Abstract
Objective
IL-35 is an anti-inflammatory cytokine, which inhibits immune responses by inducing regulatory T cells and regulatory B cells, and suppressing effector T cells and macrophages. It remains unknown whether atherogenic stimuli induce IL-35, and whether IL-35 inhibits atherogenic lipid-induced endothelia cell (EC) activation and atherosclerosis. EC activation induced by hyperlipidemia stimuli including lysophosphatidylcholine (LPC) is considered as an initiation step for monocyte recruitment and atherosclerosis. In this study, we examined the expression of IL-35 during early atherosclerosis, and the roles and mechanisms of IL-35 in suppressing LPC-induced EC activation.
Approach and Results
Using microarray and ELISA, we found that IL-35 and its receptor are significantly induced during early atherosclerosis in the aortas and plasma of apolipoprotein E (ApoE) knockout mice, an atherosclerotic mouse model, as well as in the plasma of hypercholesterolemic patients. In addition, we found that IL-35 suppresses LPC-induced monocyte adhesion to human aortic ECs (HAEC). Furthermore, our RNA-Seq analysis shows that IL-35 selectively inhibits LPC-induced EC activation-related genes such as ICAM-1. Mechanistically, using flow cytometry, mass spectrometry, electron spin resonance analyses, and CHIP-Seq analyses, we found that IL-35 blocks LPC-induced mitochondrial reactive oxygen species (mtROS), which are required for the induction of site-specific histone 3 lysine 14 (H3K14) acetylation, increased binding of pro-inflammatory transcription factor AP-1 in the promoter of ICAM-1, and induction of ICAM-1 transcription in HAEC. Finally, IL-35 cytokine therapy suppresses atherosclerotic lesion development in ApoE knockout mice.
Conclusions
IL-35 is induced during atherosclerosis development and inhibits mtROS-H3K14ac-AP-1-mediated EC activation.
Keywords: inteleukin-35, mitochondrial reactive oxygen species, histone acetylation, endothelial cell activation, atherosclerosis
Introduction
Cardiovascular disease (CVD) remains a leading cause of fatality in well-developed countries. As a chronic autoimmune inflammatory disease, atherosclerosis involves both the innate and adaptive arms of the immune response that mediate the initiation, progression, and ultimate thrombotic complications of atherosclerosis1. We and others have reported that hyperlipidemia, along with other CVD stressors, such as hyperglycemia, chronic kidney disease, obesity, and hyperhomocysteinemia, promote atherosclerosis via several mechanisms. These mechanisms include endothelial cell (EC) activation and injury2–5; monocyte recruitment and differentiation6, 7; decreased regulatory T cells (Tregs)8–10; impaired vascular repair ability of bone marrow-derived progenitor cells11–13; increased migration and proliferation of vascular smooth muscle cells14, 15, and high fat-induced adipocyte hypertrophy16. However, the underlying mechanisms of how Tregs inhibit vascular response during atherosclerosis remain not fully understood.
CD4+ T helper cells (Th) play essential roles in regulating inflammation and immune responses via differentiation into various Th functional subsets, including Th1, Th2, Th17, Th9, Th22, follicular Th, and regulatory T cells (Tregs)17. The majority of Th cell functions are fulfilled via the secretion of various cytokines, which can play a dual role in regulating atherogenesis18. Proinflammatory and Th1-related cytokines such as interleukin-1 (IL-1) promote the development and progression of atherosclerosis19, 20. However, Tregs-related anti-inflammatory cytokines such as interleukin 10 (IL-10) and transforming growth factor-β (TGF-β) exert clear anti-atherogenic activities21, 22. Recently, IL-35 has been identified as a novel immunosuppressive/anti-inflammatory cytokine23. IL-35 is a dimeric protein with two subunits, IL-35A and Epstein-Barr virus induced 3 (EBI3)24, 25. IL-35 is secreted by Tregs, regulatory B cells (Bregs) 26, dendritic cells27, and to less extent by endothelial cells, smooth muscle cells and monocytes28. We have previously reported that in contrast to another immunosuppressive cytokine TGF-β, IL-35 is not constitutively expressed in tissues but rather induced by proinflammatory stimuli23, 29 and inhibits lipopolysaccharide (LPS)-induced endothelial cell activation29; and that IL-35 is a newly proposed homeostasis-associated molecular pattern30. However, an important issue of whether IL-35 is induced during chronic atherogenesis development remains unknown.
Lysophosphatidylcholines (LPCs) are a group of bioactive, proinflammatory lipid molecules and a newly proposed conditional danger-associated molecular pattern31, which are critically involved in the development of atherosclerosis32. It has been suggested that LPC-induced EC activation is an initiation step of atherogenesis4. During early hyperlipidemia, LPCs are induced and activate EC to produce adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), which mediate the adhesion and migration of leukocyte into the intima33, contributing to atherosclerosis development. We have shown previously that IL-35 could inhibit lipopolysaccharide-induced EC activation process during acute inflammatory responses in sepsis29. However, the issues of whether IL-35 could suppress hyperlipidemia stimuli-induced EC activation during chronic inflammation; and by what mechanisms IL-35 suppresses EC activation remain unclear.
Epigenetic regulations in the cell include DNA methylation, non-coding RNAs, and post translational modification of histone proteins such as acetylation, methylation, phosphorylation, ubiquitylation, and SUMOylation34–36. Key post translational modifications of histones include methylation and acetylation35. Histone acetylation favors gene transcription, and is considered as a hallmark of transcriptionally active regions. The addition of acetyl groups on histone tails facilitate gene transcription by neutralizing the histone charges, thus weakening histone-DNA interaction, relaxing the chromatin structure, and allowing related transcription factors to bind to gene promoters37. It has been shown that proatherogenic stimuli oxidized low density lipoprotein (oxLDL) induces histone 3 (H3) and H4 acetylation globally, which contributes EC activation by upregulating IL-8 and monocyte chemoattractant protein-1 (MCP-1/CCL2) expression38. However, since there are at least 12 histone acetylation sites in the H3 histone tails alone39, it remains unknown whether site-specific histone acetylation events contribute to hyperlipidemia-induced EC activation.
