Abstract
Objective
von Willebrand factor (VWF) is synthesized by endothelial cells and megakaryocytes, and is known to contribute to atherosclerosis. In vitro studies suggest that platelet-derived VWF (Plt-VWF) is biochemically and functionally different from endothelial cell-derived VWF (EC-VWF). We determined the role of different pools of VWF in the pathophysiology of atherosclerosis.
Approach and Results
Using bone marrow (BM) transplantation, we generated chimeric Plt-VWF, EC-VWF, and Plt-VWF mice lacking ADAMTS13 in platelets and plasma (Plt-VWF/ADAMTS13−/−) on apolipoprotein-deficient (Apoe−/−) background. Controls were chimeric Apoe−/− mice transplanted with BM from Apoe−/− mice (wild-type; WT) and Vwf −/−Apoe−/− mice transplanted with BM from Vwf −/−Apoe−/− mice (VWF-KO). Susceptibility to atherosclerosis was evaluated in whole aortae and cross sections of the aortic sinus in female mice fed a high fat “Western” diet for 14 weeks. VWF-KO, Plt-VWF, and Plt-VWF/ADATS13−/− mice exhibited reduced plaque size characterized by smaller necrotic cores, reduced neutrophil and monocytes/macrophages content, decreased MMP9, MMP2, and CX3CL1-positive area and abundant interstitial collagen (P<0.05 versus WT or EC-VWF mice). Atherosclerotic lesion size and composition was comparable between WT or EC-VWF mice. Together these findings suggest that EC-VWF, but not Plt-VWF, promotes atherosclerosis exacerbation. Furthermore, intravital microscopy experiments revealed that EC-derived VWF, but not Plt-derived VWF, contributes to platelet and leukocyte adhesion under inflammatory conditions at the arterial shear rate.
Conclusions
EC-VWF, but not Plt-VWF, contributes to VWF-dependent atherosclerosis by promoting platelet adhesion and vascular inflammation. Plt-VWF even in the absence of ADAMTS13, both in platelet and plasma, was not sufficient to promote atherosclerosis.
Keywords: von Willebrand factor, plaque, platelet, and endothelial cell
Introduction
von Willebrand factor (VWF) is a large multimeric protein that plays an important role in hemostasis and thrombosis. VWF is stored as ultra large (ULVWF) multimers or high molecular weight multimers (HMWM) in endothelial Weibel-Palade bodies and platelet α-granules.1 Upon secretion into circulation and under shear stress, anchored hyperactive ULVWF multimers on the endothelium are cleaved by ADAMTS13 (A Disintegrin And Metalloprotease with Thrombospondin type I repeats-13) into smaller less active VWF multimers that support hemostasis and thrombosis.2 VWF present in plasma is derived from both endothelial cells (~80–85% via a constitutive secretory pathway or regulated secretion in response to secretagogues such as TNF and histamine) and platelets (~15–20 % from activated platelets).3, 4
Several murine studies suggest that VWF/ADAMTS13 axis modulates vascular inflammation in addition to thrombosis in experimental models.5–13 For example, ADAMTS13 deficiency in mice exacerbates myocardial, and brain ischemia/reperfusion injury by promoting thrombosis and inflammatory processes,7, 8, 11, 14 whereas VWF deficiency had a protective effect.7, 11, 14, 15 Similarly, ADAMTS13 deficiency in mice exacerbates atherosclerosis,9, 10, 16 whereas VWF deficiency reduces atherosclerosis in mice and pigs.9, 12, 17 Although the role of VWF in atherosclerosis is well established, the relative contribution of platelet-derived VWF (Plt-VWF) versus endothelial cell-derived VWF (EC-VWF) in atherosclerotic lesion progression is not yet elucidated. Notably, several in vitro studies suggest that there are biochemical and functional differences between platelet-derived VWF (Plt-VWF) and endothelial cell-derived VWF (EC-VWF). For example, Plt-VWF differs in glycosylation profile, particularly with a reduction in N-linked sialic acid expression when compared to EC-VWF.18 Although platelets contain ADAMTS13, the authors suggested that due to different glycosylation profile, Plt-VWF is resistant to ADAMTS13 cleavage.18 In contrast, other in vitro studies have shown that Plt-derived ULVWF multimers are only observed when platelets are activated or lysed in the presence of EDTA.19, 20 There is also a functional difference between EC-VWF and Plt-VWF. Although binding affinities to collagen are comparable between EC-VWF and Plt-VWF, Plt-VWF binds with higher affinity to the surface of thrombin-stimulated platelets when compared with EC-VWF.21, 22 On the other hand, EC-VWF binds with higher affinity to platelet GPIbα when compared to Plt-VWF.22
