Abstract
OBJECTIVE
To evaluate temporal trends in use of antihypertensive medications during delivery hospitalizations complicated by preeclampsia and risk of maternal stroke over the same time period.
METHODS
The Perspective database was used to perform a retrospective cohort study evaluating antihypertensive drugs dispensed during delivery hospitalizations complicated by preeclampsia from 2006 to the first quarter of 2015. Medications evaluated included nifedipine, hydralazine, and oral and intravenous labetalol. Adjusted models for receipt of antihypertensive agents accounting for demographic and hospital factors were created. Hospital-level rates of antihypertensive administration for women with severe preeclampsia were analyzed. Risk of stroke during delivery hospitalization was evaluated.
RESULTS
A total of 239,454 patients with preeclampsia were included in the analysis including 126,595 women with mild, 31,628 with superimposed, and 81,231 with severe preeclampsia. Overall, 105,409 women received a hypertensive agent. From 2006 to 2014, for all patients with preeclampsia, receipt of oral labetalol increased from 20.3% to 31.4%, intravenous labetalol from 13.3% to 21.4%, hydralazine from 12.8% to 16.9%, nifedipine from 15.0% to 18.2%, and more than one medication from 16.5% to 25.8%. The proportion of patients with preeclampsia receiving any antihypertensive medication rose from 37.8% in 2006 to 49.4% in 2015. In adjusted models, temporal trends retained significance. Rates of antihypertensive administration for severe preeclampsia varied significantly by hospital. For severe preeclampsia, the risk for stroke decreased from 13.5 per 10,000 deliveries in 2006–2008 (n=27) to 9.7 in 2009–2011 (n=25) to 6.0 in 2012–2014 (n=20) (p=0.02).
CONCLUSION
Use of multiple antihypertensive agents to treat preeclamptic women increased over the study period for women with mild, superimposed, and severe preeclampsia. There was substantial hospital variation in use of antihypertensive agents. This trend was associated with decreased risk of maternal stroke.
INTRODUCTION
Population-based data has demonstrated a doubling of the risk of stroke associated with hypertensive diseases of pregnancy between 1994 and 2011.1 Severe range blood pressure, and in particular systolic blood pressure of 160 mmHg or higher, may be associated with pregnancy-associated stroke.2,3 Data from the Hospital Corporation of America (HCA) demonstrated decreased risk for fatal hypertension-related intracranial hemorrhage associated with protocolized, timely administration of antihypertensive medication.4 Goals of antihypertensive therapy also include prevention of renal failure and injury and cardiovascular morbidity (specifically congestive heart failure and myocardial ischemia).5 The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy supports administration of antihypertensive agents for preeclampsia with severe features with sustained blood pressure of 160 mmHg or 110 mmHg systolic and diastolic, respectively, with additional recommendations for treatment of postpartum hypertension and women with chronic hypertension. First line agents for treatment of acute hypertension include intravenous labetalol, intravenous hydralazine, and oral nifedipine.5 The National Partnership for Maternal Safety has disseminated a bundle to aid in timely treatment of severe hypertension.6
In the setting of these recommendations and evolving evidence demonstrating the benefit of treating pregnancy-associated hypertension, there is limited research on how practice patterns are changing and specifically to what degree antihypertensive agents are being used to treat hypertensive episodes. How temporal trends, patient factors, and hospital-level effects may affect use of hypertensive agents is not well characterized. Additionally, there are little data on the degree to which treatment with antihypertensive agents is associated with the improved outcomes.
Given that hypertension is a leading cause of maternal mortality and severe morbidity7 and that there are knowledge gaps in how management is evolving, the primary objective of this study was to characterize antihypertensive use during delivery hospitalizations complicated by preeclampsia. We hypothesized that use of antihypertensive agents has increased concurrent with expanding evidence and supportive clinical recommendations. A secondary objective of this analysis was to characterize risk for pregnancy-associated stroke for women with a preeclampsia diagnosis.
MATERIALS AND METHODS
The Perspective database was used for this retrospective cohort analysis. Perspective is maintained by Premier Incorporated (Charlotte, NC) and includes patient demographics, hospital characteristics, and discharge diagnosis codes, as well as medications and devices administered during acute care hospitalizations. Perspective reports 100% of hospitalizations for individual hospitals. Ninety-five quality assurance and validation checks are performed on data prior to being used for research.8 Perspective is routinely used for research on trends on medications and device use during delivery hospitalizations.9–15 The discharges included in the Perspective database account for approximately 15% of all inpatient hospital stays annually in the entire United States. The Columbia University Institutional Review Board deemed the study exempt given that all data are deidentified.
Women included in this analysis were admitted for a delivery hospitalization with an associated preeclampsia diagnosis from January 2006 through March 2015. Patients with preeclampsia were identified based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes and subcategorized as mild, severe, and superimposed (ICD-9-CM codes 642.4x, 642.5x, and 642.7x, respectively). Reports of sensitivity for ICD-9-CM codes for preeclampsia range from 69 to 84%.16–18 Delivery hospitalizations were identified based on billing and procedure codes that ascertain more than 95% of deliveries.19 Among patients with preeclampsia, we analyzed whether patients received the following antihypertensive medications: oral labetalol, intravenous labetalol, nifedipine, and intravenous hydralazine. We evaluated temporal trends in rates of individual antihypertensive use within this cohort: (i) for all patients with preeclampsia, and (ii) individually for mild, severe, or superimposed preeclampsia. Temporal trends are reported through 2014 given that only data for the first quarter of 2015 was available.