Thus, despite significant progress, several important knowledge gaps exist: First, the expression profile of IL-35 in atherosclerosis remains unknown; and second, the roles and detailed molecular mechanisms of IL-35 in hyperlipidemic stimuli-induced aortic EC activation and atherogenesis development is unclear. In this study, we hypothesized that IL-35 is induced during atherosclerosis development, and plays an anti-atherosclerosis role. Using RNA-sequencing (Seq), chromatin immunoprecipitation (CHIP)-Seq, mass spectrometry, electron spin resonance, and several other biochemical assays, we found that IL-35 is an atherosclerosis-induced anti-inflammatory cytokine, which inhibits hyperlipidemic stimuli-induced EC activation and atherosclerosis. Moreover, we identified a novel mitochondrial reactive oxygen species (mtROS)-driven site-specific histone 3 lysine 14 (H3K14) acetylation mechanism in driving EC activation gene transcription, which could be suppressed by IL-35. Thus, a previously uncharacterized novel pathophysiological pathway linking “three dots” including endogenous metabolite danger signal LPC as we proposed31, 40, mitochondria-derived metabolites (mtROS), and nucleus signaling is uncovered, which is important for hyperlipidemia-induced EC activation. Our in-depth analysis of the roles of IL-35 in suppressing EC activation and atherosclerosis development has provided novel avenues for therapeutic treatments for vascular inflammation and other CVDs.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
1. IL-35 receptor subunit transcripts are increased in atherosclerotic ApoE−/− mouse aortas; and plasma IL-35 cytokine is increased in patients with hypercholesterolemia and ApoE−/− mice
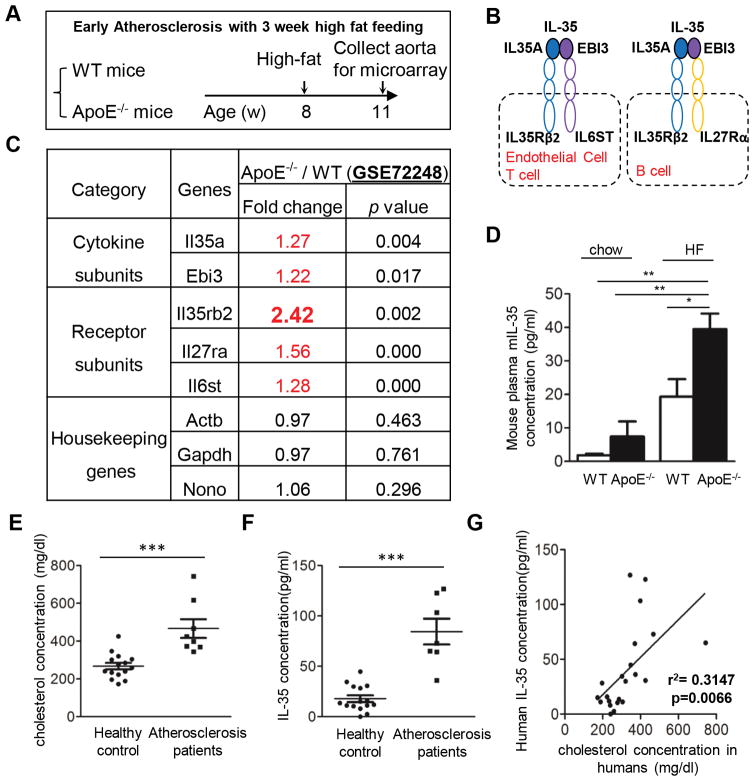
We have shown previously that IL-35 is a responsive anti-inflammatory cytokine which is induced in several vascular cell types in response to inflammatory cytokine stimulation and during acute inflammatory response in endotoxemia23, 29, 30. In this study, we hypothesized that IL-35 is also induced in the development of atherosclerosis, which is a chronic inflammatory disease. To test this hypothesis, firstly we examined the gene expression of IL-35 and its receptor in the aortas of apolipoprotein E knockout (ApoE−/−) mice in comparison of those of wild-type (WT) mice using microarray analysis (Figure 1A). It has been reported that the engagement of IL-35 with different receptor subunits depends on the cell type studied30, 41 (Figure 1B). Regardless of the receptor composition, our results showed that both IL-35 subunits (Il35a and Ebi3) and all the potential IL-35 receptor subunits are significantly induced in the aortas of ApoE−/− mice in comparison to those of WT mice (Figure 1C). Notably, IL-35 receptor subunit IL35rb2 was highly induced when comparing to the IL-35 itself, indicating that the aorta tissue is a target rather than a source of IL-35 during early atherosclerosis development. This result is supported by the findings of increased IL-35 levels in the plasma of ApoE−/− mice fed with either chow diet or high fat diet, compared with those of WT mice (Figure 1D). We also examined IL-35 protein expression in the plasma samples from patients with hypercholesterolemia and healthy controls, as judged by their plasma cholesterol concentrations (Figure 1E). We found that the levels of IL-35 are significantly induced in the hypercholesterolemic patients; and that increased IL-35 levels are positively correlated with cholesterol concentrations (Figures 1F and 1G). Taken together, these results suggest that hyperlipidemia conditions upregulate IL-35 cytokine expressions in the blood, which could then signal in the aortic cells by heightened IL-35 receptor expression.
Figure 1. IL-35 receptor subunits are increased in atherosclerotic ApoE−/− mouse aortas; and plasma IL-35 cytokine is increased in patients with atherosclerosis and ApoE−/− mice.
A. Microarray design. Wild-type (WT) and apolipoprotein E knockout (ApoE−/−) mice were fed with high fat (HF) diet for 3 weeks (w) starting from 8-week old. The RNAs of mouse aortas were collected, reverse-transcribed and subjected to the Affymetrix microarray analysis. B. Schematic representation of the subunits and receptor of IL-35. C. Fold change of the expression of IL-35-related genes in the aortas of ApoE−/− mice and WT mice detected by microarray experiments. n=5 mice in each group. D. The ELISA was performed for the detection of mouse IL-35 in the plasma samples from WT mice and ApoE−/− mice fed with high fat diet for 12 weeks (WT: n=7; ApoE−/− : n=8) or normal chew (WT: n=3; ApoE−/−: n=4). E. Plasma cholesterol levels were measured in the plasma samples from same patients (n=8) and healthy controls (n=15). F. The ELISA was performed for the detection of human IL-35 in the plasma samples from patients with hypercholesterolemia (n=7) and healthy controls (n=15). G. Positive correlation between plasma cholesterol levels and IL-35 levels (simple linear regression analysis) in patient samples. For all panels, data are expressed as mean ± SEM.*, p<0.05, **, p<0.01, ***, p<0.001. HF, high fat; WT, wild-type; ApoE−/−, apolipoprotein E knockout.