Herein, we sought to determine the relative contribution of platelet-derived VWF versus endothelial cell-derived VWF pool in the pathophysiology of atherosclerosis. Using reciprocal bone marrow transplantation (BMT), we generated chimeric Plt-VWF and EC-VWF mice on the Apoe-deficient background. We found that EC-VWF, but not Plt-VWF, contributes to atherosclerotic lesion progression by promoting platelet adhesion and vascular inflammation.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Characterization of chimeric mice expressing Plt-derived VWF and EC-derived VWF on Apoe−/− background
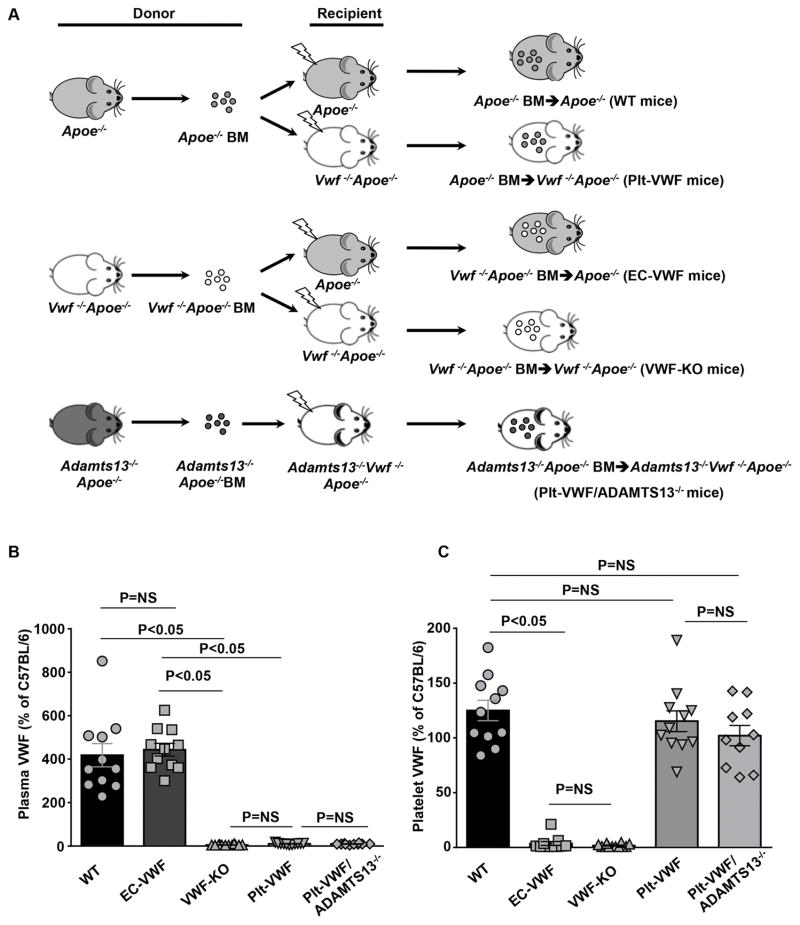
In the remainder of the manuscript: 1) irradiated Apoe−/− mice reconstituted with BM from Apoe−/− donors (Apoe−/−-BM→ Apoe−/− mice) are controls and are referred as wild-type (WT) mice (source of VWF: endothelial cells and platelets), 2) irradiated Apoe−/− mice reconstituted with bone marrow from Vwf −/−Apoe−/− donors (Vwf −/−Apoe−/−-BM→ Apoe−/− mice) are referred as EC-VWF mice (express VWF in endothelial cells, but lack VWF in platelet), 3) irradiated Vwf −/−Apoe−/− mice reconstituted with BM from Vwf −/−Apoe−/− donors (Vwf −/−Apoe−/−-BM→ Vwf −/−Apoe−/− mice) are referred as VWF-KO mice, 4) irradiated Vwf −/−Apoe−/− mice reconstituted with bone marrow from Apoe−/− donors (Apoe−/−-BM→ Vwf−/−Apoe−/− mice) are referred as Plt-VWF mice (have VWF in platelets, but lack VWF in endothelial cells), and 5) irradiated Adamts13−/−Vwf−/−Apoe−/− mice reconstituted with bone marrow from Adamts13−/−Apoe−/− donors (Adamts13−/−Apoe−/− -BM→ Adamts13−/−Vwf−/−Apoe−/− mice) are referred as Plt-VWF/ADAMTS13−/− mice (ADAMTS13 deficient in plasma and platelet plus lack VWF in endothelial cells) (Figure 1A). Complete blood counts were comparable suggesting that BMT did not affect the number of BM-derived blood cells (Table SI). To confirm successful engraftment of bone marrow, the relative content of VWF in plasma and platelets was quantified by ELISA. Plasma VWF levels were comparable between WT mice (414.5 ± 53.5 %,) and EC-VWF mice (439.9 ± 29.0 %, Figure 1B). Notably, plasma VWF levels in Apoe−/− mice are relatively higher to WT levels. No detectable levels of plasma VWF were found in VWF-KO mice, whereas a minor amount of plasma VWF (Figure 1B) was found in Plt-VWF (6.7 ± 1.1 %) and Plt-VWF/Adamts13−/− mice (5.6 ± 0.9 %). Plt-derived VWF levels were comparable between WT, Plt-VWF mice and Plt-VWF/ADAMTS13−/− mice (Figure 1C). No detectable level of Plt-derived VWF was found in VWF-KO or EC-VWF mice (Figure 1C).
Figure 1.
Scheme and characterization of chimeric mice, which express VWF either in megakaryocytes or endothelial cells. A, Schematic representation of BMT protocol. Reciprocal bone marrow (BM) from Apoe−/− or Vwf −/− Apoe−/− mice were transplanted into either Apoe−/− or Vwf −/−Apoe−/− recipient mice to generate 4 experimental groups of mice: 1) WT mice: source of VWF is platelets and endothelial cells, control, 2) EC-VWF mice: source of VWF is endothelial cells, 3) VWF-KO mice: lack VWF in platelets and endothelial cells, 4) Plt-VWF mice: source of VWF is platelets, 5) Plt-VWF/ADAMTS13−/− mice: source of VWF is platelets but are deficient for ADAMTS13 in both platelets and plasma. B, Plasma VWF levels. N=10–12 mice/group. C, Platelet VWF levels. N=10–12 mice/group. Data are presented as mean ± SEM. Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test. NS: non-significant.
