Abstract
Objective
Hematopoietic-derived cells have been reported in heart valves but remain poorly characterized. Interestingly, recent studies reveal infiltration of leukocytes and increased macrophages in human myxomatous mitral valves. Nevertheless, timing and contribution of macrophages in normal valves and myxomatous valve disease (MVD) are still unknown. The objective is to characterize leukocytes during postnatal heart valve maturation and identify macrophage subsets in MVD.
Approach and results
Leukocytes are detected in heart valves after birth and their numbers increase during postnatal valve development. Flow cytometry and immunostaining analysis indicate that almost all valve leukocytes are myeloid cells, consisting of at least 2 differentially localized macrophage subsets and dendritic cells. Beginning a week after birth, increased numbers of CCR2+ macrophages are present, consistent with infiltrating populations of monocytes, and macrophages are localized in regions of biomechanical stress in the valve leaflets. Valve leukocytes maintain expression of CD45 and do not contribute to significant numbers of endothelial or interstitial cells. Macrophage lineages were examined in aortic and mitral valves of Axin2 KO mice that exhibit myxomatous features. Infiltrating CCR2+ monocytes and expansion of CD206-expressing macrophages are localized in regions where modified heavy chain hyaluronan is observed in myxomatous valve leaflets. Similar colocalization of modified hyaluronan and increased numbers of macrophages were observed in human MVD.
Conclusions
Our study demonstrates the heterogeneity of myeloid cells in heart valves and highlights an alteration of macrophage subpopulations, notably an increased presence of infiltrating CCR2+ monocytes and CD206+ macrophages, in MVD.
Keywords: heart valve, leukocyte, macrophage, myxomatous heart valve, heart valve disease
Introduction
Proper heart valve function relies on highly organized extracellular matrix (ECM) consisting of layers rich in fibrillar collagens, glycosaminoglycans (GAG) and elastin1. Pathological myxomatous valves display disrupted ECM, characterized by thickening of leaflets with GAG accumulation, along with disorganization and excessive deposition of collagen fibers, resulting in impaired valve closure and valve regurgitation2. Valve leaflets are populated with valve interstitial cells (VICs) that produce ECM and mediate ECM remodeling during development or disease. Hematopoietic-derived cells have been detected in heart valves and are proposed to have synthetic properties of VICs, such as producing collagen type 1a13,4. More recently, increased inflammatory cells composed of macrophages and T cells have been described in human myxomatous mitral valves2,5. Likewise, cytokines, chemokines, and chemokine receptors are upregulated in human diseased leaflets5, indicative of immune cell infiltration in human myxomatous valves. Macrophage numbers are also increased in the filamin A-deficient murine model of myxomatous valve disease (MVD)6. Moreover, we recently described that inflammation precedes pathogenic ECM remodeling in Axin2 knockout (KO) mice, another murine model of MVD7. Yet, immune cell contributions to heart valve development and MVD are not well-characterized.
In cardiovascular development and disease, bone marrow-derived and tissue-resident immune cells are present in normal hearts and increase with disease or after injury8,9. While roles of macrophages in cardiac regeneration and myocardial fibrosis have been extensively studied10,11, much less is known about macrophage lineages in heart valve development, remodeling, or homeostasis. Macrophage subtypes are involved in development and exert important homeostatic activity in numerous organ systems12. They also play key roles in tissue repair following injury. For instance, macrophages affect fibroblasts through paracrine activity, playing a crucial role in ECM remodeling during development13, while aberrant response to inflammation promotes excessive ECM deposition during fibrosis14. Recent studies have indicated that macrophages are more heterogeneous than previously assumed, and different subsets achieve distinct functions, which can be detrimental or beneficial to pathogenesis14. In the murine aorta, macrophages are present in the adventitia, and myeloid cells contribute to multiple stages of lesion formation and disease progression in atherosclerosis9,15. However, analogous roles in normal heart valves and MVD have not been previously determined.
In the current study, we sought to determine leukocyte subtypes and localization during postnatal heart valve development and also to evaluate macrophage subsets in Axin2 KO mice that exhibit myxomatous valve characteristics7. Our analyses revealed that most leukocytes present in normal heart valves are macrophages and dendritic cells that do not differentiate into endothelial cells or VICs. Macrophage subsets are differentially localized in heart valve leaflets, and the increased numbers of CCR2+ monocytes and CD206+ macrophages in murine myxomatous disease supports a role for infiltrating cells in tissue remodeling in diseased heart valves. Similar ECM abnormalities and increased numbers of macrophages were observed in human MVD tissue samples.
Materials and methods
Detailed Materials and Methods are available in the online-only Supplement.
