Abstract
The evidence is strong that biological functions contained in high-density lipoproteins (HDL) are anti-atherogenic. These functions may track with HDL-cholesterol or apoA1 concentration to explain the strongly inverse risk curve for cardiovascular disease (CVD). Moreover, there are harmful as well as protective HDL subspecies in regard to CVD which could be responsible for paradoxical responses to HDL-directed treatments.
Recent metabolic studies show that apoA1-containing HDL is secreted into the circulation as mostly spherical cholesterol ester rich lipoproteins that span the HDL size range. Most of the flux of apoA1 HDL into and out of the circulation occurs in these spherical cholesterol-replete particles. Discoidal cholesterol-poor HDL comprises a minority of HDL secretion. We propose that much cholesterol in reverse cholesterol transport enters and exits medium and large size HDL without changing a size category, and its flux may be estimated provisionally from holoparticle clearance of cholesterol ester rich HDL. A accurate framework for metabolism of HDL is essential to finding steady-state biomarkers that reflect HDL function, in vivo.
Whereas cholesterol efflux from cells to mainly discoidal HDL, mediated by ABCA1, predicts CVD, cholesterol transfers to spherical HDL also can be measured and may be relevant to protection against atherosclerosis.
We propose several investigative paths on which human HDL biology may be investigated leading to convenient biomarkers of HDL quality and function having potential not only to improve risk prediction but also to more accurately target drug treatments.
We hold the view that high-density lipoproteins (HDL) are important biological entities that protect against atherosclerosis and cardiovascular disease (CVD). However, HDL is not simply a carrier of cholesterol taken from cells for redistribution and removal from the body, but rather HDL is a complex constellation of many proteins and phospholipids with diverse physiochemical properties and metabolic actions. These proteins are organized into HDL subspecies, a lipoprotein particle system just starting to be understood in terms of structure, function and relation to disease.1-6 The view that HDL participates in fundamental biological processes that are relevant to health powers a very large research effort by many colleagues. The question is how can new information on HDL quality and function be tested in relation to risk of CVD and how can HDL functions be converted into clinical tests that assess future cardiovascular disease or can be used in the evaluation of new drug developments? In this paper, we approach this question by first reviewing HDL structure and metabolism, emphasizing new developments that modify long-held concepts of reverse cholesterol transport. Implicit in use of HDL cholesterol as a predictor of CVD is the expectation that the concentration of HDL cholesterol coincides with anti-atherogenic metabolic pathways that are effective in reverse cholesterol transport and hence prevention of atherosclerosis. Failure of treatments that increase HDL cholesterol to reduce incidence of CVD7-12 suggest that parts are missing in our understanding of HDL metabolism and how it connects to HDL cholesterol concentration and closely related measurements such as apolipoprotein A1 and HDL particle number.
HDL: Its raison d’etre?
Cells cannot metabolize cholesterol to smaller molecules generating energy, as they can with other lipids. When a cell experiences a higher cholesterol content than optimal for cellular functions, it must transfer excess cholesterol to the outside, a process called cholesterol efflux. This is accomplished by a lipoprotein system in which HDL is a main actor, interacting with cellular cholesterol transporters, partly independent of and partly engaging with the apoB lipoproteins, chylomicrons, VLDL and LDL.13-17
HDL is a particle composed of a coat of phospholipid surrounding a core of mainly esterified cholesterol. Most HDL in plasma is spherical, in a size range accommodating substantial variation in its cholesterol content. The larger the HDL particle, the more cholesterol ester it has. About 10% of HDL is discoidal, having minimal amounts of cholesterol ester. HDL has proteins on its surface which carry out various biological functions. The main protein on HDL is apolipoprotein A1 (apoA1) which lends structural stability to the particle and stimulates efflux of cholesterol from cells to HDL, enlarging the particles. Protein molecules other than apoA1 could influence size and form of HDL. 18-23 Finally, the phospholipid in the HDL coat has biological activity that can be potent. 24,25 Overall, HDL is a vast system of particles heterogeneous in size, shape, and amount of cholesterol, and in type of proteins and phospholipids. HDL carries out diverse functions, which pertain to atherosclerosis but also has other biological actions such as hemostasis, inflammation, anti-oxidation, and innate immunity.
HDL, identified by the presence of apoA1 and a size range, circulates in plasma for two to four days. Cholesterol taken up by HDL is delivered to the liver for excretion in the bile17, to the intestine for transintestinal cholesterol efflux (TICE) 26, and to organs that make steroid hormones from cholesterol. This movement of cholesterol from cells to HDL redistributes cholesterol among tissues. Some of it is excreted by the liver into the bile, and this process is often called “reverse cholesterol transport”, using intricate molecular machinery shown in studies mainly in mouse models and in cell culture. Protection against atherosclerosis by reducing macrophage cholesterol content in the vasculature thus might be viewed as an epiphenomenon of the general need to regulate cellular cholesterol.
Each HDL particle could have several functions or a focused role via functional speciation. Although we view cholesterol export and transfer as a central feature of HDL metabolism, these other functions could have been involved in the evolution and persistence of the HDL system. ApoL1 is an example of an HDL associated protein that has an established function to destroy trypanosomes, and is therefore also called, trypanosome lytic factor. 27 In general, we still do not know which of the many proteins and functions of HDL have a meaningful relation to CVD and other diseases.
There is even divergence in the definition of HDL. Traditionally, HDL is defined as a lipoprotein that contains apoA1 but not apoB. This is reasonable from the perspective of reverse cholesterol transport because apoA1 is the most effective apolipoprotein in stimulating ABCA1-mediated cholesterol efflux from cells. An alternative definition is simply that HDL is a protein-phospholipid complex in a specified size range smaller than the apoB lipoproteins.23 Any protein-lipid complex can satisfy this definition. This concept is just being explored, and we do not know how it yet pertains to HDL function and relation to disease. Nonetheless, the distribution of the HDL proteome among sizes is similar for HDL prepared by presence of phospholipid23 or apoA1.21
HDL reverse cholesterol transport: Can it be measured?
Several research groups have accomplished steps to establish a method to measure flux of cholesterol from cells into HDL and out of the circulation, in vivo.28-31 Since HDL size is determined mostly by its cholesterol ester content, size expansion indicates cholesterol uptake and esterification, and size contraction indicates net transfer of cholesterol to apoB lipoproteins and to liver and intestine where it may leave the body or be resecreted into the circulation. The flux rate of HDL clearance from the circulation is another component of cholesterol excretion and reverse cholesterol transport. Each of these three reverse cholesterol transport fluxes can be measured by apoA1 flux from small to large HDL, large to small HDL, and irreversible HDL removal. 32 These measurements are obtained from metabolic tracer studies that endogenously label apoA1 in HDL. Another approach is to inject radiolabelled cholesterol nanoparticles and follow their transport into HDL, to apoB lipoproteins, and fecal excretion.31 These studies are not suitable for large-scale clinical use. But they could be used to vet treatments that are designed to improve reverse cholesterol transport such as CETP inhibitors or LCAT agonists. Ideally, as data accumulate, a steady state HDL measurement might be found suitable to represent reverse cholesterol transport flux.
Knowing how HDL is metabolized as it circulates over days is indispensible to understanding how HDL conducts the process of reverse cholesterol transport, and to identify biomarkers. More fundamentally, the very concept of reverse cholesterol transport needs to be validated against atherosclerosis and CVD ---- a goal thus far elusive.