EC-derived VWF, but not platelet-derived VWF, exacerbates atherosclerosis in aorta and aortic sinus
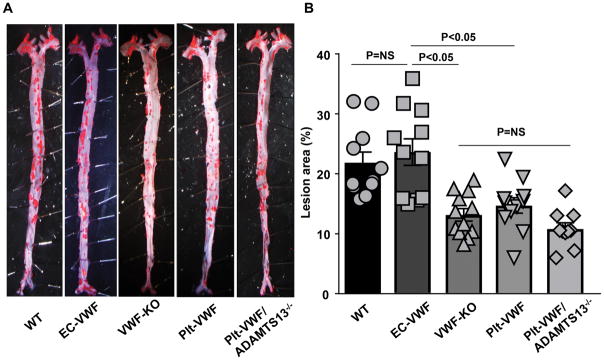
To define the relative contribution of Plt-derived VWF versus EC-derived VWF pool in the pathophysiology of atherosclerosis, EC-VWF and Plt-VWF female mice with controls (WT and VWF-KO) were fed a high-fat “Western” diet for 14 weeks. We measured total cholesterol and triglyceride levels in all the groups fed a high fat “Western” diet for 14 weeks. Total cholesterol and triglyceride levels were comparable among groups (Figure SI). Next, we compared the extent of atherosclerotic lesion progression in whole aortae by staining with Oil Red O and quantifying en face lesion area. Lesion areas were comparable between EC-VWF (22.8 ± 2.3 %) and WT mice (21.8 ± 1.8 %; Figure 2AB). Similar to previous reports,9, 12 VWF-KO mice exhibited significantly reduced lesions area (12.7 ± 1.1 %, P<0.05) when compared with either EC-VWF or WT mice. Atherosclerotic lesion area was comparable between Plt-VWF (14.7 ± 1.3 %) and VWF-KO mice (Figure 2AB). These results suggest that EC-derived VWF, but not Plt-derived VWF, contributes to accelerated atherosclerosis in the aorta. Next, we determined whether Plt-VWF, in the absence of ADAMTS13 in plasma and platelets, is able to promote atherosclerosis comparable to EC-VWF. Lesion areas were comparable in Plt-VWF mice (14.7 ± 1.3%) and Plt-VWF/ADAMTS13−/− mice (11.4 ± 1.0 %) but significantly decreased when compared to WT mice (21.8 ± 1.8 %) or EC-VWF mice (22.8 ± 2.3 %, P<0.05, Figure 2A and B). This result suggests that Plt-VWF even in the absence of ADAMTS13 was not sufficient to promote atherosclerosis similar to EC-VWF.
Figure 2.
EC-VWF promotes atherosclerotic lesion formation in the aorta. Female mice from all the groups were fed a high fat “Western” diet for 14 weeks. A, Oil red O staining of the representative aortae from each group. B, Quantification of en face lesion area. Each dot represents a single mouse. N=10–12 mice/group. Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test. NS: non-significant.
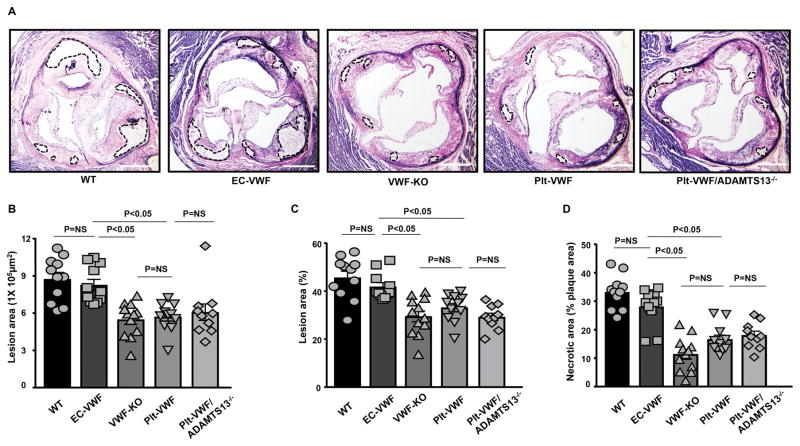
Next, we quantified the cross-sectional area of atherosclerotic lesions in the aortic sinus using the VerHoeffs/Van Gieson staining method. We found that the mean lesion areas (in μm2 or %) in the aortic sinus were comparable between VWF-KO (29.5 ± 2.2 %), Plt-VWF (33.2 ± 1.8 %) and Plt-VWF/ADAMTS13−/− mice (29.3 ± 1.6 %), but significantly decreased when compared to EC-VWF (41.0 ± 1.3 %) or WT mice (45.6 ± 2.6 %, P<0.05, Figure 3 A–C). Furthermore, necrotic area (unstained acellular area, %) was comparable between VWF-KO, Plt-VWF and Plt-VWF/ADAMTS13−/− mice, but significantly decreased when compared to EC-VWF or WT mice (P<0.05, Figure 3D).
Figure 3.
EC-VWF promotes atherosclerotic lesion formation in the aortic sinus. Female mice from all the groups were fed a high fat “Western” diet for 14 weeks. A, Representative photomicrographs of VerHoeffs/Van Geison-stained aortic sinuses. Scale bar = 500 μm. B & C, Quantification of the lesion in the cross-section area of aortic sinuses. D, Necrotic core (%) defined as acellular area and demarcated by dashed lines in panel A. Each dot represents a single mouse. N=10–12 mice/group. Statistical analysis: Kruskal-Wallis test followed by uncorrected Dunn’s test. NS: non-significant.
