Abstract
Objective
Fibronectin containing extra domain A (Fn-EDA) is an endogenous ligand of toll like-receptor 4 (TLR4) and is abundant in the extracellular matrix (ECM) of advanced atherosclerotic lesions in human and mice. Irrespective of gender, deletion of Fn-EDA reduces early atherosclerosis in apolipoprotein-deficient (Apoe−/−) mice. However, the contribution of Fn-EDA in advanced atherosclerosis remains poorly characterized. We determined the contribution of Fn-EDA in advanced atherosclerotic lesions of aged (1-year-old) Apoe−/− mice.
Approach and Results
Plaque composition was determined in the innominate artery, a plaque instability site that is known to mimic several histological features of vulnerable human plaques. Female Apoe−/−, Fn-EDA−/−Apoe−/−, TLR4−/−Apoe−/− and Fn-EDA−/−TLR4−/−Apoe−/− mice were fed a high-fat “Western” diet for 44 weeks. Fn-EDA−/−Apoe−/− mice exhibited reduced plaque size characterized by smaller necrotic cores, thick fibrous caps containing abundant vascular smooth muscle cells (VSMCs) and collagen, reduced CD68/MMP9-positive content, less accumulation of MMP-cleaved ECM aggrecan, and decreased VSMC and macrophage apoptosis (P<0.05 versus Apoe−/− mice). Together these findings suggest that Fn-EDA induces plaque destabilization. Deletion of TLR4 reduced histological features of plaque instability in Apoe−/− mice but did not further reduce features of plaque destabilization in Fn-EDA−/−Apoe−/− mice, suggesting that TLR4 may contribute to Fn-EDA-induced plaque destabilization. Fn-EDA potentiated TLR4-dependent MMP9 expression in bone marrow-derived macrophages, suggesting that macrophage TLR4 may contribute to Fn-EDA-mediated plaque instability.
Conclusions
Fn-EDA induces histological features of plaque instability in established lesions of aged Apoe−/− mice. The abundance of Fn-EDA in advanced atherosclerotic lesions may increase the risk of plaque destabilization.
Keywords: Fibronectin-EDA, plaque destabilization, innominate artery, advanced atherosclerosis
Subject code list: Atherosclerosis: [145] Genetically altered mice, Basic science research
Introduction
Despite recent advances, the molecular and cellular mechanisms that contribute to atherosclerotic plaque instability/vulnerability are not entirely understood. Studies have found that extracellular matrix (ECM) remodeling takes place during atherosclerotic plaque progression.1 In healthy human arteries, the endothelium resides on an ECM that is mainly composed of collagen type IV and laminin, with largely devoid of cellular fibronectin containing extra domain A (Fn-EDA).2–4 In contrast, the ECM of atherosclerotic arteries is enriched in Fn-EDA in both humans and mice.2–4
Fn plays a pivotal role in several cellular and biological processes.5 Fn gene undergoes alternative splicing at three sites:6 Extra Domain A (EDA), Extra Domain B (EDB), and the Type III Homologies Connecting Segment (IIICS).6 FN exists in two forms in humans and mice: 1) Plasma Fn, which lacks the alternatively spliced EDA and EDB and is synthesized by hepatocytes; 2) Cellular Fn (cFn), which contains either the EDA and EDB, or both. cFn is synthesized by fibroblasts and other cells, including endothelial cells and vascular smooth muscle cells (VSMCs).5 Alternative splicing of EDA and EDB is developmentally regulated. Inclusion of EDA and EDB in cFn increases during embryonic development, but decreases substantially after birth.7, 8 Fn-EDA plays a role in several biological and pathological processes, including wound healing,9, 10 vascular hypertension,11 and fibrotic disorders of the lung, liver, and skin,12–14
Previously, we and others have reported that deletion of the EDA exon of the FN gene reduces early atherosclerotic plaque progression, suggesting a role for Fn-EDA in progression of early atherosclerosis.15–17 The contribution of Fn-EDA in advanced human atherosclerosis remains poorly characterized.18 A murine study suggested that abundant Fn-EDA expression may paradoxically stabilize advanced plaques,17 In contrast, several lines of evidence support the hypothesis that increased Fn-EDA in advanced atherosclerotic plaques may induce plaque destabilization by potentiating the toll like-receptor 4 (TLR4) innate immune signaling pathway in macrophages or other cells: 1) Fn-EDA is an endogenous ligand of TLR4 19 that colocalizes with macrophage TLR4 in human and murine atherosclerotic plaques.16 2) TLR4 contributes to Fn-EDA-mediated early atherosclerosis exacerbation in 5-month old male and female Apoe−/− mice.16 3) Fn-EDA promotes MMP9 gene activity in THP-1 19 and synovial cells,20 and MMP9 is known to induce histological features of plaque destabilization.21
In the current study, we used aged (1-year-old) Apoe−/−, Fn-EDA−/−Apoe−/−, TLR4−/−Apoe−/− and Fn-EDA−/−TLR4−/−Apoe−/− mice to determine the contribution of the Fn-EDA/TLR4 axis in advanced atherosclerotic lesions. Histological features of plaque composition were studied in the innominate artery, a well-established site of plaque instability that is known to exhibit histological and gene expression characteristics similar to vulnerable human plaques.22, 23 We found that Fn-EDA induces features of plaque destabilization in advanced lesions of the innominate artery.
Materials and Methods
Materials and Methods are available in the online-only supplement.
