Abstract
Objective
Copper (Cu) transporter ATP7A is required for full activation of extracellular SOD (SOD3), which is secreted from vascular smooth muscle cells (VSMCs) and anchors to endothelial cell surface to preserve endothelial function by scavenging extracellular superoxide. We reported that ATP7A protein expression and SOD3 activity are decreased in insulin-deficient type1 diabetes mellitus (T1DM) vessels, thereby inducing superoxide-mediated endothelial dysfunction, which are rescued by insulin treatment. However, it is unknown regarding the mechanism by which insulin increases ATP7A expression in VSMCs and whether ATP7A downregulation is observed in T2DM mice and human in which insulin-Akt pathway is selectively impaired.
Approach and Results
Here we show that ATP7A protein is markedly downregulated in vessels isolated from T2DM patients as well as those from high fat diet-induced or db/db T2DM mice. Akt2 activated by insulin promotes ATP7A stabilization via preventing ubiquitination/degradation as well as translocation to plasma membrane in VSMCs, which contributes to activation of SOD3 that protects against T2DM-inudced endothelial dysfunction. Downregulation of ATP7A in T2DM vessels is restored by constitutive active Akt or PTP1B−/− T2DM mice which enhance insulin-Akt signaling. Immunoprecipitation, in vitro kinase assay and mass spectrometry analysis reveal that insulin stimulates Akt2 binding to ATP7A to induce phosphorylation at Ser1424/1463/1466. Furthermore, SOD3 activity is reduced in Akt2−/− vessels or VSMCs, which is rescued by ATP7A overexpression.
Conclusion
Akt2 plays a critical role in ATP7A protein stabilization and translocation to plasma membrane in VSMCs, which contributes to full activation of vascular SOD3 that protects against endothelial dysfunction in T2DM.
Keywords: Type 2 DM, endothelial dysfunction, ATP7A, Akt2, vascular smooth muscle cells
Introduction
Oxidative stress contributes to diabetes mellitus (DM)-induced endothelial dysfunction, which is one of the most common causes of cardiovascular morbidity and mortality1, 2. The major cellular defense against superoxide (O2•−) is superoxide dismutases (SODs) which consists of the cytoplasmic Cu (copper)/Zn SOD (SOD1), the mitochondrial MnSOD (SOD2), and the extracellular SOD (SOD3). SOD3 is highly expressed in the vasculature, which is mainly secreted from vascular smooth muscle cells (VSMCs)3, and is anchored to endothelial cell surface through binding to the heparin sulfate proteoglycan, collagen and fibrin-54. By contrast, SOD3 in endothelial cells is epigenetically silenced3–6. Of note, the R213G polymorphism in the SOD3 gene, which reduces binding to the endothelial surface and increases serum SOD3 levels, is correlated with cardiovascular risk7, while plasma SOD3 levels are altered in diabetic patients8, 9. Studies using SOD3−/− mice showed that vascular SOD3 plays an essential role in preserving endothelial function by scavenging extracellular O2•− and increasing NO bioavailability in hypertension10, 11, and aging12.
Cu-transporting/exporting ATPase (ATP7A) is essential not only for activation of secretory Cu enzymes such as SOD3, but also for exporting excess Cu to regulate intracellular bioavailable Cu4, 13. To achieve this, ATP7A translocates from the trans-Golgi network (TGN) to the plasma membrane or cytoplasmic vesicle, thereby exporting Cu or delivering Cu to the secretory Cu enzymes13. ATP7A trafficking is regulated by post-translational modification processes such as glycosylation14 and phosphorylation15. We demonstrated that ATP7A is involved in platelet-derived growth factor-stimulated VSMC migration via regulating lysyl oxidase (LOX) activity and Rac116. We also reported that ATP7A protein expression is decreased in vessels of insulin-deficient type1 diabetes mellitus (T1DM) mice, thereby reducing SOD3 activity and inducing excess O2•−-mediated endothelial dysfunction, which are rescued by insulin treatment. However, it is unknown regarding the molecular mechanism by which insulin increases ATP7A expression in VSMCs as well as whether ATP7A protein is downregulated in insulin-resistant T2DM mice and patients.
Insulin resistance in T2DM has been characterized by selective impairment of Akt dependent pathways without altering ERK pathways17–19. Vascular tissues express two Akt isoforms (Akt1 and Akt2) with below limit of detection of Akt3 as shown in previous reports20. Importantly, role of Akt1 and Akt2 in insulin resistance and blood vessel function seems to be different. Akt2−/− mice (in particular, male) have phenotypes such as insulin resistance and a DM-like syndrome21, while insulin-mediated Akt and eNOS phosphorylation are intact in Akt1−/− mice22. Although impaired Akt-eNOS pathway in endothelial cells from T2DM has been reported17–19, the role of Akt in VSMCs for T2DM-induced endothelial dysfunction and its relationship with SOD3 remain unclear. In the present study, we show that ATP7A protein is markedly downregulated in microvessels isolated from adipose tissues of T2DM patients as well as mesenteric arteries and aorta of T2DM (high fat diet-induced, and db/db) mice. Using various genetically approach (gene delivery of SOD3 as well as SOD3−/−, Akt1−/−, Akt2−/−, and ATP7A transgenic (ATP7A-Tg) mice), we provide the novel evidence that Akt2 phosphorylates ATP7A and plays an important role in insulin-induced ATP7A protein stabilization and plasma membrane translocation in VSMCs, which contributes to full expression of SOD3 activity. Thus, restoring the Akt2-ATP7A-SOD3 axis is potential therapeutic strategy for treatment of diabetes vascular complications.
Materials and Methods
Materials and methods are available in the online-only Data Supplement
Results
Selectively impaired Akt signaling contributes to decrease in ATP7A expression in T2DM vessels
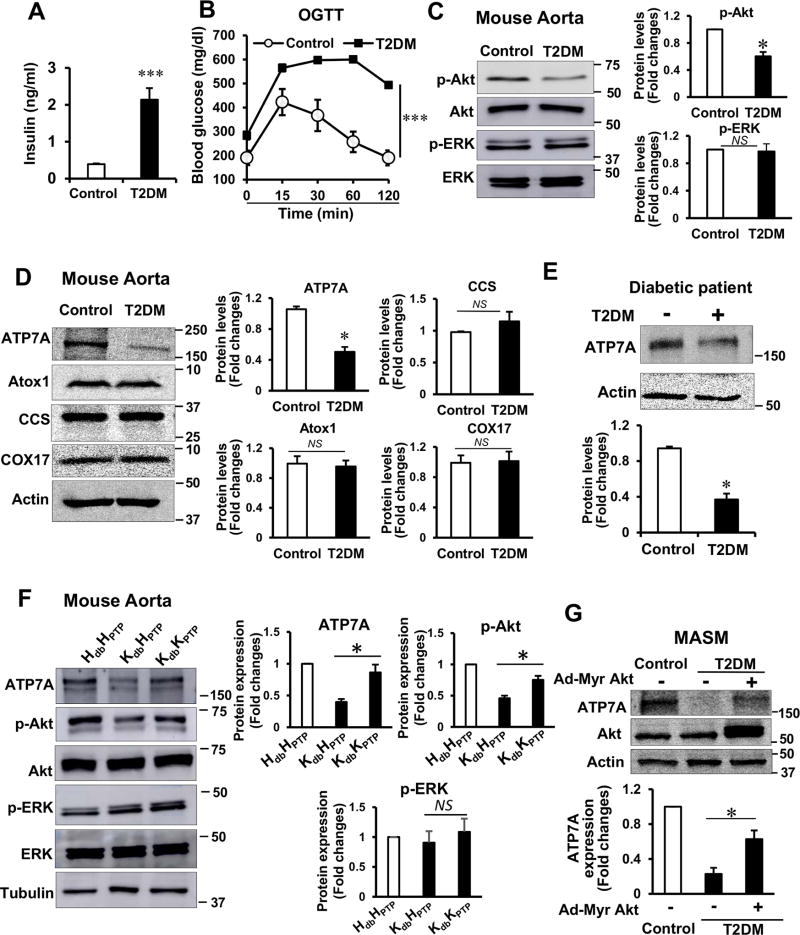
To determine the role of ATP7A-SOD3 axis in T2DM mice with compensatory hyperinsulinemia and selectively impaired insulin-Akt pathway, we used T2DM mice that were induced by a high-fat diet feeding (HFD) combined with a single injection of low dose streptozotocin (STZ). This is a well-established animal model of T2DM which show insulin resistance and compensatory hyperinsulinemia23–25. Combination of low dose STZ with HFD helps to develop reproducible and advanced degree of type 2 diabetic strain23–25. After 4 months of HFD, body weight, plasma levels of triglycerides, total cholesterol, HDL and LDL were significantly higher in T2DM than control mice (Table I in the online-only Data Supplement). T2DM mice exhibited a significant increase in the epididymal, retroperitoneal, mesenteric fat, and brown adipose tissue fat pads over control mice (Figure I in the online-only Data Supplement). Plasma insulin level was significantly higher in T2DM than control mice (Figure 1A), which was associated with a significant decrease in insulin sensitivity (Figure 1B). We also confirmed that insulin resistance assessed by HOMA-IR (calculated by using the fasted blood glucose and insulin levels)26 was significantly increased in T2DM mice (Table I in the online-only Data Supplement) which show impaired insulin-induced vasorelaxation and reduced NO2−/NO3− level in vascular tissue of T2DM mice compared to control mice (Figure IG and IH in the online-only Data Supplement). These findings indicate that we successfully generated T2DM mice.