Univariate associations between clinical, hospital, and demographic variables and receipt of antihypertensive medications were evaluated using the chi-squared test. Adjusted risk ratios (RR) for receipt of any antihypertensive medication with 95% confidence intervals (CI) as measures of effect accounting for demographic, hospital, and preeclampsia diagnosis were derived from fitting a log-linear generalized estimating equations regression model that accounts for the effect of patients clustered within hospitals. Patient demographic characteristics included: year of discharge, maternal age, marital status, race (white, black, other), and payer status. Hospital characteristics included bed size, geographic region, teaching status, and urban or rural location. Specific preeclampsia diagnosis (severe, mild, or superimposed) was included in the model. We additionally performed a sensitivity analysis to address potential confounding given that the hospitals included in Perspective change from year to year and temporal trends could be secondary to inclusion of specific hospitals; to address this changing sampling frame, we repeated both the adjusted and unadjusted analysis restricted to hospitals that contributed data for the entire study period. Given maternal disparities and increased risk for maternal mortality among black women,7 we performed an additional analysis stratified for receipt of any antihypertensive by race (black, white, other) over the study period. Given that uptake of clinical recommendations may be different for teaching versus non-teaching centers, we also report on temporal trends in receipt of antihypertensive agents for preeclamptics based on this factor.
To determine whether risk for pregnancy associated stroke for patients with preeclampsia changed over the study period we analyzed the number of strokes that occurred restricted to ICD-9-CM codes with high sensitivity ascertained in non-obstetric validation studies (ICD-9-CM 430, 431, 434.×1, and 436) as well as ICD-9-CM code 674.0 which we have found to be highly sensitive in pregnancy related stroke in our institution.20–24 Because pregnancy associated stroke is a relatively rare event occurring during approximately 34 per 100,000 pregnancies,25,26 we divided the study into three time periods: 2006–2008, 2009–2011 and 2012–2014 and compared risk using the chi-squared test with the numerator as stroke cases and the denominator as all delivery hospitalizations. Risk for stroke in 2015 was not reported given that only the first quarter was available. We performed this analysis for all women with preeclampsia and women with a diagnosis of severe preeclampsia alone. Lastly, to evaluate variation in use of antihypertensive agents in the setting of severe preeclampsia across hospitals we restricted the analysis to centers that treated ≥50 patients with severe preeclampsia over the study period and calculated hospital-level rates of antihypertensive use for this subset of patients. All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Overall 239,454 patients with preeclampsia were included in the analysis. Of these, 126,595 patients had mild, 31,628 patients had superimposed, and 81,231 patients had severe preeclampsia (Table 1). The proportion of patients with severe and superimposed relative to mild preeclampsia increased over the study period with severe preeclampsia accounting for 36.9% of cases in 2015 compared to 30.9% of cases in 2006. In 2006 10.3% and 30.9% of patients received diagnoses of superimposed and severe preeclampsia respectively compared to 15.7% and 36.9% in 2015. Superimposed preeclampsia was more common in the South, among black women, women ≥35 years of age, and in teaching compared to nonteaching hospitals. Severe preeclampsia was also more common in teaching compared to nonteaching hospitals as well as hospitals with larger bed sizes (>600 beds).