Results
Leukocytes are present in heart valves after birth
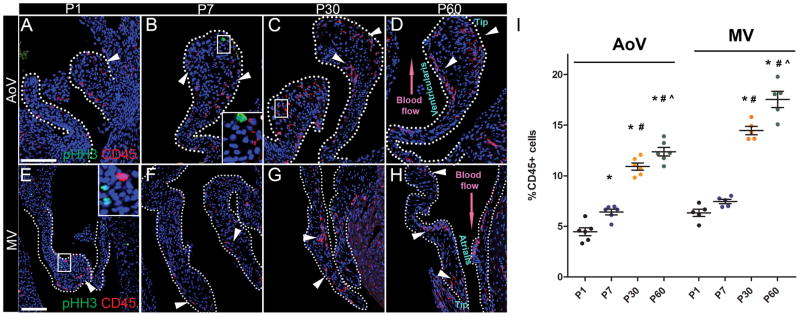
Previous studies have reported that leukocytes are present in adult mouse heart valves as indicated by CD45 expression3,4. Here, the presence of leukocytes in developing heart valves was examined by immunostaining for CD45 in neonatal and young adult heart valves at postnatal day 1 (P1), P7, P30, and P60. At birth, leukocytes represent about 5% of heart valve cells in aortic (AoV) and mitral (MV) valve leaflets (Fig. 1A, E). By 60 days, leukocyte numbers are increased to about 12% and 17% of cells in AoV and MV, respectively (Fig. 1D, H, I). Interestingly, the largest increase is observed between P7 and P30 when numbers of leukocytes double (Fig. 1I). To determine whether cell proliferation accounted for the observed increase in leukocytes, phospho-histone H3 (pHH3) staining was performed. Although some pHH3-positive cells (green) are detected in P1 (Fig. 1E) and P7 heart valves (Fig. 1B), pHH3 reactivity was not detected in CD45+ cells (red). At later stages, cell proliferation is rarely observed in juvenile (P30) (Fig. 1C, G) and young adult heart valves (P60) (Fig. 1D, H). Notably, pHH3 expression is not detected in CD45+ leukocytes at any stage. Together, these data indicate a postnatal increase of leukocytes in AoV and MV leaflets, which is not the result of leukocyte proliferation.
Figure 1. Leukocyte (CD45+) numbers are increased independent of cell proliferation during postnatal heart valve remodeling.
A – H: Representative pHH3 (green) and CD45 (red) immunostaining of P1, P7, P30 and P60 WT AoV sections and P1, P7, P30 and P60 WT MV sections. Arrowheads indicate CD45+ cells with undetectable pHH3 staining. White dashed outlines denote AoV or MV leaflets. High magnification of boxed insets are shown in B and E. I: The number of CD45 positive cells/total nuclei (%) was quantified in AoV leaflet sections (n=6) and MV leaflets section (n=5). Data are reported as dot plots where each dot represents a biological sample and the horizontal bar indicates the mean ± SEM. * p < 0.05 vs P1, # p < 0.05 vs P7 and ^ p < 0.05 vs P30 determined with one-way ANOVA. Scale bar =100μm.
In neonates and juveniles, CD45+ leukocytes are localized in two distinct parts of heart valve leaflets, similar to adult heart valves. One of the leukocyte populations is present subjacent to endothelial cells on the ventricularis side of the AoV commissure and similarly on the atrialis of the MV, adjacent to blood flow. The other concentration of leukocytes is located in the distal tip of AoV and MV leaflets (Fig. 1D, H). It is possible that these locations of enhanced biomechanical stress may be more amenable to leukocyte infiltration during the cardiac cycle.
Leukocytes do not contribute to endothelial and valve interstitial cell lineages
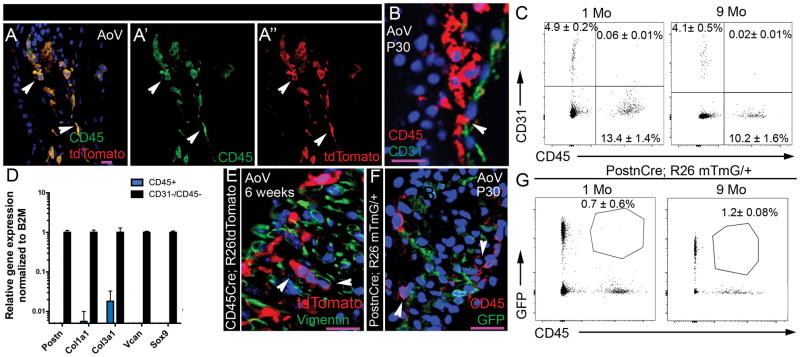
Heart valve leaflets are composed primarily of valve interstitial cells (VICs) surrounded by a layer of endothelial cells, and previous reports implicate hematopoietic-derived cells as a source of VICs3,4. To investigate whether CD45+ cells contribute to endothelial and/or interstitial cell populations in the heart valves, CD45-expressing cells were irreversibly labeled for cell lineage studies using CD45Cre;R26tdTomato reporter mice. Endogenous expression of CD45 was compared to CD45Cre-mediated tdTomato expression to determine if CD45+ lineage cells differentiate into CD45-negative valve interstitial or endothelial cells (Fig. 2A). Completely overlapping expression of tdTomato staining (Fig. 2A, A″) with CD45 immunostaining (Fig. 2A′) indicates that hematopoietic-derived cells maintain CD45 expression in juvenile heart valve leaflets. Although closely apposed to endothelial cells, CD45+ cells do not express CD31 and are not located in the endothelium (Fig. 2B). Flow cytometric analyses further confirmed that leukocytes (CD45+) and endothelial (CD31+) cells are distinct populations in juvenile (1Mo) and adult (9Mo) pooled AoV and MV leaflets (Fig. 2C). Only a minimal (0.06%) percentage of cells express both CD45 and CD31 and this number was not significantly changed with age (Fig. 2C). Thus, CD45+ lineage cells maintain CD45 expression in mature valve leaflets and do not contribute to significant numbers of valve endothelial cells.
Figure 2. Leukocytes do not contribute to significant numbers of endothelial and valve interstitial cell lineages.