Figure 1. Selectively impaired Akt signaling contributes to the decrease in ATP7A expression in T2DM vessels.
T2DM was induced by 16 week of high fat diet combined with single injection of low dose streptozotocin (STZ). A, plasma insulin concentrations in non-fasting T2DM and control mice (n=20). B, Blood glucose levels in control and T2DM mice fasted overnight before OGTT (n=6). C, pAkt, pERK levels and their total proteins in T2DM and control mouse aorta (n=4). D, ATP7A protein, but not other copper trafficking proteins, is decreased in T2DM mouse aorta (n=5). E, ATP7A protein expression is decreased in microvessels from adipose tissue of diabetic patient (n=7–8). The blot is representative of 7 control and 8 diabetic independent patients. Densitometric analysis are shown below. F, decrease in ATP7A protein expression in aortas from genetically-induced T2DM db/db mice is rescued in those from T2DM crossed with PTP1B−/− which enhances insulin Akt signaling (n= 4). H or K indicates heterozygote or knockout mice, respectively. The db and PTP indicate db/db and PTP1B, respectively. G, Mouse aortic SMC (MASM) isolated from control and T2DM mice were transfected with Adenovirus-myristoylated Akt. Lysates were used to measure ATP7A, Akt and actin protein expression (n=4). Results are presented as mean ± SEM. *p<0.05, ***p<0.001, NS, not significant.
Insulin resistance in T2DM has been characterized by selective impairment of Akt pathways without altering other branches, including MAP kinase pathways17–19. Consistent with this, phosphorylation of Akt, but not that of ERK, was significantly decreased in aortas of T2DM mice (Figure 1C). In these T2DM vessels, protein expression of ATP7A, but not other Cu transport proteins Atox1, CCS and COX17, was markedly decreased (Figure 1D). Similar results were also obtained in mesenteric arteries and aortas from genetically-induced T2DM db/db mice (Figure IIA and IIB in the online-only Data Supplement). Of note, ATP7A protein expression was also significantly downregulated in microvessels from adipose tissue of diabetic patients (Figure 1E). Furthermore, decreased ATP7A expression in aortas and cultured VSMCs from T2DM mice was rescued in T2DM mice crossed with PTP1B−/− mice which enhances insulin-Akt signaling (Figure 1F) or by overexpression of constitutive-active Akt (Figure 1G). These results suggest that impaired Akt signaling contributes to decrease in ATP7A expression in T2DM vessels or VSMCs.
Cu transporter ATP7A expression is decreased in T2DM vessels, which results in decreased SOD3 activity and endothelial dysfunction
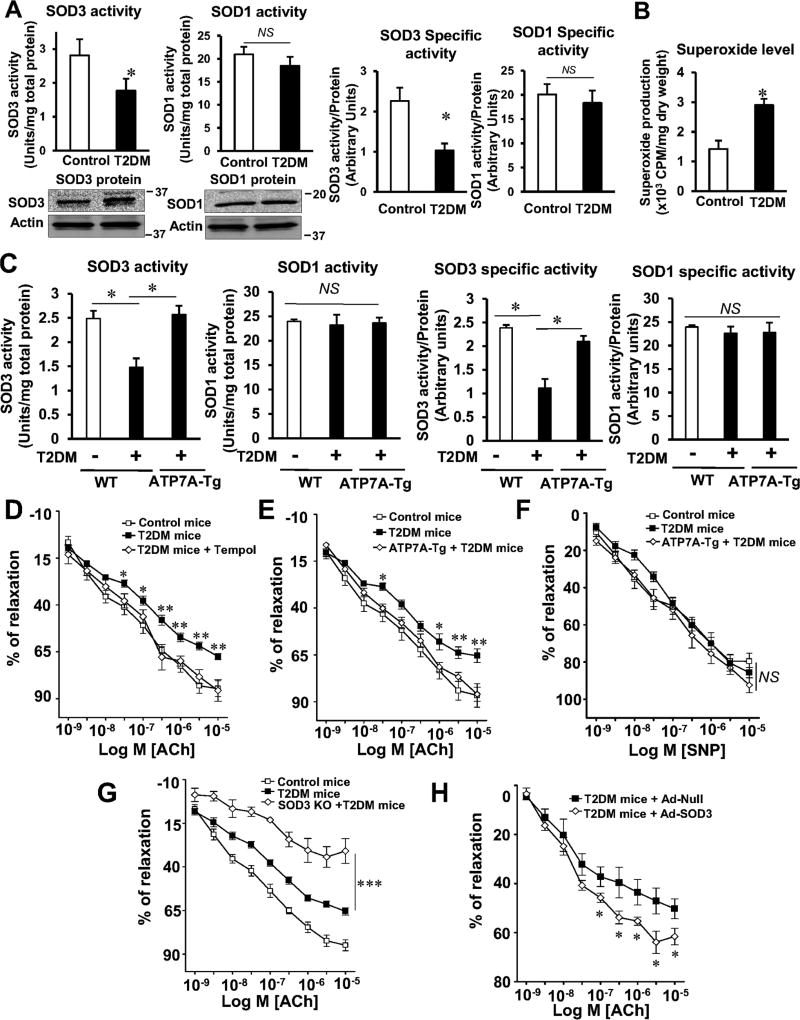
Figure 2A shows that SOD3 activity was decreased while SOD3 protein level was increased in the aorta of T2DM mice compared to control mice. By contrast, SOD1 activity and protein were not changed. Thus, the specific activity of SOD3, as determined by the ratio of activity to protein, was markedly decreased in T2DM vessels. These results suggest that decrease in ATP7A protein contributes to increase in inactive SOD3 protein (Figure 2A). In addition, decrease in SOD3 activity in T2DM vessels was associated with a marked increase in O2•− production (Figure 2B) and impaired acetylcholine (ACh)-induced endothelium-dependent vasorelaxation (EDR) (Figure 2D, 2E, 2G and 2H). Importantly, endothelial dysfunction in T2DM mice was rescued by SOD mimetic Tempol (Figure 2D), or adenoviral gene transfer of SOD3 (Figure 2H), and further enhanced in SOD3−/− T2DM mice (Figure 2G). Furthermore, decreased SOD3 total or specific activity as well as impaired EDR in T2DM mice were significantly restored in diabetic ATP7A-Tg mice (Figure 2C and 2E). We confirmed overexpression of ATP7A in diabetic ATP7A-Tg mice (Figure III in the online-only Data Supplement). Of note, sodium nitroprusside (SNP)-induced endothelium-independent vasorelaxation was not different between T2DM vessels and control groups (Figure 2F and Figure IVA and IVB in the online-only Data Supplement). Given that decreased SOD3 activity results in O2•− production in vessels10, 11, 27, 28, these results suggest that decreased ATP7A expression in T2DM vessels resulted in decreased SOD3 activity, thereby promoting O2•− production and endothelial dysfunction.