Revising our understanding of the physiology of human HDL
The canonical model of HDL metabolism, illustrating reverse cholesterol transport (Figure 1A, top panel), begins with secretion of a small discoidal protein-phospholipid complex, approximately 7 nm, called prebeta-1, because of its migration on 2-dimensional gel electrophoresis. Prebeta-1 has been the hypothesized nascent particle that interacts at the cell surface of peripheral tissues such as macrophages to activate cholesterol efflux, and then progressively expands with the transferred cholesterol to larger spherical HDL known as alpha 3, 2, and 1 HDL in ascending size ranges. These large HDL transfer their cholesterol esters to hepatocytes by mechanisms that include SR-B1 or to apoB lipoproteins using CETP, regenerating a smaller HDL that repeats the process. Eventually HDL are cleared with their cholesterol load by holoparticle uptake by the liver33 , and the intestine by TICE.26 Surprisingly, this straightforward metabolic pathway had not been demonstrated in vivo in humans, although it is inferred from studies of infusion of partially depidated HDL in monkeys, some of which is converted to larger HDL, in vivo. 34 Finally, recombinant LCAT infusion in humans increased conversion of small to large HDL, consistent with reverse cholesterol transport. 35
Figure 1. Classical and new models for HDL-mediated reverse cholesterol transport.
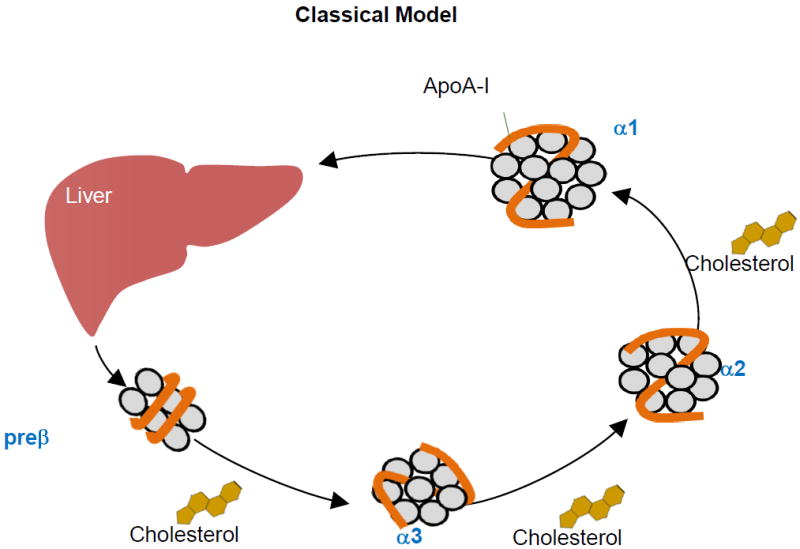
A. Top Panel. In the classical model, the liver secretes prebeta HDL as the nascent HDL particle. The prebeta HDL circulates in plasma growing in size as it takes up cholesterol from cells. When it is large, it can deliver its cholesterol ester to the liver by holoparticle or selective uptake
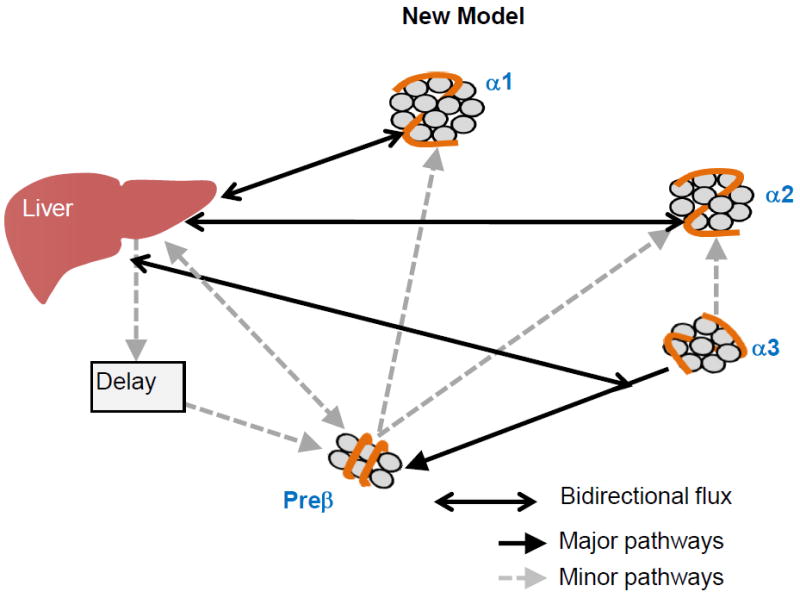
B. Bottom Panel. In the new model described in Mendivil et al32, the liver secretes all sizes of HDL which circulate for 2-3 days, not changing in size category, and then are cleared by the liver. Medium size HDL (a3) additionally undergoes size contraction and remodeling to become prebeta HDL. Minor metabolic pathways depict size expansion of prebeta and a3 HDL to large (a2) and very large (a1) HDL.
We developed a method to study HDL metabolism in vivo across its 4 main size subfractions, prebeta1, alpha3, alpha2, and alpha1, expecting to establish in humans this canonical model.32 Instead, the data compelled us toward a model of HDL metabolism that requires secretion into plasma of all sizes of HDL simultaneously which circulate mainly within their secreted size for 2-4 days before they are cleared (Figure 1B bottom panel). These findings are supported by many reports that cultured cells secrete HDL in a wide range of sizes with no evidence of precursor-product relationships. 36-44 Cynomolgous monkeys secrete a range of sizes of HDL. 34, 45
We identified size expansion as a minor pathway that connects prebeta with alpha 2 and alpha 1.32 Contraction of HDL, perhaps as a consequence of particle fusion and remodeling, could be identified only from alpha 3 to prebeta. Therefore much of the metabolism of HDL occurs within specific relatively stable size ranges.
Implications of new metabolic pathways for reverse cholesterol transport, and for HDL infusion therapy
These findings provoke fundamental questions about the function of HDL. First, if HDL is secreted throughout its size range, and mainly remains within the secreted size range as it circulates, then how does reverse cholesterol transport take place? One possibility stems from the structure of spherical (alpha) HDL which includes 4 or 5 apoA1 molecules arranged as a trefoil.46 The trefoil can twist and untwist, contracting and expanding the HDL particle, altering its core cholesterol ester content. Trefoil models allow about a 1nm diameter range, representing about a 10% change. Figure 2 shows sizes of LpA-I (HDL that contains apoA1 but not apoA2) that each have four molecules of apoA1. The trefoil permits cholesterol transfers in and out of HDL, but does not change a size category, such as from alpha-2 to alpha-1. Preliminary results suggest that two molecules of apoA2 can substitute for one molecule of apoA1 to preserve the trefoil (WS Davidson, communication), important because 60% to 70% of HDL has both proteins. Alternatively, or in addition, size expansion and contraction between conventional size categories could be done by HDL subspecies that contain proteins that stimulate cholesterol efflux and particle expansion. Our ongoing work is identifying HDL containing apoE, a subspecies comprising a minority of total HDL 5, that engages size expansion as a major pathway (Morton AM et al, presented at ATVB 2016) as suggested by studies in vitro.47-50
Figure 2.
