Abstract
PURPOSE
Over half of all cancer patients receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy experience chemotherapy-induced peripheral neuropathy (CIPN), which includes numbness, tingling, pain, cold sensitivity, and motor impairment in the hands and feet. CIPN is a dose-limiting toxicity, potentially increasing mortality. There are no FDA-approved drugs to treat CIPN, and behavioral interventions such as exercise are promising yet understudied. This secondary analysis of our nationwide phase III randomized controlled trial of exercise for fatigue examines (1) effects of exercise on CIPN symptoms, (2) factors that predict CIPN symptoms, and (3) factors that moderate effects of exercise on CIPN symptoms.
METHODS
Cancer patients (N=355, 56±11 years, 93% female, 79% breast cancer) receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy were randomized to chemotherapy or chemotherapy plus Exercise for Cancer Patients (EXCAP©®). EXCAP is a standardized, individualized, moderate-intensity, home-based, six-week progressive walking and resistance exercise program. Patients reported CIPN symptoms of numbness and tingling and hot/coldness in hands/feet (0–10 scales) pre- and post-intervention. We explored baseline neuropathy, sex, age, body mass index, cancer stage, and cancer type as possible factors associated with CIPN symptoms and exercise effectiveness.
RESULTS
Exercise reduced CIPN symptoms of hot/coldness in hands/feet (−0.46 units, p=0.045) and numbness and tingling (−0.42 units, p=0.061) compared to the control. Exercise reduced CIPN symptoms more for patients who were older (p=0.086), male (p=0.028), or had breast cancer (p=0.076).
CONCLUSIONS
Exercise appears to reduce CIPN symptoms in patients receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy. Clinicians should consider prescribing exercise for these patients.
Keywords: CIPN, exercise, neuropathy
Introduction
Over half of all cancer patients receiving chemotherapy regimens that include taxanes, platinum-based agents, vinca alkaloids, thalidomide, or bortezomib experience chemotherapy-induced peripheral neuropathy (CIPN) [1]. CIPN typically affects the hands and feet and involves sensory symptoms such as numbness, tingling, and pain, including neuropathic pain from cold stimulation (e.g., feelings of hot/coldness); motor symptoms like cramping, difficulty handling small objects, and issues with gait and balance; and autonomic symptoms related to orthostatic hypotension [1–3]. CIPN is a dose-limiting toxicity, potentially increasing mortality [4]. It also interferes with daily activities such as buttoning clothes, writing, and typing, and it reduces quality of life [5]. Half of all CIPN patients do not recover six months after completing chemotherapy [1], and many require years to recover, if they recover at all [6].
The etiology of CIPN is not entirely clear [7] but appears to involve inflammation [8, 9] and damage to mitochondria in peripheral sensory neurons [10]. The neuropathic pain component of CIPN appears to involve changes in sensory pathways of the spinal cord, thalamus, and regions of the cortex such as the somatosensory cortex and the insula [11]. These changes in the central nervous system might exacerbate symptoms of peripheral nerve damage [12].
Treatments for CIPN are extremely limited and require further study [3, 13]. A systematic review of 48 randomized controlled trials (RCTs) testing drugs to prevent or treat CIPN concluded that none of the eleven drugs prevented CIPN, and, of the seven drugs tested to treat CIPN, only duloxetine could be recommended [14]. However, duloxetine has not been shown to reduce numbness or completely eliminate CIPN pain [15], and it causes dry mouth, constipation, diarrhea, and dizziness [16]. Nutritional and complementary interventions for CIPN have yielded inconsistent results, likely due to small sample sizes [17].
Fortunately, exercise may treat or prevent CIPN, as suggested by cross-sectional [18, 19] and randomized [20–22] studies in humans. One secondary analysis of an RCT in 301 breast cancer patients during chemotherapy compared three doses of exercise—without a standard care control group—for treatment of patient-reported CIPN symptoms [20]. Their results suggest that more severe CIPN symptoms tend to occur in patients who are older, less aerobically fit, and overweight or obese. Moreover, for patients who are younger, fitter, and leaner, CIPN symptoms may be treated more effectively using a larger dose of exercise (180 min/week of aerobic exercise instead of 90 min/week). Exercise may treat CIPN through changes in inflammation [23] and sensory pathways in the brain [24].
Several barriers limit our understanding of using exercise to treat CIPN. Prior studies of exercise and CIPN used supervised exercise in a gym or clinic, which can pose as a barrier for patients who have limited time or difficulty obtaining transportation [25]. The use of unsupervised interventions, such as at-home exercise, as part of self-management is important to complement and improve adherence to basic cancer rehabilitation interventions (e.g., information, medical exercise, psycho-oncology, dietetics) [26]. Additionally, there is limited information on which patients benefit most from exercise in terms of CIPN symptoms (only one study [20]).
The primary aim of this secondary analysis was to examine the effects of a six-week, at-home, unsupervised exercise program conducted during chemotherapy on patient-reported CIPN symptoms compared to standard care for chemotherapy. The secondary aims were to explore factors that predict CIPN symptoms and the effectiveness of exercise by assessing demographics, physical fitness, and cancer characteristics. Specifically, we explored whether the effects of exercise on CIPN symptoms were moderated by three established risk factors for CIPN: (1) baseline neuropathy [1], (2) age [20], and (3) body mass index (BMI) [20], and three exploratory variables: (1) sex, (2) cancer stage, and (3) cancer type. We used data from our phase-III nationwide RCT designed to study fatigue in response to six weeks of either exercise during chemotherapy or standard care for chemotherapy. We identified 355 cancer patients in this sample receiving neurotoxic chemotherapy regimens (i.e., containing taxane-, platinum-, or vinca alkaloid-based drugs). We hypothesized that exercise during chemotherapy would reduce CIPN symptoms compared to standard care for chemotherapy.