Table 1.
Patient demographics for study cohort based on preeclampsia diagnosis
| Preeclampsia diagnosis | Mild | Superimposed | Severe | All patients | Receipt of any antihypertensive |
|---|---|---|---|---|---|
| All patients | 126,595 | 31,628 | 81,231 | 239,454 | 105,409 |
|
| |||||
| Year | |||||
| 2006 | 12,027 (9.5%) | 2,112 (6.7%) | 6,325 (7.8%) | 20,464 (8.5%) | 7,727 (7.3%) |
| 2007 | 12,253 (9.7%) | 2,354 (7.4%) | 6,767 (8.3%) | 21,374 (8.9%) | 8,435 (8.0%) |
| 2008 | 12,092 (9.6%) | 2,580 (8.2%) | 6,864 (8.5%) | 21,536 (9.0%) | 8,646 (8.2%) |
| 2009 | 12,840 (10.1%) | 3,113 (9.8%) | 7,685 (9.5%) | 23,638 (9.9%) | 9,994 (9.5%) |
| 2010 | 13,496 (10.7%) | 3,259 (10.3%) | 8,429 (10.4%) | 25,184 (10.5%) | 10,890 (10.3%) |
| 2011 | 14,766 (11.7%) | 3,786 (12.0%) | 9,567 (11.8%) | 28,119 (11.7%) | 12,638 (12.0%) |
| 2012 | 15,911 (12.6%) | 4,149 (13.1%) | 11,048 (13.6%) | 31,108 (13.0%) | 14,312 (13.6%) |
| 2013 | 15,700 (12.4%) | 4,652 (14.7%) | 11,213 (13.8%) | 31,565 (13.2%) | 14,942 (14.2%) |
| 2014 | 14,349 (11.3%) | 4,577 (14.5%) | 10,869 (13.4%) | 29,795 (12.4%) | 14,529 (13.8%) |
| 2015 (1Q) | 3,161 (2.5%) | 1,046 (3.3%) | 2,464 (3.0%) | 6,671 (2.8%) | 3,296 (3.1%) |
| Hospital bed size | |||||
| <400 | 67,780 (53.5%) | 14,214 (44.9%) | 37,282 (45.9%) | 119,276 (49.8%) | 51,018 (48.4%) |
| 400–600 | 34,600 (27.3%) | 9,338 (29.5%) | 24,870 (30.6%) | 68,808 (28.7%) | 30,234 (28.7%) |
| >600 | 24,215 (19.1%) | 8,076 (25.5%) | 19,079 (23.5%) | 51,370 (21.5%) | 24,157 (22.9%) |
| Age, years | |||||
| 15–17 | 4,825 (3.8%) | 189 (0.6%) | 2,893 (3.6%) | 7,907 (3.3%) | 3,125 (3.0%) |
| 18–24 | 42,015 (33.2%) | 4,980 (15.8%) | 25,927 (31.9%) | 72,922 (30.5%) | 30,320 (28.8%) |
| 25–34 | 61,756 (48.8%) | 16,994 (53.7%) | 39,030 (48.0%) | 117,780 (49.2%) | 50,976 (48.4%) |
| ≥35 | 17,999 (14.2%) | 9,465 (29.9%) | 13,381 (16.5%) | 40,845 (17.1%) | 20,988 (19.9%) |
| Insurance status | |||||
| Private | 65,320 (51.6%) | 15,677 (49.6%) | 40,573 (50.0%) | 121,570 (50.8%) | 50,692 (48.1%) |
| Medicare | 1,001 (0.8%) | 574 (1.8%) | 877 (1.1%) | 2,452 (1.0%) | 1,254 (1.2%) |
| Medicaid | 53,462 (42.2%) | 13,780 (43.6%) | 35,049 (43.2%) | 102,291 (42.7%) | 47,746 (45.3%) |
| Uninsured | 2,340 (1.9%) | 521 (1.7%) | 1,693 (2.1%) | 4,554 (1.9%) | 2,077 (2.0%) |
| Other | 4,472 (3.5%) | 1,076 (3.4%) | 3,039 (3.7%) | 8,587 (3.6%) | 3,640 (3.5%) |
| Race | |||||
| White | 67,873 (53.6%) | 14,125 (44.7%) | 39,664 (48.8%) | 121,662 (50.8%) | 47,297 (44.9%) |
| Black | 20,883 (16.5%) | 9,610 (30.4%) | 17,004 (20.9%) | 47,497 (19.8%) | 28,068 (26.6%) |
| Other | 37,790 (29.9%) | 7,878 (24.9%) | 24,519 (30.2%) | 70,187 (29.3%) | 29,997 (28.5%) |
| Unknown | 49 (0.0%) | 15 (0.0%) | 44 (0.1%) | 108 (0.0%) | 47 (0.0%) |
| Hospital Location | |||||
| Rural | 13,418 (10.6%) | 2,404 (7.6%) | 5,754 (7.1%) | 21,576 (9.0%) | 7,923 (7.5%) |
| Urban | 113,177 (89.4%) | 29,224 (92.4%) | 75,477 (92.9%) | 217,878 (91.0%) | 97,486 (92.5%) |
| Marital Status | |||||
| Married | 57,239 (45.2%) | 14,284 (45.2%) | 35,043 (43.1%) | 106,566 (44.5%) | 43,686 (41.4%) |
| Unmarried | 54,136 (42.8%) | 13,584 (43.0%) | 36,657 (45.1%) | 104,377 (43.6%) | 49,120 (46.6%) |
| Unknown | 15,220 (12.0%) | 3,760 (11.9%) | 9,531 (11.7%) | 28,511 (11.9%) | 12,603 (12.0%) |
| Hospital Region | |||||
| Northeast | 18,475 (14.6%) | 4,547 (14.4%) | 13,146 (16.2%) | 36,168 (15.1%) | 15,619 (14.8%) |