Results
Fn-EDA/TLR4 axis induces histological features of plaque destabilization in the innominate artery
Previously, we have found that Fn-EDA contributes to early atherosclerosis exacerbation both in male and female mice.16 Because Fn-EDA-mediated early atherosclerosis exacerbation was gender-independent, herein, we used only female mice to determine the contribution of Fn-EDA/TLR4 axis in advanced atherosclerotic progression. Apoe−/−, Fn-EDA−/−Apoe−/−, TLR4−/−Apoe−/− and Fn-EDA−/−TLR4−/−Apoe−/− mice were fed a high-fat “Western” diet for 44 weeks. Irrespective of the genotype, we found that approximately 35–40% mice died after 36 weeks on high-fat “Western diet” in all the groups (Table 1). The cause of premature death remains unclear. In the mice that survived up to 50 weeks of age, plasma cholesterol and triglycerides were comparable among groups (Table 1). Plasma levels of pro-inflammatory cytokines, including IL-1β and TNFα, which are known to be elevated in atherosclerotic lesions, were similar among groups (Table 1). Deletion of Fn-EDA or TLR4 in Apoe−/− mice did not affect body weight or complete blood counts (not shown), which are in agreement with previous studies.16
Table 1.
Comparison of total cholesterol, triglyceride levels, inflammatory cytokines in the plasma and lesion size in female aged mice fed a high-fat “Western” diet for 44 weeks.
| Apoe−/− | Fn-EDA−/−Apoe−/− | TLR4−/−Apoe−/− | Fn-EDA−/− TLR4−/−Apoe−/− | |
|---|---|---|---|---|
| Premature death | 7/19 | 6/18 | 8/19 | 7/19 |
| Cholesterol (mg/dl) | 1087±183.6 | 1324±117.9 | 1190±176.5 | 1128±148.5 |
| Triglyceride (mg/dl) | 165.8±48.32 | 151.52±40.63 | 145.6±60.2 | 161.8±55.7 |
| TNF-α (ng/ml) | 45.12±6.91 | 31.88±5.35 | 31.08±3.84 | 30.36±6.76 |
| IL1-β (ng/ml) | 50.68±20.94 | 42.83±29.57 | 52.76±19.86 | 42.34±53.94 |
| Lesion area ×103 (μm2) | 305.73±67.47 | 232.64±33.73* | 205.31±38.2* | 197.96±36.65* |
| Lumen area ×103 (μm2) | 142.77±42.9 | 220.42±34.78* | 227.4±60.5* | 234.66±56.41* |
| Vessel area X103 (μm2) | 423.49±52.73 | 457.20±43.31 | 432.71±78.59 | 472.59±91.26 |
Values are represented as means ± SD. N=11–12/group.
P<0.05 versus Apoe−/− mice. Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test.
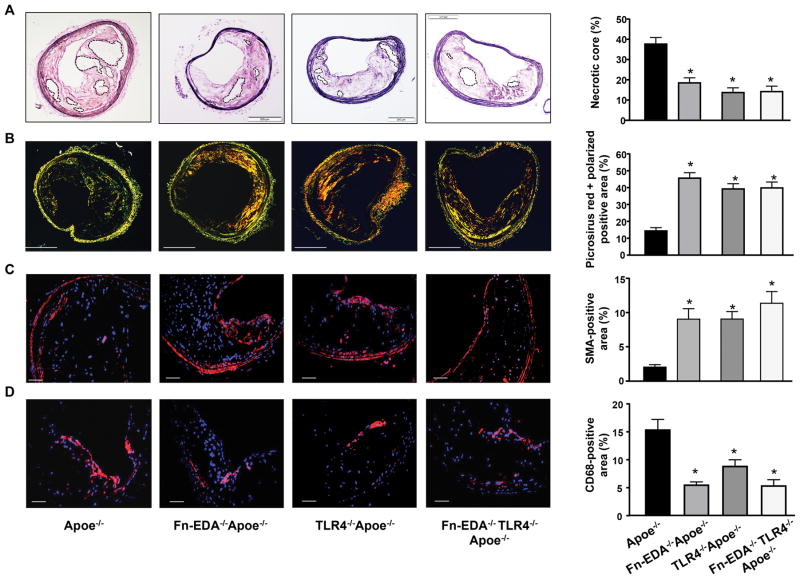
We next analyzed plaque size and composition by histology (explained in Figure SI) and immunohistochemistry. Compared to Apoe−/− mice, Fn-EDA−/−Apoe−/− mice exhibited reduced total lesion area (Table 1) characterized by smaller necrotic core area and increased VSMC (SMA positive-cells) and collagen content (picrosirius staining) (P<0.05, Figure 1A–C). We have observed that the content of CD68-positive monocytes/macrophages in 1-year-old Apoe−/− mice is markedly (but not completely) less when compared to atherosclerotic lesions in 5-month-old Apoe−/− mice (not shown). The accumulation of CD68-positive cells was significantly reduced in Fn-EDA−/−Apoe−/− mice when compared to Apoe−/− mice (P<0.05, Figure 1D). Higher magnification images showed CD68-positive cells lining the lateral xanthoma (Figure SII). Moreover, CD68-positive cells were abundant in the necrotic core and ECM surrounding the necrotic core (Figure SIII). To determine whether the decrease in CD68-positive monocyte/macrophage cells within lesions was due to a reduction in macrophage recruitment or macrophage proliferation, we quantified Ki67 (a marker of proliferation) and CD68-positive cells within the lesion. The number of CD68/Ki67-positive cells was comparable between groups (Figure SIV) suggesting that ECM rich in Fn-EDA does not influence macrophage proliferation, but may affect monocyte recruitment. We next characterized CD68-positive monocytes/macrophages and mannose receptor (MR)-positive macrophages within lesions. MR-positive area was comparable among genotypes. The ratio of CD68 positive area to MR-positive area was reduced in Fn-EDA−/−Apoe−/− mice compared to Apoe−/− mice (P<0.05, Figure SV).
Figure 1.