Figure 2. Decreased ATP7A expression in T2DM vessels contributes to reduced SOD3 activity, resulting in impaired endothelium-dependent relaxation.
A, Activities of SOD3 and SOD1 in T2DM or control mice aorta were measured by inhibition of cytochrome c reduction by xanthine/xanthine oxidase. Con A-Sepharose chromatography was used to isolate SOD3 from tissue homogenates. Protein levels of SOD1, SOD3 and actin (bottom). Specific activity of SOD1 and SOD3 were determined by the ratio of activity to relative amount of protein (n=4). B, Aortic superoxide production in T2DM and control mice was measured by a lucigenin-enhanced chemiluminescence (5 µmol/L) (n=6). C, Decreased SOD3 activity in T2DM mice aorta is restored in T2DM ATP7A overexpressing mice. Activity and specific activity of SOD3 and SOD1 were assayed, as described (n=4). D, E, F, G, and H, Isometric tension of mesenteric resistance arteries from T2DM with SOD mimetic or SOD3 gene transfer (D and H), transgenic mice overexpressing ATP7A with T2DM (E and F), SOD3KO with T2DM (G), or control mice was measured using a wire myograph. Vasodilation was evoked by ACh and SNP after preconstriction with phenylephrine (1–5 µmol/L) in the presence and absence of cell-permeable SOD mimetic tempol (1 mmol/L) (D), or after injection of adeno-SOD3 or adeno-null (control) (H) (n=5–8). Results are presented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, NS, not significant.
Insulin increases ATP7A protein stability by inhibiting ubiquitination and degradation in a PI3K/Akt-dependent manner in VSMCs
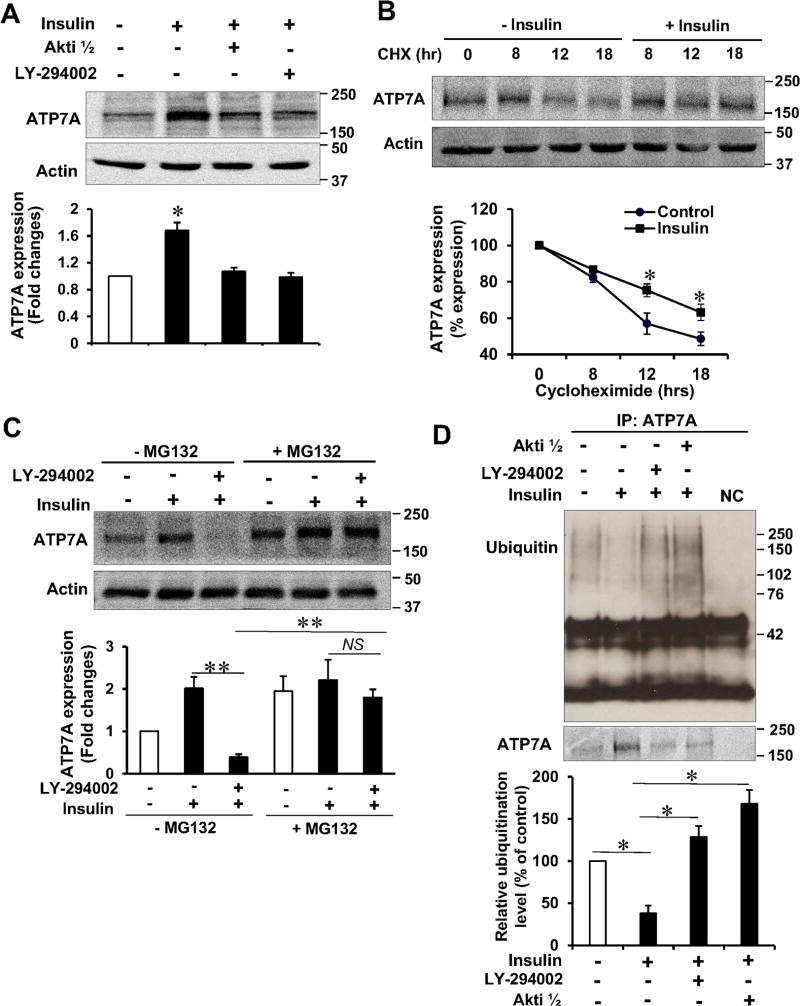
We then examined the mechanism by which insulin increases ATP7A protein expression and found that insulin stimulation in VSMC increased ATP7A protein expression without affecting ATP7A mRNA in a dose- and time-dependent manner (Figure V in the online-only Data Supplement). Furthermore, insulin-induced ATP7A protein expression was blocked by specific PI3K or Akt inhibitors, or dominant negative Akt, but not by MEK or JNK inhibitors (Figure 3A, Figure VI in the online-only Data Supplement). These results suggest that Akt mediates insulin-induced ATP7A expression in VSMCs and that decreased ATP7A protein expression in T2DM vessels is partly due to the impaired insulin-Akt pathway. We next examined whether insulin regulates ATP7A protein stability and found that the half-life of ATP7A was significantly prolonged by insulin in the presence of protein synthesis inhibitor cycloheximide (Figure 3B). Proteasome inhibitor MG132 prevented the PI3K inhibitor-induced attenuation of insulin-induced increase in ATP7A protein expression (Figure 3C). Furthermore, insulin-induced attenuation of ATP7A ubiquitination was reversed by the PI3K or Akt inhibitors (Figure 3D). These results suggest that insulin stimulation increases ATP7A protein stability by inhibiting ATP7A protein degradation/ubiquitination in an Akt-dependent manner.
Figure 3. Insulin increases ATP7A protein stability by inhibiting ubiquitination and degradation in a PI3K/Akt-dependent manner in VSMCs.
A, rat aortic SMCs (RASMs) were incubated with insulin (10 nM, 16 hrs) in the presence of PI3K inhibitor, LY-294002 (20 µM) or Akt inhibitor, Akti1/2 (5 µM) and then used to measure ATP7A protein expression (n=4). B, RASMs were incubated with insulin (10 nM) or cycloheximide (CHX, 10 nM) and then used to measure ATP7A protein expression (n=3). C, RASMs were incubated with insulin (10 nM, 16 hrs) with or without LY-294002 (20 µM) or inhibitor of proteosomal degradation, MG132 (20 µM) and then used to measure ATP7A protein exression (n=4). D, RASMs were incubated with insulin (10 nM, 16 hrs) with or without LY-294002 (20 µM) or Akt inhibitor, Akti 1/2 (5 µM) and immunoprecipitated with anti-ATP7A, followed by immunoblotted with anti-ubiquitin antibody (n=4). Bottom, averaged data for ATP7A ubiquitination (n=4). Results are presented as mean ± SEM. *p<0.05, **p<0.01, NS, not significance.