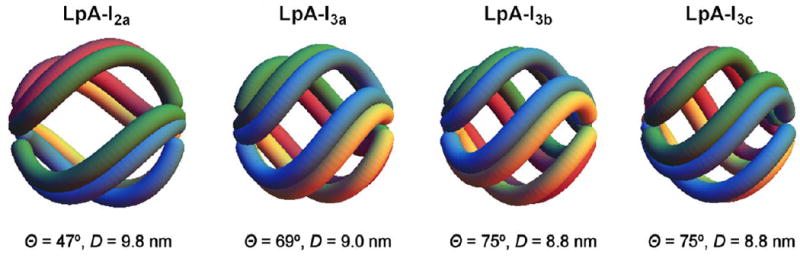
Trefoil model of HDL showing twisting and untwisting throughout a 1 nm size range. In this way, an HDL can take up and deliver cholesterol ester without a change in size category. Figure provided by W. Sean Davidson.
Second, for what purpose does the liver and intestine secrete cholesterol-rich HDL? Secretion of large HDL could be another means by which hepatocytes and enterocytes regulate their cholesterol content, because size of nascent HDL depends on the amount of cholesterol and phospholipid in domains in the endoplasmic reticulum and plasma membrane that are recruited by ABCA1 in HDL assembly and secretion.36 Proteins other than apoA1 tend to be localized in part, not all, of the size range of HDL, some involved in lipid and lipoprotein metabolism and others having other functions such as in thrombosis, inflammation and oxidation (Figure 3).21,23 Some of these proteins are secreted into plasma on apoA1-containing HDL at the same time as apoA1 secretion rather than formed while circulating in plasma.21 Structurally, the subspecies may need a certain size to accommodate specific proteins, essentially the protein component driving the size of the secreted particle.
Figure 3.
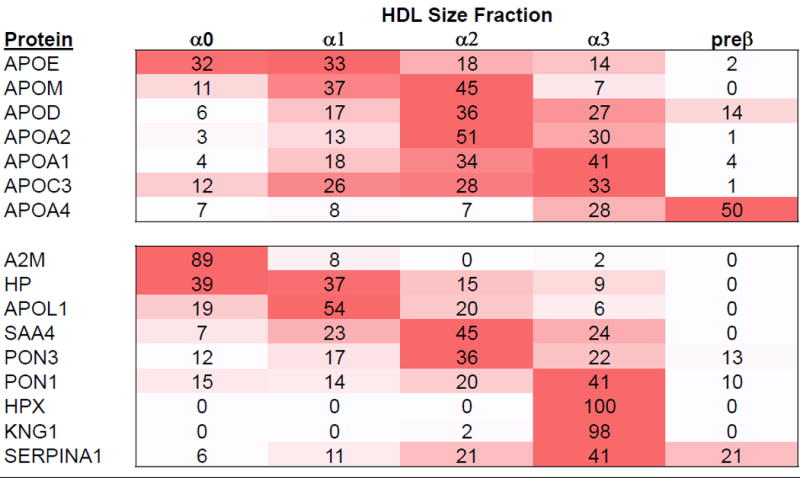
Relative distribution across 5 HDL size fractions of 7 apolipoproteins that are involved in lipid metabolism and 9 proteins having other functions. Illustration provided by A. Andraski from data in Singh S, Andraski A et al 21. Average of 3 participants.
Third, the hypothesis that reverse cholesterol transport occurs within the larger HDL sizes downgrades the uniqueness of prebeta-1 as the nascent HDL particle that fills with and empties cholesterol to perform reverse cholesterol transport. Trials to date of HDL prebeta infusion therapy have failed to reverse coronary atherosclerosis,51,52 although additional trials of variations of prebeta HDL are in progress.53 We note that the early trial of infusion of ApoA1 Milano prebeta HDL particles, reported in 2003, widely viewed as delivering positive results, was actually a negative trial.52 The positive interpretation came from analyzing change from baseline in percent atheroma volume in just the treated group using a paired test. But the valid test in a randomized clinical trial is a 2-sample test that compares change in the HDL infusion group to that of the control group. The p-value for that test, reported in the article, was far from statistically significant, p=0.29. The default interpretation of this circumstance is that the therapy is not effective. It is also possible that the trial was inadequately powered, which would require a follow-up trial with a larger sample. The future of HDL infusion therapy may venture to larger size HDL subspecies that have specific proteins in addition or in place of apoA1 that stimulate steps in reverse cholesterol transport.
HDL biomarkers to predict CVD: Is there as yet a causal biomarker?
HDL cholesterol, apoA1, and particle number
HDL cholesterol and apoA1 concentrations are both inversely associated with risk of CVD in large, prospective studies of individuals free of existing CVD.54 Importantly, the two measures for HDL are strongly correlated, (p=0.8 or greater), suggesting that for the most part that the cholesterol and apoA1 concentrations reflect the same thing and show somewhat similar associations with risk of CVD. In the prospective EPIC-Norfolk study of individuals free of existing CVD, the relative risk of CHD per standard deviation higher HDL-cholesterol was 0.78 (95% CI: 0.70-0.87) and for apoA1 it was 0.79 (95% CI: 0.71-0.87) in models adjusted for age, sex, smoking, BMI, and alcohol intake.55 However, additional adjustment for other lipid markers, such as LDL cholesterol, triglycerides, or mutual inclusion of HDL cholesterol and apoA1 in the same model, can influence the strength of these associations, and the association of apoA1 may be more robust than HDL cholesterol to such comprehensive model adjustments.55 Further, HDL cholesterol may not have a completely linear association with CHD-risk, as the pattern of the association appears to reach a plateau at very high HDL cholesterol levels (>75 mg/dL for men and > 90 for women).55,56
HDL particle concentration (sometimes called “particle number” or HDL-P) can be estimated by nuclear magnetic resonance (NMR) spectroscopy, which analyzes the lipid signals of HDL size populations. Plasma apoA1 is highly correlated with HDL particle number (r=0.74) ,57 but the majority of studies compare HDL-P to HDL cholesterol and not with apoA1 concentrations. Studies that include association analyses of both HDL cholesterol and HDL-P have highlighted some important considerations. For instance, in the MESA study, HDL cholesterol and HDL-P were similarly associated with 65% lower risk of CHD among those in the top quartile, compared to the lowest quartile. However, when mutually adjusted, only HDL-P remained inversely associated with risk of CHD.58 In the Dallas Heart Study and the Women’s Health Study, HDL-cholesterol and HDL-P were slightly less correlated (r=0.52 and 0.47, respectively).57,59 In the Dallas Heart Study, HDL-P was significantly inversely associated with cardiovascular events even after adjustment for HDL cholesterol. 59 HDL cholesterol trended toward an association with cardiovascular events, which became null after adjusting for HDL-P. However, it should be mentioned that there were only 132 events in the study, making conclusions from this type of complex multivariable analysis insecure. In contrast, in the Women’s Health Study, HDL-cholesterol was more strongly inversely associated with CHD risk than HDL-P in the multivariable-adjusted models.57 Unfortunately, the adjusted relative risk for apoA1 with risk of CHD was not reported.
HDL cholesterol and HDL-P have also been compared across race and ethnic groups. Interestingly, African American men have been reported to have higher HDL cholesterol levels compared to non-black men, whereas such differences were not observed for HDL-P.60 In this study, HDL-P was strongly inversely associated with risk of CHD in both blacks and non-black participants, whereas HDL cholesterol was statistically significant only in non-blacks.
In summary, complex considerations of study design, multivariable modeling choices, and measurement error in the assessment of HDL cholesterol, HDL-P, and apoA1 must be encumbered when discussing the extent to which any of the measures are superior in their capacity to reflect the cardioprotective functions of HDL and prediction of CVD.