| Midwest | 22,507 (17.8%) | 5,029 (15.9%) | 13,378 (16.5%) | 40,914 (17.1%) | 17,227 (16.3%) |
| South | 61,239 (48.4%) | 17,480 (55.3%) | 40,287 (49.6%) | 119,006 (49.7%) | 55,069 (52.2%) |
| West | 24,374 (19.3%) | 4,572 (14.5%) | 14,420 (17.8%) | 43,366 (18.1%) | 17,494 (16.6%) |
| Teaching | |||||
| Non-teaching | 70,905 (56.0%) | 14,409 (45.6%) | 39,191 (48.3%) | 124,505 (52.0%) | 53,868 (51.1%) |
| Teaching | 55,690 (44.0%) | 17,219 (54.4%) | 42,040 (51.7%) | 114,949 (48.0%) | 51,541 (48.9%) |
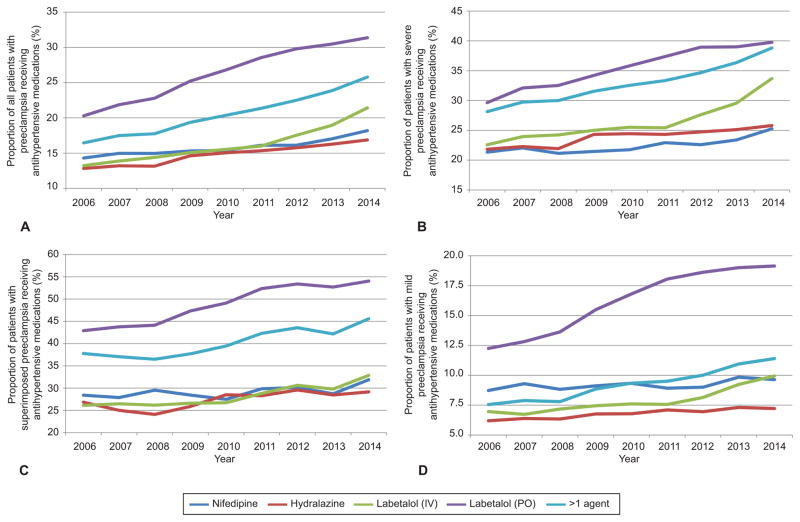
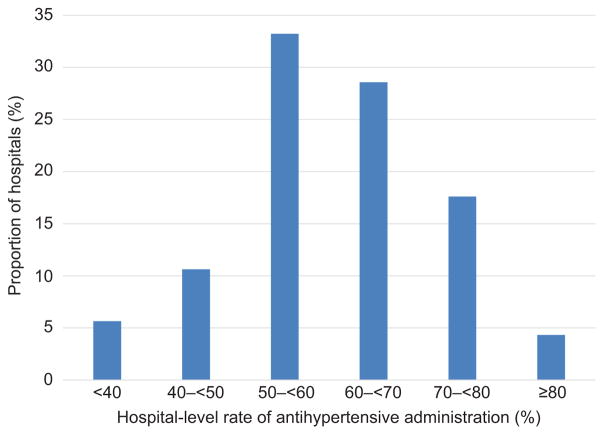
Over the course of the study the proportion of patients with preeclampsia receiving any antihypertensive medication rose from 37.8% of patients in 2006 to 49.4% in 2015, (p<0.01)(Table 1). Receipt of individual hypertensive agents (intravenous labetalol, oral labetalol, nifedipine, hydralazine) by year for all preeclamptic patients and by specific preeclampsia diagnosis: mild, superimposed, or severe (Figure 1). For all patients with preeclampsia receipt of four individual medications increased over the study period from 2006 to 2014 (Figure 1A): oral labetalol from 20.3% to 31.4%, intravenous labetalol from 13.3% to 21.4%, hydralazine from 12.8% to 16.9%, nifedipine from 15.0% to 18.2%, and more than one medication from 16.5% to 25.8%. Patients with severe preeclampsia (Figure 1B) also received medications more often over the study period: between 2006 and 2014 use of intravenous labetalol increased 11.1%, oral labetalol 10.1%, hydralazine 4.0%, nifedipine 3.9%, and more than one agent 10.7% (absolute increases in administration). For superimposed preeclampsia (Figure 1C) use of intravenous labetalol increased 6.8%, oral labetalol 11.1%, hydralazine 2.3%, nifedipine 3.5%, and more than one agent 7.8% (absolute increases in administration). Increases in antihypertensive use among mild preeclamptic women were smaller (Figure 1D) with use of intravenous labetalol increasing 3.0%, oral labetalol 7.0%, hydralazine 1.0%, nifedipine 0.9%, and more than one agent 3.9% over the same period (absolute increases in administration). Table 2 demonstrates the number of patients each year with each preeclampsia diagnosis receiving each medication. Individual hospital rates of antihypertensive administration to patients with severe preeclampsia differed significantly (Figure 2). The median hospital-level rate of antihypertensive administration to patients with severe preeclampsia was 60.0%, with the 25th and 75th percentiles 52.7% and 67.9% respectively.
Figure 1.
Rates of administration of antihypertensive medications for all patients with preeclampsia (A), and patients with severe (B), superimposed (C), and mild preeclampsia (D). Medication use from 2015 is not included as only a quarter of data is available for that year. Rates of administration differed significantly by year for labetalol (intravenous [IV] and oral [PO]), nifedipine, hydralazine, and >1 agent for mild, superimposed, severe, and all patients with preeclampsia (P<.01).
Table 2.
Administration of Antihypertensive Medications by Preeclampsia Diagnosis
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All preeclampsia (n) | |||||||||||
|
| |||||||||||
| All patients | 20464 | 21374 | 21536 | 23638 | 25184 | 28119 | 31108 | 31565 | 29795 | 6671 | 239454 |
| Nifedipine | 2926 | 3199 | 3220 | 3617 | 3876 | 4531 | 5018 | 5377 | 5419 | 1263 | 38446 |
| IV labetalol | 2708 | 2966 | 3098 | 3560 | 3912 | 4497 | 5452 | 5979 | 6382 | 1589 | 40143 |
| Oral labetalol | 4152 | 4675 | 4909 | 5958 | 6745 | 8024 | 9271 | 9621 | 9348 | 2043 | 64746 |