Insulin promotes ATP7A translocation to the plasma membrane where SOD3 colocalizes with ATP7A in an Akt- and Cu-dependent manner in VSMCs
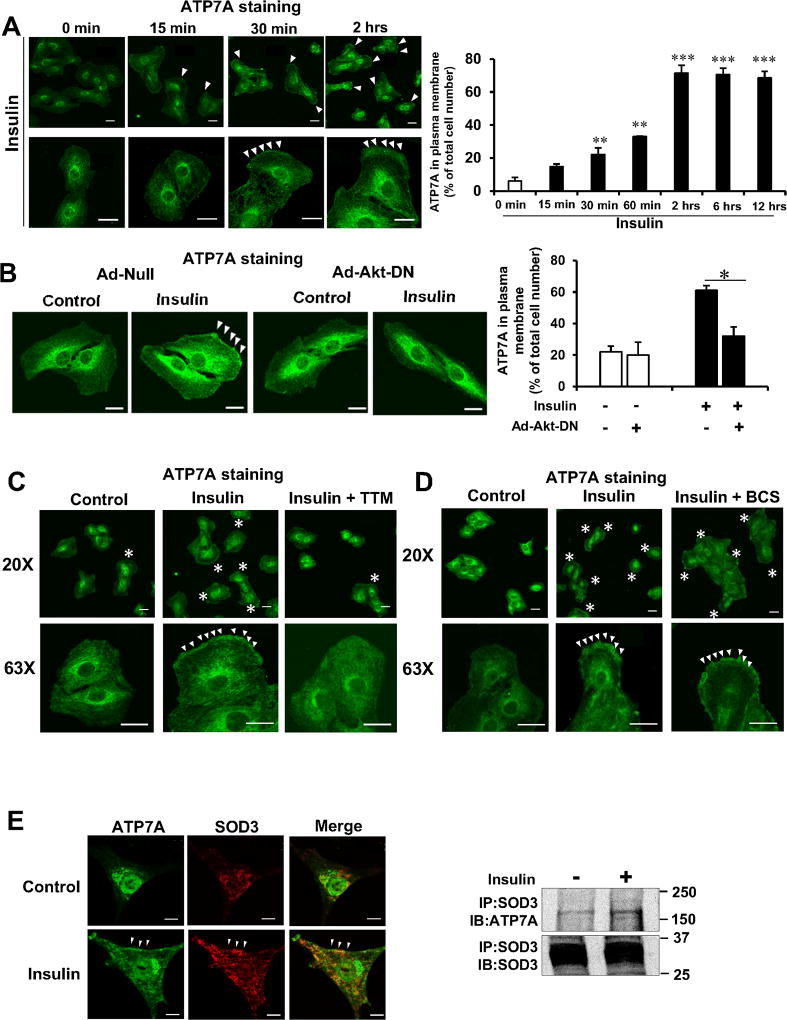
We next examined effects of insulin stimulation on subcellular localization of ATP7A in VSMCs and found that insulin promoted ATP7A translocation from the TGN to the plasma membrane within 30 min with a peak at 2 hrs in VSMCs (Figure 4A). Insulin-induced ATP7A translocation was inhibited by dominant negative-Akt or cell permeable Cu chelator TTM, but not by cell impermeable Cu chelator BCS (Figure 4B, 4C, and 4D). Immunofluorescence and co-immunoprecipitation analysis revealed that insulin increased ATP7A binding to SOD3 at the plasma membrane in VSMCs (Figure 4E). Furthermore, insulin-induced ATP7A translocation was associated with a decrease in intracellular Cu contents, which might be at least due to increased Cu export or increased Cu-bound SOD3 section (Figure VII in the online-only Data Supplement). These results suggest that Akt is also involved in insulin-stimulated ATP7A translocation from TGN to the plasma membrane where SOD3 might obtain catalytic cofactor Cu from ATP7A.
Figure 4. Insulin increases ATP7A translocation to the plasma membrane, where SOD3 colocalizes with ATP7A in an Akt- and Cu-dependent manner in VSMCs.
A–D, RASMs were stimulated with 10 nM of insulin for indicated time (A). RASMs infected with adenovirus expressing Akt-dominant negative (Ad-Akt-DN) or Ad.null (control)(B) or treated with the cell permeable Cu chelator TTM (10 nM, 24 hrs)(C) or treated with cell impermeable Cu chelator, BCS (200 µM, 72 hrs)(D) were stimulated with 10 nM insulin for 2 hrs. These cells were stained with anti-ATP7A antibody, and images were taken at 5 different fields/well from 3 different experiments. Graph represents quantification of plasma membrane ATP7A positive cells. Results are presented as mean± SEM. *p<0.05, **p<0.01, ***p<0.001. E, Immunofluorescence analysis showing the co-localization of ATP7A and SOD3 at plasma membrane (left) or co-immunoprecipitation of SOD3 and ATP7A (right) in human aortic smooth muscle cells (HASMs) stimulated with or without insulin (10 nM, 2 hrs) (n=4). Negative control staining lacking the primary antibodies in each staining is shown in Figure XIV in the online-only Data Supplement. Bar represents 20 µm.
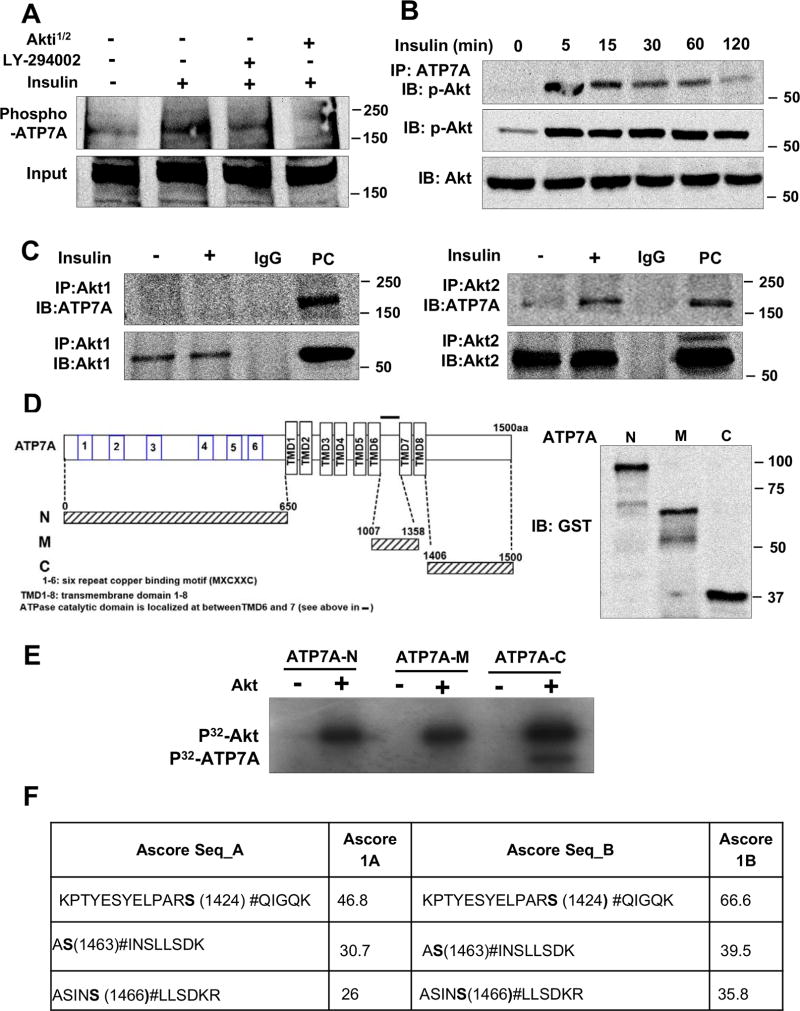
Insulin promotes Akt2-dependent phosphorylation of ATP7 in VSMCs
Since Akt-dependent phosphorylation is shown to stabilize Akt substrates by decreasing ubiquitination29, we next examined if Akt directly phosphorylates ATP7A. Figure 5A shows that insulin stimulation increased phosphorylation of ATP7A, which was inhibited by Akt or PI3K inhibitors in VSMCs. Furthermore, insulin significantly increased binding of p-Akt to ATP7A within 5 min, which continued at least for 2 hrs (Figure 5B). Furthermore, insulin significantly increased binding of Akt2, but not Akt1, to ATP7A in VSMCs (Figure 5C), suggesting that insulin induces Akt2-dependent phosphorylation of ATP7A in VSMCs.
Figure 5. Insulin increases Akt2 binding to ATP7A as well as ATP7A phosphorylation in VSMCs.