HDL concentration may not indicate effective reverse cholesterol transport
If HDL concentration indicates a causal relationship to CVD, then HDL should perform reverse cholesterol transport more effectively as its plasma concentration increases. But this supposition appears counterintuitive from a metabolic standpoint. The first step of reverse cholesterol transport, transferring cholesterol from cells to HDL, which would increase HDL-cholesterol concentration, needs to be paired with an equal or stronger second step to transfer the cholesterol in HDL to the liver, directly or via apoB lipoproteins, which would decrease HDL cholesterol concentration. Carrying the argument further, a low HDL cholesterol concentration could reflect better reverse cholesterol transport than a high HDL cholesterol level if it reflects a steady state of fast removal of cholesterol obtained from cells by HDL. Indeed, the SR-B1 mutation, reducing selective uptake of cholesterol from HDL by the liver, is associated with higher HDL cholesterol and higher CVD prevalence.61
Conversely, CETP inhibition increases the concentration of HDL cholesterol but has had no effect or actually increases CVD. 7-9 However, the most recent trial of CETP inhibition, conducted in over 30,000 patients with CVD who were receiving intensive statin therapy found that the group treated with CETP inhibitor, anacetrapib, achieved 43 mg/dL higher HDL cholesterol than the placebo group and the rate ratio for an coronary event was 9% lower (0.91; 0.85-0.97).10 As intriguing as this new finding is for the promise of HDL cholesterol drug treatments, in order for the trial results to fully support the concept that HDL cholesterol (or apoA1 or HDL-P concentrations) are indicative of reverse cholesterol transport, the relative difference in HDL cholesterol levels of 104% between treatment and placebo group should have reflected a much greater difference in coronary event risk. In addition, anacetrapib decreased LDL-cholesterol by 41%, which could have accounted for most if not all of the reduced risk of CVD. A recent genetic study suggested that the mechanism by which CETP inhibition affects CVD is in its effects on apoB lipoproteins rather than on HDL.62
HDL and triglyceride-rich lipoproteins
Often-mentioned is the hypothesis that HDL cholesterol predicts CVD only because it is a marker for plasma triglycerides and atherogenic triglyceride-rich apoB lipoproteins. There is less intra-individual biological variability in HDL cholesterol than triglycerides, which would favor a stronger relation with CVD for HDL cholesterol than triglycerides. However, while there is a moderate inverse correlation between HDL-cholesterol and triglyceride concentrations, e.g. -0.40 in the Women’s Health Initiative cohort,57 the correlation between triglycerides and apoA1 concentration is weaker (-0.09), but nonetheless apoA1 is as strong or stronger predictor of CVD-risk as HDL cholesterol. Adjustment for triglycerides does not eliminate the significant association of HDL cholesterol with CVD. Thus, it seems unlikely that atherogenic triglyceride-rich apoB lipoproteins account for the increased risk associated consistently with low HDL cholesterol. Just like HDL, the apoB lipoproteins exist in subspecies based on proteins such as apoC3 or apoE, and many others. These subspecies differ in metabolism63-65 and in relation to CVD.66,67 It remains possible that a low HDL cholesterol concentration is a biomarker for an especially atherogenic VLDL or LDL subspecies.
Why then are HDL cholesterol, apoA1, and HDL-P such strong and independent predictors of CVD? Our hypothesis is that HDL ordinarily contains one or more anti-atherogenic components, subspecies or functions that track with its plasma concentration but are not affected by HDL cholesterol raising treatments or mutations that do not target the protective factors. In this framework, HDL cholesterol, apoA1, and HDL-P are biomarkers for causal anti-atherogenic components or traits in HDL but so far treatments are misdirected.
HDL size
Associations between HDL size and CVD vary depending on the method used to determine HDL size. Some studies have shown that levels of large HDL are inversely associated with CHD68,69, but other studies have found that levels of both large and small HDL predict reduced CHD incidence70-72 or that HDL size determination does not improve CHD risk prediction beyond that provided by HDL cholesterol or apoA1 measurements.55,73 In addition, high levels of the small pre-β1 HDL have been identified in CHD patients, but pre-β1 HDL did not independently predict CHD in models that included the large alpha-1, which was the independent predictor among the sizes.74-76 Adjustment for LDL cholesterol concentration may affect the relation of HDL size to CVD. HDL is a complex family of lipoproteins which may not result in the same constellation of subspecies when isolated according to different methods. For example, a large size HDL prepared by size exclusion chromatography does not have the same population of subspecies or components as a large HDL prepared by ultracentrifugation.77 Presently, HDL size has not been convincingly demonstrated to aid in either risk prediction or the evaluation of HDL-raising treatments.55, 70-73, 75,76 Furthermore, it is not intuitively clear that a preponderance of large or small HDL reflects strong or weak reverse cholesterol transport. Finally, anti-thrombotic, anti-inflammatory and anti-oxidative proteins each localize in a portion of the overall HDL size range that may be affected by preparation techniques (Figure 3).21,23,77
Genetic variation affecting HDL-cholesterol (Mendelian randomization studies)
Epidemiologic studies that have explored the risk of CVD events among carriers of genetic variants that are associated with lifelong differences in HDL cholesterol levels have generally not found lower risk of CVD among individuals that are genetically predisposed to the high HDL cholesterol levels.61,78 These Mendelian randomization studies are more often derived from genome-wide association studies (GWAS) that have identified many genetic loci that are associated with HDL-cholesterol or apoA1 levels. 78-81 Of hundreds of identified single nucleotide variants (SNPs), a genetic risk score is then compiled after limiting this to SNPs that have exclusive effects on HDL cholesterol, i.e. no association with triglycerides or LDL cholesterol. These studies consistently report that the genetically determined HDL cholesterol is not associated with risk of CAD. 78-81
Limitations for these genetic observation studies include the summation of a high number of genetic variants for which we have only very little knowledge about their function. We may argue that because of the complexity of HDL metabolism, we should sum variants from different loci to capture the biology. However, as each variant explains only very little of the variation in HDL cholesterol levels, and the complexity of HDL metabolism involves interactions with both triglycerides and LDL cholesterol (that is, most genetic variants will exert pleiotropic lipid effects), restricting to genetic variants with an exclusive HDL cholesterol association may miss some of the most important functional aspects of the HDL pathway.
Further, the Mendelian randomization approach, having thus far focused on HDL cholesterol or apoA1 levels, may not capture the functional properties of HDL that play a role in the disease pathology. In fact, the clear null findings in Mendelian randomization studies of HDL cholesterol may be considered another strong argument for the continued investigations into the complexity of the protein and phospholipid constellation that makes up HDL. In contrast, Mendelian randomization studies of LDL cholesterol, which is more simply defined by a single apoB protein per particle, have been strong and straightforward in supporting the role of LDL cholesterol in atherosclerosis.78, 79, 81
Cholesterol efflux from cells to HDL: the first biomarker of HDL function predicting CVD
Current HDL research is emphasizing the development and evaluation of assays that reflect functional properties of HDL rather than its plasma concentration. Cholesterol efflux measures the ability of HDL to receive cholesterol from macrophages, remodeling and enlarging the particles. Cholesterol efflux has been measured in several epidemiological settings.