Patients and Methods
Study design
This was a secondary analysis of an RCT (ClinicalTrials.gov NCT00924651) designed to assess the effects of exercise on fatigue. Briefly, the trial was conducted and analyzed through the University of Rochester Cancer Center (URCC) National Cancer Institute (NCI) Community Oncology Research Program (NCORP) Research Base across 20 community oncology practices in the United States from 2009–2016. Participants were randomly assigned to receive six weeks of (1) standard care for chemotherapy or (2) standard care for chemotherapy plus exercise. Allocation was concealed from coordinators until after participant registration, and concealed from participants until baseline assessments were complete. It was not possible to blind participants or researchers due to the nature of the intervention. Each institutional review board approved the study before participants were enrolled. All participants provided written informed consent. As part of the pre- and post-intervention assessments, participants completed questionnaires, daily diaries, and wore a pedometer (Walk 4 Life Classic; Oswego, IL).
Study participants
To be eligible for the parent RCT, patients must have (1) been ≥21 years, (2) had a primary diagnosis of cancer other than leukemia, without distant metastasis, (3) been chemotherapy naïve, (4) started chemotherapy after enrollment and been scheduled for at least six weeks of chemotherapy with treatment cycles of either two, three, or four weeks; (5) had a Karnofsky Performance Status ≥70, (6) been able to read English, (7) not received concurrent radiation therapy, (8) not had physical limitations that contraindicate participation in a low- to moderate-intensity home-based walking and progressive resistance program as determined by the patient’s oncologist, who had full knowledge of the provided exercise program, and (9) not been identified as in the active or maintenance stage of exercise behavior as assessed by the Exercise Stages of Change [27]. This secondary analysis was restricted to patients receiving neurotoxic chemotherapy (taxane-, platinum-, or vinca alkaloid-based drugs).
Exercise intervention
Exercise for Cancer Patients (EXCAP©®) was designed by American College of Sports Medicine (ACSM)-certified exercise scientists at the University of Rochester Medical Center. The intervention consisted of an EXCAP kit, which includes a manual, pedometer, and three resistance bands. The intervention was delivered via one 60-minute session by an NCORP clinical research associate in the oncology clinic on the first day of chemotherapy. Clinical research associates, with no professional exercise qualifications, received brief training in the delivery of EXCAP by ACSM-certified exercise professionals. EXCAP conformed with ACSM guidelines for exercise prescription [28].
The first component of EXCAP was a walking prescription intended to provide low to moderately intense aerobic exercise (60–85% of heart rate reserve) daily for the 6-week intervention. Before randomization, patients wore pedometers and recorded steps for four consecutive days. Patients received an individually tailored, progressive walking prescription for the next six weeks based on their baseline average daily steps and were encouraged to increase the total number of steps walked daily by 5–20% each week.
The second part of the exercise program was a therapeutic band prescription designed to provide low to moderately intense resistance exercise (3–5 rated perceived exertion (RPE) scale [28]) daily for the 6-week intervention. Patients were given three color-coded bands with varying levels of resistance (red=medium, green=heavy, blue=extra heavy) and a list of ten band exercises (squat, side bend, leg extension, leg curl, chest press, row, calf raise, overhead press, biceps curl, triceps extension) and four optional band exercises (front raise, lateral raise, internal rotation, external rotation). Patients received an individually tailored, progressive therapeutic band prescription for six weeks based on their optimal baseline band color, number of sets, and number of repetitions; the prescription included instruction on proper band use, safety, and exercise mechanics. Patients were encouraged to progressively increase the total number of sets and repetitions (maximum of 4 sets of 15 repetitions) as well as band resistance each week.
Standard care control condition
Control participants completed all study assessments and were followed by study staff in the exact same manner as the exercise participants. Control participants were offered the exercise intervention after all assessments were complete.
Measures
Clinical and demographic information were collected from medical records and study-specific forms. Exercise adherence was reported daily using (1) steps from a pedometer, (2) minutes of resistance exercise, and (3) RPE where 1=no exertion and 10=maximal exertion [28]. Patients reported their CIPN symptoms: (1) numbness and tingling and (2) hot/coldness in hands/feet, both rated on a 0–10 scale, where 0=not present and 10=as bad as you can imagine, during the last seven days. Validity and reliability have been demonstrated for similar scales of numbness and tingling for cancer patients [29, 30]. At the end of the study, participants completed a feedback survey.
Adverse events
Adverse events were monitored by the URCC Data Safety Monitoring Committee. All unexpected, serious, life-threatening, and fatal adverse events were reported.
Statistical analyses
All analyses used the intention-to-treat approach. Analyses were performed using R [31], SAS Studio v.3.6, and JMP v.13 (SAS Institute Inc.; Cary, NC, USA). All outcomes were examined with two-tailed tests at α=0.05. Due to the exploratory nature of this work, we did not adjust for multiple comparisons and we highlighted any trend-level effects (p<0.1). To test demographic factors and CIPN symptom change scores, we used t-tests and χ2-tests for continuous and nominal characteristics, respectively. We used linear regression to model post-intervention CIPN symptoms using study arm and hypothetical risk factors: baseline neuropathy, age, BMI, sex, cancer stage (I, II, or III; nominal), and cancer type (breast or other; nominal). To assess moderation, we estimated the interaction between each hypothetical risk factor and study arm.