| Hydralazine | 2624 | 2822 | 2830 | 3457 | 3787 | 4313 | 4904 | 5137 | 5025 | 1043 | 35942 |
| >1 medication | 3366 | 3738 | 3822 | 4574 | 5128 | 6000 | 6999 | 7531 | 7684 | 1740 | 50582 |
|
| |||||||||||
| Severe preeclampsia (n) | |||||||||||
|
| |||||||||||
| All patients | 6325 | 6767 | 6864 | 7685 | 8429 | 9567 | 11048 | 11213 | 10869 | 2464 | 81231 |
| Nifedipine | 1350 | 1492 | 1451 | 1649 | 1833 | 2194 | 2496 | 2622 | 2745 | 634 | 18466 |
| IV labetalol | 1428 | 1621 | 1664 | 1921 | 2151 | 2432 | 3050 | 3313 | 3663 | 934 | 22177 |
| Oral labetalol | 1875 | 2173 | 2233 | 2629 | 3019 | 3575 | 4303 | 4373 | 4323 | 954 | 29457 |
| Hydralazine | 1381 | 1508 | 1505 | 1869 | 2060 | 2327 | 2733 | 2817 | 2804 | 612 | 19616 |
| >1 medication | 1780 | 2012 | 2060 | 2425 | 2744 | 3191 | 3830 | 4076 | 4219 | 966 | 27303 |
|
| |||||||||||
| Superimposed preeclampsia (n) | |||||||||||
|
| |||||||||||
| All patients | 2112 | 2354 | 2580 | 3113 | 3259 | 3786 | 4149 | 4652 | 4577 | 1046 | 31628 |
| Nifedipine | 593 | 645 | 760 | 871 | 880 | 1112 | 1224 | 1324 | 1439 | 319 | 9167 |
| IV labetalol | 548 | 609 | 664 | 800 | 852 | 1057 | 1242 | 1364 | 1468 | 369 | 8973 |
| Oral labetalol | 1015 | 1124 | 1458 | 1587 | 1965 | 2204 | 2429 | 2468 | 536 | 15690 | 904 |
| Hydralazine | 544 | 582 | 609 | 781 | 907 | 1047 | 1207 | 1298 | 1316 | 248 | 8539 |
| >1 medication | 783 | 855 | 927 | 1144 | 1268 | 1565 | 1781 | 1931 | 2062 | 445 | 12761 |
|
| |||||||||||
| Mild preeclampsia (n) | |||||||||||
|
| |||||||||||
| All patients | 12027 | 12253 | 12092 | 12840 | 13496 | 14766 | 15911 | 15700 | 14349 | 3161 | 126595 |
| Nifedipine | 983 | 1062 | 1009 | 1097 | 1163 | 1225 | 1298 | 1431 | 1235 | 310 | 10813 |
| IV labetalol | 732 | 736 | 770 | 839 | 909 | 1008 | 1160 | 1302 | 1251 | 286 | 8993 |
| Oral labetalol | 1373 | 1487 | 1552 | 1871 | 2139 | 2484 | 2764 | 2819 | 2557 | 553 | 19599 |
| Hydralazine | 699 | 732 | 716 | 807 | 820 | 939 | 964 | 1022 | 905 | 183 | 7787 |
| >1 medication | 803 | 871 | 835 | 1005 | 1116 | 1244 | 1388 | 1524 | 1403 | 329 | 10518 |
Figure 2.
Hospital-level rates of administration of any hypertensive medication to patients with severe preeclampsia. Each bar represents the proportion of hospitals in the study (n=301) by hospital-level rate of antihypertensive administration to patients with severe preeclampsia. Only hospitals with ≥50 patients are included in the figure.
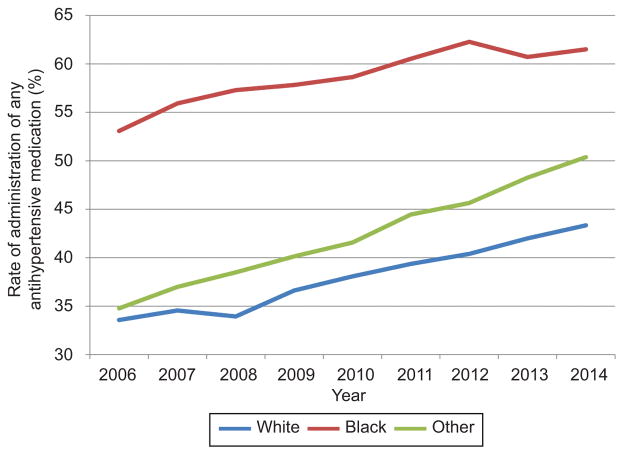
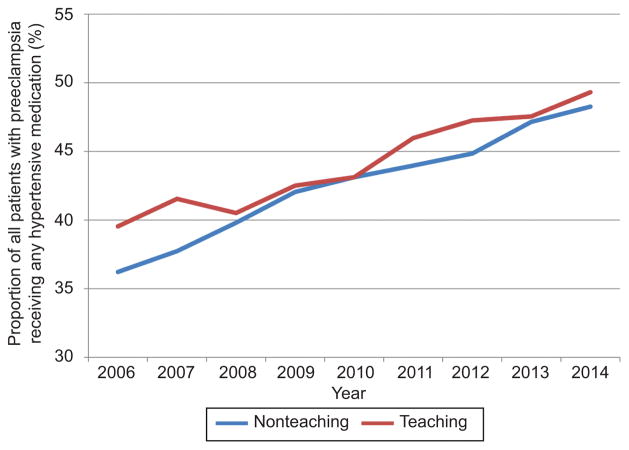
In the adjusted analysis, the likelihood of antihypertensive use increased over the study period with an adjusted risk ratio (aRR) of 1.19 and 95% confidence interval (95% CI) in 2015 with 2006 as a reference (Table 3). Severe preeclampsia and superimposed preeclampsia were associated with the largest increase in likelihood of antihypertensive receipt (aRR 2.19 95% CI 2.12–2.26, aRR 2.32 95% CI 2.23–2.41 with mild preeclampsia as a reference, respectively. Other significant factors included black compared to white race (aRR 1.30 95% CI 1.27–1.33) and maternal age ≥35 compared to age 18–24 (aRR 1.17 95% CI 1.15–1.19). For the sensitivity analysis, when the cohort was restricted only to centers contributing data for the entire study period temporal trends and adjusted analyses demonstrated similar results (not shown). In evaluating antihypertensive agents by race, black women were most likely to receive medication with receipt of any hypertensive increasing from 53.1% to 60.9% over the study period, compared to 33.6% to 45.6% for white women (Figure 3). Rates of antihypertensive receipt were similar at teaching and non-teaching hospitals (Figure 4).
Table 3.