Fn-EDA/TLR4 axis promotes histological features of plaque destabilization in the innominate artery of aged Apoe−/− mice. All mice were females and fed a high-fat “Western” diet for 44 weeks starting at the age of 6 weeks. Left side shows representative cross-sections of lesions. A, Necrotic area (demarcated by dashed lines). B, Collagen content (Picrosirus red) as analyzed by polarization microscopy. C, VSMCs (SMA-positive). D, Monocytes/macrophages (CD68-positive). Right side shows quantification of necrotic area, picrosirus red polarized-positive area, SMA-positive area and CD68-positive area. Quantification was calculated as a percent of total plaque area. Data represent mean ± SEM. Value for each mouse represents a mean of 4 serial sections (each section approximately 60 μm apart). *P<0.05 versus Apoe−/− mice. N=11–12 mice/group. Statistical analysis: Figure 1A, C, and D; Parametric one-way ANOVA followed by Sidak’s multiple comparisons test, Figure 1B; Kruskal-Wallis test followed by uncorrected Dunn’s test. Scale bar= A & B; 200 μm, C & D; 50 μm.
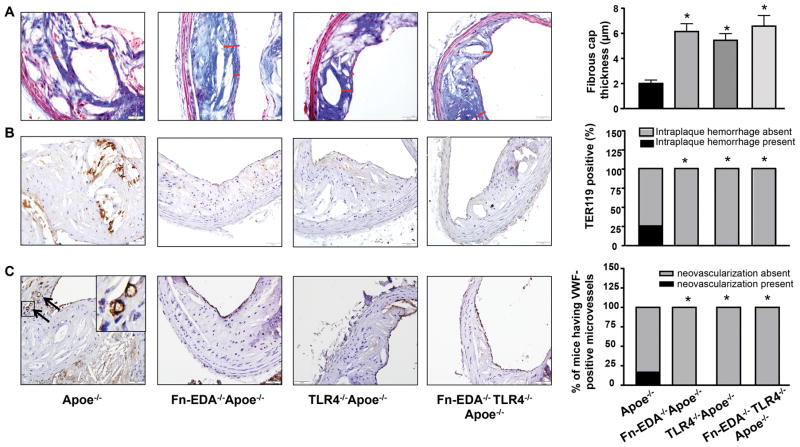
We next analyzed other histological features of plaque destabilization, including calcification, thrombus in the lumen, intraplaque hemorrhage, fibrous cap thickness, fibrous cap breaks and neovascularization. Calcification was comparable in the lesions of Fn-EDA−/−Apoe−/− mice and Apoe−/− mice (Figure SVI). Thicker fibrous caps were present around the necrotic cores in Fn-EDA−/−Apoe−/− mice compared to Apoe−/− mice (Figure 2A). Although thrombus in the lumen of the innominate artery was absent in both groups, intraplaque hemorrhage (TER119-positive) was observed in 25% (3/12) of Apoe−/− mice, but not in Fn-EDA−/−Apoe−/− mice (Figure 2B). Occasionally fibrous cap breaks were found in Apoe−/− mice (3/12) and not in Fn-EDA−/−Apoe−/− mice, but they were not associated with thrombus or intraplaque hemorrhage (Figure SII). Neovascularization (oval and VWF-positive) was only observed in Apoe−/− mice (2/12); however, it was absent in Fn-EDA−/−Apoe−/− mice (Figure 2C). Since Fn-EDA forms fibrous networks, we speculated that Fn-EDA might influence total Fn deposition within the plaques. To our surprise, total Fn deposition was comparable between Apoe−/− and Fn-EDA−/−Apoe−/− mice (Figure SVII). Together, these results suggest that Fn-EDA induces histological features of plaque destabilization in advanced lesions of Apoe−/− mice.
Figure 2.
Fibrous cap, intraplaque hemorrhage, and neovascularization in advanced innominate artery lesions of aged Apoe−/− mice. Left side shows representative cross-sections of lesions. A, Fibrous cap (demarcated by the red line) was calculated as the thickness of fibrous tissue overlaying the necrotic core. B, Intraplaque hemorrhage (TER119-positive RBCs). C, Neovascularization (VWF-positive, indicated by arrow). Insert shows higher magnification image. Right side shows quantification of fibrous cap, % incidence of mice having intraplaque hemorrhage and neovascularization within plaques. Data represent mean ± SEM. Value for each mouse represents a mean from 4 serial sections (each section approximately 60 μm apart). *P<0.05 versus Apoe−/− mice. N=11–12 mice/group. Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test (Figure 2A) and Fisher exact test (Figure 2B & 2C).
Because Fn-EDA is an endogenous ligand for TLR4, we hypothesized that TLR4 might contribute to Fn-EDA-mediated plaque destabilization. TLR4−/−Apoe−/− mice showed reduced plaque size (Table 1) characterized by reduced necrotic core area accompanied by increased collagen and vascular SMC content, thick fibrous caps, reduced CD68-positive monocyte/macrophage content, and absence of intraplaque hemorrhage and neovascularization (P<0.05 versus Apoe−/− mice, Figure 1 & 2). We next compared atherosclerotic lesions in Fn-EDA−/−Apoe−/− and Fn-EDA−/−TLR4−/−Apoe−/− mice, to determine whether the reduced features of plaque destabilization in Fn-EDA−/−Apoe−/− mice is mediated via TLR4. Lesion areas and histological features as mentioned above were comparable between Fn-EDA−/−TLR4−/−Apoe−/− mice and Fn-EDA−/−Apoe−/− mice (Figure 1 & 2 and Table 1), suggesting that TLR4 may contribute to Fn-EDA-mediated plaque destabilization.