In cross-sectional studies, the capacity of HDL to stimulate cholesterol efflux is inversely associated with carotid intima–media thickness,82 and with prevalent coronary artery disease.82,83 However, because the measurement of cholesterol efflux occurred after the CVD event, the results are prone to reverse causation. Thus, there was an urgent need for studies that followed participants for incident disease. The first such prospective studies were mostly convenience samples of participants that were referred for coronary angiography. In the Cleveland Clinic study higher cholesterol efflux capacity was paradoxically associated with higher risk of myocardial infarction and stroke,83 whereas a large study in Austria also of patients that were referred for angiography reported an inverse association between cholesterol efflux and risk of CVD mortality.84 Beyond reverse causation, the complicated confounder structure in such patient samples where medications for hypercholesterolemia are prevalent make it difficult to interpret and reconcile these results. For example, the lack of association between LDL cholesterol and risk of CVD in the Cleveland Clinic study may hint that considerable residual negative confounding may be obscuring the relationships.
Importantly, two prospective studies in populations free of underlying CVD, the Dallas Heart Study and the UK EPIC Norfolk Study, both found strong inverse associations between cholesterol efflux measured using two different methodological approaches (reviewed below) and cardiovascular events, which persisted even when HDL cholesterol was held constant by entering it into the statistical model.59,85 However, in the Dallas Heart Study,59 HDL cholesterol level was not statistically significantly associated with risk of CVD by itself. In the absence of a strong association between HDL cholesterol and CVD, it cannot be expected that adjustment for HDL cholesterol would influence results. When using this approach to judge the “superiority” of one factor over the other, the precision, biological variation and predictive value of each measurement are important considerations.
Recently, efflux was studied in a case-control analysis nested in a primary prevention trial of a high potency statin, rosuvastatin 20 mg.86 Efflux, measured at the start of the trial, was not a predictor of incident CVD in models either adjusted for age and treatment group (OR =0.84; 95% CI: 0.55-1.27), or that added CVD risk factors and LDL cholesterol and triglycerides (OR= 0.75; 0,47-1.21). Efflux, measured at one year while the participants were taking rosuvastatin, significantly predicted coronary events. However, efflux did not significantly predict coronary events after adjustment for HDL cholesterol, apoA1, or HDL-P measured either at baseline or after 1 year of statin treatment. Change in efflux from baseline to one year also was not associated with incident CVD events. In most analyses in this study, HDL cholesterol, apoA1 and HDL-P were significant or near-significant predictors of coronary events, not affected by additional adjustment for efflux.
In summary, the novelty and appeal of HDL mediated cholesterol efflux, as a predictor of CVD, is that it actually connects HDL with its most widely accepted property. In most studies, HDL cholesterol or another measure of HDL concentration is moderately correlated with efflux, e.g. R=0.5-0.6. However, results among several studies as yet do not agree whether efflux significantly predicts CVD independently of concentrations of HDL cholesterol, HDL-P or apoA1.
The multiple pathways of HDL-mediated macrophage cholesterol efflux
Cellular efflux of cholesterol to HDL occurs by both specific receptor-mediated transport (via ABCA1, ABCG1, and SR-BI) and aqueous diffusion.87 ABCA1 promotes unidirectional cholesterol flux most strongly to lipid-free apoA1 and less well to spherical HDL.88-90 ABCG1 and SR-B1 promote cholesterol efflux to spherical HDL but not to lipid-free apoA1.91,92 Aqueous diffusion constitutes another major pathway of cholesterol efflux from macrophages.93 and may account for a large subset of cholesterol efflux in vivo.94
Each of these four mechanisms can be studied, although only total efflux has been used in epidemiological studies pertaining to prediction of CVD. Total efflux is measured by incubating a mouse macrophage cell line, J774, with plasma from which apoB lipoproteins have been removed. Before incubation with samples, the cells are loaded with labelled unesterified cholesterol and treated with cyclic AMP which upregulates ABCA1. In most but not all studies the cells are treated with an inhibitor of ACAT to increase further their content of unesterified cholesterol which increases the efflux process. These treatments enhance ABCA1 mediated efflux as a component of total efflux. Cholesterol that effluxed from the macrophages is accepted not only by HDL but also by albumin present in the apoB depleted sera, which makes the test partly nonspecific to HDL function.83
Cholesterol efflux from cells to HDL specifically by ABCG1, SR-B1, or passive diffusion has not been studied in relation to future risk of CVD in an observational setting. If our hypothesis is correct, that large size HDL engage in reverse cholesterol transport more so than small discoidal HDL, then ABCG1 and SR-B1 efflux may have an even more important role for atherosclerosis than than ABCA1 mediated efflux, as the latter only effluxes cholesterol to the small HDL particles.
Fundamental questions on cholesterol efflux
First, the four types of efflux have not yet been compared in terms of their respective strength of association with risk of future CVD. The proportion of efflux that occurs through each of the four pathways in vitro and in vivo is unknown. In other words, does total efflux, measured in vitro, accurately model what occurs in vivo? Second, does cholesterol efflux explain the strong prediction of CVD by HDL cholesterol? Third, ideally, the efflux property of HDL would be reduceable to a simple compositional measurement in HDL. An HDL test that measures a core function of HDL like efflux, could be used widely if it strongly predicts CVD independent of the easily and cheaply measured HDL cholesterol.
HDL proteins: subspecies and proteomics
The tremendous compositional heterogeneity of the HDL proteome reflects our emerging understanding of the diverse functions of HDL. The proteins that are present on HDL have great importance for the downstream metabolism and function of HDL by mediating its interactions with receptors, enzymes and other proteins. Further, the HDL proteome is modifiable, and has been shown to be heavily affected by inflammation such as in cardiovascular disease and infections.95-98
Myeloperoxidase-oxidized HDL
One protein that has been found of importance to HDL’s anti-inflammatory properties and capacity to accept cholesterol is the immunogenic epitope of myeloperoxidase (2-OH group of Trp72).99 Thus far, apoA1 with the myeloperoxidase epitope (MPO-oxidized HDL) is elevated in patients with cardiovascular disease. The MPO-oxidized HDL subtype is only found at very low concentration in human plasma (≈0.007%). Further investigation in prospective studies is warranted in order to determine its potential as a diagnostic and therapeutic target for cardiovascular disease.
HDL containing apoC3
Ongoing research has also focused on apoC3 as a potentially important protein that may modulate HDL function. ApoC3 is present on 6-15% of HDL. The concentration of HDL that has apoC3 is associated with higher, not lower, risk of CHD.3 (Figure 4, top panel) In four prospective studies of middle-aged participants free of CVD, the relative risk of developing CHD over a period of 10-14 years was 0.76 (95% CI = 0.70 to 0.83) for each standard deviation higher HDL lacking apoC3 and 1.09 (95% CI= 1.01 to 1.18) for HDL containing apoC3. The heterogeneity test for difference between these HDL subspecies for CHD risk is significant (P < 0.02). The relative risk of CHD per each standard deviation of total HDL cholesterol was 0. 0.80 (95% CI = 0.74 to 0.87).Therefore, the risk of CHD for total HDL cholesterol becomes more inverse (protective) when the harmful apoC3 containing subspecies is removed.
Figure 4.