EC-derived VWF, but not platelet-derived VWF, promotes vascular inflammation in aortic sinus of Apoe−/− mice
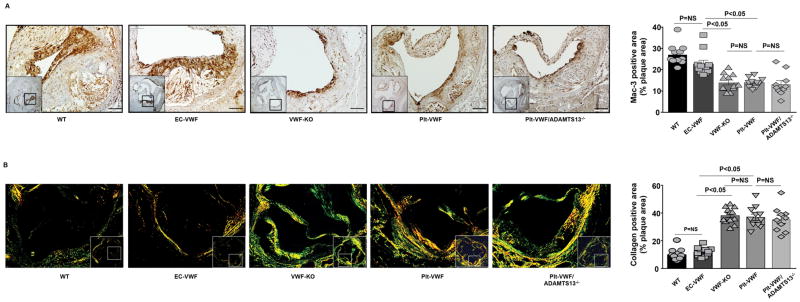
To test the hypothesis that EC-VWF promotes vascular inflammation within atherosclerotic lesions, we quantitated macrophage and neutrophil infiltration within lesions in the aortic sinus by immunohistochemistry. We found that the Mac-3 positive area (μm2) were comparable between VWF-KO, Plt-VWF and Plt-VWF/ADAMTS13−/− mice, but significantly decreased when compared to EC-VWF or WT mice (P<0.05, N=10–12 mice/group, Figure 4A). Not only was the absolute Mac-3 positive area increased in EC-VWF and WT mice (Figure SII), but the percent of the total lesion area occupied by Mac-3 positive cells was also significantly higher in EC-VWF or WT mice when compared to VWF-KO or Plt-VWF or Plt-VWF/ADAMTS13−/− mice (P<0.05, Figure 4A). Next, we measured neutrophil content within lesions. We observed that the content of Ly6G-positive neutrophils in Apoe−/− mice fed a high fat “Western diet” for 14 weeks is markedly (but not completely) less when compared to Apoe−/− mice fed a high fat “Western diet” for 4 weeks (not shown). However, it was still possible to quantify Ly6G-positive cells among all groups. The accumulation of Ly6G-positive cells was comparable between WT and EC-VWF mice but significantly higher when compared with VWF-KO or Plt-VWF or Plt-VWF/ADAMTS13−/− mice (Figure SIII). Next, we measured the interstitial collagen content. Picrosirius red staining of lesions in the aortic sinus revealed similar collagen content in EC-VWF and WT mice but significantly decreased when compared with VWF-KO or Plt-VWF or Plt-VWF/ADAMTS13−/− mice (Figure 4B).
Figure 4.
EC-VWF mice, but not Plt-VWF mice, exhibit increased macrophage infiltration and reduced interstitial collagen within atherosclerotic lesions. A, Left-hand side: Higher magnification images showing representative photomicrographs stained for macrophages (Mac-3 positive cells stained as brown) and counterstained with hematoxylin (blue) from the boxed region. Scale bar = 100 μm. Right-hand side: Quantification of macrophages in the cross-section area of aortic sinuses. N=10–12 mice/group. Statistical analysis: Kruskal-Wallis test followed by uncorrected Dunn’s test. B, Left-hand side: Higher magnification images showing representative photomicrographs stained for collagen from the boxed region and visualized by a polarization microscope. Right-hand side: Quantification of positive collagen area (%) in the cross-section area of aortic sinus. N=10–12/group. Value for each mouse represents a mean of 16 fields from 4 serial sections. Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test. Each dot represents a single mouse. NS: non-significant.
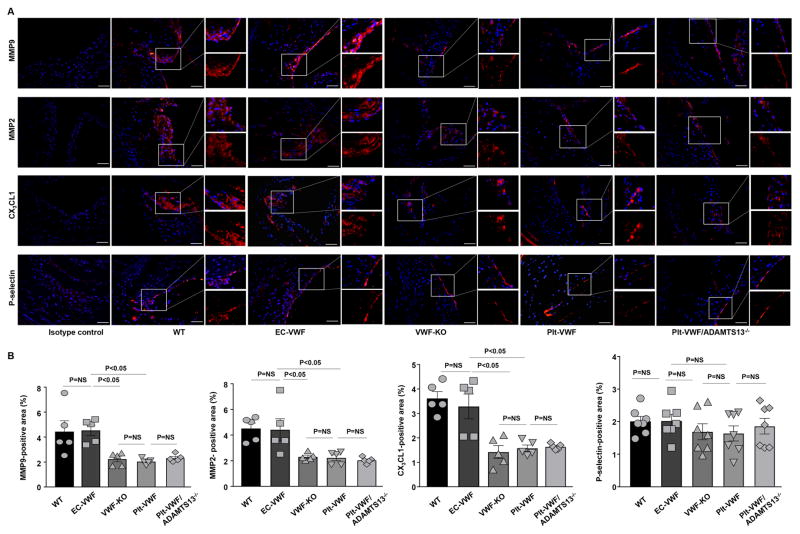
Next, we analyzed other histological features of vascular inflammation including MMP9, MMP2, chemokine CX3CL1 (known to contribute to platelet adhesion via VWF) and P-selectin, all known modulators of leukocyte infiltration within lesions in the aortic sinus by immunohistochemistry. We found that MMP9-positive, MMP2-positive, and CX3CL1-positive area were comparable between VWF-KO, Plt-VWF and Plt-VWF/ADAMTS13−/− mice, but significantly reduced when compared to EC-VWF or WT mice (P<0.05, Figure 5). Although there was a trend towards a decreased P-selectin-positive area in VWF-KO, Plt-VWF and Plt-VWF/ADAMTS13−/− mice when compared with WT or EC-VWF mice, it was not statistically significant (Figure 5). Together, these results suggest that EC-VWF by promoting vascular inflammation contributes to atherosclerosis exacerbation.
Figure 5.
EC-VWF mice, but not Plt-VWF mice, exhibit increased MMP9, MMP2, and CX3CL1 expression within atherosclerotic lesions. A, Representative immunofluorescence images of sections from different strains of chimeric mice stained with MMP9, MMP2. CX3CL1, and P-selectin. Nuclei were visualized with DAPI stain. Boxed region is further magnified to show MMP9-positive area, MMP2-positive area, CX3CL1-positive area and P-selectin-positive area. B, Quantification of MMP9-positive area, MMP2-positive area, CX3CL1-positive area and P-selectin-positive area. Data represent mean ± SEM. Each dot represents a single mouse. Value for each mouse represents a mean from 4 serial sections (each section approximately 60 μm apart). Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test. Scale bar = 50 μm. NS: non-significant.