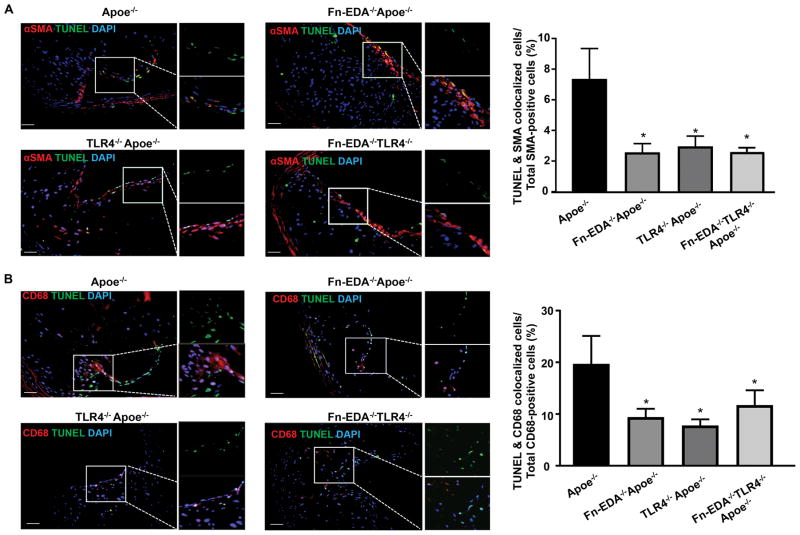
Fn-EDA/TLR4 axis promotes apoptosis in advanced plaques
Cellular apoptosis is known to promote plaque necrosis in advanced lesions.24 We quantified cellular apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method. We observed a decrease in TUNEL/SMA-positive cells in advanced lesions of Fn-EDA−/−Apoe−/− mice when compared to Apoe−/− mice (P<0.05, Figure 3A). Furthermore, we found decreased TUNEL/CD68-positive cells in Fn-EDA−/−Apoe−/− mice when compared to Apoe−/− mice (P<0.05, Figure 3B). The possibility that some of the TUNEL/CD68-positive cells could be derived from VSMCs could not be ruled out. Previous studies have shown that nearly 50% of cells in advanced atherosclerotic plaques in mice and humans are derived from VSMCs.25, 26 Together, these results suggest that ECM enriched in Fn-EDA promotes VSMC and macrophage apoptosis in advanced lesions. To determine the molecular mechanism by which Fn-EDA promotes apoptosis, we focused on TLR4. Apoptosis in SMA-positive and CD68-positive cells were comparable between TLR4−/−Apoe−/−, Fn-EDA−/−TLR4−/−Apoe−/− and Fn-EDA−/−Apoe−/− mice but significantly decreased when compared to Apoe−/− mice (P<0.05, Figure 3AB). Together, these results suggest that TLR4 may contribute to Fn-EDA-mediated apoptosis in advanced lesions of aged Apoe−/− mice.
Figure 3.
FN-EDA promotes VSMCs and macrophage apoptosis in advanced innominate lesions via TLR4. Left-side show representative immunofluorescence images of sections stained with A, TUNEL, and αSMA. B, TUNEL and CD68. Nuclei were visualized with DAPI stain. Boxed region in A & B is magnified to show colocalization of TUNEL/αSMA-positive cells (A) and TUNEL/CD68-positive cells (B). Right side shows quantification of TUNEL/αSMA-positive cells and TUNEL/CD68-positive cells. Data represent mean ± SEM. *P<0.05 versus Apoe−/− mice. N=7–8 mice/group. Value for each mouse represents a mean from 4 serial sections (each section approximately 60 μm apart). Statistical analysis: Figure 3A; Kruskal-Wallis test followed by uncorrected Dunn’s test. Figure 3B; Parametric one-way ANOVA followed by Sidak’s multiple comparisons test. Scale bar= 50 μm.
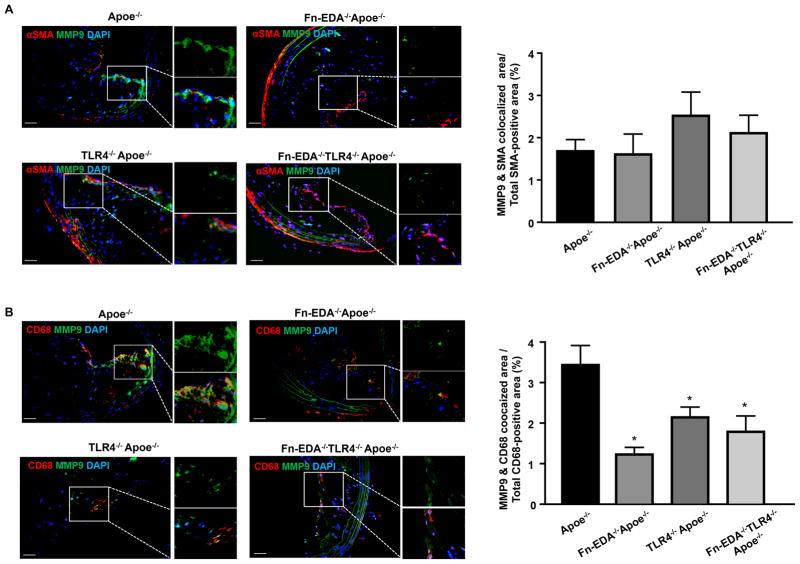
Fn-EDA/TLR4 axis mediates MMP9 potentiation in macrophages
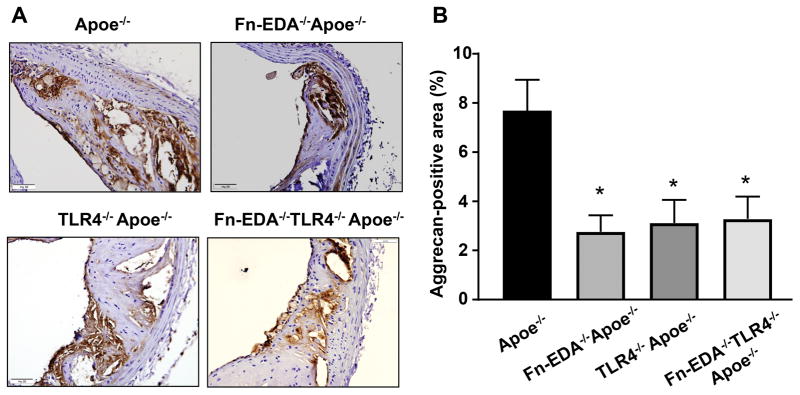
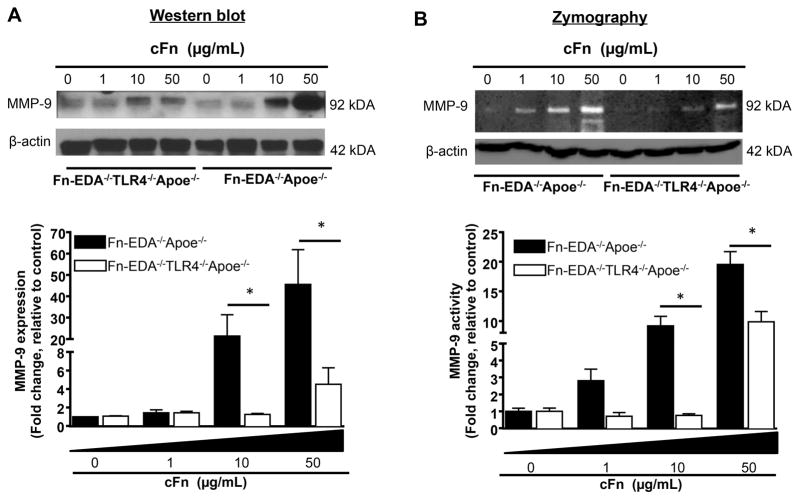
Monocytes/macrophages were present in the ECM surrounding the necrotic core (Figure SIII). MMPs expressed by VSMCs and monocytes/macrophages are known to degrade and remodel the ECM. Fn-EDA is known to potentiate MMP9 activity in vitro.19, 20 Therefore, we focused on MMP9 expression within plaques by immunohistochemistry. The percent of the MMP9/SMA-positive area was comparable between Apoe−/− and Fn-EDA−/−Apoe−/− mice (Figure 4A). However, Fn-EDA−/−Apoe−/− mice