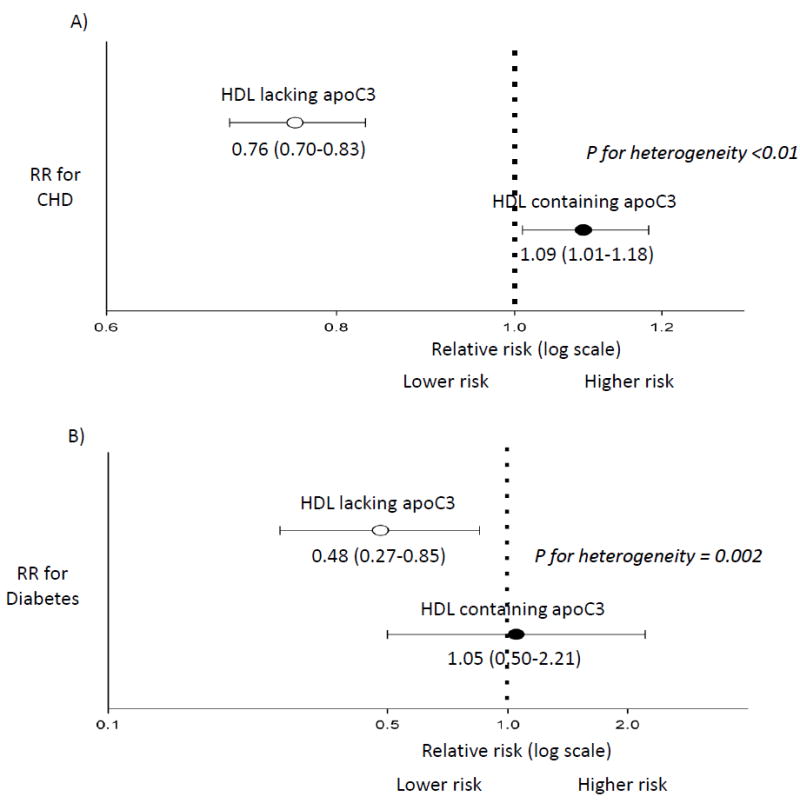
HDL containing apoC3 (black dots) and HDL lacking apoC3 (white dots) differ in their association with risk of CHD and type 2 diabetes in prospective studies.
Relative Risk (RR) of CHD [panel A] and diabetes [panel B] when comparing extreme quintiles of HDL containing or not containing apoC3. Multivariable-adjusted models included: age, sex, smoking, education, body mass index, alcohol intake, and hypertension. P-heterogeneity indicates that the the RRs significantly differ for the two HDL subspecies when modeled continuously.
A. CHD: Meta-analysis of four prospective studies totaling 2,997 incident cases, nested within the Nurses’ and Health Professionals Follow-Up studies, the Diet, Cancer and Health Study, and the Multi-Ethnic Study of Atherosclerosis. 3
B. Diabetes: The Diet, Cancer and Health Study, N= 434 cases, 3,101 controls. 101
We found that the presence of apoC3 on HDL modifies the association of HDL with risk factors for CHD. HDL containing apoC3 was associated with metabolic risk factors such as diabetes, obesity and blood glucose. In contrast, HDL lacking apoC3 was associated with favorable levels of these risk factors.3,5,100
These findings on HDL apoC3 and CHD were recently extended to the risk of incident diabetes. Concentrations of HDL containing or lacking apoC3 were differentially associated with risk of diabetes (p for heterogeneity = 0.002) (Figure 4, bottom panel).101 The relative risk of diabetes for top vs bottom quintiles of HDL cholesterol lacking apoC3 was 0.48 (95% CI: 0.27, 0.85), p for trend = 0.002, whereas HDL cholesterol containing apoC3 was unassociated with risk (relative risk top vs bottom quintile = 1.05; 95% CI: 0.50, 2.21; p for trend = 0.44). The association with incidence of diabetes for HDL cholesterol lacking apoC3 was stronger than that for total HDL cholesterol (RR = 0.60; 95% CI: 0.35, 1.03; p for trend = 0.04).
These findings on apoC3 demonstrate the importance of the protein cargo of HDL, and the ability to identify functionally distinct subtypes based on it. It may be that HDL subtypes defined on the basis of other proteins may reveal even greater functional differences, and potentially lead to early detection of individuals at high risk of cardiometabolic disease.
HDL lipidome
A vast array of phospholipids comprise the coat of the HDL particle. The type of phospholipid used to reconstitute HDL particles affects its anti-atherogenic and anti-inflammatory properties. 25 The organization of specific phospholipids on the HDL surface and interactions with proteins are not well understood. Sphingosine-1-phosphate (S1P) is a bioactive phospholipid with pleiotrophic effects on endothelial cell migration, nitric acid formation, adhesion molecule abundance, and inflammation.24,102,103 ApoM, a lipocalin on HDL, associates with S1P and serves as a chaparone for its effects on cells.103 The relation between apoM and S1P is noteworthy as an interacting pair of protein and phospholipid on HDL. ApoM has a complex relation to atherosclerosis in diverse mouse models. 24
Direction for progress on finding a causal biomarker of atheroprotective HDL function
The complexities of HDL metabolism offer a huge challenge to researchers to discover findings important to understand human biology and to develop them into clinical tools. But it should be a welcome challenge. We list some possible directions for research.
Kinetic measures of reverse cholesterol transport in vivo such as size expansion, contraction and removal from circulation. Actual measures of cholesterol flux itself in reverse cholesterol transport. While kinetics can be studied only in small samples in tracer metabolism study, kinetic findings could be a reference point for developing and calibrating surrogate biomarkers for HDL reverse cholesterol transport. The obvious advantage to HDL metabolic measurements is that they show what is actually occurring in the human body, without the inherent uncertainty in studies of nonhuman or cell culture systems.
Development of a surrogate biomarker for HDL reverse cholesterol transport. Such a biomarker could be total efflux or one or more of its four components: diffusion, SR-B1, ABCG1 and ABCA1. When we learn to what extent these mechanisms operate in humans, we may be closer to the goal of devising a surrogate which may be calibrated to metabolism in vivo.
Search for HDL subspecies that are high effluxers because of specific protein content. These could be steady-state surrogates for efflux.
Search for causal components in circulating HDL that explain robust independent CVD risk prediction.
HDL subspeciation based on protein or phospholipid content, e.g. HDL with apoC3 or apoE. Individual subspecies as risk markers or combination of subspecies into an index.
For all of these new biomarkers, there will be a need to develop them into a simple test for HDL function that can be done in a routine clinical lab in order for it to be of diagnostic value in the management of patients.
Table 1.
Measures of HDL concentration, quality or function evaluated in observational studies.