Results
Participant flow (Figure 1)
Figure 1.
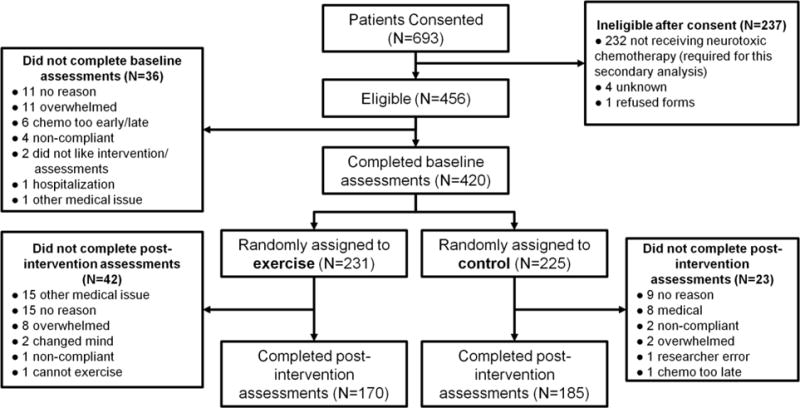
CONSORT diagram of study participants.
This secondary analysis included all 456 patients receiving neurotoxic chemotherapy regimens (taxane-, platinum-, or vinca alkaloid-based chemotherapy) from our parent RCT. From the 420 patients who completed baseline assessments, 355 patients (85%) also completed post-intervention assessments (170 exercisers, 185 controls). The most common reasons for incomplete data were because participants were overwhelmed, had some type of medical issue, or offered no reason. Patients were more likely to drop out of the study— completing only baseline assessments—if they were in the exercise arm (p=0.010), were older (p=0.014), reported greater fatigue at baseline (p=0.019), or had limited education (no high school/GED; p=0.0003) while controlling for gender, BMI, race, marital status, cancer site, cancer stage, chemotherapy type, Karnofsky Performance Status, and other patient reported symptoms (numbness/tingling, hot/coldness in hands/feet, distress, pain, quality of life; all rated 0–10).
Baseline characteristics (Table 1)
Table 1.
Participant demographics and characteristics.
| Characteristic | Control | Exercise | Total | Test for Difference Between Control and Exercise | ||
|---|---|---|---|---|---|---|
| Testa | Statistic | p | ||||
| Total participants | 185 | 170 | 355 | |||
| Female sex | 174 | 155 | 329 | χ2 | 1.42 | 0.234 |
| Age, years (mean ± std. dev) | 55.9 ± 9.7 | 55.6 ± 11.8 | 55.8 ± 10.8 | t | 0.35 | 0.729 |
| Body mass index, kg/m2 (mean ± std. dev.)a | 29.9 ± 6.1 | 29.0 ± 6.3 | 29.5 ± 6.2 | t | −1.70 | 0.090 |
| Race | χ2 | 0.42 | 0.519 | |||
| White | 155 | 148 | 303 | |||
| Non-White | 30 | 22 | 52 | |||
| Employment | χ2 | 1.81 | 0.405 | |||
| Employed outside the house | 110 | 94 | 204 | |||
| Self-employed / homemaker | 19 | 17 | 36 | |||
| Unemployed | 51 | 57 | 108 | |||
| Marital status | χ2 | 0.06 | 0.810 | |||
| Married or long-term committed relationship | 117 | 111 | 228 | |||
| Divorced, separated, single, widowed | 60 | 49 | 109 | |||
| Education | χ2 | 2.28 | 0.320 | |||
| At least some college | 114 | 117 | 231 | |||
| High school/GED degree | 57 | 41 | 98 | |||
| No high school or GED degree | 5 | 2 | 7 | |||
| Cancer typeb | χ2 | 4.24 | 0.374 | |||
| Breast | 152 | 129 | 281 | |||
| Lymphoma | 5 | 13 | 18 | |||
| Colon | 10 | 8 | 18 | |||
| Lung | 7 | 5 | 12 | |||
| Other | 11 | 15 | 26 | |||
| Cancer stage | χ2 | 1.42 | 0.701 | |||
| Stage I | 52 | 47 | 99 | |||
| Stage II | 87 | 73 | 160 | |||
| Stage III | 37 | 36 | 73 | |||
| Stage IV | 3 | 7 | 10 | |||
| Not reported | 6 | 7 | 13 | |||
| Previous treatmentc | χ2 | 0.16 | 0.691 | |||
| Previous surgery | 158 | 147 | 305 | |||
| Previous radiation therapy | 3 | 4 | 7 | |||
| Previous hormone therapy | 4 | 7 | 11 | |||
| Neurotoxic chemotherapy type | ||||||
| Only taxanes | 118 | 100 | 218 | χ2 | 0.07 | 0.794 |
| Only platinums | 18 | 21 | 39 | χ2 | 0.002 | 0.965 |
| Only vinca alkaloids | 5 | 14 | 19 | χ2 | 4.25 | 0.039 |
| Taxanes and/or platinums | 180 | 156 | 336 | |||
| Taxanes, platinums, and/or vinca alkaloids | 185 | 170 | 355 | |||
| Time since end of first radiation or surgery for cancer, weeks (mean ± std. dev) | 5.7 ± 10.0 | 12.1 ± 60.9 | 8.8 ± 43.1 | t | 1.11 | 0.266 |
| Karnofsky performance status (mean ± std. dev) | 94.9 ± 7.0 | 94.3 ± 7.1 | 94.6 ± 7.0 | t | −0.60 | 0.552 |
| Baseline patient-reported neuropathy | ||||||
| Numbness and tingling (0–10) | 1.1 ± 2.0 | 0.7 ± 1.7 | 0.9 ± 1.9 | t | −0.92 | 0.360 |
| Hot/coldness in hands/feet (0–10) | 0.9 ± 2.0 | 0.8 ± 1.8 | 0.8 ± 1.9 | t | −0.04 | 0.970 |
Statistical tests includes t-test or χ2 test
Other cancer types include endometrial, ovary, testes, uterine, brain, cervical, fallopian tube, head or neck, kidney, pancreas, and peritoneum.