Unadjusted and adjusted models for receipt of any antihypertensive medication
| RR | 95% CI | aRR | 95% CI | |
|---|---|---|---|---|
| Year | ||||
| 2006 | 1.00 | Reference | 1.00 | Reference |
| 2007 | 1.05 | 1.02, 1.07 | 1.04 | 1.02, 1.07 |
| 2008 | 1.06 | 1.04, 1.09 | 1.05 | 1.01, 1.09 |
| 2009 | 1.12 | 1.09, 1.15 | 1.10 | 1.05, 1.14 |
| 2010 | 1.15 | 1.12, 1.17 | 1.11 | 1.06, 1.16 |
| 2011 | 1.19 | 1.16, 1.22 | 1.14 | 1.09, 1.19 |
| 2012 | 1.22 | 1.19, 1.24 | 1.15 | 1.10, 1.21 |
| 2013 | 1.25 | 1.23, 1.28 | 1.17 | 1.11, 1.23 |
| 2014 | 1.29 | 1.26, 1.32 | 1.18 | 1.11, 1.24 |
| 2015 (1Q) | 1.31 | 1.27, 1.35 | 1.19 | 1.12, 1.27 |
| Hospital bed size | ||||
| <400 | 1.00 | Reference | 1.00 | Reference |
| 400–600 | 1.03 | 1.02, 1.04 | 1.06 | 0.96, 1.18 |
| >600 | 1.10 | 1.09, 1.11 | 1.03 | 0.90, 1.18 |
| Age, years | ||||
| 15–17 | 0.95 | 0.92, 0.98 | 0.96 | 0.94, 0.99 |
| 18–24 | 1.00 | Reference | 1.00 | Reference |
| 25–34 | 1.04 | 1.03, 1.05 | 1.05 | 1.04, 1.06 |
| ≥35 | 1.24 | 1.22, 1.25 | 1.17 | 1.15, 1.19 |
| Insurance status | ||||
| Medicare | 1.23 | 1.18, 1.28 | 1.03 | 1.00, 1.07 |
| Medicaid | 1.12 | 1.11, 1.13 | 1.04 | 1.03, 1.06 |
| Private | 1.00 | Reference | 1.00 | Reference |
| Uninsured | 1.09 | 1.06, 1.13 | 1.02 | 0.99, 1.06 |
| Other | 1.02 | 0.99, 1.04 | 1.00 | 0.95, 1.04 |
| Race | ||||
| White | 1.00 | Reference | 1.00 | Reference |
| Black | 1.52 | 1.50, 1.54 | 1.30 | 1.27, 1.33 |
| Other | 1.10 | 1.09, 1.11 | 1.08 | 1.06, 1.10 |
| Unknown | 1.12 | 0.90, 1.39 | 0.92 | 0.76, 1.11 |
| Hospital Location | ||||
| Rural | 0.82 | 0.81, 0.84 | 0.96 | 0.89, 1.03 |
| Urban | 1.00 | Reference | 1.00 | Reference |
| Marital Status | ||||
| Married | 1.00 | Reference | 1.00 | Reference |
| Unmarried | 1.15 | 1.14, 1.16 | 1.06 | 1.04, 1.07 |
| Unknown | 1.08 | 1.06, 1.09 | 0.97 | 0.90, 1.05 |
| Hospital Region | ||||
| Northeast | 1.00 | Reference | 1.00 | Reference |
| Midwest | 0.98 | 0.96, 0.99 | 0.96 | 0.85, 1.10 |
| South | 1.07 | 1.06, 1.09 | 0.92 | 0.81, 1.03 |
| West | 0.93 | 0.92, 0.95 | 1.02 | 0.87, 1.20 |
| Preeclampsia diagnosis | ||||
| Mild preeclampsia | 1.00 | Reference | 1.00 | Reference |
| Severe preeclampsia | 2.27 | 2.25, 2.30 | 2.19 | 2.12, 2.26 |
| Superimposed preeclampsia | 2.58 | 2.55, 2.61 | 2.32 | 2.23, 2.41 |
| Teaching | ||||
| Non-teaching | 1.00 | Reference | 1.00 | Reference |
| Teaching | 1.04 | 1.05 | 1.09 | 0.98, 1.22 |
RR, risk ratio; aRR, adjusted risk ratio; CI, confidence interval. The adjusted model included all factors included in the table: year, hospital bed size, maternal age, insurance status, race, hospital location, marital status, hospital region, preeclampsia diagnosis, and hospital teaching status.
Figure 3.
Rates of administration of any antihypertensive medication (intravenous or oral labetalol, nifedipine, and hydralazine) for all patients with preeclampsia by year and race (white, black, other). Medication use from 2015 is not included as only a quarter of data is available for that year.
Figure 4.
Rates of administration of any antihypertensive medication (intravenous or oral labetalol, nifedipine, and hydralazine) for all patients with preeclampsia by year and hospital teaching status. Medication use from 2015 is not included as only a quarter of data is available for that year.
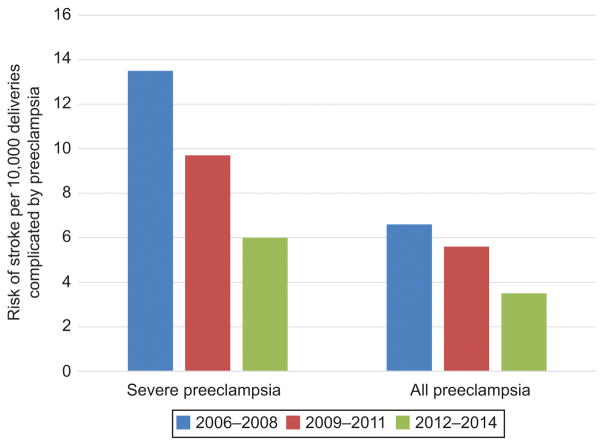
Risk for pregnancy-associated stroke over the study period for women with severe preeclampsia and any preeclampsia decreased (Figure 5). For severe preeclampsia, the risk for stroke was highest from 2006 to 2008 (13.5 cases per 10,000 deliveries, n=27) and decreased to 9.7 per 10,000 deliveries in 2009 to 2011 (n=25) and 6.0 per 10,000 deliveries in 2012 to 2014 (n=20) (p=0.02) (Table 4). For all patients with preeclampsia, the risk for stroke decreased from 6.6 to 5.6 to 3.5 per 10,000 deliveries, respectively, over the same study period (p=0.02).