| Biomarker/measure | Quantity, quality or function of HDL the measure reflects | Findings | Hypothesized mechanisms | Conceptual Limitations, Need for further study |
|---|---|---|---|---|
|
| ||||
| HDL-cholesterol | Cholesterol concentration associated with HDL | High HDL-c strong inverse predictor of CVD. May reach a plateau effect and results may not be uniform across race/ethnic groups. Inversely associated with dyslipidemia and metabolic syndrome. | The higher the HDL-c, the higher the flux of cholesterol out of the body. | No clear evidence for hypothesized mechanism. Heterogeneous system of HDL particles. Some components may be adverse, some beneficial. |
|
| ||||
| ApoA1, HDL particle number (HDL-P) | ApoA1: concentration of principal protein in HDL, close estimate of HDL particle concentration. | Both measures strongly inversely associated risk of CVD. | The higher the apoA1 or HDL-P concentration, the higher the amount of cholesterol it carries out of the body. | No clear evidence for hypothesized mechanism. Heterogeneous system of HDL particles. Some components may be adverse, some beneficial. |
| HDL-P: estimated by NMR from lipid content of HDL. | ||||
|
| ||||
| HDL size | HDL divided into size categories. | The findings on associations between HDL size and CVD vary depending on the method used to determine HDL size. | Small HDL and the largest HDL size category may reflect less active reverse cholesterol transport. Or, large HDL reflects good reverse cholesterol transport. | No clear evidence for hypothesized mechanisms. Metabolically diverse HDL subspecies and proteins localize to specific size ranges. |
| Whether smallest pre-β1 HDL is independently associated with risk of CHD is unclear. | ||||
|
| ||||
| Cholesterol efflux capacity | The capacity of apoB depleted serum to accept cholesterol from macrophages. Several methods in use, results moderately correlated. Methods used for epidemiology studies accentuate efflux by ABCA1 to free and lipid-poor apoA1. | Efflux of cholesterol from macrophages is inversely associated with prevalent atherosclerosis and CVD. Also inversely associated with risk of CVD in most prospective studies in participants initially free of CVD. No consensus whether efflux predicts CVD independently of HDL cholesterol, apoA1 or HDL-P. | Rationale comes from hypothesis that the main in vivo efflux is mediated by ABCA1 to lipid-poor and free apoA1, not to HDL. Results also include efflux to serum albumin. | No clear evidence that most reverse cholesterol transport occurs by ABCA1-mediated cholesterol efflux. Contribution of diffusion, SR-B1, and ABCG1 to transfer of macrophage cholesterol to larger sizes of HDL is unknown. Unclear if current efflux methods model efflux that occurs in vivo. |
|
| ||||
| MPO-oxidized HDL | Epitope of myeloperoxidase (2-OH group of Trp72) on HDL. | HDL with the myeloperoxidase epitope is elevated in patients with CVD | When the immunogenic epitope of myeloperoxidase (2-OH group of Trp72) is found on HDL, HDL’s anti-inflammatory properties and capacity to accept cholesterol is diminished. | HDL with MPO epitope has not been investigated in a prospective setting of participants free of CVD yet. |
|
| ||||
| HDL subspecies according to presence or absence of one or more proteins, such as HDL containing or lacking apoC3 | ApoA1 or cholesterol concentration of HDL that contains or lacks apoC3; or another protein. | In prospective studies in participants free of CVD, HDL that has apoC3 is adversely associated with CHD. In contrast, HDL lacking apoC3 is strongly inversely associated with risk of CHD, more so than the total HDL. Concerning type 2 diabetes, HDL containing apoC3 is not associated whereas HDL lacking apoC3 is associated with lower incidence. | It is hypothesized that the tremendous compositional heterogeneity of the HDL proteome reflects many subspecies of HDL with differential anti-thrombotic, anti-inflammatory, and anti-oxidant effects depending on the associated proteins. The proteins associated with HDL have great importance for the downstream metabolism and function of HDL by mediating the interactions with receptors, enzymes and other proteins. | Studies in diverse populations needed. Proteomic analysis of HDL subspecies in relation to CVD. |
Highlights.
Recent metabolic studies show that apoA1-containing HDL is secreted into the circulation as mostly spherical cholesterol ester rich lipoproteins that span the HDL size range. We propose that much cholesterol in reverse cholesterol transport enters and exits medium and large size HDL without changing a size category, and its flux may be estimated provisionally from holoparticle clearance of cholesterol ester rich HDL.
We hypothesize that HDL ordinarily contains one or more anti-atherogenic components, subspecies or functions that track with its plasma concentration but are not affected by HDL cholesterol raising treatments or mutations that do not target the protective factors.
New measures that reflect functional or biochemical properties of HDL may be more relevant than plasma total HDL concentration to elucidate the role of HDL in health and disease. For example, an HDL subspecies containing apoC3 is associated with higher risk of cardiovascular disease and type 2 diabetes.
Discovery is needed of novel measures of HDL functional quality not only pertaining to reverse cholesterol transport but also to other vascular pathways involved in atherogenesis and inflammation. Such knowledge may help understand the paradoxical findings of trials of drugs that elevate HDL-cholesterol, and provide more functional HDL targets for diagnosis and future drug targeting.
Acknowledgments
None
Sources of funding: NHLBI-NIH (# R01HL095964) and American Diabetes Association (# 1-15-JF-30).
Abbreviations
- ApoA1
apolipoprotein A1
- ApoC3
apolipoprotein C3
- CETP
Cholesterol Ester Transfer Protein
- HDL-P
HDL particle concentration
- CVD
cardiovascular disease
- CHD
coronary heart disease
Footnotes
Disclosures: The authors are inventors on US patents owned by Harvard University pertaining to use of assays for the apoC-III defined HDL subspecies.
References
- 1.Alaupovic P. Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzym. 1996;263:32–60. doi: 10.1016/s0076-6879(96)63004-3. [DOI] [PubMed] [Google Scholar]
- 2.Cheung MC, Albers JJ. Distribution of high density lipoprotein particles with different apoprotein composition: particles with A-I and A-II and particles with A-I but no A-II. J Lipid Res. 1983;23:747–53. [PubMed] [Google Scholar]
- 3.Jensen MK, Aroner SA, Mukamal KJ, Furtado JD, Post WS, Tsai MY, Tjønneland A, Polak JF, Rimm EB, Overvad K, McClelland RL, Sacks FM. HDL Subspecies Defined by Presence of Apolipoprotein C-III and Incident Coronary Heart Disease in Four Cohorts. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.031276. Originally published November 21 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon SM, Deng J, Tomann AB, Shah AS, Lu LJ, Davidson WS. Multi-dimensional co-separation analysis reveals protein-protein interactions defining plasma lipoprotein subspecies. Mol Cell Proteomics. 2013;12:3123–34. doi: 10.1074/mcp.M113.028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talayero B, Wang L, Furtado JD, Carey VJ, Bray GA, Sacks FM. Obesity favors apolipoprotein E and CIII-containing high-density lipoprotein subfractions associated with risk of heart disease. J Lipid Res. 2014;55:2167–2177. doi: 10.1194/jlr.M042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Gordon SM, Zhu X, Deng J, Swertfeger DK, Davidson WS, Lu LJ. Network-based analysis on orthogonal separation of human plasma uncovers distinct high density lipoprotein complexes. J Proteome Res. 2015;14:3082–3094. doi: 10.1021/acs.jproteome.5b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 9.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933–42. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 10.The HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 11.The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 12.The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 13.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–20. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenson RS, Brewer HB, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rye K-A, Barter PJ. Regulation of high-density lipoprotein metabolism. Circ Res. 2014;114:143–156. doi: 10.1161/CIRCRESAHA.114.300632. [DOI] [PubMed] [Google Scholar]