EC-derived VWF, but not platelet-derived VWF promotes platelet and leukocyte adhesion to activated endothelium of Apoe−/− mice
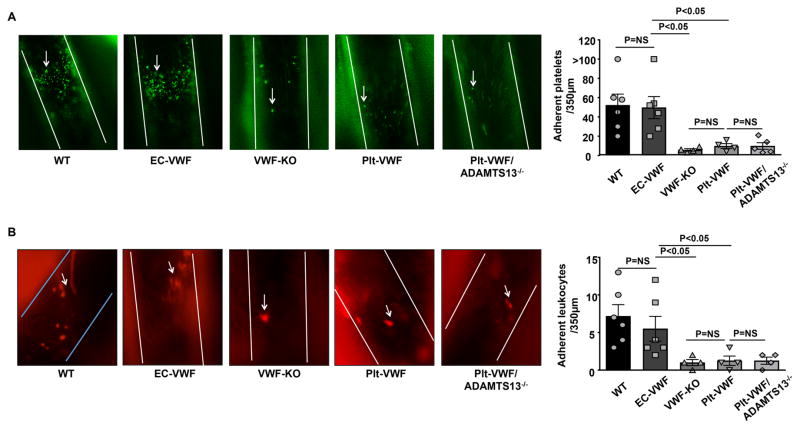
Platelets are known to recruit leukocytes and play a key role in the onset and progression of atherosclerosis.23,24 To define the relative contribution of Plt-derived VWF versus EC-derived VWF pool in recruiting quiescent platelets and leukocytes in vivo, we quantified platelet and leukocyte adhesion under the inflammatory condition at the arterial shear rate. Chimeric mice fed a chow diet were challenged with TNF, and the common carotid artery and carotid bifurcation (atheroprone) were visualized for platelet and leukocyte adhesion after 3.5 hours by intravital microscopy. We found that the number of adherent platelets (adherent for >30 s) and leukocytes were comparable between Plt-VWF-KO, VWF-KO and Plt-VWF/ADAMTS13−/− mice, but significantly decreased when compared to WT or EC-VWF mice (Figure 6). The number of adherent platelets and leukocytes were comparable between WT and EC-VWF mice. These results suggest that EC-derived VWF, but not Plt-derived VWF, contributes to platelet and leukocyte adhesion under inflammatory conditions.
Figure 6.
EC-VWF mice exhibit increased platelet and leukocyte adhesion in carotid vessels under inflammatory conditions. All mice were challenged with recombinant TNF 3.5 hours before visualization of platelet and leukocyte adhesion. A, Left-hand side shows representative photomicrographs of adhering platelets (>30 s) at the common carotid artery and carotid bifurcation, a lesion-prone site, as visualized by intravital upright microscope. Magnification= 100X. The right-hand side shows quantification of the number of adherent fluorescent-labeled platelets (calcein green, AM labeled). B, Left-hand side shows representative photomicrographs of adhering leukocytes (Rhodamine 6G labeled) at the common carotid artery and carotid bifurcation. The right-hand side shows quantification of adhered leukocytes. Magnification= 200X. White lines delineate the arteries in A and B. Arrows indicate adherent platelet and leukocyte. Data represent mean ± SEM. Each dot represents a single mouse. Statistical analysis: Parametric one-way ANOVA followed by Sidak’s multiple comparisons test. NS: non-significant.
Discussion
The results presented herein provide novel insights on the relative importance of different pools of VWF in the pathophysiology of atherosclerosis. The key findings of the study are: 1) EC-VWF, but not Plt-VWF, contributes to VWF-dependent atherosclerosis most likely by promoting platelet adhesion and vascular inflammation. 2) Plt-VWF even in the absence of ADAMTS13 in the platelets and plasma was not able to promote atherosclerosis.
The first evidence on the role of VWF in atherosclerosis was demonstrated in 1978 utilizing pigs with von Willebrand’s disease (VWD).17 These findings were further confirmed at the genetic level using either Vwf−/−LDLR−/− or Vwf−/−Apoe−/− mice,9, 12 suggesting a protective effect of complete VWF deficiency in atherosclerotic lesion development. However, the relative contribution of different pools of VWF in the pathophysiology of atherosclerosis was never investigated. We determined the differential role of Plt-VWF and EC-VWF in the progression of atherosclerosis because of functional differences between them. Although binding affinities to collagen are comparable between Plt-VWF and EC-VWF, Plt-VWF binds with higher affinity to the surface of thrombin-stimulated platelets when compared with EC-VWF.21, 22 Other studies suggested that Plt-derived VWF binds efficiently to activated αIibβ3 and αIIb contributes to atherosclerosis.22, 23, 25, 26 In contrast, Plt-derived VWF was demonstrated to be less capable of binding to GPIbα when compared with EC-VWF.22 We and others have recently found that Plt-VWF contributed to transient thrombus growth in experimental models of thrombosis.27, 28 Based on these studies, we speculated that Plt-VWF might alone be sufficient to promote atherosclerosis exacerbation. To our surprise, we found that EC-VWF, but not Plt-VWF, solely contributes to atherosclerotic lesion progression in both aorta and aortic sinus. We found that atherosclerotic lesions in the EC-VWF were not only larger but more inflammatory (increased Ly6G-positive and Mac3-positive content, increased MMP9 and MMP2 content) associated with the larger necrotic area and reduced interstitial collagen when compared to Plt-VWF mice. Furthermore, we found an increase in chemokine fractalkine (CX3CL1) in the lesions of EC-VWF mice when compared to Plt-VWF mice. CX3CL1 was shown to mediate platelet adhesion and leukocyte recruitment via endothelial VWF.29 Together, these studies suggest that EC-VWF, but not platelet VWF, exacerbate atherosclerosis by promoting vascular inflammation.