Figure 5.
Risk of stroke per 10,000 delivery hospitalizations over three three-year periods: 2006–2008, 2009–2011, and 2012–2014 for patients with severe preeclampsia and all women with a preeclampsia diagnosis. The differences in risk noted for severe preeclampsia and all preeclampsia were statistically significant with P values of .017 and .016, respectively.
Table 4.
Stroke incidence for women with any preeclampsia and severe preeclampsia
| Stroke events (n) | Per 10,000 deliveries | 95% CI | |
|---|---|---|---|
| Severe preeclampsia | |||
|
| |||
| 2006–8 | 27 | 13.5 | 9.3, 19.7 |
| 2009–11 | 25 | 9.7 | 6.6, 14.3 |
| 2012–14 | 20 | 6.0 | 3.9, 9.3 |
|
| |||
| All preeclampsia | |||
|
| |||
| 2006–8 | 42 | 6.6 | 4.9, 9.0 |
| 2009–11 | 43 | 5.6 | 4.1, 7.5 |
| 2012–14 | 32 | 3.5 | 2.5, 4.9 |
DISCUSSION
This analysis demonstrated increasing use of antihypertensive agents during delivery hospitalizations complicated by preeclampsia. The rates of use of intravenous labetalol, oral labetalol, nifedipine, and hydralazine as well as use of multiple agents all increased for women with mild, superimposed, and severe preeclampsia over the study period. This data supports that research evidence supporting the benefit of antihypertensive use4 may be becoming integrated into practice as patients benefiting from antihypertensive management are increasingly identified by providers and hospital protocols. Given that the American College of Obstetricians and Gynecologists’ (ACOG) Task Force on Hypertension report was released in November 2013 and recommendations from the National Partnership were released this year, further changes in clinical practice may be occurring.5,6 While the ACOG Task Force report likely affected trends late in the study period, these data support that anti-hypertensive use was already increasing prior to release of ACOG recommendations. That use of both intravenous and oral medications increased suggests that medical management of hypertension in preeclamptic patients is changing comprehensively. While misclassification between severe and mild preeclampsia is a potential concern, increased use of antihypertensive agents for women with mild preeclampsia may represent providers treating more lower range blood pressures more frequently, particularly with oral agents prior to discharge.
Important findings from our analysis include that, particularly for women with severe preeclampsia, providers use intravenous and oral labetalol preferentially. With expanded use of these medications appropriate patient selection and use of other agents for women with conditions such as asthma, ischemic heart disease, and heart failure5 may maximize benefit and minimize risk. In this analysis the effect of individual hospitals was an important determinate in medication receipt for severe preeclampsia. Rates of medication receipt varied significantly and further investigation is indicated to what degree protocol, guideline, and bundle adoption can facilitate uniformly high-quality care that minimizes variation.
In interpreting this analysis there are several important limitations to consider. First, because this study was limited to administrative data, we were not able to obtain detailed information on how hospital factors such as protocols, guidelines, and bundle implementation may have affected management of preeclampsia and outcomes. Second, our ability to characterize patient care is limited given that we do not have patient data on vital signs and time to hypertensive administration. Even though a drug may have been ordered and administered, we cannot determine whether care occurred in a timely, optimal fashion and whether treatment in a specific time interval improved outcomes. For nifedipine we are not able to determine if medication is being administered acutely for severe range hypertension or preemptively for longer-term blood pressure control. Furthermore, because we are not able to review blood pressure we are not able to evaluate risk for hypotension with antihypertensive administration and potential effects on the fetus, nor are we able to determine specific blood pressure readings prior to stroke. Third, we cannot establish a causal relationship between antihypertensive administration (or lack thereof) and risk for stroke. While data from the HCA supports a relationship between timely administration of antihypertensive agents and decreased risk, we are not able to determine whether patients with stroke received hypertensive management prior to their event per the recommendations of ACOG and others and we are not able to evaluate hospital level implementation of protocols.5,6,27 Fourth, because we do not have outpatient data, we are unable to evaluate the degree to which prenatal outpatient management and use of antihypertensive agents may have affected use of these medications in the hospital. Fifth, because we do not have blood pressure information we do not have a true denominator for how many patients should have been treated. Sixth, because many patients were coded with nonspecific pregnancy related stroke codes, for a significant proportion of the study it is unclear whether women were diagnosed with a hemorrhagic or thrombotic stroke. Seventh, because discharge diagnoses are used, this study does not have information on whether stroke occurred antepartum, intrapartum, or postpartum during a delivery hospitalization. Eight, because ascertainment of obesity is extremely poor in administrative data this factor was not included in our analysis. Ninth, use of multiple agents may represent sequential medication administration (for example, a new agent being utilized after a suboptimal response from a prior agent) or use of multiple concurrent agents (such as a longer acting medication for maintenance along with an acute agent for severe hypertension); this analysis cannot differentiate these clinical scenarios. Tenth, in some cases hydralazine may be administered orally. In our query, in the vast majority instances hydralazine was administered intravenously; however, there were a few instances where route of administration was not documented specifically. For this reason our reported rates of intravenous hydralazine administration could be slightly overestimated.
Strengths of the study include a large patient population powered to assess rare outcomes such as stroke, a long study period, geographically and clinically diverse hospital settings, and the ability to query use of specific antihypertensive medications.