A, RASMs treated with PI3K inhibitor, LY-294002 (20 µM) or Akt inhibitor, Akti 1/2 (5 µM) and were stimulated with 10 nM insulin for 2 hrs. Phosphoprotein were purified by column and then immunoblotted (IB) with anti-ATP7A-antibody (n=3). B, RASMs treated with 10 nM insulin for indicated time were immunoprecipitated (IP) with anti-ATP7A antibody, followed by IB with p-Akt and total Akt antibody (n=3). C, RASMs stimulated with insulin (10 nM) for 2 hrs were IP with anti-Akt1 or anti-Akt2, followed by IB with anti-ATP7A antibody (n=3). D, Domains contained in the GST-ATP7A fusion construct (left). Molecular masses of various construct in kDa (right). E, Various GST-ATP7A construct (N-, Middel (M)- C-Terminus ATP7A-GST) (2 ug) were incubated with 10 µCi [γ-32P] ATP with or without recombinant Akt. [γ-32P]-incorporated GST-ATP7A protein was visualized by autoradiography. F, Peptides identified in C-Terminus ATP7A by mass spectrometry containing phosphorylated serine amino acids. Three phosphorylation sites were identified.
Akt directly phosphorylates ATP7A in vitro
To identify the Akt phosphorylation sites in ATP7A, we performed an in vitro kinase assay using recombinant active Akt and the GST-tagged various ATP7A protein fragments [N-terminal (N), Middle (M), C-terminal (C)], which are based on in silico analysis of the amino acid sequence and Cu sensitive phosphorylation sites in ATP7A (Figure 5D)15. We found that Akt phosphorylated ATP7A at C-terminus, but not at N- or middle-terminus (Figure 5E). Mass spectrometry analysis identified three Akt-dependent serine phosphorylation sites in C-terminus ATP7A: S1424, S1463 and S1466 with ascore value higher than 18, which was confidently assigned (Figure 5F, Figure VIII in the online-only Data Supplement). There was significant reduction in Akt-induced phosphorylation of ATP7A mutants (S1424A, S1463A, S1466A) compared with WT-ATP7A-C (data not shown). Furthermore, alignment of the vertebrate ATP7A C-terminal region shows complete conservation of these three phosphorylation sites (Figure VIIIC in the online-only Data Supplement). Thus, these results indicate that insulin increased Akt binding to ATP7A to induce phosphorylation at S1424/S1463/S1466.
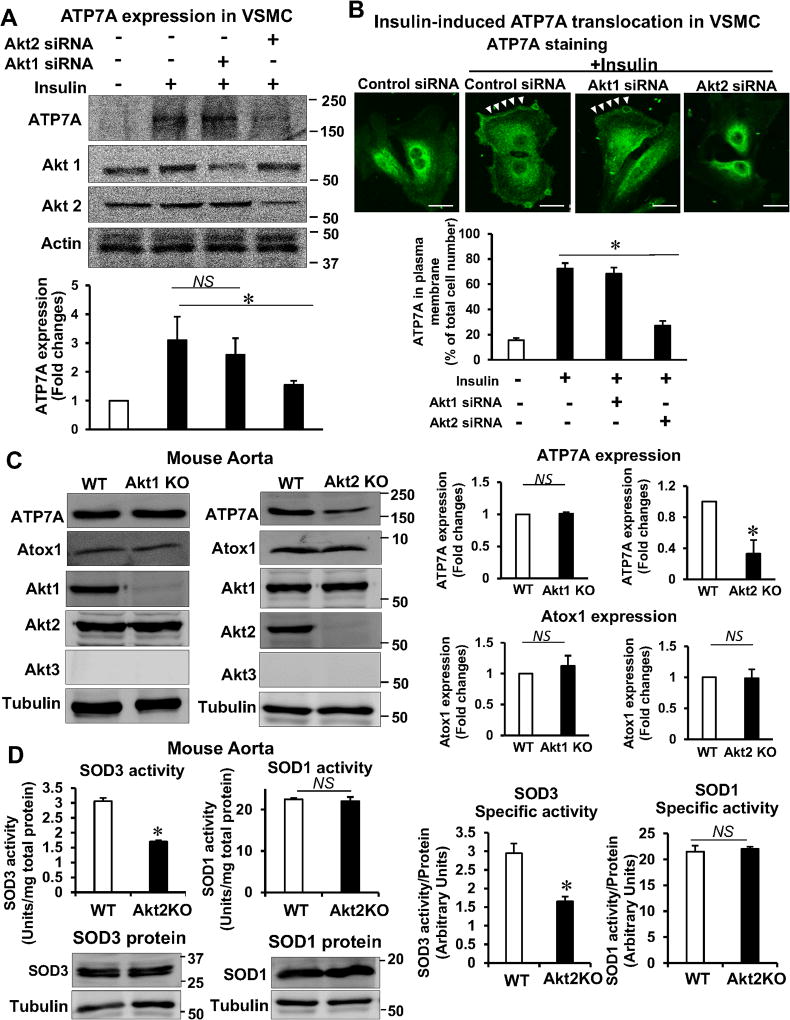
Akt2 is involved in ATP7A protein expression and ATP7A translocation to membrane in VSMCs, which contributes to SOD3 activity
Since it is shown that role of Akt1 and Akt2 in insulin resistance and blood vessel function is different, we next examined the role of Akt isoform in VSMCs for endothelial dysfunction and its relationship with SOD3. Consistent with our results that Akt2 induces phosphorylation of ATP7A, insulin-induced ATP7A protein expression in VSMCs was inhibited by Akt2 siRNA, but not Akt1 siRNA (Figure 6A). We then examined the relationship among ATP7A, Akt2, and SOD3 activity and found that insulin-induced specific activity of SOD3 secreted in VSMCs culture medium was decreased in Akt2-depleted VSMCs, which was restored in ATP7A-Tg VSMCs (Figure IX in the online-only Data Supplement). These results suggest that Akt2-mediated ATP7A protein expression contributes to full activation of SOD3 in VSMCs. Next, we examined the role of Akt isoforms in ATP7A translocation and found that insulin-induced ATP7A translocation to the plasma membrane was markedly inhibited by Akt2 siRNA-treated VSMCs or in Akt2−/− VSMCs, but not by either Akt1 siRNA-treated VSMCs or Akt1−/− VSMCs (Figure 6B and Figure X in the online-only Data Supplement). These results suggest that Akt2 is also involved in insulin-induced ATP7A translocation to the membrane in addition to ATP7A protein expression. To examine in vivo role of Akt2 for the ATP7A-mediated SOD3 activity, we used female Akt2−/− mice which were neither insulin-resistant nor diabetic, in contrast to male Akt2−/− mice which have T2DM phenotype21. We found that ATP7A protein expression, but not ATP7A mRNA, was significantly decreased in aorta from female Akt2−/− mice, but not Akt1−/− mice (Figure 6C, Figure XI in the online-only Data Supplement). We confirmed these findings using mesenteric arteries (data not shown). Figure 6D showed that the activity of SOD3, but not that of SOD1, was significantly decreased in female Akt2−/− mice compared to WT mice. By contrast, protein expression of SOD3 and SOD1 was not changed. Thus, the specific activity of SOD3, but not SOD1, was markedly decreased in vessels of female Akt2−/− mice (Figure 6D). We also confirmed these findings in male Akt2−/− mice (Figure XII in the online-only Data Supplement). Thus, these results suggest that Akt2-mediated ATP7A protein stabilization contributes to full activation of SOD3 in blood vessels.
Figure 6. Akt2 is involved in ATP7A protein expression and ATP7A translocation to membrane in VSMCs, which contributes to SOD3 activity.