2. IL-35 suppresses pro-atherogenic lysophosphatidylcholine (LPC)-induced human aortic endothelial cell activation by inhibiting ICAM-1 expression
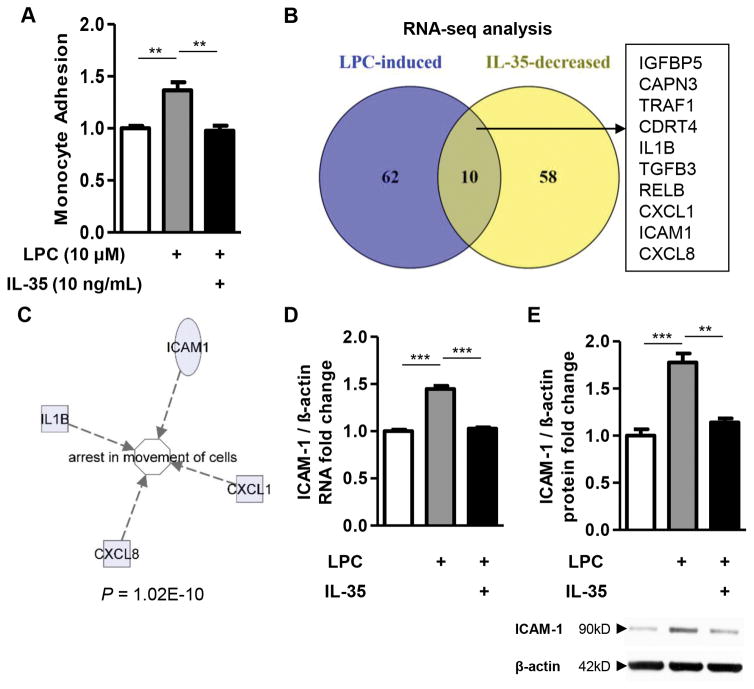
Next, we hypothesized that IL-35 could inhibit lysophosphatidylcholine (LPC)-induced EC activation process, which is an initiation step of atherogenesis. The results showed that IL-35 could completely abolish non-stimulated human peripheral blood monocyte adhesion to LPC-treated HAECs (Figure 2A). The results allowed us to argue that IL-35 could inhibit LPC-induced EC activation by inhibiting the gene expression of adhesion molecules and chemokines/cytokines. To test this hypothesis, we performed RNA-Seq experiments using the following three RNA samples including: a) control HAECs; b) HAECs stimulated with LPC; and c) HAECs stimulated with LPC and IL-35. We found that among the genes that were significantly induced by LPC in HAECs, IL-35 could significantly inhibit the expression of 10 genes, including insulin like growth factor binding protein 5 (IGFBP5), calpain 3 (CAPN3), tumor necrosis factor receptor associated factor 1 (TRAF1), CMT1A duplicated region transcript 4 (CDRT4), IL1B, TGF-β3 (TGFB3), NF-kB subunit RELB, chemokine (C-X-C motif) ligand 1 (CXCL1), intercellular adhesion molecule 1 (ICAM1), and CXCL8 (Figure 2B and Supplemental Tables 1 and 2). Strikingly, the Ingenuity Pathway Analysis revealed that “arrest in movement of cells” is the most significantly enriched pathway, with four out of these ten IL-35-inhibited genes in this pathway (p = 1.02E-10, Figure 2C and Supplemental Table 3). Using EC adhesion molecule ICAM-1 as prototype for LPC-induced but IL-35-suppressed EC activation molecules, we performed both RT-PCR and Western blots, and confirmed that IL-35 significantly inhibited LPC-induced ICAM-1 RNA and protein expression (Figures 2D and E). Taken together, these results strongly support the notion that IL-35 suppresses LPC-induced EC activation by inhibiting EC activation-related genes such as ICAM-1.
Figure 2. IL-35 suppresses pro-atherogenic lysophosphatidylcholine (LPC)-induced human aortic endothelial cell activation by inhibiting ICAM-1 expression.
A. Effects of IL-35 on LPC-induced monocyte adhesion to human aortic endothelial cells (HAECs). HAECs were treated with LPC (10μM) with or without IL-35 (10ng/ml) for 4 hours, after which the adhesion of non-stimulated, fluorescence-labeled human peripheral blood mononuclear cells to stimulated HAECs was quantified (n=4). B to E. HAECs were treated with LPC (10μM) with or without IL-35 (10ng/ml) for 18 hours, RNA-Seq (B, shown as Venn diagram, pooled samples of 5 in each group), Ingenuity Pathway Analysis of IL-35-regulated genes (C), RT-PCR (D, n=3), and Western blots (E, n=3) were performed. For all panels, data are expressed as mean ± SEM. NS, not significant, **, p<0.01, ***, p<0.001. LPC, lysophosphatidylcholine
3. IL-35 suppresses LPC-induced mtROS, which are required for site-specific histone acetylation of H3K14
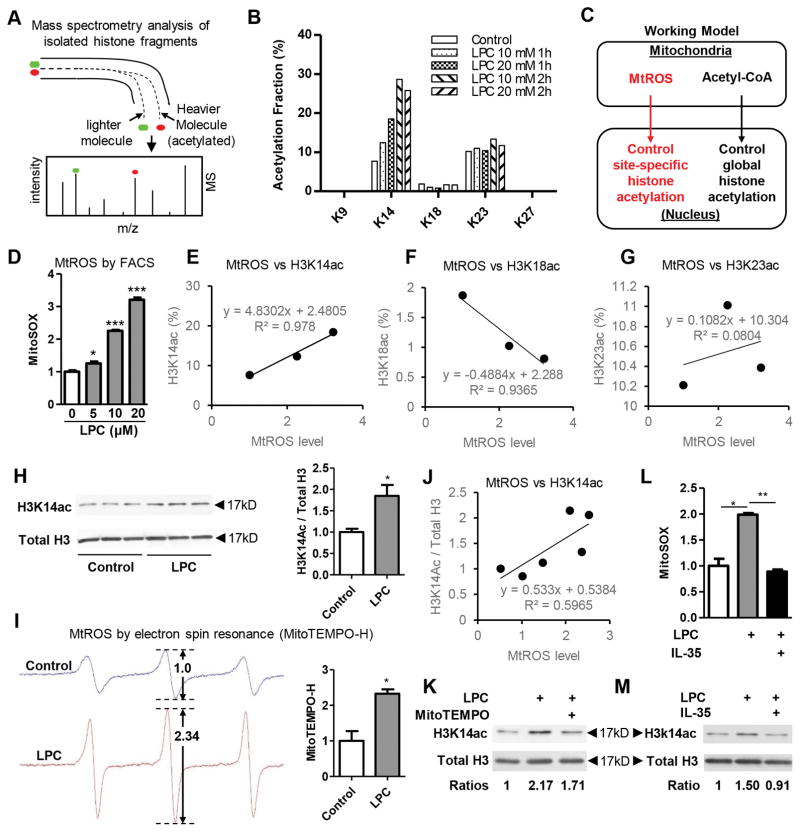
Endothelial cell activation process is accompanied by massive chromatin remodeling process, which is required for the stable transcriptional induction of EC activation-related gene expression42. Histone acetylation favors gene transcription and is considered as a hallmark of transcriptionally active regions. The addition of acetyl groups on histone tails was considered to facilitate gene transcription by neutralizing the histone charges, thus weakening histone-DNA interaction and relaxing the chromatin structure36, 37. Our recent report showed that among 164 histone modification enzymes in ApoE−/− mouse aortas, atherosclerosis downregulated as many as 8 histone deacetylases (HDACs), upregulated only one histone acetyltransferase 1 (HAT1) but none of HDACs, suggesting that in atherogenic process site-specific histone acetylation may be a major event36. Thus, we hypothesized that during EC activation process elicited by hyperlipidemia stimulation LPC, chromatin remodeling of EC activation genes such as ICAM-1 occurs presumably via increased HAT-mediated specific histone acetylation, which could be inhibited by IL-35. To test this hypothesis, we applied site-specific histone acetylation screening by high-performance liquid chromatography coupled with mass spectrometer (HPLC-MS) method43, 44 (Figure 3A). We screened for 8 different lysine acetylation positions on histone H3 including lysine 9 (K9), K14, K18, K23, K27, K36, K37 and K64 simultaneously (Supplemental Figure 1A). The results showed that LPC treatment (10μM) for 2 hours (h) specifically induces H3K14 acetylation (H3K14ac) in HAECs in a dose- and time- dependent manner (Figure 3B), and to a lesser degree H3K23 acetylation (Supplemental Figure 1B). The acetylation levels of H3K9 and H3K18 were not significantly changed by LPC treatment, and the acetylation levels of K27, K36, K37, and K64 did not reach the detection limits, and were not plotted.