GED, general educational development
The study participants were primarily middle-aged, overweight, married women with at least some college education and employed outside of the house. Most patients had early-stage breast cancer, received taxane chemotherapy, and reported mild neuropathy at baseline. There were no significant baseline differences between patients in the exercise and control conditions.
Intervention adherence
At baseline, there were no significant differences between exercise and control conditions in terms of daily steps (exercisers 4,171 steps/day, controls 4,413 steps/day; p=0.444) or minutes of resistance band exercise (both groups reported none). After the intervention, exercisers increased their average daily steps by 649 (approximately 0.32 miles) and walked significantly more steps than control participants (4,820 vs. 4,285, respectively; p=0.019, who decreased their average daily steps by 129 (approximately 0.06 miles). Thus, exercisers walked approximately 0.27 miles per day more than controls while receiving chemotherapy at post-intervention. For resistance exercise, 77% of exercise participants reported performing at least some resistance exercise during the study. These sessions were on average 28.4 min long with an RPE of 4.0 and were performed 3.5 days/week. Exercise contamination was minimal in control participants. Specifically, only 7% of controls reported any resistance exercise during the study, and, on average, these participants exercised only 3 times during the 6-week study. Thus, exercisers performed significantly more days of resistance band exercise than controls (average of 3.5 days/week vs. 0.5; p<0.001).
Effects of exercise on CIPN symptoms (Table 2, Figure 2)
Table 2.
Testing the effect of exercise on chemotherapy-induced peripheral neuropathy.
| Pre-intervention | Post-intervention | Change score (Post minus Pre)a | Main effect of exercise (vs. control)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | N | Mean | 95% CI | N | Mean | 95% CI | N | p | Coefficient | Coefficient 95% CI | p | |
| Numbness and tingling | −0.42 | −0.85, 0.02 | 0.061 | ||||||||||
| Exercise | 0.72 | 0.46, 0.98 | 170 | 1.10 | 0.80, 1.40 | 170 | 0.38 | 0.04, 0.71 | 170 | 0.027 | |||
| Control | 1.06 | 0.78, 1.35 | 185 | 1.64 | 1.30, 1.98 | 185 | 0.58 | 0.20. 0.95 | 185 | 0.003 | |||
| Hot/coldness in hands/feet | −0.46 | −0.01, −0.91 | 0.045 | ||||||||||
| Exercise | 0.76 | 0.49, 1.03 | 170 | 1.14 | 0.81, 1.46 | 170 | 0.38 | 0.06, 0.70 | 170 | 0.022 | |||
| Control | 0.90 | 0.61, 1.18 | 185 | 1.67 | 1.30, 2.04 | 185 | 0.77 | 0.42, 1.13 | 185 | <0.0001 | |||
Note: Both numbness and tingling and hot/coldness in hands/feet were rated on a scale from 0–10.
Two-tailed t-test against zero.
Testing the main effect of exercise (vs. control) on post-intervention CIPN controlling for pre-intervention CIPN using regression. Negative coefficients indicate exercise reduced CIPN symptom severity compared to the control condition.
Figure 2.
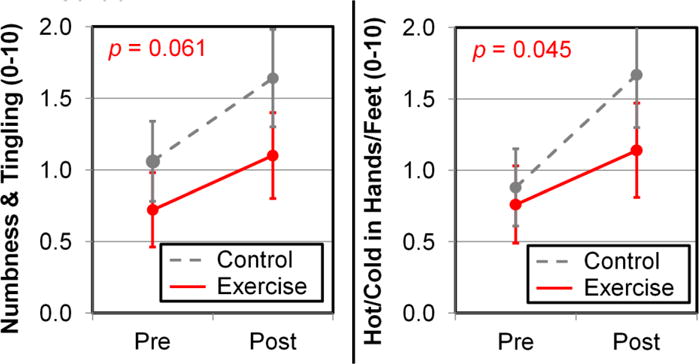
Exercise reduces the severity of CIPN symptoms per patient-reported numbness and tingling (left; trend-level effect) and hot/coldness in hands/feet (right). Error bars show 95% confidence intervals from 170 exercise patients and 185 control patients. The p-values correspond to differences in exercise and control conditions from regression (Table 2).
At baseline, patients in both conditions reported mild neuropathy. Collapsing across conditions, the average numbness and tingling was 0.90 (95% CI=0.71, 1.09) and the average hot/coldness in hands/feet was 0.83 (CI=0.63, 1.03). In terms of prevalence, 29.6% of patients reported any numbness and tingling (i.e., >0) and 25.4% reported any hot/coldness in hands/feet.