A: Representative CD45 immunostaining and Cd45Cre-mediated dTomato expression of AoV sections from 6 weeks old CD45-Cre;R26tdTomato mouse. Note coincidence of CD45 immunostaining and RFP expression (yellow). A′: endogenous CD45 expression (green), A″: CD45Cre-mediated tdTomato expression (red). B: Representative CD45 and CD31 staining of P30 WT AoV. Arrowhead indicates that CD45+ cells are adjacent to but not within the endothelium. C: Flow cytometric analyses of CD31 and CD45 labeled cells from 1 and 9 months in pooled AoV and MV leaflet cell preparations. The percent of CD45+, CD31+ or CD45+;CD31+ cells among live gated cells is indicated (n=4 for each stage, mean ± SEM). D: Periostin (Postn), Collagen type 1a1 (Col1a1), Collagen type 3a1 (Col3a1), Versican (Vcan) and Sox9 expression was measured by qPCR in CD45+ cells and compared to expression in VICs (CD31-/CD45−) set to 1 from sorted cells of P30 AoV and MV leaflets (n=3, mean ± SEM). E: Representative vimentin (green) and tdTomato staining of AoV section from 6 weeks old CD45Cre;R26tdTomato mouse. Arrowheads indicate that CD45+ cells are not positive for vimentin. F: Representative CD45 (red) and GFP staining of AoV section from P30 PostnCre;R26mTmG/+ mouse. Arrowheads indicate that CD45+ cells are not of the PostnCre lineage. G: Flow cytometric analyses of CD45 and GFP labeled cells from 1 and 9 months AoV and MV leaflets of PostnCre;R26mTmG/+ mice. The percent of CD45+ cells that are GFP+ is shown (n=3, mean ± SEM). Scale bar =25μm.
CD45+ cells are localized in the valve interstitium, predominantly at the distal tips of developing AoV and MV. To determine if CD45+ cells exhibit ECM expression characteristic of VICs as previously reported3, cells from dissociated AoV and MV leaflets from 1-month-old mice were subjected to FACS to separate leukocytes (CD45+ cells) and VICs (CD45− and CD31− cells). Sorted CD45+ and CD45−;CD31− cell populations were analyzed for expression of genes encoding major ECM components of the heart valves, including, periostin (Postn), collagen type 1a1 and type 3a1 (Col1a1, Col3a1) and versican (Vcan), as well sox9, which encodes a VIC transcription factor, by qPCR. As expected, robust expression of VIC markers was detected in CD45−;CD31− cells (Fig. 2D). In contrast, CD45+ leukocytes express extremely low levels of Col3a1, and all other VIC genes are barely expressed in leukocytes. Absence of vimentin staining in CD45+ lineage cells (Fig. 2E) demonstrates further that leukocytes do not exhibit VIC properties. Lineage tracing of PostnCre expressing cells confirmed that CD45 is not expressed in Postn lineage cells in juvenile heart valve leaflets (Fig. 2F). Finally, flow cytometric analyses reaffirmed that Postn-expressing cells (GFP+) are distinct from leukocytes in 1-month and 9-month-old heart valve leaflets. Indeed, only 0.7% ± 0.6% of CD45+ cells express Postn, a percentage that is not significantly increased with age (Fig. 2G). Thus, leukocyte lineages are distinct from heart valve endothelial or interstitial cell populations during heart valve homeostasis.
Heart valve leukocytes are predominantly macrophages and dendritic cells
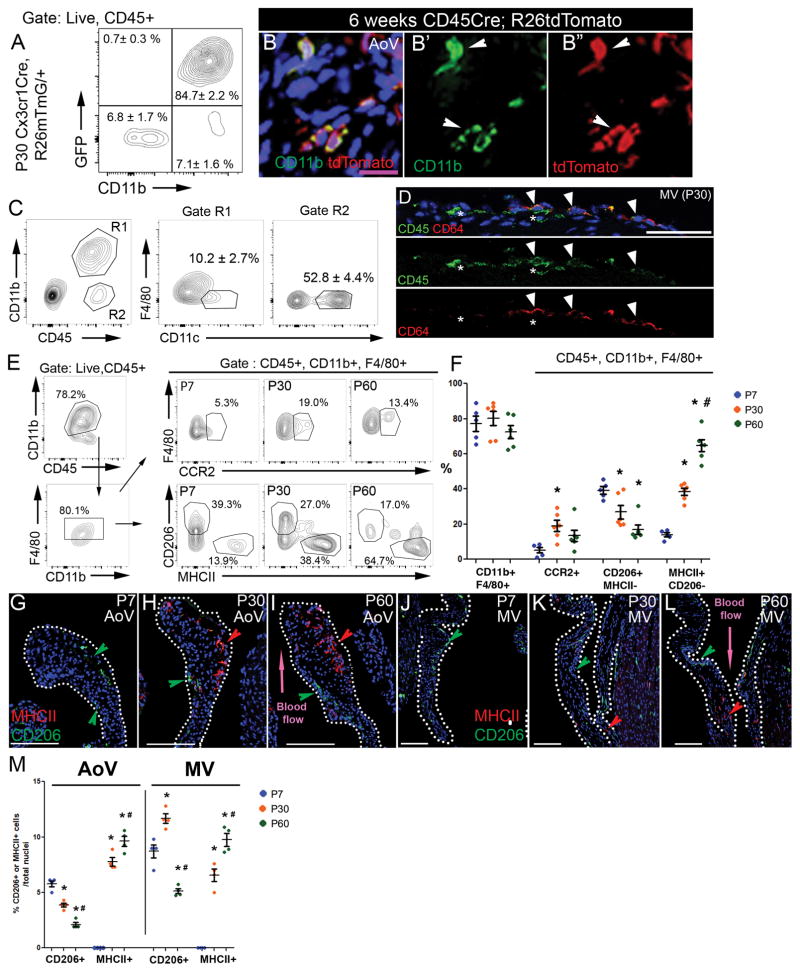
As CD45 is a pan-leukocyte marker, we used additional lineage markers to characterize which immune cell types are present in heart valves. The majority of leukocytes within the ventricles and conduction system atrioventricular node of mouse hearts express chemokine (C-X3-C motif) receptor 1 (Cx3cr1), an indicator of myeloid-derived lineages8,16. Similarly, upon examination of juvenile pooled AoV and MV leaflets from Cx3Cr1Cre;R26mTmG mice, the vast majority of CD45+ leukocytes (84.7± 2.2%) were GFP-positive, indicative of Cx3Cr1-expressing origin (Fig. 3A). Moreover, >99% of the GFP positive cells sorted from Cx3Cr1Cre;R26mTmG valve leaflets express CD11b, indicative of myeloid lineage identity (Fig. 3B′, 3B″).