had decreased MMP9/CD68-positive area (%) when compared to Apoe−/− mice (P<0.05, Figure 4B). The MMP9/CD68- positive area (%) was comparable between TLR4−/−Apoe−/−, Fn-EDA−/−TLR4−/−Apoe−/− and Fn-EDA−/−Apoe−/− mice. These results suggest that TLR4 may contribute to Fn-EDA-mediated increased MMP9 expression in monocytes/macrophages (Figure 4B). The possibility that Fn-EDA potentiates other MMPs cannot be ruled out. To determine whether the increase MMP9 expression influences the accumulation of cleaved ECM constituents, we assessed MMP-cleaved aggrecan by immunohistochemistry. MMP-cleaved aggrecan-positive area was comparable between TLR4−/−Apoe−/−, Fn-EDA−/−TLR4−/−Apoe−/− and Fn-EDA−/−Apoe−/− mice, but decreased when compared to Apoe−/− mice (P<0.05, Figure 5). Together, these results suggest that the Fn-EDA/TLR4 axis, by potentiating MMP9 activity in macrophages covering the fibrous cap, may degrade ECM. To confirm that the Fn-EDA/TLR4 axis indeed potentiates MMP9 activity in macrophages, we performed cell culture experiments in vitro. Bone marrow-derived macrophages from Fn-EDA−/−Apoe−/− and Fn-EDA−/−TLR4−/−Apoe−/− mice were stimulated for 24 hours in the presence of exogenous human cellular Fn (cFn; 0–50 μg/mL), which contains the EDA. At doses of 10 and 50 μg/mL of cFn, both immunoblotting and gelatin zymography revealed a significant linear increase in MMP9 expression and activity in cFn-treated macrophages from Fn-EDA−/−Apoe−/− mice compared to control macrophages (P<0.05, Figure 6). This linear increase in MMP9 expression and activity levels was not observed in macrophages from Fn-EDA−/−TLR4−/−Apoe−/− mice treated with Fn-EDA up to a dose of 10 μg/mL. At the highest cFn concentration (50 μg/mL), we observed an increase in MMP9 activity in macrophages from Fn-EDA−/−TLR4−/−Apoe−/− mice, suggesting a pleiotropic effect, but it was significantly lower than in Fn-EDA−/−Apoe−/− mice (P<0.05, Figure 6). Together these results indirectly indicate that Fn-EDA/TLR4 axis may promote histological features of plaque destabilization by potentiating MMP activity in macrophages.
Figure 4.
Fn-EDA potentiates MMP9 expression in monocytes/macrophages of advanced lesions via TLR4. Left-side show representative immunofluorescence images of sections from 1-year old mice stained with A, MMP9 and αSMA. B, MMP9 and CD68. Nuclei were visualized with DAPI stain. Boxed region in A & B is magnified to show colocalization of MMP/SMA-positive area (A) & MMP9/CD68-positive area (B). Right side shows quantification of MMP/SMA-positive area and MMP9/CD68-area. Data represent mean ± SEM. Value for each mouse represents a mean from 4 serial sections (each section approximately 60 μm apart). *P<0.05 versus Apoe−/− mice. N=7–8 mice/group. Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test. Scale bar= 50 μm.
Figure 5.