VWF deficiency in mice results in a defect in Weibel-Palade body formation, which results in lower P-selectin expression and defect in leukocyte recruitment in an experimental model of acute inflammation when fed a chow diet.30 Is it possible that decreased P-selectin expression in endothelial cells of chimeric Plt-VWF mice, which lack EC-VWF, may mask the effect of Plt-VWF in atherosclerotic lesion development? We think that such a possibility is minimal based on following observations. First, the absence of VWF reduces P-selectin dependent leukocyte rolling in mesenteric venules of mice fed a chow diet but not an atherogenic diet.12 This result suggests that increased P-selectin synthesis and direct plasma membrane deposition under atherogenic conditions is less dependent on regulated secretion of Weibel-Palade bodies.12 Second, immunohistochemical staining of the aortic sinus revealed that P-selectin expression was comparable between EC-VWF and Plt-VWF mice fed a high a high-fat “Western” diet for 14 weeks. Third, reduction in the atherosclerotic lesion in P-selectin−/−LDLR−/− mice was not observed in female mice fed a high-fat diet for 8 weeks.31 Herein; we have used female mice fed a high fat “Western” diet for 14 weeks.
Platelets contain ADAMTS13,20, 32 in addition to ADAMTS13 present in plasma. A previous study suggested that Plt-VWF is resistant to ADAMTS13 cleavage in vitro because of different glycosylation profile.18 Based on these observations, we speculated that in the absence of ADAMTS13, VWF/ULVWF released from platelets might be sufficient to support atherosclerotic lesion progression by promoting leukocyte infiltration. To test this, we transplanted irradiated Vwf−/−Adamts13−/−Apoe−/− mice with BM from Adamts13−/−Apoe−/− donors so that VWF released after platelet activation is not cleaved by ADAMTS13. To our surprise, we found that atherosclerotic lesion progression in the aorta and aortic sinus was comparable in Plt-VWF/ADAMTS13−/− mice to Plt-VWF or VWF-KO mice. Results from these chimeric studies suggest that under physiological conditions Plt-VWF by itself, even in the absence of ADAMTS13, does not contribute to atherosclerosis.
Platelets bound to the activated endothelium are known to promote vascular inflammation, and thereby play a key role in the pathophysiology of atherosclerosis.23,24 We found that EC-VWF, but not platelet-VWF, markedly increased platelet and leukocyte adhesion in atheroprone carotid vessels under inflammatory conditions. These findings are in agreement with other studies, which have shown that EC-VWF released from activated endothelium can recruit quiescent platelets and leukocytes, though at low shear rate similar to condition that might occur in the regions of aorta and aortic sinus.6, 13, 33, 34 Another study showed that quiescent platelets adhere to endothelium via endothelial VWF get activated and can support leukocyte rolling at high shear stress in vitro via leukocyte P-selectin glycoprotein ligand-1 (PSGL-1) and platelet P-selectin.35 Activated platelets are known to promote acute inflammation by stimulating endothelium36 and accelerate atherosclerosis. Several studies suggest that in addition to vascular inflammation, hypercoagulability also contributes to the pathophysiology of atherosclerosis.37, 38 The possibility that endothelial VWF/platelet interactions may promote atherosclerosis exacerbation by enhancing hypercoagulability, in addition to vascular inflammation cannot be ruled out.
Despite animal studies that strongly support the role of VWF in atherosclerosis, the evidence of protective effect of VWD in humans is weak.39–44 Autopsies showed that VWD patients were not protected from atherosclerosis.42–44 On the other hand patients with more severe hemophilia A or VWD had fewer atherosclerotic plaques compared to patients with less severe coagulopathy.45 The possibility that VWD patients have some detectable level of VWF activity in plasma (Ristocetin cofactor activity; ~30–50 IU/dL, whereas the normal range is 50–200 IU/dL) that might be sufficient to promote atherosclerosis cannot be ruled out. We note that there is one study, which demonstrated that patients with type 3 severe VWD (completely lack VWF) are not protected from atherosclerosis. However, the overall plaque burden in the study population was less than 20% most likely because of inclusion of the young subjects (mean age 35 years).46 More clinical studies are needed to further clarify the role of VWF in promoting atherosclerosis in humans.
In conclusion, the present study identifies that EC-VWF, but not Plt-VWF, as a key player that promotes atherosclerosis in the aorta and aortic sinus of Apoe−/− mice, a clinical-relevant model of atherosclerosis.
Supplementary Material
Highlights.
VWF is synthesized in endothelial cells and megakaryocytes.
Endothelial cell-derived VWF, but not platelet-derived, contributes to VWF-dependent atherosclerosis in apolipoprotein E deficient mice by promoting platelet adhesion and vascular inflammation.
Platelet-derived VWF even in the absence of ADAMTS13 (both in the platelets and plasma) was not sufficient to promote atherosclerosis in apolipoprotein E deficient mice.
Acknowledgments
Sources of funding
A.K.C lab is supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health grants (R01 HL118246 and R01 HL118742) and by innovative grant 16IRG27490003 from American Heart Association.
Non-standard Abbreviations and Acronyms
- VWF
von Willebrand factor
- ULVWF
Ultra large von Willebrand factor
- ADAMTS13
A Disintegrin And Metalloprotease with Thrombospondin type I repeats-13
- Plt-VWF
Platelet-derived von Willebrand factor
- EC-VWF
Endothelial cell-derived von Willebrand factor
- WT
Wild-type
- VWF-KO
VWF-Knock out
- BMT
Bone marrow transplantation
- TNF
Tumor necrosis factor
Footnotes
Disclosures
None.