Figure 3. IL-35 suppresses LPC-induced mtROS, which are required for site-specific histone acetylation of H3K14.
A. Principle of mass spectrometry analysis of isolated histone fragments. B. After treatment of human aortic endothelial cells (HAECs) with LPC for different doses (10μM/20μM) and different time points (1 hour/2 hour), histones were purified. After propionylation and trypsin digestion, the acetylation fractions on individual lysine residues on histone H3 were measured by HPLC-MS method. C. Working model of communications between mitochondria and nucleus through means of mtROS and acetyl-CoA. D. After loading with MitoSOX (5 μM) for 10 min, HAECs were treated with LPC (10 μM, 16:0) for different doses for 1 hour and flow cytometry analysis was performed to quantify mtROS (n=3). E to G. Correlation analyses between mtROS and site-specific histone acetylation from the data of panel B and D. H. HAECs were treated with LPC (10μM) for 2 hours and protein was collected for Western blot analysis. Quantitation was shown in the right (n=3). I. Aliquots from the same samples from panel H were also used to measure mtROS by electron spin resonance by MitoTEMPO-H. Quantitation was shown in the right (n=3). J. Correlation analyses between mtROS and site-specific histone acetylation from the data of panel H and I. K. HAECs were treated with LPC (10μM) or LPC (10μM) plus MitoTEMPO-H (1μM) for 2 hours and protein was collected for Western blot analysis. L. HAEC are preloaded with MitoSOX (5μM) for 10 minutes and washed three times with PBS afterwards. Then, HAEC are treated with LPC (10μM) with or without IL-35 (10ng/ml). After 1 hour of stimulation, cells are collected for FACS analysis. Data are presented as fold change of percentage of MitoSOX positive cells (n=4). M. HAECs were treated with LPC (10μM) with or without IL-35 (10ng/ml) for 2 hours, proteins are collected for western blot analysis. For all panels, data are expressed as mean ± SEM. NS, not significant, *, p<0.05, ***, p<0.001. MtROS, mitochondrial reactive oxygen species.
It has been reported previously that mitochondria serve as upstream regulators of global histone acetylation by providing the substrate in the form of acetyl-coA45 (Figure 3C). Since we have previously reported that mitochondrial ROS (mtROS)46 mediate LPC-induced EC activation4, we hypothesized that mitochondria-derived mtROS signaling provides additional layer of histone regulation by site-specific regulation of histone H3 (Figure 3C). We found that LPC dose-dependently increase mtROS in HAEC, as we have reported previously4 (Figure 3D). Importantly, the induction of mtROS was positively associated with the induction of H3K14ac, but not H3K18ac or H3K23ac (Figure 3E to G). To consolidate these findings, we used two different methods, Western Blots and electron spin resonance, to simultaneously measure H3K14ac and mtROS, respectively, in the same group of cells. Similar to the results obtained from the analyses of mass spectrum and flow cytometry, we found that LPC could significantly induce H3K14ac and mtROS in HAECs (Figure 3H and I); and that the induction of mtROS was positively associated with H3K14ac increase (Figure 3J). Furthermore, when mtROS inhibitor MitoTEMPO is applied, LPC-induced H3K14ac is inhibited (Figure 3K), suggesting that mtROS is located upstream of the histone H3 acetylation, and specifically mediate LPC-induced H3K14ac. Remarkably, IL-35 could completely inhibit LPC-induced mtROS and H3K14ac (Figure 3L and M), suggesting that inhibition of H3K14ac by IL-35 probably act via suppressing LPC-induced mtROS first. Collectively, we unveiled a new mitochondria-to-nucleus communication pathway during EC activation, which could be blocked by IL-35.