Fn-EDA promotes MMP-cleaved aggrecan accumulation in advanced plaques. A, Representative immunofluorescence images of sections from 1-year old mice stained with anti-aggrecan antibody. B, Quantification of MMP-cleaved aggrecan-positive area. Data represent mean ± SEM. N=7–8 mice/group. Value for each mouse represents a mean from 4 serial sections (each section approximately 60 μm apart). *P<0.05 versus Apoe−/− mice. Statistical analysis: Kruskal-Wallis test followed by uncorrected Dunn’s test. Scale bar= 50 μm.
Figure 6.
Dose-dependent effect of exogenous cFn on MMP9 activity in macrophages. Pooled bone marrow-derived macrophages from Fn-EDA−/−Apoe−/− and Fn-EDA−/−TLR4−/−Apoe−/− mice (n=5 mice/group) were stimulated in the presence of cFN (0–50 μg/mL) for 24 hours. A, Top panel shows representative Western blots showing MMP9 expression and β-actin (loading control). Bottom panel represents quantification of the intensity of MMP9 to β-actin. N = 5 experiments/group. B, Top panel shows MMP9 activity (gelatin zymography). The same amount of protein was loaded on another gel and stained for β-actin (loading control). The bottom panel shows quantification of the intensity of MMP9 normalized to β-actin in panel A. Data is presented as mean ± SD. *P<0.05. N = 5 experiments/group. Statistical analysis: Two way ANOVA followed by Holm-Sidak multiple comparison tests.
Discussion
The findings of our study performed in aged Apoe−/− mice suggest that an ECM microenvironment enriched in Fn-EDA may increase the risk of plaque destabilization in advanced atherosclerotic lesions, and that Fn-EDA induces plaque instability in part via a TLR4 pathway. These findings in EDA- and TLR4-deficient Apoe−/− mice may have clinical significance for the following reasons: 1) Fn-EDA is abundantly expressed in the ECM of advanced atherosclerotic plaques in humans;16, 18 2) Two clinical studies have shown a positive correlation between TLR4 expression on plaque monocytes and the clinical stages of atherosclerosis in patients with unstable angina and myocardial infarction.27, 28
We chose to study the innominate artery of aged Apoe−/− mice to elucidate the contribution of Fn-EDA in advanced atherosclerosis because, unlike other animal models of atherosclerosis, the innominate artery mimics several histological features of vulnerable human plaques, including large necrotic cores, thin fibrous caps, and intraplaque hemorrhage.22, 23 However, this model has some limitations because some features observed in vulnerable human plaques, such as denuded endothelium and large occlusive thrombi, are rarely present.29, 30 Despite these limitations, there is a consensus that of all currently available murine models of atherosclerosis, the innominate artery is the most informative model to elucidate the mechanisms and factors that may contribute to increased risk of plaque destabilization.
We found that genetic deletion of Fn-EDA in one-year-old Apoe−/− mice significantly reduced plaque size, characterized by a decreased necrotic core, reduced CD68-positive cell content, decreased VSMC and macrophage apoptosis, and absence of intraplaque hemorrhage, implicating Fn-EDA as a mediator of destabilization of advanced plaques. In contrast, while our studies were in progress, another study by Pulkazhi Venu et al. suggested that Fn-EDA may paradoxically promote plaque stabilization.17 In that study, the authors observed decreased atherosclerosis in the aortic sinus of 5-month-old Fn-EDA−/−Apoe−/− mice, a finding that is consistent with current findings. Surprisingly, however, the authors reported that the smaller atherosclerotic lesions in Fn-EDA−/−Apoe−/− mice exhibited features of plaque instability characterized by increased macrophage content, increased MMP2 and MMP9 expression, and reduced collagen gene expression. Based on these findings, the authors concluded that Fn-EDA promotes atherosclerosis but stabilizes plaque. This was a surprising finding because we have reported previously that decreased atherosclerosis in the aortic sinus of 5-month old male and female Fn-EDA−/−Apoe−/− mice is associated with reduced macrophage content.16 Several other studies have shown that macrophages promote atherosclerosis and that Fn-EDA is known to potentiate MMP9 expression.19, 20, 24, 31 The explanation for the apparent discrepancies between the study by Pulkazhi Venu et al. in 5-month old mice and our current results in one-year-old mice remains unclear but could be related to the different sites of atherosclerosis and ages of the mice studied. Notably, Pulkazhi Venu et al. did not determine several relevant parameters of plaque destabilization including necrotic core, apoptosis, and intraplaque hemorrhage.17
In our opinion, the data reported herein provide novel mechanistic insights beyond the study reported by Pulkazhi Venu Venu et al. for the following reasons. First, we determined the contribution of Fn-EDA in advanced lesions of the innominate artery of aged mice, a plaque instability site that mimics several histological features of vulnerable human plaques. The choice of experimental animal model is important because it is well established that the mechanisms of plaque progression governing different stages of atherosclerosis are distinct. For example, macrophage apoptosis in early atherosclerosis is protective but detrimental to advanced lesions.32 Second, we performed a comprehensive histological analysis of plaque stability that included assessment of the necrotic area, intraplaque hemorrhage, fibrous cap thickness, and neovascularization. Third, we