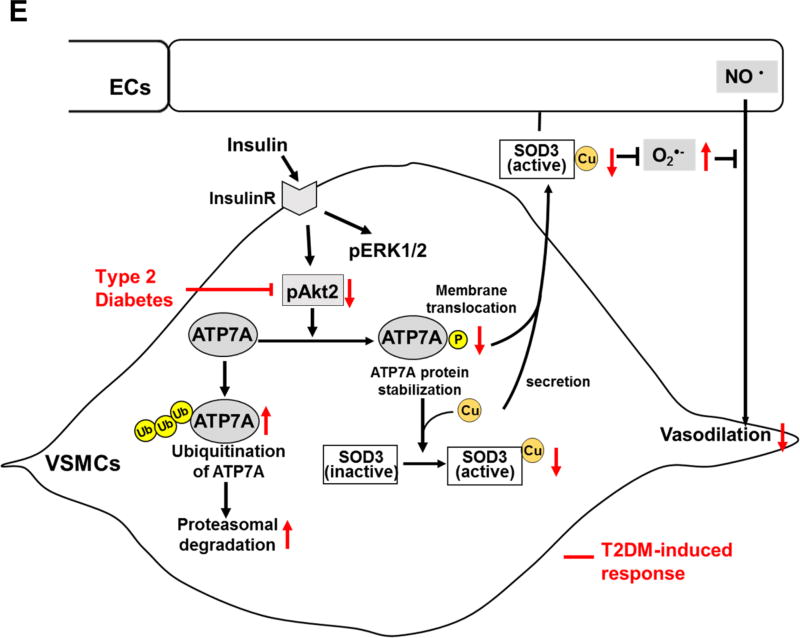
A and B, RASMs transfected with control or Akt1 or Akt2 siRNA were stimulated with 10 nM insulin for 2 hrs (B) or 16 hrs (A). In A, lysates were used to measure ATP7A protein expression (n=4). In B, cells were stained with anti-ATP7A. Small white stars point to the plasma membrane. Fluorescence images were analyzed as described in Figure 4. Negative control staining lacking the primary antibodies in each staining is shown in Figure XIV in the online-only Data Supplement. C, Protein expression for ATP7A and Atox1 in aortas of female Akt1 or Akt2 KO mice (n=4). D, Specific activity of SOD1 and SOD3 was determined by the ratio of activity to relative amount of protein (n=5). Activities and protein levels of SOD3 and SOD1 in homogenates from female Akt2 KO or WT mice aorta were assayed as described in Figure 2. Results are presented as mean ± SEM. *p<0.05. NS, not significant. E, Proposed model for the protective role of the insulin-Akt2-ATP7A-SOD3 pathway against T2DM-induced endothelial dysfunction. Decreased ATP7A expression in vessels from T2DM with selective impairment of insulin/Akt signaling contributes to decreased SOD3 activity, resulting in increased O2•− production and endothelial dysfunction. Mechanistically, insulin increases Akt2 binding to ATP7A to induce phosphorylation, which may increase ATP7A protein expression via preventing proteasomal degradation as well as ATP7A translocation to the plasma membrane, which contributes to full activation of SOD3 in VSMC and preserves endothelial function.
DISCUSSION
In this study, we provide the novel evidence that: 1) ATP7A protein is markedly downregulated in microvessels isolated from adipose tissues of T2DM patients as well as mesenteric arteries and aorta of T2DM (high fat diet-induced, and db/db) mice; 2) insulin-induced Akt2 activation, which is selectively impaired in T2DM, is required for ATP7A stabilization via preventing ubiquitination/degradation as well as ATP7A trafficking to plasma membrane in VSMCs, which induces full activation of SOD3 that protects against endothelial dysfunction in T2DM; 3) downregulation of ATP7A in vessels or VSMCs from T2DM is rescued by constitutive active Akt or PTP1B−/− T2DM mice which enhance insulin-Akt signaling; 4) Akt2 activated by insulin binds to ATP7A to induce phosphorylation of C-terminus at Ser1424/1463/1466. Thus, these new findings provide novel mechanistic insights into how SOD3 activity is decreased in T2DM blood vessels via impaired Akt2-ATP7A pathway, which contributes to endothelial dysfunction.
The functional significance of impaired ATP7A-SOD3 axis in endothelial dysfunction in T2DM is demonstrated by the results that the reduced ACh-induced endothelium dependent vasorelaxation (EDR) in T2DM vessels was rescued by SOD mimetic tempol or gene transfer of SOD3, while it was enhanced in SOD3−/− mice. In contrast, sodium nitroprusside (SNP)-induced endothelium-independent vasorelaxation was not different between T2DM vessels and control groups. EDR of mesenteric arteries in mice depends on not only NO but also endothelium-dependent hyperpolarizing factor including H2O230 which is resistant to NOS inhibitor L-NAME but sensitive to calcium-activated K+ channel inhibitors (both Apamin (small conductance calcium-activated K-channel inhibitor) and Charybdotoxin (intermediate and large conductance calcium-activated K-channel inhibitor)30. Relative contribution of NO- and EDH-mediated component to EDR is inversely proportional to mesenteric arterial diameter31. In the current study, we used 1st order (~230 µm internal diameter) mesenteric arteries showing that L-NAME markedly inhibits ACh-induced EDR. Indeed, we verified that NO dependent component is predominant in the 1st order mesenteric arteries compared to the 2nd order ones, while EDH component is larger in the 2nd order mesenteric arteries compared to the 1st order ones (Data not shown), confirming our methodology to measure EDR. Of note, Stepp and colleagues reported that NO component, but not EDH component, is impaired in the 2nd order mesenteric arteries from T2DM db/db mice, which is rescued by SOD mimetic32–34. Taken together, our study suggests that reduced SOD3 activity is associated with increased O2•− production, which results in reduced NO bioavailability and impaired NO-dependent vasorelaxation in the T2DM mice. The potential sources of O2•− in vessels from T2DM will include mitochondrial electron transport chain and NADPH oxidases24, 35. Thus, whether disrupted balance between the antioxidant systems through the ATP7A-SOD3 pathway and O2•− generating systems may contribute to EC dysfunction in T2DM should be clarified in future study.
The present study also shows that phosphorylation of Akt, but not that of ERK, is selectively reduced in T2DM blood vessels or VSMCs and that decrease in ATP7A expression and Akt phosphorylation in T2DM mice are rescued by constitutive-active Akt or PTP1B−/− T2DM mice which enhance Akt phosphorylation compared to T2DM32. Akt isoforms consist of three families including Akt1, Akt2, and Akt3 which have distinct roles36, and vascular tissues mainly express Akt1 and Akt2 with below limit of Akt3 as shown in our study and previous reports20. Note that Akt2 is expressed in aorta and femoral artery of mice20 and plays a key role in insulin signaling21. In this study, both male and female Akt2−/− mice which are insulin resistant and not insulin resistant, respectively21, but not Akt1−/− mice, show significant decrease in ATP7A protein expression and SOD3 activity, which are rescued in ATP7A-Tg mice. Thus, these results suggest that insulin-Akt2 pathway plays an important role in increasing the ATP7A-SOD3 axis in blood vessels, which protects against diabetic endothelial dysfunction.
Mechanistically, using Akt2 siRNA and Akt2−/−VSMCs or vessels, we demonstrate that Akt2 activated by insulin, which is impaired in T2DM, is required for stabilizing ATP7A protein by preventing ubiquitination/degradation as well as for ATP7A trafficking from TGN to plasma membrane where it binds to SOD3. Co-immunoprecipitation, in vitro kinase assay and LC-MS/MS analysis reveal that insulin promotes Akt2 binding to ATP7A, thereby phosphorylating C-terminus of ATP7A at S1424/S1463/S1466. Previous reports suggest that Akt-mediated phosphorylation of substrate proteins prevents ubiquitination/proteosomal degradation, thereby enhancing stabilization29, 37, and that phosphorylation of ATP7A regulates its trafficking required for its Cu transport function15, 38, 39. Furthermore, it is shown that Cu-dependent serine phosphorylation of ATP7B is required for preventing its degradation as well as for Cu-induced trafficking40. We also found that cell-permeable Cu chelator, TTM, but not cell-impermeable Cu chelator BCS, blocked insulin-induced ATP7A trafficking. Consistently, previous reports show that Cu-induced ATP7A or ATP7B phosphorylation and trafficking require Cu binding to cytoplasmic regions of these proteins at N-terminus15 in a BCS-independent manner41. Taken together, these findings indicate that Akt2 directly binds to ATP7A to induce its phosphorylation, which may prevent ubiquitination/degradation of ATP7A and promote translocation of ATP7A to plasma membrane where SOD3 might obtain Cu via ATP7A, thereby increasing the full SOD3 activity.