Figure 3. Leukocytes are predominantly dendritic cells and myeloid cells, that include distinct CD206- and MHCII-expressing macrophage populations, in P30 heart valves.
A: Flow cytometric analyses of CD45, CD11b, and GFP labeled cells from P30 AoV and MV leaflets of Cx3cr1Cre;R26 mTmG/+ mice were used to calculate the percent expressing cells (n=6, mean ± SEM). B: Representative CD11b (green) and tdTomato staining of AoV section from 6 weeks old CD45-Cre;R26tdTomato mouse. B′: CD11b immunostaining, B″: CD45-driven expression of tdTomato (RFP). Arrowheads indicate double positive cells (yellow). C: Representative flow cytometric analyses with myeloid dendritic cells markers (CD11b+, CD11c+, F4/80−) and non-myeloid dendritic cells markers (CD11b−, CD11c+) from isolated cells of P30 AoV and MV WT leaflets (n=4, mean ± SEM). D: Representative immunostaining of CD64 (red) and CD45 (green) cells. Note CD64+ (arrowheads) and CD64− (asterisks) cells. E: Representative flow cytometric analyses with myeloid (CD11b, CCR2) and macrophage markers (F4/80, CD206, MHCII) from isolated single live cells of P7, P30, and P60 WT AoV and MV leaflets. F: The percent of indicated population averaged from n=5 (P7) or n=6 (P30, P60) AoV and MV leaflets is represented as a dot for each individual with mean ± SEM. G – L: Representative CD206 (red arrowhead) and MHCII (green arrowhead) immunostaining of P7, P30, and P60 AoV and MV sections. M: The number of CD206 or MHCII positive cells/total nuclei was quantified in AoV leaflet sections (n=4) and MV leaflet sections (n=4) at P7, P30, and P60. * p < 0.05 vs P7 and # p < 0.05 vs P30 determined with one-way ANOVA. Purple scale bar =25μm, white scale bar =100μm.
As dendritic cells have been observed in heart valves17, cells from P30 valve leaflets were sorted to further delineate myeloid subsets (Fig 3C). First, live single cells were gated for CD45 and CD11b (Supplemental Fig. I) to distinguish myeloid (Fig. 3C, Gate R1) from non-myeloid cells (Fig. 3C, Gate R2). Within the non-myeloid CD11b- compartment, dendritic cells account for more than 50% of the cells (Fig. 3C, Gate R2), while the remainder contribute to other immune cell populations, such as lymphocytes. Among CD11b+ myeloid cells, only 10.2 ± 2.7% were dendritic cells (CD11c+; F4/80−) (Fig. 3C, Gate R1). The remainder consisted of macrophages (F4/80+, CD11c+), further supported by the presence of CD64+ cells by immunohistochemistry (Fig. 3D). Thus, myeloid lineage cells in P30 valves consist of both macrophages (more than 60% of CD45+ cells) and dendritic cells (approximately 20% of CD45+ for both myeloid and non-myeloid dendritic cells).
Flow cytometric analysis was performed on living singlet cell preparations gated on CD45 and CD11b (Supplemental Fig. I) to evaluate the macrophage cell types in pooled P7, P30 or P60 AoV and MV leaflets. At all stages, approximately 80% of leukocytes were labeled with CD11b, among which 80% were double positive for macrophage markers, CD11b and F4/80 (Fig. 3E, F), which is comparable to immunostaining. Interestingly, the percent of macrophages (CD45+, CD11b+, F4/80+) present in heart valves was similar from P7 to P60 (Fig. 3F). However, the percent of recruited macrophages (F4/80+, CCR2+) was significantly increased between P7 and P30 (Fig. 3E, F), the period with the highest increase of leukocyte number in heart valves (Fig 1I). The F4/80+ macrophage population is heterogeneous, composed predominantly of CD206+ cells at P7 and an MHCII+ population that increases with age (Fig. 3E, F). The age-dependent differences in macrophage cell types were confirmed by immunohistochemistry, whereby both the AoV and MV exhibited a switch from a predominant CD206+ macrophage population at P7 to a more heterogeneous population at P30, consisting of both CD206+ and MHCII+ subtypes (Fig. 3G–L). MV leaflets also exhibited a higher percent of CD206+ macrophages than that of AoV (Fig. 3M). Interestingly, these two macrophage subtypes display specific localization in AoV, with CD206+ cells located on the flow side, while MHCII+ cells are mostly found in the tip of adult P60 AoV leaflet (Fig. 3I, L). Although other minor immune cell populations, such as lymphocytes and neutrophils, remain to be characterized, macrophages and dendritic cells account for most of the leukocytes found in heart valve leaflets.