At post-intervention, patients in each condition reported more severe CIPN symptoms, as expected after six weeks of neurotoxic chemotherapy. For numbness and tingling, the change for exercisers was 0.38 (CI=0.04, 0.71, p=0.027) with 36.5% of patients reporting any CIPN post-intervention. The change for controls was greater: 0.58 (CI=0.20, 0.95, p=0.003) with 49.2% of patients reporting any CIPN post-intervention (Table 2). For hot/coldness, the change for exercisers was 0.38 (CI=0.06, 0.70, p=0.022) with 33.5% of patients reporting any CIPN post-intervention, and the change for controls was greater: 0.77 (CI=0.42, 1.13, p<0.0001) with 45.4% of patients reporting any CIPN post-intervention (Table 2). At post-intervention, participants in the exercise condition reported less severe CIPN symptoms than participants in the control condition by nearly 0.5 units on the 0–10 scales, as assessed by numbness and tingling (coefficient=−0.42, CI=−0.85, 0.02, p=0.061; a trend-level effect) and hot/coldness in hands/feet (coefficient=−0.46, CI=−0.01, −0.91, p=0.045; Table 2; Figure 2).
Factors that predict post-intervention CIPN symptoms (Table 3)
Table 3.
Testing moderators of the effect of exercise on chemotherapy-induced peripheral neuropathy (N=278).
| Main effect on post-intervention CIPN symptoms | Moderating the effect of exercise on post-intervention CIPN symptoms | |||||
|---|---|---|---|---|---|---|
| Coefficienta | Coefficient’s 95% CI | p | Coefficient | Coefficient’s 95% CI | p | |
| Numbness and tingling | ||||||
| Intercept | 0.16 | −2.92, 3.24 | 0.917 | – | – | – |
| Study arm (control vs. exercise) | 0.35 | −1.87, 2.56 | 0.757 | – | – | – |
| Baseline numbness and tingling | 0.35 | 0.14, 0.56 | 0.002 | 0.13 | −0.15, 0.41 | 0.365 |
| Sex (female vs. male) | 1.86 | 0.12, 3.60 | 0.036 | −0.60 | −3.06, 1.85 | 0.630 |
| Age (years) | −0.0001 | −0.03, 0.03 | 0.993 | 0.04 | −0.01, 0.09 | 0.086 |
| BMI (kg/m2) | 0.02 | −0.04, 0.08 | 0.533 | −0.003 | −0.08, 0.08 | 0.934 |
| Cancer stage (II vs. I) | −0.73 | −1.57, 0.11 | 0.088 | 0.50 | −0.66, 1.66 | 0.393 |
| Cancer stage (III vs. II) | 0.69 | −0.25, 1.63 | 0.149 | −0.23 | −1.59, 1.13 | 0.741 |
| Cancer type (breast vs. other) | −1.54 | −2.55, −0.52 | 0.003 | 0.41 | −1.16, 1.98 | 0.608 |
| Hot/coldness in hands/feet | ||||||
| Intercept | −3.61 | −6.91, −0.32 | 0.032 | – | – | – |
| Study arm (control vs. exercise) | 1.78 | −0.57, 4.13 | 0.137 | – | – | – |
| Baseline hot/coldness in hands/feet | 0.58 | 0.37, 0.79 | <0.0001 | 0.10 | −0.18, 0.37 | 0.489 |
| Sex (female vs. male) | 3.53 | 1.69, 5.38 | 0.0002 | −2.92 | −5.52, −0.32 | 0.028 |
| Age (years) | 0.01 | −0.02, 0.04 | 0.597 | 0.002 | −0.05, 0.05 | 0.920 |
| BMI (kg/m2) | 0.05 | −0.01, 0.12 | 0.083 | −0.04 | −0.13, 0.04 | 0.301 |
| Cancer stage (II vs. I) | −0.001 | −0.88, 0.88 | 0.999 | 0.28 | −0.93, 1.50 | 0.646 |
| Cancer stage (III vs. II) | 0.86 | −0.12, 1.84 | 0.084 | 0.38 | −1.04, 1.80 | 0.599 |
| Cancer type (breast vs. other) | −1.50 | −2.56, −0.45 | 0.005 | 1.49 | −0.16, 3.14 | 0.076 |
Note: Both numbness and tingling and hot/coldness in hands/feet were rated from 0–10. This regression models post-intervention CIPN (either numbness and tingling or hot/coldness in hands/feet) using study arm (exercise vs. control) and other variables through their direct effects on CIPN and their effects on the potency of exercise. If a variable predicts the effect of exercise on post-intervention CIPN, then it is irrelevant to interpret their main effect (by itself) on post-intervention CIPN.
Positive coefficients on main effects indicate more severe CIPN symptoms for the underlined group or for increasing the value of the variable (e.g., age, BMI).
Negative coefficients on moderating effects indicate more severe CIPN symptoms when exercising for the underlined group or for increasing the value of the variable (e.g., age, BMI).
Exploratory analyses revealed several factors that predicted greater increases in CIPN symptoms: baseline neuropathy, female sex, and non-breast cancer (all based on both CIPN scales). Two factors exhibited trend-level effects (p<0.1) in the prediction of post-intervention CIPN symptoms: advanced stage cancer (stage II vs. stage I for numbness and tingling, and stage III vs. stage II for hot/coldness in hands/feet) and higher BMI (based on hot/coldness in hands/feet).
Factors that moderate the effect of exercise on CIPN symptoms (Table 3)
For numbness and tingling, there was a trend-level effect that older patients benefitted more from exercise than younger patients (p=0.086; Figure 3). For hot/coldness in hands/feet, male patients exhibited a better response from exercise than female patients (p=0.028), and patients with breast cancer exhibited a trend for a better response from exercise compared to patients with other cancer types (p=0.076).
Figure 3.
