, age, race, BMI, current smoker, diabetes, hypertension;
Mean difference in the annualized change in %WV in units of %/year per 1-SD increase in the lipid variable;
Variable was log-transformed prior to inclusion in the model to reduce right-skewness.
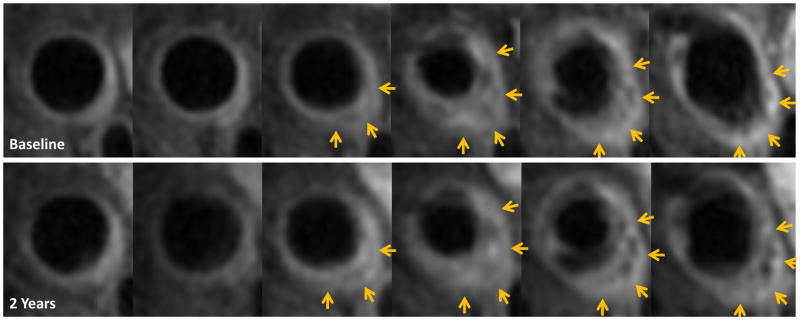
Figure 2 presents an MRI example of carotid plaque progression with elevated Lp(a) at both baseline (263.6 nmol/L) and on-study (159.4 nmol/L), other on-study lipids were well controlled, particularly, on-study LDL-C was 45 mg/dL.
Figure 2.
Example of carotid plaque progression in a subject with elevated Lp(a).
The top panel shows 6 consecutive post-contrast T1-weighted images (2 mm thickness) of the left common carotid artery up to the bifurcation. The lower panel presents the matching locations 2 years later and a noticeable plaque volume progression as indicated by the yellow arrows compared to baseline. This example is from an AIM-HIGH participant who was a current smoker, had a history of coronary artery disease with a prior coronary bypass surgery and on standard of care for secondary prevention, including statin therapy >5 years prior to enrollment in the study. At baseline, body mass index (BMI) = 30, BP = 112/70 mmHg, total cholesterol (TC) = 140 mg/dl, triglycerides (TG) = 203 mg/dl, low density lipoprotein cholesterol (LDL-C) = 64 mg/dl, high density lipoprotein cholesterol (HDL-C) = 35 mg/dl, apolipoprotein B (ApoB) = 92 mg/dl, apolipoprotein A1 (apoA1) = 120 mg/dl, Lp(a) = 263.6 nmol/L [>95 percentile of Lp(a) distribution]. The subject was randomized to simvastatin and placebo for extend-release niacin in AIM-HIGH. On-study lipids showed that TC = 115 mg/dl, TG = 148 mg/dl, LDL-C = 45 mg/dl, HDL-C = 40 mg/dl, ApoB = 64 mg/dl, ApoA1 = 130 mg/dl, Lp(a) = 159.4 nmol/L [>90 percentile of Lp(a) distribution].
Clinical risk factors and other lipid values between subjects with higher baseline Lp(a) (≥ median of 32 nmol/L) and lower baseline Lp(a) (<32 nmol/L) were compared (Supplemental Table S3). Non-white subjects were more likely to have elevated Lp(a) (p=0.048). Those with higher Lp(a) at baseline tended to have lower total-C (p=0.030), LDL-C (p=0.043), and triglycerides (p=0.048) at 1 year. Baseline %WV was similar between the two groups (median: 40 vs. 41%, p=0.52) and there was no significant correlation between %WV and baseline Lp(a) (Spearman’s rho = −0.04, p=0.60).
Discussion
Our study showed that despite intensive lipid therapy, aimed at aggressively lowering LDL-C to <70 mg/dL, carotid atherosclerosis continued to progress as assessed by carotid MRI. Previous studies [10,11] have demonstrated that progression of carotid plaque volume as assessed by 3D ultrasound significantly predicted cardiovascular events including stroke, TIA, MI and death. Engelen and colleagues showed that subjects with >10% of carotid plaque progression over 1 year were considered to have an increased risk for future events [11]. However, the clinical implication of the observed carotid atherosclerosis progression at mean 0.2% and up to 3.7% per year as assessed by MRI would require further investigations.