provide additional mechanistic insight into how Fn-EDA may contribute to plaque instability. We found that Fn-EDA promotes VSMC and macrophage apoptosis. Increased apoptosis in advanced lesions contributes to lesion size and enlargement of the necrotic core because of defective efferocytosis.32 Fourth, we provide a molecular mechanism by which Fn-EDA may induce plaque destabilization. We found that deletion of TLR4 reduced features of plaque instability including necrotic area, macrophage content, VSMC, and macrophage apoptosis and intraplaque hemorrhage in Apoe−/− mice, but had no additional effect in Fn-EDA−/−Apoe−/− mice, suggesting that Fn-EDA may induce plaque destabilization via TLR4. The following observations further support the conclusion that the Fn-EDA/TLR4 axis may contribute to plaque instability: 1) TLR4 interacts with Fn-EDA,19, 20, 33 2) TLR4 is upregulated in circulating monocytes in patients with unstable angina and at sites of plaque rupture in patients with acute myocardial infarction.27, 28 3) Lentivirus-mediated knockdown of both TLR2 and TLR4 genes in 5 month-old Apoe−/− mice reduces the plaque vulnerability index in a collar-induced atherosclerosis model.34 4) TLR4 contributes to Fn-EDA mediated thrombosis and inflammation in experimental models of thrombosis and acute stroke.31, 33, 35 5) TLR4 contributes to Fn-mediated atherosclerotic lesion progression in 5-month-old Apoe−/− mice.16
Our studies also suggest a mechanistic link between Fn-EDA, TLR4, and MMP9 in plaque destabilization. TLR4 contributes to outward arterial remodeling,36 a process that involves higher MMP9 expression and excessive ECM degradation. Other studies have suggested an association between MMP9 expression and atherosclerotic plaque destabilization.21, 37 Herein, we found that Fn-EDA/TLR4 axis promotes an increase in CD68-positive cells expressing MMP9 and increased accumulation of MMP-cleaved aggrecan (an ECM constituent) in the fibrous cap region around the necrotic core. Together, these observations suggest that the Fn-EDA/TLR4 axis, by potentiating MMP9 activity in macrophages, may contribute to degradation and remodeling of ECM, and, thereby, increase the risk of plaque destabilization. Previously, we have shown that exogenous cFn (which contains EDA) potentiates the phosphorylation of NFκB p65 via TLR4 in macrophages.16 Here, we found that exogenous cFn also potentiated MMP9 activity in bone marrow-derived macrophages via TLR4. Based on the studies reported herein, we propose a mechanistic model in which infiltrating CD68 monocytes/macrophages or resident macrophages interact with Fn-EDA in the ECM niche of advanced atherosclerotic lesions, potentiating MMP9 activity to degrade and remodel ECM components in the fibrous cap, thereby increasing the risk of plaque destabilization. The possibility that other MMPs such as MMP2 may contribute to Fn-EDA/TLR4-mediated plaque destabilization cannot be excluded. Although our studies indicate that TLR4 signaling significantly contributes to Fn-mediated plaque destabilization, it remains possible that some of the proinflammatory effects of Fn-EDA could be partially mediated by the leukocyte integrins α4β1 and α9β1, which are known to have binding sites within the EDA domain.38 Additional complex studies will be required to determine if disruption of Fn-EDA-integrin interactions in vivo prevents monocyte recruitment and subsequent atherosclerotic lesion progression and stability.
Our studies have some limitations. First, Fn-EDA-mediated histological features of plaque destabilization were not associated with luminal thrombus, which is a characteristic feature of vulnerable human plaques.29, 30 Notably, reproducible thrombus in the lumen has not been observed by several groups in the advanced lesions in the murine innominate artery.21, 22 Second, the present study did not define the specific cell types that contribute to Fn-EDA-mediated plaque destabilization in advanced lesions. Additional studies utilizing endothelial-specific and vascular SMC-specific deletion of Fn-EDA will be required to define the cellular source of Fn-EDA that mediates plaque destabilization. Third, TIMPs are known to regulate MMP9 activity. We did not analyze the activity of TIMP1 and TIMP2, although in vitro studies suggest that absence of TIMP1 and TIMP2 does not enhance proteolytic auto-activation of MMP9 in macrophages, implicating the existence of other independent mechanisms that regulate MMP9.21
In summary, our studies unequivocally support a causal connection between Fn-EDA present in advanced atherosclerotic plaques and increased MMP9 activity in macrophages that may increase the risk of plaque destabilization. These observations suggest that interventions targeting Fn-EDA may lessen plaque progression and reduce plaque destabilization.
Supplementary Material
Highlights.
Fibronectin containing extra domain A (Fn-EDA) is abundant in the extracellular matrix of advanced atherosclerotic lesions in human and mice.
The contribution of Fn-EDA in advanced atherosclerosis remains poorly characterized.
We provide evidence that Fn-EDA induces histological features of plaque instability in advanced lesions of aged Apoe−/− mice.
Our studies suggest that Fn-EDA present in advanced atherosclerotic plaques may increase the risk of plaque destabilization.
Acknowledgments
Sources of funding
This work was supported by National Institutes of Health grants R01HL118246 and R01HL118742, and American Heart Association, Innovative Research grant # to16IRG27490003 A.K.C, and NIH grant P01 HL062984 to S.R.L.