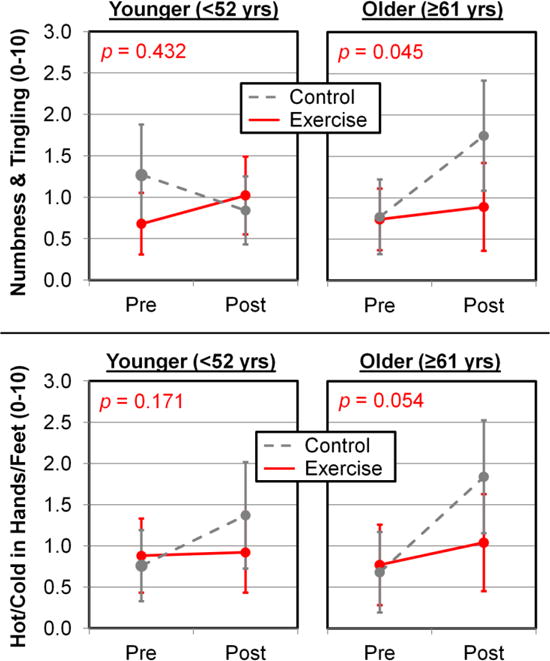
Exercise works particularly well in reducing CIPN symptoms for older patients. CIPN symptoms were assessed using patient-reported numbness and tingling (top) and hot/coldness in hands/feet (bottom). Error bars show 95% confidence intervals from 108 patients in the youngest tertile (59 exercisers, 49 controls) and 109 patients in the oldest tertile (53 exercisers, 56 controls).
Adverse events
During the study, five participants had grade 3–5 adverse events (3 non-serious, 2 serious), including lymphopenia, neutropenia, and multi-organ failure. All adverse events were unrelated to the exercise intervention.
Participant feedback
After completion of this study, patients reported very positive experiences of exercising during chemotherapy. Specifically, 72% of exercisers reported that this study changed their opinions of regular exercise during chemotherapy, with 92% of those patients indicating a more positive view of exercise during chemotherapy. Additionally, 94% of exercisers said they would recommend EXCAP to other patients receiving chemotherapy.
Discussion
Our results suggest that six weeks of exercise during chemotherapy—compared to chemotherapy without exercise—reduced the prevalence and severity of CIPN symptoms, as assessed by patient reports of numbness and tingling and hot/coldness in hands/feet. All patients reported worse CIPN after six weeks of neurotoxic chemotherapy, as expected, but patients randomized to the exercise group showed significantly smaller increases in CIPN prevalence and severity. The effects of exercise on CIPN symptoms were small to modest, but likely clinically significant, as evidenced by a reduction of 0.5 units on a 0–10 symptoms severity scale, considered noticeable and meaningful to patients, and by a reduction of prevalence from approximately half of patients in the control group to approximately one third of patients in the exercise group. Exploratory analyses implicated several variables that may predict the severity of CIPN symptoms and the effectiveness of exercise: baseline neuropathy, sex, cancer stage, cancer type, BMI, and age. Our attrition of 15% is consistent with other large Phase-III nationwide RCTs in cancer patients (e.g., [32]) and superior to other supportive oncology clinical trials (18-study-average attrition of 26% [33]).
This study confirms and extends prior work on the effects of exercise on CIPN. Our results are consistent with cross-sectional evidence that more physical activity [18, 19] and larger muscle volume [34] is associated with less severe CIPN symptoms. Moreover, our results extend prior RCTs of exercise for CIPN [20–22] by using a home-based exercise intervention, comparing chemotherapy with vs. without exercise, and utilizing a larger sample size than prior RCTs of exercise for CIPN (N=355 here vs. N=301 [20], N=61 [21], and N=30 [22]). Our results suggesting that exercise treats CIPN better for older patients are consistent with results that older patients require less exercise to treat CIPN [20]. Indeed, perhaps the exercise dose delivered here was sufficient for older patients but not younger patients.
Several possible mechanisms may underlie the beneficial effects of exercise on CIPN symptoms. First, exercise reduces chronic inflammation [23], and inflammation appears to play a role in the etiology and treatment of CIPN [8, 9]. Second, exercise changes how sensations from the hands, feet, and rest of the body are processed by the brain [24], specifically by the thalamus, sensorimotor cortex, and insula, which are all part of interoceptive brain circuitry [35]. Exercise-induced changes in the brain might counteract central sensitization associated with neuropathic pain [11], a feature of CIPN [1, 2], and thus may alleviate CIPN symptoms independent of the peripheral causes of CIPN [12]. These ideas can also explain our observation that exercise may treat CIPN symptoms better in older patients. Specifically, normal aging impairs the function [36, 37] and structure [38, 39] of interoceptive brain circuitry, thus potentially making the brain more vulnerable to the effects of chemotherapy, thereby exacerbating symptoms of CIPN. Moreover, exercise protects interoceptive brain circuitry from normal age-related declines [40], thus potentially protecting older brains from the effects of chemotherapy, thereby mitigating symptoms of CIPN.
This study has several noteworthy strengths. First, our large sample (N=355) drawn from multiple locations across the United States enhances the precision and generalizability of our results. Second, whereas most clinical trials are performed at academic medical centers, our study suggests that exercise is effective in community oncology clinics, where the majority of cancer patients are treated [41]. In addition, this home-based, unsupervised exercise intervention complements traditional face-to-face exercise interventions because it can save time and does not require transportation to a gym. Finally, our use of a standard care control group helps inform clinical recommendations and adds scientific rigor in studies of the exercise and CIPN.