References
- 1.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von willebrand protein in weibel-palade bodies of human endothelial cells. The Journal of cell biology. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. Adamts-13 rapidly cleaves newly secreted ultralarge von willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 3.Tsai HM, Nagel RL, Hatcher VB, Seaton AC, Sussman II. The high molecular weight form of endothelial cell von willebrand factor is released by the regulated pathway. British journal of haematology. 1991;79:239–245. doi: 10.1111/j.1365-2141.1991.tb04528.x. [DOI] [PubMed] [Google Scholar]
- 4.Sporn LA, Chavin SI, Marder VJ, Wagner DD. Biosynthesis of von willebrand protein by human megakaryocytes. J Clin Invest. 1985;76:1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. Von willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan AK, Kisucka J, Brill A, Walsh MT, Scheiflinger F, Wagner DD. Adamts13: A new link between thrombosis and inflammation. J Exp Med. 2008;205:2065–2074. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. Von willebrand factor-cleaving protease adamts13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, Dietrich B, Rottensteiner H, Scheiflinger F, Wagner DD. Protective anti-inflammatory effect of adamts13 on myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5217–5223. doi: 10.1182/blood-2012-06-439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi C, Ahmad A, Wilson KM, Chauhan AK. Adamts13 modulates atherosclerotic plaque progression in mice via a vwf-dependent mechanism. J Thromb Haemost. 2014;12:255–260. doi: 10.1111/jth.12456. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi C, Khan MM, Lentz SR, Chauhan AK. Adamts13 reduces vascular inflammation and the development of early atherosclerosis in mice. Blood. 2012;119:2385–2391. doi: 10.1182/blood-2011-09-376202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi C, Motto DG, Jensen M, Lentz SR, Chauhan AK. Adamts13 deficiency exacerbates vwf-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5224–5230. doi: 10.1182/blood-2012-06-440255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Methia N, Andre P, Denis CV, Economopoulos M, Wagner DD. Localized reduction of atherosclerosis in von willebrand factor-deficient mice. Blood. 2001;98:1424–1428. doi: 10.1182/blood.v98.5.1424. [DOI] [PubMed] [Google Scholar]
- 13.Petri B, Broermann A, Li H, Khandoga AG, Zarbock A, Krombach F, Goerge T, Schneider SW, Jones C, Nieswandt B, Wild MK, Vestweber D. Von willebrand factor promotes leukocyte extravasation. Blood. 2010;116:4712–4719. doi: 10.1182/blood-2010-03-276311. [DOI] [PubMed] [Google Scholar]
- 14.Khan MM, Motto DG, Lentz SR, Chauhan AK. Adamts13 reduces vwf-mediated acute inflammation following focal cerebral ischemia in mice. J Thromb Haemost. 2012;10:1665–1671. doi: 10.1111/j.1538-7836.2012.04822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinschnitz C, De Meyer SF, Schwarz T, Austinat M, Vanhoorelbeke K, Nieswandt B, Deckmyn H, Stoll G. Deficiency of von willebrand factor protects mice from ischemic stroke. Blood. 2009;113:3600–3603. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 16.Jin SY, Tohyama J, Bauer RC, Cao NN, Rader DJ, Zheng XL. Genetic ablation of adamts13 gene dramatically accelerates the formation of early atherosclerosis in a murine model. Arterioscler Thromb Vasc Biol. 2012;32:1817–1823. doi: 10.1161/ATVBAHA.112.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuster W, Bowie EJ, Lewis JC, Fass DN, Owen CA, Jr, Brown AL. Resistance to arteriosclerosis in pigs with von willebrand’s disease. Spontaneous and high cholesterol diet-induced arteriosclerosis. J Clin Invest. 1978;61:722–730. doi: 10.1172/JCI108985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath RT, van den Biggelaar M, Byrne B, O’Sullivan JM, Rawley O, O’Kennedy R, Voorberg J, Preston RJ, O’Donnell JS. Altered glycosylation of platelet-derived von willebrand factor confers resistance to adamts13 proteolysis. Blood. 2013;122:4107–4110. doi: 10.1182/blood-2013-04-496851. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez MF, Ginsberg MH, Ruggeri ZM, Batlle FJ, Zimmerman TS. Multimeric structure of platelet factor viii/von willebrand factor: The presence of larger multimers and their reassociation with thrombin-stimulated platelets. Blood. 1982;60:1132–1138. [PubMed] [Google Scholar]
- 20.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, Ruan C, Moake JL, Dong JF. Platelet-derived vwf-cleaving metalloprotease adamts-13. J Thromb Haemost. 2005;3:2536–2544. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 21.Koutts J, Walsh PN, Plow EF, Fenton JW, 2nd, Bouma BN, Zimmerman TS. Active release of human platelet factor viii-related antigen by adenosine diphosphate, collagen, and thrombin. J Clin Invest. 1978;62:1255–1263. doi: 10.1172/JCI109246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams SB, McKeown LP, Krutzsch H, Hansmann K, Gralnick HR. Purification and characterization of human platelet von willebrand factor. British journal of haematology. 1994;88:582–591. doi: 10.1111/j.1365-2141.1994.tb05077.x. [DOI] [PubMed] [Google Scholar]
- 23.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 25.Mannucci PM. Platelet von willebrand factor in inherited and acquired bleeding disorders. Proc Natl Acad Sci USA. 1995;92:2428–2432. doi: 10.1073/pnas.92.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massberg S, Schurzinger K, Lorenz M, Konrad I, Schulz C, Plesnila N, Kennerknecht E, Rudelius M, Sauer S, Braun S, Kremmer E, Emambokus NR, Frampton J, Gawaz M. Platelet adhesion via glycoprotein iib integrin is critical for atheroprogression and focal cerebral ischemia: An in vivo study in mice lacking glycoprotein iib. Circulation. 2005;112:1180–1188. doi: 10.1161/CIRCULATIONAHA.105.539221. [DOI] [PubMed] [Google Scholar]