Non-standard Abbreviations and Acronyms
- cFN
Cellular fibronectin
- EDA
Extra domain A
- Fn-EDA
Fibronectin containing extra domain A
- TLR4
Toll-like receptor 4
- ECM
Extracellular matrix
- Apoe
Apolipoprotein E
Footnotes
Disclosures
None
References
- 1.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: Relationships to vascular disease. International journal of experimental pathology. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shekhonin BV, Domogatsky SP, Idelson GL, Koteliansky VE, Rukosuev VS. Relative distribution of fibronectin and type i, iii, iv, v collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987;67:9–16. doi: 10.1016/0021-9150(87)90259-0. [DOI] [PubMed] [Google Scholar]
- 3.Pedretti M, Rancic Z, Soltermann A, Herzog BA, Schliemann C, Lachat M, Neri D, Kaufmann PA. Comparative immunohistochemical staining of atherosclerotic plaques using f16, f8 and l19: Three clinical-grade fully human antibodies. Atherosclerosis. 2010;208:382–389. doi: 10.1016/j.atherosclerosis.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates nf-kappab activation by flow: A potential role in atherosclerosis. The Journal of cell biology. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamkun JW, Schwarzbauer JE, Hynes RO. A single rat fibronectin gene generates three different mrnas by alternative splicing of a complex exon. Proc Natl Acad Sci U S A. 1984;81:5140–5144. doi: 10.1073/pnas.81.16.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan AK, Iaconcig A, Baralle FE, Muro AF. Alternative splicing of fibronectin: A mouse model demonstrates the identity of in vitro and in vivo systems and the processing autonomy of regulated exons in adult mice. Gene. 2004;324:55–63. doi: 10.1016/j.gene.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Pagani F, Zagato L, Vergani C, Casari G, Sidoli A, Baralle FE. Tissue-specific splicing pattern of fibronectin messenger rna precursor during development and aging in rat. The Journal of cell biology. 1991;113:1223–1229. doi: 10.1083/jcb.113.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. The Journal of cell biology. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin eda exon is essential for proper skin wound healing and normal lifespan. The Journal of cell biology. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takasaki I, Chobanian AV, Mamuya WS, Brecher P. Hypertension induces alternatively spliced forms of fibronectin in rat aorta. Hypertension. 1992;20:20–25. doi: 10.1161/01.hyp.20.1.20. [DOI] [PubMed] [Google Scholar]
- 12.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type iii domain a in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: Cellular source and biological activity of the eiiia segment in rat hepatic fibrogenesis. The Journal of cell biology. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, White ES, Varga J. Fibronectineda promotes chronic cutaneous fibrosis through toll-like receptor signaling. Sci Transl Med. 2014;6:232ra250. doi: 10.1126/scitranslmed.3008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin eiiia domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 16.Doddapattar P, Gandhi C, Prakash P, Dhanesha N, Grumbach IM, Dailey ME, Lentz SR, Chauhan AK. Fibronectin splicing variants containing extra domain a promote atherosclerosis in mice through toll-like receptor 4. Arterioscler Thromb Vasc Biol. 2015;35:2391–2400. doi: 10.1161/ATVBAHA.115.306474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulakazhi Venu VK, Uboldi P, Dhyani A, Patrini A, Baetta R, Ferri N, Corsini A, Muro AF, Catapano AL, Norata GD. Fibronectin extra domain a stabilises atherosclerotic plaques in apolipoprotein e and in ldl-receptor-deficient mice. Thromb Haemost. 2015;114:186–197. doi: 10.1160/TH14-09-0790. [DOI] [PubMed] [Google Scholar]
- 18.van Keulen JK, de Kleijn DP, Nijhuis MM, Busser E, Velema E, Fijnheer R, van der Graaf Y, Moll FL, de Vries JP, Pasterkamp G. Levels of extra domain a containing fibronectin in human atherosclerotic plaques are associated with a stable plaque phenotype. Atherosclerosis. 2007;195:e83–91. doi: 10.1016/j.atherosclerosis.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain a of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 20.Saito S, Yamaji N, Yasunaga K, Saito T, Matsumoto S, Katoh M, Kobayashi S, Masuho Y. The fibronectin extra domain a activates matrix metalloproteinase gene expression by an interleukin-1-dependent mechanism. J Biol Chem. 1999;274:30756–30763. doi: 10.1074/jbc.274.43.30756. [DOI] [PubMed] [Google Scholar]
- 21.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active mmp-9 induces acute plaque disruption in apoe-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the apoe knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 23.Williams H, Johnson JL, Carson KG, Jackson CL. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein e knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:788–792. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- 24.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 26.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 27.Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–2661. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa Y, Satoh M, Itoh T, Minami Y, Takahashi Y, Akamura M. Local expression of toll-like receptor 4 at the site of ruptured plaques in patients with acute myocardial infarction. Clin Sci (Lond) 2008;115:133–140. doi: 10.1042/CS20070379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schapira K, Heeneman S, Daemen MJ. Animal models to study plaque vulnerability. Curr Pharm Des. 2007;13:1013–1020. doi: 10.2174/138161207780487575. [DOI] [PubMed] [Google Scholar]
- 30.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 31.Khan MM, Gandhi C, Chauhan N, Stevens JW, Motto DG, Lentz SR, Chauhan AK. Alternatively-spliced extra domain a of fibronectin promotes acute inflammation and brain injury after cerebral ischemia in mice. Stroke. 2012;43:1376–1382. doi: 10.1161/STROKEAHA.111.635516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 33.Prakash P, Kulkarni PP, Lentz SR, Chauhan AK. Cellular fibronectin containing extra domain a promotes arterial thrombosis in mice through platelet toll-like receptor 4. Blood. 2015;125:3164–3172. doi: 10.1182/blood-2014-10-608653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JM, Wang Y, Qi LH, Wang Y, Gao F, Ding SF, Ni M, Liu CX, Zhang C, Zhang Y. Combinatorial interference of toll-like receptor 2 and 4 synergistically stabilizes atherosclerotic plaque in apolipoprotein e-knockout mice. J Cell Mol Med. 2011;15:602–611. doi: 10.1111/j.1582-4934.2010.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhanesha N, Ahmad A, Prakash P, Doddapattar P, Lentz SR, Chauhan AK. Genetic ablation of extra domain a of fibronectin in hypercholesterolemic mice improves stroke outcome by reducing thrombo-inflammation. Circulation. 2015;132:2237–2247. doi: 10.1161/CIRCULATIONAHA.115.016540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, Strijder CF, Van Middelaar BJ, Pasterkamp G, Quax PH, De Kleijn DP. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–398. doi: 10.1161/01.CIR.0000109140.51366.72. [DOI] [PubMed] [Google Scholar]
- 37.Loftus IM, Naylor AR, Goodall S, Crowther M, Jones L, Bell PR, Thompson MM. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31:40–47. doi: 10.1161/01.str.31.1.40. [DOI] [PubMed] [Google Scholar]
- 38.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The eiiia segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.