Pro-inflammatory ECM and macrophage numbers are increased in mouse and human myxomatous heart valves
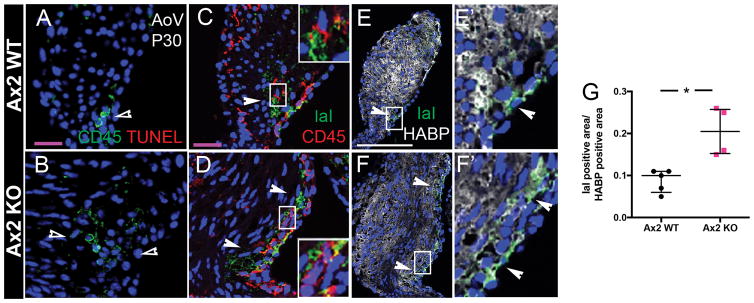
Myxomatous heart valve disease is characterized by increased deposition of GAGs, namely hyaluronan (HA), in addition to increased numbers of leukocytes and macrophages in humans2 and mice6,7. In inflammatory disease, HA is bound covalently by the heavy chain (HC) of inter-α-trypsin inhibitor (IαI) giving rise to the HC-HA complex, which functions to attract and sequester immune cells18. Axin2 KO mice display thickened heart valves with increased leukocytes7 and no evidence of cell death by TUNEL (Fig. 4A, B). IαI staining and CD45+ cells are colocalized on the flow side of Axin2 WT leaflets (Fig. 4C), and both are expanded in myxomatous AoV leaflets from Axin2 KO mice (Fig. 4D, arrowheads). The ratio of IαI to HA area is increased in Axin2 KO valves (Fig. 4E, F, G), confirming quantitatively that HC-HA complexes are increased in myxomatous leaflets.
Figure 4. Increased leukocytes and macrophages localized to regions of immunogenic hyaluronic acid remodeling in myxomatous Axin2 KO AoV.
A, B: Representative CD45 immunostaining (green) and TUNEL staining (red) of AoV section from P30 Axin2 WT and KO mice, respectively. Empty arrowheads indicate positive CD45 staining but no TUNEL labelling. C, D: Representative IαI (green) and CD45 (red) immunostaining of P30 Axin2 WT and KO AoV. Arrowheads indicate colocalization of CD45+ cells with IαI positive areas on the flow side of leaflets. E, F: Representative IαI (green) and hyaluronan binding protein (HABP; white) immunostaining of P30 Axin2 WT and KO AoV with high magnification of regions indicated by boxes (E′ F′). Arrowheads indicate IαI positive area. G: IαI positive area was measured as positive pixels and reported relative to the HABP positive area in P30 Axin2 WT (n=5) and KO (n=4) AoV leaflets. Data are reported as dot plots and interquartile ranges, where each dot represents a mouse and the horizontal bar indicates the median. * p < 0.05 determined with Mann-Whitney U test. Purple scale bar =25μm. Scale bar =100μm.
While there is accumulating evidence that human myxomatous mitral valves exhibit increased CD45 leukocytes2, specific leukocyte lineages and potential factors promoting immune cell infiltration in diseased valves are not known. The presence of pro-inflammatory ECM remodeling and macrophages was examined in human myxomatous and control mitral valves harvested and processed as previously described19. In myxomatous valves, characteristic loss of tri-laminar organization, as well as increased deposition of GAGs interspersed with fibrillar collagen, were apparent in comparison to control valve tissue as detected by Movat’s pentachrome staining (Fig. 5A, B). Relative to controls, proteoglycan expression, indicated by HAPB, is increased with MVD, as is IαI indicative of modified immunogenic ECM (Fig. 5A′, B′, C). Likewise, macrophages, as defined by CD6420, also are significantly increased in human MVD and are localized to regions of pro-inflammatory ECM remodeling (Fig 5A″, B″, D). The colocalization of increased macrophages with increased HABP and IαI expression in human MVD supports a common mechanism of myxomatous degeneration in mouse models, such as Axin2 KO mice, and human valve disease.
Figure 5. Human myxomatous mitral valves exhibit increased CD64+ macrophages localized to regions of immunogenic hyaluronic acid remodeling.