Since excess Cu is toxic13, 42, not only Cu transporter function for secretory Cu enzymes but also Cu exporter function of ATP7A plays an important role in regulating bioavailable intracellular Cu levels13. The physiological role of Cu exporter ATP7A in other systems is not merely the elimination of excess cellular Cu but the supplying adequate Cu to the developing fetus as gestation progresses41, 43 or neuronal protective mechanism42. In placental cells, insulin-induced ATP7A relocalization is proposed to supply adequate Cu to the developing fetus as gestation progresses41, 43. Thus, downregulation of ATP7A by impaired insulin-Akt2 pathway may impair Cu delivery to fetus. Indeed, it is reported that Cu metabolism is abnormal in diabetes44, 45, and that Cu chelation therapy mitigates various complications of diabetes44. The present study shows that intracellular Cu content is increased in T2DM vessels (Figure XIII in the online-only Data Supplement), which may be due to a decrease in Cu exporter function of ATP7A. This excess Cu is not bioavailable, because Cu is not properly transported to secretory Cu enzymes, and thus resulting in decreased specific SOD3 activity. Indeed, accumulated Cu caused by Cu importer CTR1 deficiency in intestinal cells do not activate Cu-dependent enzymes46. However, since some reports indicate that Cu supplementation rather restores diabetic phenotype45, role of Cu homeostasis in diabetes may differ, depending on severity or genetic background.
We and others previously reported that decrease in SOD3 activity in blood vessels contributes to endothelial dysfunction, hypertension, impaired ischemia-induced neovascularization4, 47 by overproduction of O2•− levels10, 11, 28. Moreover, SOD3 gene transfer prevents neointima formation in vascular injury model48. In addition to SOD3, Cu transporter function of ATP7A is required for activating various secretory Cu enzymes, including lysyl oxidases13, which promotes collagen cross-linking involved in vascular remodeling and wound repair. Thus, our finding that the impaired Akt2-ATP7A-SOD3 or other Cu enzymes axis may apply to other cardiovascular diseases such as hypertension, atherosclerosis, vascular remodeling and aging. Addressing this issue is important subject of future study.
In summary, the present study provides compelling evidence that ATP7A, a Cu transporter for SOD3, is markedly downregulated in T2DM vessels due to impaired insulin-Akt2 pathway in VSMCs, which contribute to decrease in SOD3 activity, excess O2•− production and subsequent impaired NO-dependent EC function. Mechanistically, Akt2 activated by insulin phosphorylates C-terminus of ATP7A, thereby promoting ATP7A protein stabilization via preventing ubiquitination/protein degradation as well as ATP7A trafficking to plasma membrane to deliver Cu to SOD3. Thus, enhancing and restoring Akt2-ATP7A-SOD3 axis is novel therapeutic strategy for treatment of diabetic vascular complications and various cardiovascular diseases which are associated with oxidative stress.
Supplementary Material
Highlights.
-
➢
Protein expression of ATP7A, a Cu transporter for SOD3, is downregulated in the vessels from type2 diabetic patients and mice with impaired insulin-Akt signaling.
-
➢
Akt2 activated by insulin phosphorylates ATP7A, thereby promoting ATP7A protein stabilization via preventing ubiquitination/protein degradation as well as ATP7A trafficking to plasma membrane in VSMCs., which contributes to full activation of SOD3 that protects against endothelial dysfunction in T2DM.
-
➢
Decreased ATP7A expression in type2 diabetic vessels or VSMCs are rescued by constitutive active Akt or PTP1B−/− diabetic mice which enhance insulin-Akt signaling.
-
➢
Restoring the newly identified Akt2-ATP7A-SOD3 axis is novel therapeutic strategy for treatment of diabetic vascular complications which are associated with oxidative stress.
Acknowledgments
We thank Mr. Ronald D. McKinney for editorial assistance. We thank Taplin Mass Spectrometry’s facility for assistance with phosphorylation site determinations. This research was supported by 5NIH R01 HL070187-14 (to T.F.), Department of Veterans Affairs Merit Review grant 2I01BX001232-05 (to T.F.), R01HL133613, R01HL116976 (to T.F., M.U.-F.), NIHR01 HL077524, HL077524-S1, R21HL112293 (to M.U.-F.), HL085614, HL095701, HL130513 (to S.A.P.), 11POST5740006 and 15SDG25700406 (to V.S.).
Non-standard Abbreviations and Acronyms
- Akt-DN
Akt-dominant negative
- BCS
Bathocuproine disulfonate
- C/LR
Caveolin enriched lipid raft
- CHX
Cycloheximide
- ATP7A
Copper transporting/exporting ATPase
- Cu
Copper
- EDR
Endothelium-dependent vasorelaxation
- HFD
High fat diet
- IB
Immunoblot
- IP
Immunoprecipitation
- ICP-MS
Inductively coupled plasma mass spectrometry
- IRS
Insulin receptor substrate
- LC – MS/MS
Liquid chromatography tandem-mass spectrometry
- LOX
Lysyl oxidase
- MS
Mass spectrometry
- MASMs
Mouse aortic smooth muscle cells
- RASMs
Rat aortic smooth muscle cells
- RT-PCR
Reverse transcription-polymerase chain reaction
- SOD
Superoxide dismutase
- TTM
Tetrathiomolybdate
- TGN
Trans-Golgi network
- VSMC
Vascular smooth muscle cell
Footnotes
Authors’ contributions
V.S. designed the study, performed experiments, analyzed data, and wrote the manuscript. N.O. and J.P.O. performed ubiquitination assay. A.M. provided some T2DM sample. S.A.P., Z.B. and V.P. provided human biopsy sample. N.H. provided Akt1 and Akt2 KO mice. D.S. provided db/db/PTP1B−/− mice. M.U.-F. and T.F. designed the overall study, analyzed data, and wrote, reviewed, and edited the manuscript. T.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 3.Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 4.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marklund SL. Expression of extracellular superoxide dismutase by human cell lines. Biochem J. 1990;266:213–219. doi: 10.1042/bj2660213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelko IN, Stepp MW, Vorst AL, Folz RJ. Histone acetylation regulates the cell-specific and interferon-gamma-inducible expression of extracellular superoxide dismutase in human pulmonary arteries. Am J Respir Cell Mol Biol. 2011;45:953–961. doi: 10.1165/rcmb.2011-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juul K, Tybjaerg-Hansen A, Marklund S, Heegaard NH, Steffensen R, Sillesen H, Jensen G, Nordestgaard BG. Genetically reduced antioxidative protection and increased ischemic heart disease risk: The copenhagen city heart study. Circulation. 2004;109:59–65. doi: 10.1161/01.CIR.0000105720.28086.6C. [DOI] [PubMed] [Google Scholar]
- 8.Kimura F, Hasegawa G, Obayashi H, Adachi T, Hara H, Ohta M, Fukui M, Kitagawa Y, Park H, Nakamura N, Nakano K, Yoshikawa T. Serum extracellular superoxide dismutase in patients with type 2 diabetes: Relationship to the development of micro- and macrovascular complications. Diabetes Care. 2003;26:1246–1250. doi: 10.2337/diacare.26.4.1246. [DOI] [PubMed] [Google Scholar]
- 9.Adachi T, Inoue M, Hara H, Maehata E, Suzuki S. Relationship of plasma extracellular-superoxide dismutase level with insulin resistance in type 2 diabetic patients. J Endocrinol. 2004;181:413–417. doi: 10.1677/joe.0.1810413. [DOI] [PubMed] [Google Scholar]