This study also has a few limitations. First, because this study was not designed to assess CIPN symptoms, we only had access to relatively simple patient-reported measures of CIPN symptoms instead of a comprehensive questionnaire or clinical assessment [42]. However, our measure of CIPN symptoms has been used successfully in other studies (e.g., [29, 30]) and its simple nature makes it more feasible to collect in a busy clinical setting. Second, we lacked information on chemotherapy dose, which might have confounded our observations, for example that exercise is more effective for CIPN symptoms in older patients if older patients received a different (e.g., lower) chemotherapy dose than younger patients and chemotherapy dose was related to exercise effectiveness. Next, we do not know how our results generalize to more severe cases of CIPN, long-term symptom severity, other types or doses of exercise, and other patient populations. Patient dropout preferentially excluded patients who were more fatigued, older, less-well educated, and randomized to exercise—these factors have been previously identified in studies of clinical trial dropout [33, 43]. Fortunately, our relatively low 15% attrition mitigates concerns of bias compared to other clinical trials in supportive care oncology [33]. Finally, this was a secondary data analysis and the probability of Type-I error was inflated due to the exploratory nature of our work.
Exercise shows promise in the treatment of CIPN and so this research should be continued, especially given the dearth of available treatments for CIPN. We need to learn more about the optimal dose of exercise (type, duration, and intensity) [20], including conducting studies with longer interventions and longer-term follow-up assessments, and we need to use a precision medicine approach to make exercise more effective by considering age, sex, race, chemotherapy type and dose, baseline fitness, etc. Second, we need a better understanding of the mechanisms of CIPN and its treatment by obtaining richer data on CIPN including patient reports, clinical assessments, physiological measures, central neural measures, and intracellular measures [7] using rigorous and proven study designs [44]. By understanding how CIPN occurs, we may be able to prevent or treat CIPN by targeting specific pathways using new or existing drugs or behavioral interventions.
In conclusion, our results suggest that home-based walking and resistance exercise during chemotherapy can reduce the severity and prevalence of CIPN symptoms, especially in older patients. Effective cancer care requires synergism between supervised and self-management interventions, and EXCAP®© (i.e., unsupervised moderate-intensity walking and resistance exercise) is a promising tool that clinicians should consider prescribing for patients receiving taxane-, platinum-, and vinca alkaloid-based drugs, especially for their geriatric patients.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Susan Rosenthal and Amber Kleckner for their support on this project. We extend special thanks to the cancer patients, as well as the research staff in the University of Rochester Cancer Center NCORP Research Base at each of the NCI NCORP affiliates who recruited and followed participants in this study. We also thank the staff of the PEAK Human Performance Laboratory and the Cancer Control Psychoneuroimmunology Laboratory. We thank the National Cancer Institute and the Division of Cancer Prevention for their funding support of this project.
Funding/Support: Funding was provided by the National Cancer Institute, including funds from NCORP (formerly CCOP) parent grant U10 CA037420, NCORP (formerly CCOP) supplement U10 CA037420, and R25 CA102618.
Footnotes
Conflict of Interest Disclosures: There are no disclosures to report by any authors.
Trial Registration: ClinicalTrials.gov, # NCT00924651, http://www.clinicaltrials.gov.
References
- 1.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155(12):2461–70. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R, E.Q.o.L. Group The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–9. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. 2017;81(6):772–781. doi: 10.1002/ana.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7(1):99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 5.Beijers A, Mols F, Dercksen W, Driessen C, Vreugdenhil G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol. 2014;12(11):401–6. doi: 10.12788/jcso.0086. [DOI] [PubMed] [Google Scholar]
- 6.Brouwers EE, Huitema AD, Boogerd W, Beijnen JH, Schellens JH. Persistent neuropathy after treatment with cisplatin and oxaliplatin. Acta Oncol. 2009;48(6):832–41. doi: 10.1080/02841860902806609. [DOI] [PubMed] [Google Scholar]
- 7.Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10(12):694–707. doi: 10.1038/nrneurol.2014.211. [DOI] [PubMed] [Google Scholar]
- 8.Lees JG, Makker PG, Tonkin RS, Abdulla M, Park SB, Goldstein D, Moalem-Taylor G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer. 2017;73:22–29. doi: 10.1016/j.ejca.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59(1):3–9. doi: 10.1016/j.cyto.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–57. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cathcart-Rake EJ, Hilliker DR, Loprinzi CL. Chemotherapy-induced neuropathy: Central resolution of a peripherally perceived problem? Cancer. 2017;123(11):1898–1900. doi: 10.1002/cncr.30650. [DOI] [PubMed] [Google Scholar]
- 13.Majithia N, Temkin SM, Ruddy KJ, Beutler AS, Hershman DL, Loprinzi CL. National Cancer Institute-supported chemotherapy-induced peripheral neuropathy trials: outcomes and lessons. Support Care Cancer. 2016;24(3):1439–47. doi: 10.1007/s00520-015-3063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman DL, Lacchetti C, Dworkin RH, Smith EM Lavoie, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, O. American Society of Clinical Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–67. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 15.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL, O. Alliance for Clinical Trials Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–67. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tham A, Jonsson U, Andersson G, Soderlund A, Allard P, Bertilsson G. Efficacy and tolerability of antidepressants in people aged 65 years or older with major depressive disorder - A systematic review and a meta-analysis. J Affect Disord. 2016;205:1–12. doi: 10.1016/j.jad.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit Rev Oncol Hematol. 2016;98:325–34. doi: 10.1016/j.critrevonc.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mols F, Beijers AJ, Vreugdenhil G, Verhulst A, Schep G, Husson O. Chemotherapy-induced peripheral neuropathy, physical activity and health-related quality of life among colorectal cancer survivors from the PROFILES registry. J Cancer Surviv. 2015;9(3):512–22. doi: 10.1007/s11764-015-0427-1. [DOI] [PubMed] [Google Scholar]