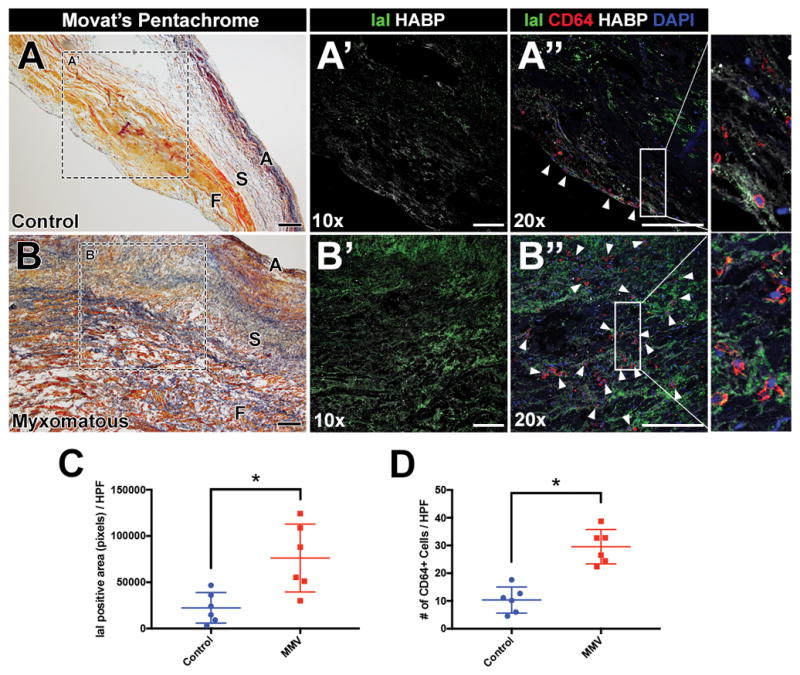
A, B: Representative human posterior leaflets of control and myxomatous mitral valves stained with Movat’s pentachrome. Valve thickening and disruption of normal stratification of matrix boundaries are observed in myxomatous leaflets versus control. A′, B′: Representative immunostaining of immunogenic hyaluronic acid remodeling (IαI; green) and hyaluronic acid binding protein (HABP; white) at 10× objective magnification. A″, B″: Representative immunostaining of IαI (green), HABP (white), and CD64 macrophages (red; arrowheads) at 20× objective magnification with magnification of boxed regions. C, D: Quantification of IαI positive area (pixels) or numbers of CD64 macrophages per high-power field (HPF) in control versus myxomatous mitral valves (n=6 per group). Data are reported as dot plots where each dot represents a biological sample and the horizontal bar indicates the mean+SD. *p < 0.05 determined with Mann-Whitney U test. A = Atrialis; S = Spongiosa; F = Fibrosa. Scale bar = 200μm
Identification of macrophage subsets in Axin2 KO mouse myxomatous heart valves
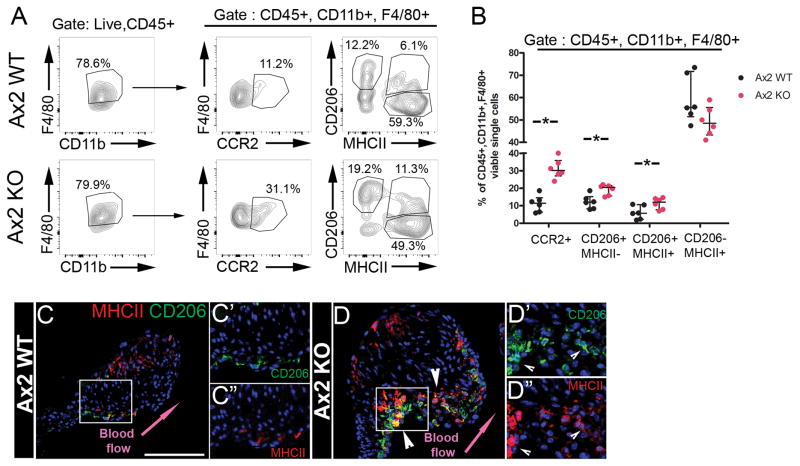
Infiltrating macrophages are predominant in sterile injury response in atherosclerosis and heart failure9, and chemokine expression is induced at early stages of MVD in Axin2 KO mice7. The presence of macrophages expressing the CCR2 monocyte surface receptor was evaluated as an indicator of myeloid cell infiltration in response to chemokine expression during MVD 21. Flow cytometric analyses of single live macrophages (CD45+, CD11b+ and F4/80+) isolated from P30 Axin2 WT and KO pooled AoV/MV leaflets (Fig. 6A) indicate a significant increase of macrophages with pro-inflammatory markers (CD45+, CD11b+, F4/80+, CCR2+) in myxomatous AoV/MV leaflets (Fig. 6B). Interestingly, the CD206+;MHCII− macrophage subset also is significantly increased in myxomatous valves, whereas the CD206-;MHCII+ macrophage subset is not changed (Fig. 6A, B). Moreover, a third smaller CD206+;MHCII+ macrophage subset present in WT leaflets also is significantly increased in myxomatous Axin2 KO leaflets (Fig. 6B). Immunostaining confirms the increased numbers of CD206+ and CD206+;MHCII+ double positive cells (Fig. 6C, D) on the flow side of the aortic valve leaflets (arrowhead in Fig. 6D), where HC-HA complexes also are localized (Fig. 4D). Altogether, these data indicate an increase of infiltrating CCR2+ monocytes and expansion of CD206+ macrophage subsets in regions of immunogenic ECM remodeling in myxomatous valve leaflets.
Figure 6. Infiltrating CCR2+ monocytes and CD206-expressing macrophage subsets are increased in P30 myxomatous aortic valves.