- 10.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 11.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: In vivo and ex vivo evidence from ecsod-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 12.Lund DD, Chu Y, Miller JD, Heistad DD. Protective effect of extracellular superoxide dismutase on endothelial function during aging. Am J Physiol Heart Circ Physiol. 2009;296:H1920–1925. doi: 10.1152/ajpheart.01342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting atpases. Physiol Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Pilankatta R, Hatori Y, Lewis D, Inesi G. Comparative features of copper atpases atp7a and atp7b heterologously expressed in cos-1 cells. Biochemistry. 2010;49:10006–10012. doi: 10.1021/bi101423j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldhuis NA, Valova VA, Gaeth AP, Palstra N, Hannan KM, Michell BJ, Kelly LE, Jennings I, Kemp BE, Pearson RB, Robinson PJ, Camakaris J. Phosphorylation regulates copper-responsive trafficking of the menkes copper transporting p-type atpase. Int J Biochem Cell Biol. 2009;41:2403–2412. doi: 10.1016/j.biocel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, Maryon EB, Kaplan JH, Ushio-Fukai M, Fukai T. Unexpected role of the copper transporter atp7a in pdgf-induced vascular smooth muscle cell migration. Circ Res. 2010;107:787–799. doi: 10.1161/CIRCRESAHA.110.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groop PH, Forsblom C, Thomas MC. Mechanisms of disease: Pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab. 2005;1:100–110. doi: 10.1038/ncpendmet0046. [DOI] [PubMed] [Google Scholar]
- 18.Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am. 2008;37:685–711. ix–x. doi: 10.1016/j.ecl.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care. 2007;10:523–530. doi: 10.1097/MCO.0b013e32819f8ecd. [DOI] [PubMed] [Google Scholar]
- 20.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase balpha is critical for ischemic and vegf-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking akt2/pkb beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, Dillmann WH. Excess protein o-glcnacylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol. 2012;303:R689–699. doi: 10.1152/ajpregu.00548.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho YE, Basu A, Dai A, Heldak M, Makino A. Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am J Physiol Cell Physiol. 2013;305:C1033–1040. doi: 10.1152/ajpcell.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Qin Z, Gongora MC, Ozumi K, Itoh S, Akram K, Ushio-Fukai M, Harrison DG, Fukai T. Role of menkes atpase in angiotensin ii-induced hypertension: A key modulator for extracellular superoxide dismutase function. Hypertension. 2008;52:945–951. doi: 10.1161/HYPERTENSIONAHA.108.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudhahar V, Urao N, Oshikawa J, McKinney RD, Llanos RM, Mercer JF, Ushio-Fukai M, Fukai T. Copper transporter atp7a protects against endothelial dysfunction in type 1 diabetic mice by regulating extracellular superoxide dismutase. Diabetes. 2013;62:3839–3850. doi: 10.2337/db12-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng J, Tamaskovic R, Yang Z, Brazil DP, Merlo A, Hess D, Hemmings BA. Stabilization of mdm2 via decreased ubiquitination is mediated by protein kinase b/akt-dependent phosphorylation. J Biol Chem. 2004;279:35510–35517. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 30.Ellinsworth DC, Sandow SL, Shukla N, Liu Y, Jeremy JY, Gutterman DD. Endothelium-derived hyperpolarization and coronary vasodilation: Diverse and integrated roles of epoxyeicosatrienoic acids, hydrogen peroxide, and gap junctions. Microcirculation. 2016;23:15–32. doi: 10.1111/micc.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceroni L, Ellis A, Wiehler WB, Jiang YF, Ding H, Triggle CR. Calcium-activated potassium channel and connexin expression in small mesenteric arteries from enos-deficient (enos−/−) and enos-expressing (enos+/+) mice. Eur J Pharmacol. 2007;560:193–200. doi: 10.1016/j.ejphar.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Ali MI, Ketsawatsomkron P, Belin de Chantemele EJ, Mintz JD, Muta K, Salet C, Black SM, Tremblay ML, Fulton DJ, Marrero MB, Stepp DW. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ Res. 2009;105:1013–1022. doi: 10.1161/CIRCRESAHA.109.206318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu S, Mintz JD, Salet CD, Han W, Giannis A, Chen F, Yu Y, Su Y, Fulton DJ, Stepp DW. Increasing muscle mass improves vascular function in obese (db/db) mice. J Am Heart Assoc. 2014;3:e000854. doi: 10.1161/JAHA.114.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JA, Larion S, Mintz JD, Belin de Chantemele EJ, Fulton DJ, Stepp DW. Genetic deletion of nadph oxidase 1 rescues microvascular function in mice with metabolic disease. Circ Res. 2017;121:502–511. doi: 10.1161/CIRCRESAHA.116.309965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, Mann GE. Vascular nad(p)h oxidase activation in diabetes: A double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez E, McGraw TE. The akt kinases: Isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarshad AA, Corcoran M, Al-Muzzaini B, Borgonovo-Brandter L, Von Euler A, Lamont D, Visa N, Percipalle P. Glycogen synthase kinase (gsk) 3beta phosphorylates and protects nuclear myosin 1c from proteasome-mediated degradation to activate rdna transcription in early g1 cells. PLoS Genet. 2014;10:e1004390. doi: 10.1371/journal.pgen.1004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petris MJ, Voskoboinik I, Cater M, Smith K, Kim BE, Llanos RM, Strausak D, Camakaris J, Mercer JF. Copper-regulated trafficking of the menkes disease copper atpase is associated with formation of a phosphorylated catalytic intermediate. J Biol Chem. 2002;277:46736–46742. doi: 10.1074/jbc.M208864200. [DOI] [PubMed] [Google Scholar]
- 39.Voskoboinik I, Fernando R, Veldhuis N, Hannan KM, Marmy-Conus N, Pearson RB, Camakaris J. Protein kinase-dependent phosphorylation of the menkes copper p-type atpase. Biochem Biophys Res Commun. 2003;303:337–342. doi: 10.1016/s0006-291x(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 40.Pilankatta R, Lewis D, Inesi G. Involvement of protein kinase d in expression and trafficking of atp7b (copper atpase) J Biol Chem. 2011;286:7389–7396. doi: 10.1074/jbc.M110.171454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer JF, Ackland ML. Hormonal regulation of the menkes and wilson copper-transporting atpases in human placental jeg-3 cells. Biochem J. 2007;402:241–250. doi: 10.1042/BJ20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlief ML, Gitlin JD. Copper homeostasis in the cns: A novel link between the nmda receptor and copper homeostasis in the hippocampus. Mol Neurobiol. 2006;33:81–90. doi: 10.1385/MN:33:2:81. [DOI] [PubMed] [Google Scholar]
- 43.La Fontaine S, Mercer JF. Trafficking of the copper-atpases, atp7a and atp7b: Role in copper homeostasis. Arch Biochem Biophys. 2007;463:149–167. doi: 10.1016/j.abb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Cooper GJ. Therapeutic potential of copper chelation with triethylenetetramine in managing diabetes mellitus and alzheimer's disease. Drugs. 2011;71:1281–1320. doi: 10.2165/11591370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Weksler-Zangen S, Jorns A, Tarsi-Chen L, Vernea F, Aharon-Hananel G, Saada A, Lenzen S, Raz I. Dietary copper supplementation restores beta-cell function of cohen diabetic rats: A link between mitochondrial function and glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2013;304:E1023–1034. doi: 10.1152/ajpendo.00036.2013. [DOI] [PubMed] [Google Scholar]
- 46.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular sod in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 48.Ozumi K, Tasaki H, Takatsu H, Nakata S, Morishita T, Koide S, Yamashita K, Tsutsui M, Okazaki M, Sasaguri Y, Adachi T, Nakashima Y. Extracellular superoxide dismutase overexpression reduces cuff-induced arterial neointimal formation. Atherosclerosis. 2005;181:55–62. doi: 10.1016/j.atherosclerosis.2005.01.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.