- 27.Dhanesha N, Prakash P, Doddapattar P, Khanna I, Pollpeter MJ, Nayak MK, Staber JM, Chauhan AK. Endothelial cell-derived von willebrand factor is the major determinant that mediates von willebrand factor-dependent acute ischemic stroke by promoting postischemic thrombo-inflammation. Arterioscler Thromb Vasc Biol. 2016;36:1829–1837. doi: 10.1161/ATVBAHA.116.307660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhenne S, Denorme F, Libbrecht S, Vandenbulcke A, Pareyn I, Deckmyn H, Lambrecht A, Nieswandt B, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. Platelet-derived vwf is not essential for normal thrombosis and hemostasis but fosters ischemic stroke injury in mice. Blood. 2015;126:1715–1722. doi: 10.1182/blood-2015-03-632901. [DOI] [PubMed] [Google Scholar]
- 29.Meyer dos Santos S, Klinkhardt U, Scholich K, Nelson K, Monsefi N, Deckmyn H, Kuczka K, Zorn A, Harder S. The cx3c chemokine fractalkine mediates platelet adhesion via the von willebrand receptor glycoprotein ib. Blood. 2011;117:4999–5008. doi: 10.1182/blood-2011-02-335471. [DOI] [PubMed] [Google Scholar]
- 30.Denis CV, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of p-selectin affects leukocyte recruitment in von willebrand factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD. Absence of p-selectin delays fatty streak formation in mice. J Clin Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki M, Murata M, Matsubara Y, Uchida T, Ishihara H, Shibano T, Ashida S, Soejima K, Okada Y, Ikeda Y. Detection of von willebrand factor-cleaving protease (adamts-13) in human platelets. Biochem Biophys Res Commun. 2004;313:212–216. doi: 10.1016/j.bbrc.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 33.Andre P, Denis CV, Ware J, Saffaripour S, Hynes RO, Ruggeri ZM, Wagner DD. Platelets adhere to and translocate on von willebrand factor presented by endothelium in stimulated veins. Blood. 2000;96:3322–3328. [PubMed] [Google Scholar]
- 34.Theilmeier G, Michiels C, Spaepen E, Vreys I, Collen D, Vermylen J, Hoylaerts MF. Endothelial von willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99:4486–4493. doi: 10.1182/blood.v99.12.4486. [DOI] [PubMed] [Google Scholar]
- 35.Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3:562–570. doi: 10.1111/j.1538-7836.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 36.Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce weibel-palade-body secretion and leukocyte rolling in vivo: Role of p-selectin. Blood. 2005;106:2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borissoff JI, Heeneman S, Kilinc E, Kassak P, Van Oerle R, Winckers K, Govers-Riemslag JW, Hamulyak K, Hackeng TM, Daemen MJ, ten Cate H, Spronk HM. Early atherosclerosis exhibits an enhanced procoagulant state. Circulation. 2010;122:821–830. doi: 10.1161/CIRCULATIONAHA.109.907121. [DOI] [PubMed] [Google Scholar]
- 38.Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 39.Bilora F, Dei Rossi C, Girolami B, Casonato A, Zanon E, Bertomoro A, Girolami A. Do hemophilia a and von willebrand disease protect against carotid atherosclerosis? A comparative study between coagulopathics and normal subjects by means of carotid echo-color doppler scan. Clin Appl Thromb Hemost. 1999;5:232–235. doi: 10.1177/107602969900500405. [DOI] [PubMed] [Google Scholar]
- 40.Bilora F, Zanon E, Casonato A, Bertomoro A, Petrobelli F, Cavraro M, Campagnolo E, Girolami A. Type iib von willebrand disease: Role of qualitative defects in atherosclerosis and endothelial dysfunction. Clin Appl Thromb Hemost. 2007;13:384–390. doi: 10.1177/1076029607303613. [DOI] [PubMed] [Google Scholar]
- 41.Sramek A, Reiber JH, Gerrits WB, Rosendaal FR. Decreased coagulability has no clinically relevant effect on atherogenesis: Observations in individuals with a hereditary bleeding tendency. Circulation. 2001;104:762–767. doi: 10.1161/hc3501.094232. [DOI] [PubMed] [Google Scholar]
- 42.Federici AB, Mannucci PM, Fogato E, Ghidoni P, Matturri L. Autopsy findings in three patients with von willebrand disease type iib and type iii: Presence of atherosclerotic lesions without occlusive arterial thrombi. Thromb Haemost. 1993;70:758–761. [PubMed] [Google Scholar]
- 43.Kernoff LM, Rose AG, Hughes J, Jacobs P. Autopsy findings in an elderly man suffering from severe von willebrand’s disease. Thromb Haemost. 1981;46:714–716. [PubMed] [Google Scholar]
- 44.Silwer J, Cronberg S, Nilsson IM. Occurrence of arteriosclerosis in von willebrand’s disease. Acta Med Scand. 1966;180:475–484. doi: 10.1111/j.0954-6820.1966.tb02860.x. [DOI] [PubMed] [Google Scholar]
- 45.Bilora F, Boccioletti V, Zanon E, Petrobelli F, Girolami A. Hemophilia a, von willebrand disease, and atherosclerosis of abdominal aorta and leg arteries: Factor viii and von willebrand factor defects appear to protect abdominal aorta and leg arteries from atherosclerosis. Clin Appl Thromb Hemost. 2001;7:311–313. doi: 10.1177/107602960100700411. [DOI] [PubMed] [Google Scholar]
- 46.Sramek A, Bucciarelli P, Federici AB, Mannucci PM, De Rosa V, Castaman G, Morfini M, Mazzucconi MG, Rocino A, Schiavoni M, Scaraggi FA, Reiber JH, Rosendaal FR. Patients with type 3 severe von willebrand disease are not protected against atherosclerosis: Results from a multicenter study in 47 patients. Circulation. 2004;109:740–744. doi: 10.1161/01.CIR.0000112567.53841.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.