A: Representative flow cytometric analyses with myeloid (CD11b), infiltrating monocyte (CCR2) and macrophage markers (F4/80, CD206, MHCII) of isolated cells of pooled AoV and MV leaflets of P30 Axin2 WT or KO mice. B: The percent of indicated populations from single live CD45+, CD11b+ and F4/80+ cells from P30 Axin2 WT (n=6) and KO (n=6) mice. C, D: Representative CD206 (red) and MHCII (green) with overlay (yellow) immunostaining of P30 Axin2 WT and KO AoV, respectively. Arrowheads indicate increased numbers and expanded area of CD206 positive and double CD206;MHCII positive cells. C′,C′, D′, D′: insets displaying individual CD206 or MHCII staining. Arrowheads indicate double positive CD206+;MHCII+ (yellow) cells. Data are reported as dot plots and interquartile ranges, where each dot represents one sample of AoV and MV leaflets pooled from 3 to 5 mice, and the horizontal bar indicates the median. * p < 0.05 determined with Mann-Whitney U test. Scale bar =100μm.
Discussion
Here, we show that leukocytes are present in murine heart valves and that their numbers increase during the first weeks after birth when valve leaflets undergo physiological ECM remodeling. These leukocytes are mostly non-myeloid dendritic cells (CD11b−, CD11c+) and myeloid cells (CD11b+) composed of 2 major macrophage (F4/80+) subsets, expressing CD206+ or MHCII+, and a small population of myeloid dendritic cells. Between P7 and P30, CCR2+ macrophages are significantly increased, indicating leukocyte infiltration of valves after birth. During heart valve homeostasis, leukocytes do not express major VIC ECM protein genes and are distinct from endothelial cells. In myxomatous valve disease in mice and humans, increased numbers of macrophages are present in regions of proteoglycan enrichment and immunogenic HA remodeling. Moreover, infiltrating CCR2+ monocyte numbers increase, along with expansion of CD206+ macrophage subsets in myxomatous valves of Axin2 KO mice. Together, these studies demonstrate alterations in macrophage lineage heterogeneity in heart valve development and myxomatous disease. Future studies are needed to determine potential roles for tissue-resident and infiltrating macrophages in normal valve remodeling and homeostasis, as well as specific contributions to MVD progression.
The presence of CD206+ macrophages in heart valves at birth until at least P7 is consistent with tissue-resident macrophages derived from embryonic progenitors originating in the yolk sac or fetal liver22. Between P7 and P30, when definitive hematopoiesis is established, macrophage subtypes shift most dramatically with a rise and differentiation of a CD206-;MHCII+ macrophage subset. This is accompanied by a decreased proportion of resident macrophages (CD206+;MHCII−), similar to macrophage transitions in the developing myocardium10. At the same time, heart valves undergo elongation and ECM remodeling, leading to maturation of trilaminar leaflets in the month after birth. Interestingly, leukocyte subpopulations are localized at the valve leaflet commissures and distal tips, regions of enhanced biomechanical stress, that could lead to adhesion and differentiation of macrophages23. Targeted manipulation of monocyte and macrophage subpopulations is needed to definitively determine if these infiltrating cells contribute to heart valve maturation after birth.
Although their functions in adult heart valve homeostasis are not known, it has been proposed that circulating bone marrow-derived CD45+ cells contribute to VICs or VECs3,4. Here, we show that leukocytes in adult heart valves maintain CD45+ expression and do not express VIC marker genes or the fibroblast proteins, vimentin and periostin. Moreover, leukocytes do not appreciably overlap with PostnCre lineage cells or CD31+ cells (<1%). These data are in contrast to previous reports of infiltrating bone marrow-derived cells contributing to resident VICs, based primarily on cell morphology and expression of ECM proteins4. The CD45+ hematopoietic cells, including multiple macrophage subtypes, are in close proximity to valve endothelial and interstitial cells, but their specific functions in ECM remodeling, repair, and homeostasis throughout life remain to be elucidated.
In myxomatous valve disease, progressive leaflet thickening and ECM dysregulation lead to long-term compromised function requiring surgical replacement or repair24. Damaged endothelial cells25 and increased expression of interleukins and chemokines, as detected in Axin2 KO mice7 and human myxomatous mitral valves5, most likely attract and promote infiltration of monocytes in diseased heart valves. In addition, hyaluronan accumulation and HC-HA complex formation may also attract leukocytes and are pro-inflammatory. The transfer of HCs from IαI to HA is catalyzed by tumor necrosis factor alpha induced protein 6 (Tnfaip6)18, which is overexpressed in human myxomatous mitral valves5 and in calcified aortic valves26, further supporting a potential role in macrophage recruitment. An active role for immune cells in human myxomatous valve disease is suspected, since macrophages are increased in regions of IαI expression in both mouse and human MVD. In addition, the increased numbers of CD206-expressing macrophages detected in mouse MVD could have a role in promoting tissue repair and regeneration, as has been demonstrated in liver27 and heart10,11. Together our studies demonstrate the heterogeneity and lineage diversity transitions of macrophages in heart valves during development and disease. Additional research is needed to determine the specific contributions of macrophage subsets to heart valve homeostasis and remodeling, as well as to determine if they are positive or negative targets for development of new therapeutic approaches in treatment or management of heart valve disease.
Supplementary Material
Highlights.
Leukocytes do not directly contribute to heart valve endothelial or interstitial cells in homeostasis
Most leukocytes present in heart valve leaflets are macrophages or dendritic cells.
Distinct macrophage subsets are differentially localized in heart valve leaflets.