4. LPC-induced H3K14ac is required for ICAM-1 gene expression by increasing the accessibility of AP-1, which is inhibited by IL-35
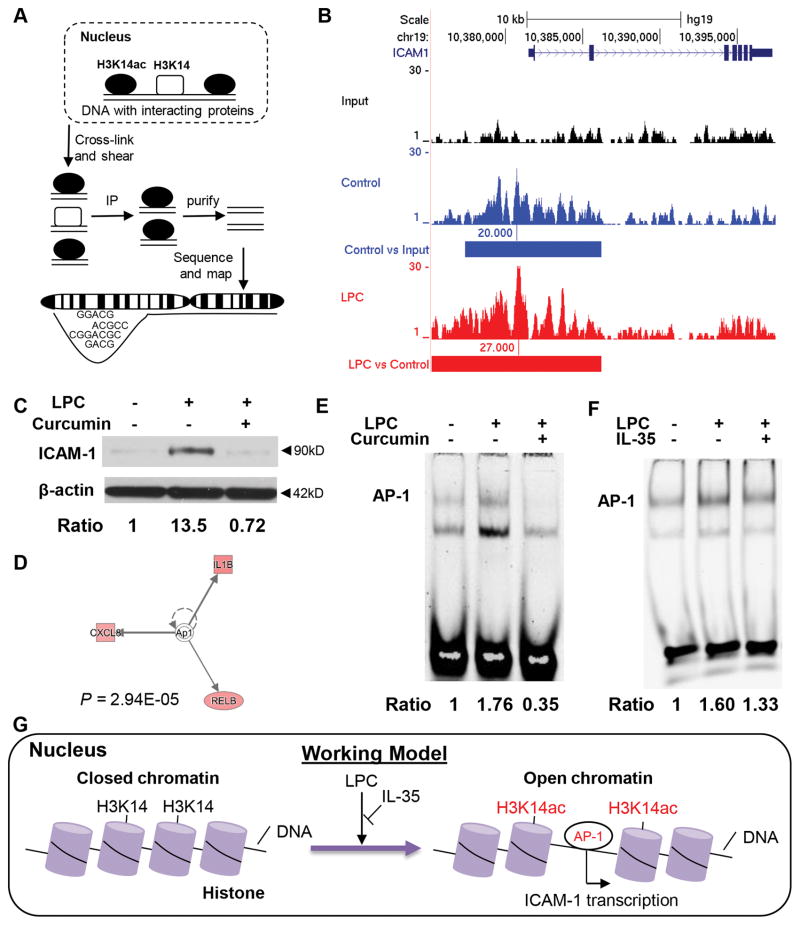
Next, we hypothesized that H3K14ac induced by LPC may result in increased binding of H3K14ac to specific EC activation-related areas in HAEC genomic DNAs. To test this hypothesis, we performed CHIP-Seq of H3K14ac in HAECs treated with vehicle or LPC (Figure 4A). The results showed that LPC further induces both stronger and wider H3K14ac signals in the promoter and enhancer regions of ICAM-1 while there are weak H3K14ac signals in the ICAM-1 promoter region under basal conditions (Figure 4B). In addition, since we reported that atherosclerosis specifically upregulates HAT expression36, when HAT inhibitor curcumin (which blocks histone acetylation) was applied, the induction of ICAM-1 by LPC was abolished (Figure 4C), suggesting that HAT-mediated H3K14ac is responsible for LPC-increased ICAM-1 upregulation.
Figure 4. LPC-induced H3K14ac is required for ICAM-1 gene expression by increasing the accessibility of AP-1, which is inhibited by IL-35.
A. Principle of CHIP-Seq of H3K14ac. B. After treatment of vehicle or LPC (10μM) for 2 hours, chromatins were collected, cross-linked, and sheared. Immunoprecipitation of H3K14ac was performed afterwards and the resulting co-immunoprecipitated DNA regions were purified, sequenced, and aligned to human genome (hg19). Unprecipitated chromatin sample was used for input control. Data were visualized using UCSC genome browser. Blue bars indicate significant difference between control and input, red bars indicate significant difference between LPC and control. Pooled samples in each group (n=5). C. HAECs were treated with LPC (10 μM) with or without Curcumin (10 μM) for 18 hours and protein was collected for Western blot analysis. D. AP-1 is predicted to be the upstream regulator of IL-35-regulated genes by Ingenuity Pathway Analysis. E. HAECs were treated with LPC (10 μM) with or without Curcumin (10 μM) for 1 hour and electrophoretic mobility shift assay (EMSA) of AP-1 was performed. F. HAECs were treated with LPC (10 μM) with/without IL-35 (10ng/mL) for 1 hour and EMSA was performed. G. A new working model of how LPC inhibits LPC-induced H3K14ac and AP-1 binding to the promoter of ICAM-1.
We have previously shown that transcription factor AP-1 mediated LPC-induced EC activation4. In addition, among the ten genes that are suppressed by IL-35 in LPC-treated HAECs, three of them were previously predicted to be the downstream targets of AP-1 pathway by Ingenuity Pathway Analysis (Figure 4D). Besides, we have shown previously that AP-1 mediates LPC-induced EC activation4. Thus, we further hypothesized that LPC-induced H3K14ac contributes to ICAM-1 upregulation and EC activation by promoting the accessibility of AP-1 to ICAM-1 promoter, which was inhibited by IL-35. Using electrophoretic mobility shift assay, we found that HAT inhibitor Curcumin, which blocks H3K14ac, completely abolished AP-1 binding to the ICAM-1 promoter AP-1 site in HAEC DNAs. Moreover, IL-35 also inhibited LPC-induced AP-1 in HAEC (Figures 4E and F). Taken together, these results indicate that IL-35 suppresses LPC-induced ICAM-1 upregulation and EC activation by suppressing mtROS-H3K14ac-mediated AP-1 binding to ICAM-1 promoter (Figure 4G).
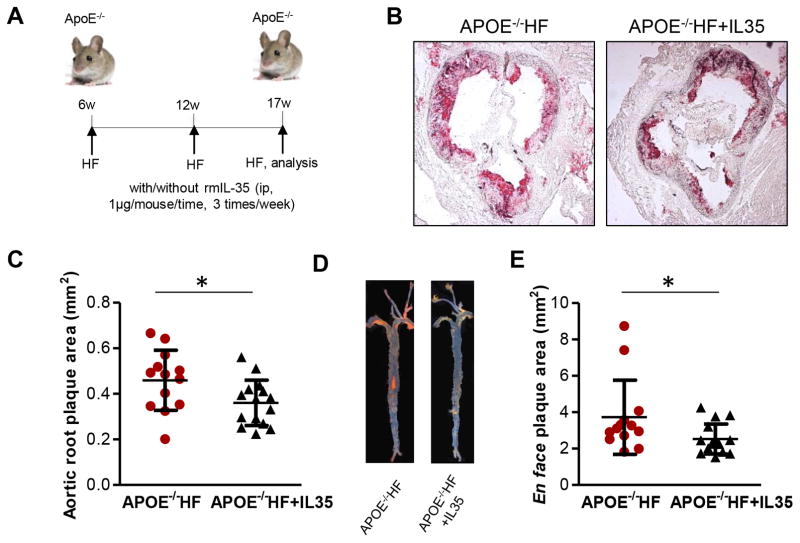
5. IL-35 inhibits the development of atherosclerosis in ApoE−/− mice
Finally, we hypothesized that IL-35 cytokine therapy could suppress atherosclerosis, at least in part due to inhibition of hyperlipidemic stimuli-promoted EC activation in ApoE−/− mice. To test this hypothesis, we adopted a reported method of cytokine therapy for atherosclerosis47. Our results showed that intra-peritoneal injection of mouse IL-35, three times per week (Figure 5A) for the last five weeks of 11-week high fat diet feeding period, significantly inhibited atherosclerosis development in ApoE−/− mice fed with high fat diet (Figures 5B to 5E). These data correlated well with a recent report, which showed similar results48, and suggested that pharmacological inhibition of endothelial activation and atherosclerosis by IL-35 could be developed into potential therapeutics against atherosclerosis development.