In summary, this analysis found use of antihypertensive agents increased among a population of women with increasing likelihood of severe and superimposed preeclampsia diagnoses. Use of antihypertensive agents increased for diagnoses of mild, moderate and severe hypertension. This trend of increased antihypertensive use was associated with a decrease in risk for pregnancy-associated stroke over the study period.
Acknowledgments
Dr. Friedman is supported by a career development award (K08HD082287) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Financial Disclosure
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. The other authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Presented at the 2018 Society for Maternal-Fetal Medicine Annual Pregnancy Meeting in Dallas, Texas, January 29-February 3, 2018.
References
- 1.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol. 2015;125:124–31. doi: 10.1097/AOG.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JN, Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–54. doi: 10.1097/01.AOG.0000151116.84113.56. [DOI] [PubMed] [Google Scholar]
- 3.Martin JN., Jr Severe systolic hypertension and the search for safer motherhood. Semin Perinatol. 2016;40:119–23. doi: 10.1053/j.semperi.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Clark SL, Christmas JT, Frye DR, Meyers JA, Perlin JB. Maternal mortality in the United States: predictability and the impact of protocols on fatal postcesarean pulmonary embolism and hypertension-related intracranial hemorrhage. American journal of obstetrics and gynecology. 2014;211:32.e1–9. doi: 10.1016/j.ajog.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 5.American College of O Gynecologists Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein PS, Martin JN, Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus Bundle on Severe Hypertension During Pregnancy and the Postpartum Period. Obstet Gynecol. 2017;130:347–57. doi: 10.1097/AOG.0000000000002115. [DOI] [PubMed] [Google Scholar]
- 7.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 8.Stulberg J, Delaney C, Neuhauser D, Aron D, Fu P, Koroukian S. Adherence to surgical care improvement project measures and the asssociation with postoperative infections. JAMA. 2010;303:2479–85. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 9.Fang M, Maselli J, Lurie J, Lindenauer P, Auerbach A. Use and outcomes of venous thromboembolism prophyalxis after spinal fusion surgery. J Thromb Haemost. 2011;9:1318–25. doi: 10.1111/j.1538-7836.2011.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritch J, Kim J, Lewin S, et al. Venous thromboembolism and use of prophylaxis among women undergoing laparoscopic hysterectomy. Obstet Gynecol. 2011;117:1367–74. doi: 10.1097/AOG.0b013e31821bdd16. [DOI] [PubMed] [Google Scholar]
- 11.Wright J, Lewin S, Shah M, et al. Quality of venous thromboembolism prophylaxis in patients undergoing oncologic surgery. Ann Surg. 2011;253:1140–6. doi: 10.1097/SLA.0b013e31821287ac. [DOI] [PubMed] [Google Scholar]
- 12.Zacharia BE, Youngerman BE, Bruce SS, et al. Quality of Postoperative Venous Thromboembolism Prophylaxis in Neuro-oncologic Surgery. Neurosurgery. 2016 doi: 10.1227/NEU.0000000000001270. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran S, Herbers P, Khoury J, et al. Is prophylactic anticoagulation for deep venous thrombosis common practice after intracerebral hemorrhage? Stroke. 2015;46:369–75. doi: 10.1161/STROKEAHA.114.008006. [DOI] [PubMed] [Google Scholar]
- 14.Kulik A, Rassen JA, Myers J, et al. Comparative effectiveness of preventative therapy for venous thromboembolism after coronary artery bypass graft surgery. Circ Cardiovasc Interv. 2012;5:590–6. doi: 10.1161/CIRCINTERVENTIONS.112.968313. [DOI] [PubMed] [Google Scholar]
- 15.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0. 9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–9. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 16.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. American journal of epidemiology. 2007;166:117–24. doi: 10.1093/aje/kwm139. [DOI] [PubMed] [Google Scholar]
- 17.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. American journal of obstetrics and gynecology. 2006;194:992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 18.Lydon-Rochelle MT, Holt VL, Nelson JC, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatric and perinatal epidemiology. 2005;19:460–71. doi: 10.1111/j.1365-3016.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuklina E, Whiteman M, Hillis S, Jameieson D, Meikle S, Posner S. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12:469–77. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 20.Jones SA, Gottesman RF, Shahar E, Wruck L, Rosamond WD. Validity of hospital discharge diagnosis codes for stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2014;45:3219–25. doi: 10.1161/STROKEAHA.114.006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiology and drug safety. 2008;17:20–6. doi: 10.1002/pds.1518. [DOI] [PubMed] [Google Scholar]
- 22.Thigpen JL, Dillon C, Forster KB, et al. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circulation Cardiovascular quality and outcomes. 2015;8:8–14. doi: 10.1161/CIRCOUTCOMES.113.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller EC, Gatollari HJ, Too G, et al. Risk of Pregnancy-Associated Stroke Across Age Groups in New York State. JAMA neurology. 2016;73:1461–7. doi: 10.1001/jamaneurol.2016.3774. [DOI] [PubMed] [Google Scholar]
- 24.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of Diagnostic Codes for Acute Stroke in Administrative Databases: A Systematic Review. PloS one. 2015;10:e0135834. doi: 10.1371/journal.pone.0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moatti Z, Gupta M, Yadava R, Thamban S. A review of stroke and pregnancy: incidence, management and prevention. Eur J Obstet Gynecol Reprod Biol. 2014;181:20–7. doi: 10.1016/j.ejogrb.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 26.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–16. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 27.Moroz LA, Simpson LL, Rochelson B. Management of severe hypertension in pregnancy. Semin Perinatol. 2016;40:112–8. doi: 10.1053/j.semperi.2015.11.017. [DOI] [PubMed] [Google Scholar]