Immunogenic ECM and macrophages are increased in human and mouse MVD.
CD206-expressing macrophages are increased in myxomatous aortic valves.
Acknowledgments
We thank Ronald J. Vagnozzi for scientific advice, Monica DeLay, Alexandra Dias and Tim Hubbell from the Research Flow Cytometry Core (CCHMC) for their technical support. We thank Aaron Petrey and Carol de la Motte (CCF) for sharing IαI-antibody. We thank Alain Colige from Laboratory of Connective Tissues Biology (GIGA, Sart-Tilman, Belgium) and M. Radermecker from Department of Cardiovascular and Thoracic Surgery and Human Anatomy (CHU-Sart-Tilman, Belgium) for sharing human mitral valve samples.
Funding
This work was supported by NIH R01 HL094319 (KEY), R01 HL127033 (JL), R35 GM119458 (TD), American Association of Anatomists Postdoctoral Fellowship Award (AH), and American Heart Association Predoctoral Fellowships 16PRE30180000 (AJK) and PRE19880008 (LJA).
Abbreviations
- AoV
Aortic Valve
- ECM
Extracellular Matrix
- HA
Hyaluronan
- IαI
Inter-α-trypsin inhibitor
- MVD
Myxomatous Valve Disease
- MV
Mitral Valve
- VEC
Valve Endothelial Cell
- VIC
Valve Interstitial Cell
Footnotes
Disclosures
None
References
- 1.Hinton RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 2.Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, Hashim S, Tellides G. Modulation of Transforming Growth Factor- Signaling and Extracellular Matrix Production in Myxomatous Mitral Valves by Angiotensin II Receptor Blockers. Circulation. 2012;126:S189–S197. doi: 10.1161/CIRCULATIONAHA.111.082610. [DOI] [PubMed] [Google Scholar]
- 3.Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An In Vivo Analysis of Hematopoietic Stem Cell Potential: Hematopoietic Origin of Cardiac Valve Interstitial Cells. Circ Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 4.Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–965. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thalji NM, Hagler MA, Zhang H, Casaclang-Verzosa G, Nair AA, Suri RM, Miller JD. Nonbiased Molecular Screening Identifies Novel Molecular Regulators of Fibrogenic and Proliferative Signaling in Myxomatous Mitral Valve Disease. Circ Cardiovasc Genet. 2015;8:516–528. doi: 10.1161/CIRCGENETICS.114.000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauls K, Toomer K, Williams K, Johnson A, Markwald R, Hajdu Z, Norris R. Increased Infiltration of Extra-Cardiac Cells in Myxomatous Valve Disease. J Cardiovasc Dev Dis. 2015;2:200–213. doi: 10.3390/jcdd2030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulin A, Moore V, James JM, Yutzey KE. Loss of Axin2 results in impaired heart valve maturation and subsequent myxomatous valve disease. Cardiovasc Res. 2017;113:40–51. doi: 10.1093/cvr/cvw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, Rodewald H-R, Rosenthal NA, Bajenoff M, Prinz M, Jung S, Sieweke MH. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swirski FK, Robbins CS, Nahrendorf M. Development and Function of Arterial and Cardiac Macrophages. Trends Immunol. 2016;37:32–40. doi: 10.1016/j.it.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K, Suzuki K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126:2151–2166. doi: 10.1172/JCI85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varol C, Mildner A, Jung S. Macrophages: Development and Tissue Specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 13.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannella KM, Wynn TA. Mechanisms of Organ Injury and Repair by Macrophages. Annu Rev Physiol. 2016;10:1–25. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 15.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Silva N, Da Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell. 2017;169:510–522. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J-H, Do Y, Cheong C, Koh H, Boscardin SB, Oh Y-S, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulin A, Deroanne CF, Lambert CA, Dumont B, Castronovo V, Defraigne J-O, Nusgens BV, Radermecker MA, Colige AC. Metallothionein-dependent up-regulation of TGF-β2 participates in the remodelling of the myxomatous mitral valve. Cardiovasc Res. 2012;93:480–489. doi: 10.1093/cvr/cvr337. [DOI] [PubMed] [Google Scholar]
- 20.Beyer M, Mallmann MR, Xue J, Staratschek-Jox A, Vorholt D, Krebs W, Sommer D, Sander J, Mertens C, Nino-Castro A, Schmidt SV, Schultze JL. High-Resolution Transcriptome of Human Macrophages. PLoS One. 2012;7:e45466. doi: 10.1371/journal.pone.0045466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72:1303–1316. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine RA, Hagége AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez-Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Mérot J, Messas E, Milan DP, Neri T, et al. Mitral valve disease-morphology and mechanisms. Nat Rev Cardiol. 2015;12:689–710. doi: 10.1038/nrcardio.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirzaie M, Meyer T, Schwarz P, Lotfi S, Rastan A, Schöndube F. Ultrastructural alterations in acquired aortic and mitral valve disease as revealed by scanning and transmission electron microscopical investigations. Ann Thorac Cardiovasc Surg. 2002;8:24–30. [PubMed] [Google Scholar]
- 26.Krishnamurthy VK, Stout AJ, Sapp MC, Matuska B, Lauer ME, Grande-Allen KJ. Dysregulation of hyaluronan homeostasis during aortic valve disease. Matrix Biol. 2017;62:40–57. doi: 10.1016/j.matbio.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.