Figure 5. IL-35 inhibits the development of atherosclerosis in ApoE−/− mice.
A. Schematic representation of rmIL-35 therapeutic model in ApoE−/− mice with atherosclerosis (rmIL-35 intraperitoneal injection (1μg/g body weight/time, 3 times/week). B. Representative picture of Oil Red O staining of the cross section of aortic sinus from ApoE−/− mice fed with high fat for 5 weeks with or without mrIL-35. C. Quantification of aortic root plaque size. n=13–15 in each group. D. Representative picture of Sudan IV en face staining of the whole aortas from ApoE−/− mice fed with high fat for 5 weeks with or without mrIL-35. E. quantitation of en face plaque size. n=13–16 in each group. HF, high fat diet; rmIL-35, recombinant mouse IL-35. For all panels, data are expressed as mean ± SEM. *, p<0.05. HF, high fat; ApoE, apolipoprotein E.
Discussion
Atherosclerosis is a chronic inflammatory autoimmune disease, which is a major pathogenic process underlying the development of coronary heart disease, stroke and peripheral vascular disease. Despite of our improved understanding of the critical role the immune system in atherosclerosis49, current anti-inflammatory strategies are not clinically effective in the prevention and treatment of atherosclerotic cardiovascular disease50. We previously reported that IL-35 is a responsive anti-inflammatory cytokine upregulated during inflammatory response, but not a constitutively expressed house-keeping cytokine23. In addition, we also reported that IL-35 is induced in endotoxemia; and it inhibits endotoxin-induced acute vascular inflammation29. In the current study, we investigated the role and molecular mechanisms underlying IL-35 in suppressing aortic endothelial activation and atherosclerosis. We have made the following findings: 1) IL-35 is upregulated during early and advanced atherosclerosis in human and mouse plasma; and the expressions of IL-35 receptor transcripts are upregulated in atherogenic ApoE−/− mouse aortas, suggesting that IL-35 is also an inflammation-responsive cytokine in atherosclerosis; 2) IL-35 suppresses the adhesion of non-stimulated primary human monocytes to LPC-treated human aortic ECs, suggesting IL-35 inhibition of endothelial activation; 3) Global transcriptomic analyses reveal that IL-35 suppresses human aortic EC activation by inhibiting the expression of EC activation-related molecules including ICAM-1; 4) Mass spectrometric analysis of histone acetylation suggests that among eight potential histone 3 (H3) potentially acetylated lysine (K) positions including K9, K14, K18, K23, K27, K36, K37 and K64, LPC epigenetically regulates EC activation by specifically inducing H3K14 acetylation, which is inhibiting by IL-35; 5) the results from CHIP-Seq experiment indicate that LPC-induced H3K14ac contributes to ICAM-1 upregulation by increasing AP-1 accessibility to ICAM-1 promoter, which is suppressed by IL-35; and 6) Treatment of ApoE−/− mice with IL-35 cytokine significantly inhibits atherosclerosis development, presumably partially by inhibiting endothelial activation.
Previous studies have shown that mitochondria could communicate with the nucleus and globally regulate histone acetylation events by providing the substrates for histone acetyltransferases in the form of acetyl-CoA45. Nevertheless, site-specific histone acetylation regulation by the mitochondria must exist to regulate specific epigenetic events. To our knowledge, our study is the first report to indicate that mitochondria, in the form of mtROS, could specifically remodel the chromatin architecture in EC activation by contributing to site-specific histone acetylation events. Thus, mitochondria could both globally regulate histone acetylation by regulating the levels of acetyl-CoA and fine-tune specific histone events by signaling through mtROS (Figure 3C).
As we recently analyzed, histone acetylation is regulated by orchestrated effects of 31 histone acetyltransferases and 18 histone deacetylases36, which work either by themselves or cooperatively to acetylate/deacetylate specific site(s) on histones. Nevertheless, such “histone codes” remain not fully understood; and we speculate that LPC-induced mtROS might regulate the activity of certain histone modification enzymes. This notion was supported by our recent data mining report, which analyzed the expressions of 164 histone modification enzymes and showed that in metabolic diseases including atherosclerosis, the expressions of most histone deacetylases are downregulated while only one histone acetyltransferase (HAT1) is upregulated, suggesting a role of site-specific histone acetylation in atherogenesis36. Future experiments in characterizing the specific roles of different histone acetylation enzymes in EC activation and atherosclerosis are warranted.
Our results for the first time demonstrated that IL-35 can suppress EC activation by inhibiting mtROS generation. Since mtROS also contribute to inflammatory cytokine production and innate immune responses in macrophages and T cells51–53 besides mediating aortic endothelial cell activation4, it would be interesting to examine whether the proposed pathway holds true in these immune cells in the future. As we pointed out previously, mtROS levels are determined by the rates of both mtROS production and disposal46. Several factors can also regulate mtROS levels including: a) mitochondrial membrane potential Δψm; b) metabolic state of mitochondria; c) O2 concentration, mitochondrial mass; d) mitochondrial fusion; e) transcription factors p53, and STAT3; f) class III nicotine adenine dinucleotide (NAD+) dependent deacetylases including sirtuin 12; g) cytokines including TNF-α; and h) non-mitochondrial ROS sources54. In addition, our new paper reported that LPC-induced, ATP synthesis-uncoupled mitochondrial proton leak and calcium influx promote mtROS generation4, 5. Thus, future studies also need to address whether IL-35 specifically modulate some, if not all, of these mtROS regulating factors to inhibit mtROS; and whether other anti-inflammatory cytokines, such as IL-10 and TGF-β, could inhibit mtROS.
STAT3, initially identified as a transcription factor that regulates gene expression in response to cytokines such as IL-6 and IL-10, has recently been found to modulate mtROS through mechanisms independent of its nuclear factor activity, but dependent on its ability to directly modulate the activity of the electron transport chain (ETC)55, 56. It has been shown that STAT3 is present in the mitochondrial matrix, and deficiency of STAT3 in murine hearts leads to decreased activities of complexes I and II while increasing mtROS at complex I56. Since STAT3 serves as downstream signaling molecule of IL-35, it is tempting to speculate that IL-35-induced STAT3 might “talk” to the mitochondria to suppress mtROS production. By modulating mitochondrial ETC activity and mtROS generation, STAT3 could link IL-35 signaling pathway to cellular metabolism. We are in the process to examine this potential.