- 19.Stevinson C, Steed H, Faught W, Tonkin K, Vallance JK, Ladha AB, Schepansky A, Capstick V, Courneya KS. Physical activity in ovarian cancer survivors: associations with fatigue, sleep, and psychosocial functioning. Int J Gynecol Cancer. 2009;19(1):73–8. doi: 10.1111/IGC.0b013e31819902ec. [DOI] [PubMed] [Google Scholar]
- 20.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Vallerand JR, Adams SC, Proulx C, Dolan LB, Wooding E, Segal RJ. Subgroup effects in a randomised trial of different types and doses of exercise during breast cancer chemotherapy. Br J Cancer. 2014;111(9):1718–25. doi: 10.1038/bjc.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihorst G, Herich L, Grussinger V, Gollhofer A, Bertz H. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25(2):493–9. doi: 10.1093/annonc/mdt568. [DOI] [PubMed] [Google Scholar]
- 22.Zimmer P, Trebing S, Timmers-Trebing U, Schenk A, Paust R, Bloch W, Rudolph R, Streckmann F, Baumann FT. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2017 doi: 10.1007/s00520-017-3875-5. [DOI] [PubMed] [Google Scholar]
- 23.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 24.Holschneider DP, Yang J, Guo Y, Maarek JM. Reorganization of functional brain maps after exercise training: Importance of cerebellar-thalamic-cortical pathway. Brain Res. 2007;1184:96–107. doi: 10.1016/j.brainres.2007.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–61. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 26.McCorkle R, Ercolano E, Lazenby M, Schulman-Green D, Schilling LS, Lorig K, Wagner EH. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin. 2011;61(1):50–62. doi: 10.3322/caac.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus BH, V, Selby C, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63(1):60–6. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 28.American College of Sports Medicine. ACSM’s Guidelines for exercise testing and prescription. Baltimore, MD: Lippincott, Williams, & Wilkins; 2010. [Google Scholar]
- 29.Mendoza TR, Zhao F, Cleeland CS, Wagner LI, Patrick-Miller LJ, Fisch MJ. The validity and utility of the M. D. Anderson Symptom Inventory in patients with breast cancer: evidence from the symptom outcomes and practice patterns data from the eastern cooperative oncology group. Clin Breast Cancer. 2013;13(5):325–34. doi: 10.1016/j.clbc.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159(2):327–33. doi: 10.1007/s10549-016-3939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 32.Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Palesh OG, Chandwani K, Reddy PS, Melnik MK, Heckler C, Morrow GR. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233–41. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui D, Glitza I, Chisholm G, Yennu S, Bruera E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2013;119(5):1098–105. doi: 10.1002/cncr.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshikawa T, Takano M, Miyamoto M, Yajima I, Shimizu Y, Aizawa Y, Suguchi Y, Moriiwa M, Aoyama T, Soyama H, Goto T, Hirata J, Suzuki A, Sasa H, Nagaoka I, Tsuda H, Furuya K. Psoas muscle volume as a predictor of peripheral neurotoxicity induced by primary chemotherapy in ovarian cancers. Cancer Chemother Pharmacol. 2017 doi: 10.1007/s00280-017-3395-5. [DOI] [PubMed] [Google Scholar]
- 35.Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF. Evidence for a Large-Scale Brain System Supporting Allostasis and Interoception in Humans. Nature Human Behaviour. 2017:1. doi: 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalsa SS, Rudrauf D, Tranel D. Interoceptive awareness declines with age. Psychophysiology. 2009;46(6):1130–6. doi: 10.1111/j.1469-8986.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendes WB. Weakened links between mind and body in older age: The case for maturational dualism in the experience of emotion. Emotion Review. 2010;2(3):240–244. [Google Scholar]
- 38.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the "default network" in normal aging. Cereb Cortex. 2008;18(8):1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 39.Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–44. doi: 10.1016/j.neuroimage.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang P, Fang R, Li BY, Chen SD. Exercise-Related Changes of Networks in Aging and Mild Cognitive Impairment Brain. Front Aging Neurosci. 2016;8:47. doi: 10.3389/fnagi.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berkeley Research Group. Impact on Medicare Payments of Shift in Site of Care for Chemotherapy Administration. 2014 [Google Scholar]
- 42.McCrary JM, Goldstein D, Boyle F, Cox K, Grimison P, Kiernan MC, Krishnan AV, Lewis CR, Webber K, Baron-Hay S, Horvath L, Park SB, I.F.D.w. party Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (CIPN): a systematic review and Delphi survey. Support Care Cancer. 2017 doi: 10.1007/s00520-017-3772-y. [DOI] [PubMed] [Google Scholar]
- 43.Nam S, Dobrosielski DA, Stewart KJ. Predictors of exercise intervention dropout in sedentary individuals with type 2 diabetes. J Cardiopulm Rehabil Prev. 2012;32(6):370–8. doi: 10.1097/HCR.0b013e31826be485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gewandter JS, Freeman R, Kitt RA, Cavaletti G, Gauthier LR, McDermott MP, Mohile NA, Mohlie SG, Smith AG, Tejani MA, Turk DC, Dworkin RH. Chemotherapy-induced peripheral neuropathy clinical trials: Review and recommendations. Neurology. 2017;89(8):859–869. doi: 10.1212/WNL.0000000000004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