Unlike earlier imaging studies that have demonstrated combinations of lipid therapies with statin, niacin and or bile acid sequestrants lead to regression or slow progression of atherosclerosis either in coronary arteries [12–15] or in carotid [16,17], there was no significant difference in carotid atherosclerosis progression between the 2 treatment groups in AIM-HIGH. This inconsistency may be potentially attributed to several important differences between the earlier studies and AIM-HIGH. First, patients in AIM-HIGH have been treated with statins and had a mean LDL-C of 74 mg/dL at baseline while subjects in earlier studies were lipid treatment naïve or with higher LDL-C levels. Second, AIM-HIGH compared the combination therapy to statin, not to placebo as in most earlier studies. By the AIM-HIGH study design, the difference in achieved LDL-C between treatment arms on-study was small, 62 mg/dL in the simvastatin plus ERN group and 67 mg/dL in the simvastatin plus placebo group. Finally, maximimum CV benefits from niacin are associated with a significant decrease of triglyceride-rich VLDL, their remnants and dense LDL particles [18]; however, the AIM-HIGH subjects did not have high TG at baseline (a mean of 161 mg/dL). More importantly, the current study results indicate that carotid atherosclerosis continue to progress despite LDL-C lowering to 62 mg/dL and lower LDL-C target is needed in order to stop or reverse atherosclerosis progression as demonstrated in GLAGOV [19] showing that adding evolocumab to statin therapy further lowers LDL-C to a mean of 36.6 mg/dL and reduces coronary atheroscleroma burden.
Importantly, we found that elevated Lp(a) levels were independent predictors of increases in carotid atherosclerosis burden. This fiding is consistent with observations from other AIM-HIGH [6] and epidemiological and genetic association studies showing a continuous and independent association between Lp(a) and cardiovascular disease [3,4,20, 21]. In AIM-HIGH, Lp(a) levels were predictive of residual CV risk with baseline and on-study Lp(a) levels being significantly associated with CV events both in the statin group (baseline HR 1.24, p=0.002 and on-study HR 1.21, p=0.017) and in the statin+ERN group (baseline HR 1.25, p=0.001 and on-study HR 1.18, p=0.028), suggesting that elevated Lp(a) levels continue to impact CVD risk in patients on intensive lipid therapy with well treated LDL-C levels [6]. In a meta-analysis of 18 population studies, Danesh et al. demonstrated that compared to the group with the bottom third of Lp(a) levels, the group with the top third had a hazard ratio of 1.6 (95% CI 1.41–1.8) for CHD death or nonfatal MI [1]. A number of hypothesized mechanisms have been proposed for the contributions of Lp(a) to atherothrombotic vascular diseases, including impairing fibrinolysis, promoting cell recruitment to vessel wall, acceleration of macrophage form cell formation and contributing to increased levels of pro-inflammatory oxidized phospholipids [22–25]. There have been mixed reports on the association of Lp(a) levels with atherosclerosis burden and progression. In a cohort of young stroke patients, Nasr et al. showed that for each standard deviation increase in Lp(a), the risk of carotid atherosclerosis increased significantly in the second and third tertiles of Lp(a) compared with the first (OR 1.89 and 2.96, respectively) [5]. We previousely reported that Lp(a) was positively associated with high-risk plaque features including intraplaque hemorrhage, mural thrombus, or surface defects [7] and with plaque vascularity [8] in AIM-HIGH. On the other hand, SATURN investigators recently reported that Lp(a) levels were not associated with coronary atheroma progression as measured by IVUS in CAD patients on long-term maximal intensity statin therapy with a mean LDL-C of 60 mg/dL [26].