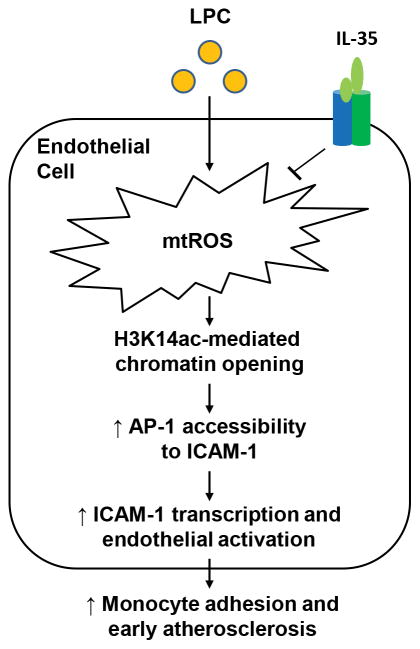
To summarize, we propose a new working model of mtROS-H3K14ac-mediated EC activation as IL-35 targeted pathway: 1) atherogenesis not only upregulates proatherogenic factors but also increases anti-atherogenic factors including IL-35, suggesting that IL-35 serves as an important homeostasis-maintaining regulator. However, such protective mechanism from IL-35 eventually fails as hyperlipidemia condition worsens and overcomes the protection. Thus, we believe that exogenous supplementation of IL-35 is a feasible approach in the treatment of cardiovascular diseases similar to a recent report on CANTOS trial with anti-IL-1β antibody Canakinumab57; and that combinational anti-inflammatory therapies might result in even better outcome against CVD; 2) mtROS generation is an upstream target for IL-35 suppression of mtROS-H3K14ac-AP-1-ICAM-1-mediated endothelial activation; and 3) as a prototypic conditional DAMP that we reported recently31, LPC-induced EC activation is mediated by potentially non-specific mtROS followed by highly specific H3K14 acetylation pathway. Our results are supported by a recently published paper, which reported that IL-35 could inhibit atherosclerosis by enhancing Treg-mediated immune suppression48. We believe that IL-35 plays an anti-atherogenic role by targeting multiple cell types, including ECs and Tregs. Additional experiments that dissect the precise mechanisms of IL-35-dependent regulation of anti-atherogenic response are needed, and this could eventually lead to identification of novel therapeutic targets for suppressing endothelial activation and atherosclerosis (Figure 6).
Figure 6. Working Model.
During early atherosclerosis, LPC-induced mtROS lead to increased H3K14ac, thus facilitating the nuclear binding of AP-1 and ICAM-1 transcription, contributing to EC activation, monocyte adhesion, and atherosclerosis development. IL-35 could block atherosclerosis development by suppressing LPC-induced EC activation through inhibiting mtROS-H3K14ac-AP-1 pathway.
Supplementary Material
Highlights.
IL-35 is induced during atherosclerosis and suppresses atherosclerosis development, suggesting that IL-35 serves as an important homeostasis-maintaining regulator.
Mitochondria, in the form of mtROS, could specifically remodel the chromatin architecture in EC activation by contributing to site-specific histone acetylation events.
MtROS is an upstream target for IL-35 suppression of mtROS-H3K14ac-AP-1-ICAM-1-mediated endothelial activation.
Acknowledgments
Funding Sources
This work was partially supported by the National Institutes of Health Grants to XFY and HW and American Heart Association Scientist Development Grant 17SDG33671051 to PF.
Non-standard Abbreviations and Acronyms
- ECs
Endothelial cells
- HAECs
Human aortic endothelial cells
- LPC
Lysophosphatidylcholine
- MtROS
mitochondrial reactive oxygen species
- HF
high fat
Footnotes
Disclosures: None
References
- 1.Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Seminars in immunopathology. 2009;31:5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin Y, Li X, Sha X, et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao Y, Cheng Z, Li X, Chernaya V, Wang H, Yang XF. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction- a novel mechanism for maintaining vascular function. Journal of hematology & oncology. 2014;7:80. doi: 10.1186/s13045-014-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Fang P, Li Y, et al. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler Thromb Vasc Biol. 2016;36:1090–1100. doi: 10.1161/ATVBAHA.115.306964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Fang P, Yang WY, Chan K, Lavallee M, Xu K, Gao T, Wang H, Yang X. Mitochondrial ros, uncoupled from atp synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells. Can J Physiol Pharmacol. 2016:1–6. doi: 10.1139/cjpp-2016-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 7.Fang P, Zhang D, Cheng Z, Yan C, Jiang X, Kruger WD, Meng S, Arning E, Bottiglieri T, Choi ET, Han Y, Yang XF, Wang H. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63:4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory t cells control the development of atherosclerosis in mice. Nature medicine. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 9.Xiong Z, Yan Y, Song J, Fang P, Yin Y, Yang Y, Cowan A, Wang H, Yang XF. Expression of tctp antisense in cd25(high) regulatory t cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408. doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang WY, Shao Y, Lopez-Pastrana J, Mai J, Wang H, Yang XF. Pathological conditions re-shape physiological tregs into pathological tregs. Burns Trauma. 2015:3. doi: 10.1186/s41038-015-0001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du F, Zhou J, Gong R, Huang X, Pansuria M, Virtue A, Li X, Wang H, Yang XF. Endothelial progenitor cells in atherosclerosis. Front Biosci. 2012;17:2327–2349. doi: 10.2741/4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YF, Ren LN, Guo G, Cannella LA, Chernaya V, Samuel S, Liu SX, Wang H, Yang XF. Endothelial progenitor cells in ischemic stroke: An exploration from hypothesis to therapy. Journal of hematology & oncology. 2015;8:33. doi: 10.1186/s13045-015-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YF, Huang X, Li X, et al. Caspase-1 mediates hyperlipidemia-weakened progenitor cell vessel repair. Front Biosci (Landmark Ed) 2016;21:178–191. doi: 10.2741/4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monroy MA, Fang J, Li S, Ferrer L, Birkenbach MP, Lee IJ, Wang H, Yang XF, Choi ET. Chronic kidney disease alters vascular smooth muscle cell phenotype. Front Biosci (Landmark Ed) 2015;20:784–795. doi: 10.2741/4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer LM, Monroy AM, Lopez-Pastrana J, Nanayakkara G, Cueto R, Li YF, Li X, Wang H, Yang XF, Choi ET. Caspase-1 plays a critical role in accelerating chronic kidney disease-promoted neointimal hyperplasia in the carotid artery. J Cardiovasc Transl Res. 2016 doi: 10.1007/s12265-016-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virtue A, Johnson C, Lopez-Pastrana J, et al. Microrna-155 deficiency leads to decreased atherosclerosis, increased white adipose tissue obesity, and non-alcoholic fatty liver disease: A novel mouse model of obesity paradox. The Journal of biological chemistry. 2017;292:1267–1287. doi: 10.1074/jbc.M116.739839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mai J, Wang H, Yang XF. Th 17 cells interplay with foxp3+ tregs in regulation of inflammation and autoimmunity. Front Biosci. 2010;15:986–1006. doi: 10.2741/3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 19.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein e-knockout mice. Cardiovascular research. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circulation research. 2001;89:E41–45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 21.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein e knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine & growth factor reviews. 2006;17:487–499. doi: 10.1016/j.cytogfr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. Il-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7:e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collison LW, Vignali DA. Interleukin-35: Odd one out or part of the family? Immunological reviews. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine il-35 contributes to regulatory t-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 26.Shen P, Roch T, Lampropoulou V, et al. Il-35-producing b cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon KO, van der Kooij SW, Vignali DA, van Kooten C. Human tolerogenic dendritic cells produce il-35 in the absence of other il-12 family members. Eur J Immunol. 2015;45:1736–1747. doi: 10.1002/eji.201445217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi J, Leung PS, Bowlus C, Gershwin ME. Il-35 and autoimmunity: A comprehensive perspective. Clin Rev Allergy Immunol. 2015 doi: 10.1007/s12016-015-8468-9. [DOI] [PubMed] [Google Scholar]