Treatment with ERN significantly lowered Lp(a) levels by 21% in the AIM-HIGH main trial [9] and 25% in the Carotid MRI substudy cohort, this reduction did not translate into reduction in CV events [9] or atherosclerosis progression. A similar Lp(a) reduction can be achieved with PCSK9 inhibition using evolocumab and alirocumab, with 25.5% achieved in OSLER [27] and 29.3% in ODYSSEY [28], in addition to substantial LDL-C lowering. Although FOURIER [29] showed significant CV event reduction in patients with CV disease and receiving statin therapy, Lp(a) reduction by evolocumb did not add apparent benefit to LDL-C lowering in a population with low Lp(a) at baseline [29]. Whether Lp(a) lowering with PCSK9 inhibition independently contributes to the reductions of coronary atherosclerosis and CV events in patients with elevated Lp(a) remains unknown. On the other hand, aggressive lowering Lp(a) levels by 73% with apheresis in addition to statin therapy has been shown to provide incremental CV event reduction in subjects with established vascular disease [30]. Promising result from the recent development in antisense therapy by inhibiting apo(a) mRNA translation showed that Lp(a) can be decreased by >80% [31]. Future studies are needed to determine the potential of aggressively lowering Lp(a), in addition to LDL-C reduction, on atherosclerosis burden and CV event risk.
Finally, in this hypothesis-generating analysis, while we found a significiant association between Lp(a) and plaque growth, we did not find significant association between baseline Lp(a) levels and baseline carotid atherosclerosis burden. This could be due to AIM-HIGH participants begin enrolled based established CV disease, reducing the range of plaque burden of subjects at baseline.
This study has a number of limitations. The MRI substudy cohort had several diffences compared to the entire AIM-HIGH cohort, potentially limiting the generalizability of these results to the broader population. However, while statistically significant, the actual differences in median age (2 years), race (4%), and body mass index (1 kg/m2) were relatively small in absolute terms. More notable is that those in the MRI substudy were less likely than the remainder of the AIM-HIGH cohort to be diabetic (27% vs. 34%), potentially because eGFR > 60 mL/min/1.73m2 was required due to the injection of a gadolinium-based contrast agent. The MRI substudy was also much more likely to be hypertensive (82% vs. 71%), though the reason for this is unclear. Within the MRI substudy cohort, there was an imbalance in Lp(a) levels at baseline between the mono- and combination therapy groups, which was mitigated by adjusting for treatment group throughout the analysis. Lastly, of the lipid variables, Lp(a) was the only one found to be significantly associated with %WV change. It is possible that the sample size of 152 subjects afforded insufficient statistical power to detect associations with other lipid variables, so they should be further evaluated in larger studies.
In conclusion, in patients under intensive LDL-C lowering to <70 mg/dL, carotid atherosclerosis continued to progress as assessed by carotid MRI. Elevated Lp(a) levels independently predicte carotid atherosclerosis progression.
Supplementary Material
Highlights.
Carotid atherosclerosis continued to progress during lipid therapy with targeted LDL-C<70 mg/dl.
This progression was not effected by adding extended-release niacin to simvastatin.
Lp(a) levels independently predicted carotid atherosclerosis progression.
Acknowledgments
Sources of funding: The carotid MRI sub-study was funded by R01 HL088214. MR imaging coils were provided by GE Healthcare and Philips Healthcare.
Abbreviations
- Lp(a)
Lipoprotein (a)
- CVD
cardiovascular disease
- MRI
magnetic resonance imaging
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoprotein cholesterol
- TC
total cholesterol
- TG
triglycerides
- ApoB
apolipoprotein B
- apoA1
apolipoprotein A1
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes
- HDL-C
high-density lipoprotein cholesterol
- ERN
extended release niacin
- %WV
percent wall volume
Footnotes
Disclosures: Mr. Hippe reports reports grants from GE Healthcare, Philips Healthcare, and Toshiba America Medical Systems outside the submitted work.