- 29.Sha X, Meng S, Li X, Xi H, Maddaloni M, Pascual DW, Shan H, Jiang X, Wang H, Yang XF. Interleukin-35 inhibits endothelial cell activation by suppressing mapk-ap-1 pathway. J Biol Chem. 2015;290:19307–19318. doi: 10.1074/jbc.M115.663286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Fang P, Yang WY, Wang H, Yang X. Il-35, as a newly proposed homeostasis-associated molecular pattern, plays three major functions including anti-inflammatory initiator, effector, and blocker in cardiovascular diseases. Cytokine. 2017 doi: 10.1016/j.cyto.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Li YF, Nanayakkara G, Shao Y, Liang B, Cole L, Yang WY, Li X, Cueto R, Yu J, Wang H, Yang XF. Lysophospholipid receptors, as novel conditional danger receptors and homeostatic receptors modulate inflammation-novel paradigm and therapeutic potential. J Cardiovasc Transl Res. 2016;9:343–359. doi: 10.1007/s12265-016-9700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YF, Li RS, Samuel SB, Cueto R, Li XY, Wang H, Yang XF. Lysophospholipids and their g protein-coupled receptors in atherosclerosis. Frontiers in bioscience. 2016;21:70–88. doi: 10.2741/4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vestweber D. How leukocytes cross the vascular endothelium. Nature reviews Immunology. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 34.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 35.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Shao Y, Chernaya V, Johnson C, Yang WY, Cueto R, Sha X, Zhang Y, Qin X, Sun J, Choi ET, Wang H, Yang XF. Metabolic diseases downregulate the majority of histone modification enzymes, making a few upregulated enzymes novel therapeutic targets-“sand out and gold stays”. J Cardiovasc Transl Res. 2016;9:49–66. doi: 10.1007/s12265-015-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Pastrana J, Shao Y, Chernaya V, Wang H, Yang XF. Epigenetic enzymes are the therapeutic targets for cd4(+)cd25(+/high)foxp3(+) regulatory t cells. Transl Res. 2015;165:221–240. doi: 10.1016/j.trsl.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dje N’Guessan P, Riediger F, Vardarova K, Scharf S, Eitel J, Opitz B, Slevogt H, Weichert W, Hocke AC, Schmeck B, Suttorp N, Hippenstiel S. Statins control oxidized ldl-mediated histone modifications and gene expression in cultured human endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:380–386. doi: 10.1161/ATVBAHA.108.178319. [DOI] [PubMed] [Google Scholar]
- 39.Shao Y, Chernaya V, Johnson C, Yang WY, Cueto R, Sha X, Zhang Y, Qin X, Sun J, Choi ET, Wang H, Yang XF. Metabolic diseases downregulate the majority of histone modification enzymes, making a few upregulated enzymes novel therapeutic targets--“sand out and gold stays”. J Cardiovasc Transl Res. 2016;9:49–66. doi: 10.1007/s12265-015-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao Y, Nanayakkara G, Cheng J, Cueto R, Yang WY, Park JY, Wang H, Yang X. Lysophospholipids and their receptors serve as conditional damps and damp receptors in tissue oxidative and inflammatory injury. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, Murray PJ, Vignali DA. The composition and signaling of the il-35 receptor are unconventional. Nature immunology. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han P, Hang CT, Yang J, Chang CP. Chromatin remodeling in cardiovascular development and physiology. Circulation research. 2011;108:378–396. doi: 10.1161/CIRCRESAHA.110.224287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo YM, Andrews AJ. Quantitating the specificity and selectivity of gcn5-mediated acetylation of histone h3. PloS one. 2013;8:e54896. doi: 10.1371/journal.pone.0054896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo YM, Henry RA, Andrews AJ. A quantitative multiplexed mass spectrometry assay for studying the kinetic of residue-specific histone acetylation. Methods. 2014;70:127–133. doi: 10.1016/j.ymeth.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galdieri L, Vancura A. Acetyl-coa carboxylase regulates global histone acetylation. The Journal of biological chemistry. 2012;287:23865–23876. doi: 10.1074/jbc.M112.380519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. Journal of hematology & oncology. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. Il-33 reduces the development of atherosclerosis. The Journal of experimental medicine. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao L, Zhu J, Chen Y, Wang Q, Pan Y, Yu Q, Zhou B, Zhu H. Il-35 improves treg-mediated immune suppression in atherosclerotic mice. Exp Ther Med. 2016;12:2469–2476. doi: 10.3892/etm.2016.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virtue A, Mai Jietang, Wang Hong, Yang Xiaofeng. Lymphocytes and atherosclerosis. In: Hong Wang CP, Pratico Domenico, Jain Mukesh, Yang Xiaofeng, Sibinga Nicholas ES, editors. Atherosclerosis: Risks, mechanisms, and therapies. Hoboken, New Jersey: John Wiley and Sons, Inc; 2015. pp. 155–172. [Google Scholar]
- 50.Back M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nature reviews Cardiology. 2015;12:199–211. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 51.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in nlrp3 inflammasome activation. Nature. 2010;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 52.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in tnfr1-associated periodic syndrome (traps) The Journal of experimental medicine. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific t cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katoh H, Zheng P, Liu Y. Foxp3: Genetic and epigenetic implications for autoimmunity. J Autoimmun. 2013;41:72–78. doi: 10.1016/j.jaut.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wegrzyn J, Potla R, Chwae YJ, et al. Function of mitochondrial stat3 in cellular respiration. Science (New York, N Y. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxidants & redox signaling. 2012;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. The New England journal of medicine. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.