References
- 1.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102(10):1082–5. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Chapman MJ, Ray K, Boren J, et al. European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamstrup PR, Benn M, Tybjaerg-Hansen A, et al. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 4.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, et al. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 5.Nasr N, Ruidavets JB, Farghali A, Guidolin B, Perret B, Larrue V. Lipoprotein (a) and carotid atherosclerosis in young patients with stroke. Stroke J Cereb Circ. 2011;42(12):3616–8. doi: 10.1161/STROKEAHA.111.624684. [DOI] [PubMed] [Google Scholar]
- 6.Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62(17):1575–9. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X-Q, Hatsukami TS, Hippe DS, et al. Clinical factors associated with high-risk carotid plaque features as assessed by magnetic resonance imaging in patients with established vascular disease (from the AIM-HIGH Study) Am J Cardiol. 2014;114(9):1412–9. doi: 10.1016/j.amjcard.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien KD, Hippe DS, Chen H, et al. Longer duration of statin therapy is associated with decreased carotid plaque vascularity by magnetic resonance imaging. Atherosclerosis. 2016;245:74–81. doi: 10.1016/j.atherosclerosis.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AIM-HIGH Investigators. Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 10.Wannarong T, Parraga G, Buchanan D, et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke. 2013;44:1859–1865. doi: 10.1161/STROKEAHA.113.001461. [DOI] [PubMed] [Google Scholar]
- 11.van Engelen A, Wannarong T, Parraga G, et al. Three-dimensional carotid ultrasound plaque texture predicts vascular events. Stroke. 2014 Sep;45(9):2695–701. doi: 10.1161/STROKEAHA.114.005752. [DOI] [PubMed] [Google Scholar]
- 12.Blankenhorn DH, Nessim SA, Johnson RD, et al. Beneficial effects of combined colestipol-niacin therapy on coroniacinry atherosclerosis and coroniacinry venous bypass grafts. JAMA. 1987;257:3233–3240. [PubMed] [Google Scholar]
- 13.Brown GB, Albers JJ, Fisher LD, et al. Regression of coroniacinry artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 14.Brown GB, Zhao XQ, Chait A, Alaupovic P, Frohlich J, Albers JJ, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coroniacinry disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 15.Whitney EJ, Krasuski RA, Personius BE, et al. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann Intern Med. 2005;142(2):95–104. doi: 10.7326/0003-4819-142-2-200501180-00008. [DOI] [PubMed] [Google Scholar]
- 16.Villines TC, Stanek EJ, Devine PJ, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J Am Coll Cardiol. 2010;55(24):2721–6. doi: 10.1016/j.jacc.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AJ, Sullenberger LE, Lee HJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–7. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 18.Zambon A, Zhao XQ, Brown BG, et al. Effects of Niacin Combination Therapy With Statin or Bile Acid Resin on Lipoproteins and Cardiovascular Disease. Am J Cardiol. 2014;113(9):1494–8. doi: 10.1016/j.amjcard.2014.01.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholls SJ, Puri R, Anderson T, et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA. 2016;316(22):2373–2384. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 20.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 22.Spence JD, Koschinsky M. Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler Thromb Vasc Biol. 2012;32(7):1550–1. doi: 10.1161/ATVBAHA.112.251306. [DOI] [PubMed] [Google Scholar]
- 23.Phan BAP, Toth PP. Lipoprotein(a): epidemiology, atherogenic activity and impact on cardiovascular risk. Clin Lipidol. 2013;8(2):195–203. [Google Scholar]
- 24.van Dijk RA, Kolodgie F, Ravandi A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53(12):2773–90. doi: 10.1194/jlr.P030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leibundgut G, Scipione C, Yin H, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54(10):2815–30. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puri R, Ballantyne CM, Hoogeveen RC, et al. Lipoprotein(a) and coronary atheroma progression rates during long-term high-intensity statin therapy: Insights from SATURN. Atherosclerosis. 2017;263:137–144. doi: 10.1016/j.atherosclerosis.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N Engl J Med. 2015;372(16):1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 28.Robinson JG, Farnier M, Krempf M, et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N Engl J Med. 2015;372(16):1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 29.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 30.Jaeger BR, Richter Y, Nagel D, et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med. 2009;6(3):229–39. doi: 10.1038/ncpcardio1456. [DOI] [PubMed] [Google Scholar]
- 31.Graham MJ, Viney N, Crooke RM, et al. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57(3):340–51. doi: 10.1194/jlr.R052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.