- 16.Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–70. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–3. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 18.Rye K-A, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res. 2014;55:168–79. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575–85. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–56. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh SA, Andraski AB, Pieper B, Goh W, Mendivil CO, Sacks FM, Aikawa M. Multiple apolipoprotein kinetics measured in human HDL by high-resolution/accurate mass parallel reaction monitoring. J Lipid Res. 2016;57:714–28. doi: 10.1194/jlr.D061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends in Molecular Medicine. 2011;17:594–602. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Swertfeger DK, Li H, Robholz S, Zhu X, Shah AS, Davidson WS, Lu LJ. Mapping atheroprotective functions and related proteins/lipoproteins in size fractionated human plasma. Molecular Cell Proteomics. 2017;16:680–693. doi: 10.1074/mcp.M116.066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christoffersen K, Nielsen LB. Apolipoprotein M: bridging HDL and endothelial function. Curr Opin Lipidol. 2013;24:295–300. doi: 10.1097/MOL.0b013e328361f6ad. [DOI] [PubMed] [Google Scholar]
- 25.Schwendeman A, Sviridov DO, Yuan W, Guo Y, Morin EE, Yuan Y, Stonik J, Freeman L, Ossoli A, Thacker S, Killion S, Pryor M, Chen YE, Turner S, Remaley AT. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56:1727–1737. doi: 10.1194/jlr.M060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temel RE, Brown JM. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends in Pharmacological Sciences. 2015;36:440–448. doi: 10.1016/j.tips.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pays E, Vanhollebeke B, Vanhamme L, Paturiaux-Hanocq, Nolan DP, Perez-Morga D. The trypanolytic factor of human serum. Nature Rev Microbiol. 4:477–486. doi: 10.1038/nrmicro1428. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer and output in vivo in humans. J Lipid Res. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Kasumov T, Willard B, Li L, Li M, Conger H, Buffa JA, Previs S, McCullough A, Hazen LF, Smith JD. 2H2O-based high-density lipoprotein turnover method for the assessment of dynamic high-density lipoprotein function in mice. Arterioscler Thromb Vasc Biol. 2013;33:1994–2003. doi: 10.1161/ATVBAHA.113.301700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner S, Voogt J, Davidson M, Glass A, Killion S, Decaris J, Mohammed H, Minehira K, Boban D, Murphy E, Luchoomun J, Awada M, Neese R, Hellerstein M. Measurement of reverse cholesterol transport pathways in humans: in vivo rates of free cholesterol efflux, esterification, and excretion. J Am Heart Assoc. 2012;1:e001826. doi: 10.1161/JAHA.112.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuchel M, Raper AC, Conlon DM, Pryma DA, Freifelder RH, Poria R, Cromley D, Li X, Dunbar RL, French B, Qu L, Farver W, Su CC, Lund-katz S, Baer A, Ruotolo G, Akerblad P, Ryan CS, Xiao L, Kirchgessner TG, Millar JS, Billheimer JT, Rader DJ. A novel approach to measuring macrophage-specific reverse cholesterol transport in vivo in humans. J Lipid Res. 2017;58:752–762. doi: 10.1194/jlr.M075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendivil CO, Furtado J, Morton AM, Wang L, Sacks FM. Novel pathways of apolipoprotein A-I metabolism in high-density lipoprotein of different sizes in humans. Arterioscler Thromb Vasc Biol. 2016;36:156–65. doi: 10.1161/ATVBAHA.115.306138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rader DS. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks FM, Rudel LL, Conner A, Akeefe H, Kostner G, Baki T, et al. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J Lipid Res. 2009;50:894–907. doi: 10.1194/jlr.M800622-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shamburek RD, Bakker-Arkema R, Auerbach BJ, Krause BR, Homan R, Amar MJ, Freeman LA, Remaley AT. Familial lecithin:cholesterol acyltransferase deficiency: first in-human treatment with enzyme replacement. J Clin Lipidol. 2016;10:356–367. doi: 10.1016/j.jacl.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund-Katz S, Lyssenko NN, Nickel M, Nguyen D, Chetty PS, Weibel G, Phillips MC. Mechanisms responsible for the compositional heterogeneity of nascent high density lipoprotein. J Biol Chem. 2013;288:23150–23160. doi: 10.1074/jbc.M113.495523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji A, Wroblewski JM, Cai L, de Beer MC, Webb NR, van der Westhuyzen DR. Nascent HDL formation in hepatocytes and role of ABCA1, ABCG1, and SR-BI. J Lipid Res. 2012;53:446–455. doi: 10.1194/jlr.M017079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chisholm JW, Burleson ER, Shelness GS, Parks JS. ApoA-I secretion from HepG2 cells: evidence for the secretion of both lipid-poor apoA-I and intracellularly assembled nascent HDL. J Lipid Res. 2002;43:36–44. [PubMed] [Google Scholar]
- 39.Zheng H, Kiss RS, Franklin V, Wang MD, Haidar B, Marcel YL. ApoA-I lipidation in primary mouse hepatocytes Separate controls for phospholipid and cholesterol transfers. J Biol Chem. 2005;280:21612–21621. doi: 10.1074/jbc.M502200200. [DOI] [PubMed] [Google Scholar]
- 40.Kiss RS, McManus DC, Franklin V, Tan WL, McKenzie A, Chimini G, Marcel YL. The lipidation by hepatocytes of human apolipoprotein A-I occurs by both ABCA1-dependent and -independent pathways. J Biol Chem. 2003;278:10119–10127. doi: 10.1074/jbc.M300137200. [DOI] [PubMed] [Google Scholar]
- 41.Sorci-Thomas MG, Owen JS, Fulp B, Bhat S, Zhu X, Parks JS, Shah D, Jerome WG, Gerelus M, Zabalawi M, Thomas MJ. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulya A, Lee JY, Gebre AK, Thomas MJ, Colvin PL, Parks JS. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol. 2007;27:1828–1836. doi: 10.1161/ATVBAHA.107.142455. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Bortnick AE, Nickel M, Dhanasekaran P, Subbaiah PV, Lund-Katz S, Rothblat GH, Phillips MC. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high density lipoprotein particles. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- 44.Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J., Jr Molecular and cellular physiology of apolipoprotein A-I lipidation by the ATP-binding cassette transporter A1 (ABCA1) J Biol Chem. 2004;279:7384–7394. doi: 10.1074/jbc.M306963200. [DOI] [PubMed] [Google Scholar]
- 45.Colvin PL, Moriguchi E, Barrett PH, Parks JS, Rudel LL. Small HDL particles containing two apoA-I molecules are precursors in vivo to medium and large HDL particles containing three and four apoA-I molecules in nonhuman primates. J Lipid Res. 1999;40:1782–92. [PubMed] [Google Scholar]
- 46.Huang R, Silva RA, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ, Davidson WS. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol. 2011;18:416–422. doi: 10.1038/nsmb.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahley RW, Huang Y, Weisgraber KH. Putting cholesterol in its place: apoE and reverse cholesterol transport. J Clin Invest. 2006;116:1226–1229. doi: 10.1172/JCI28632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koo C, Innerarity TL, Mahley RW. Obligatory role of cholesterol and apolipoprotein E in the formation of large cholesterol-enriched and receptor-active high density lipoproteins. J Biol Chem. 1985;260:11934–11943. [PubMed] [Google Scholar]
- 49.Settasatian N, Barter PJ, Rye K-A. Remodeling of apolipoprotein E-containing spherical reconstituted high density lipoproteins by phospholipid transfer protein. J Lipid Res. 2008;49:115–126. doi: 10.1194/jlr.M700220-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Peters-Libeu CA, Newhouse Y, Hatters DM, Weisgraber KH. Model of biologically active apolipoprotein E bound to dipalmitoylphosphatidylcholine. J Biol Chem. 2006;281:1073–1079. doi: 10.1074/jbc.M510851200. [DOI] [PubMed] [Google Scholar]
- 51.Tardif JC, Ballantyne CM, Barter P, et al. Effects of the high- density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J. 2014;35:3277–86. doi: 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 53.Didichenko SA, Navdaev AV, Cukier AM, Gille A, Schuetz P, Spycher MO, Thérond P, Chapman MJ, Kontush A, Wright SD. Enhanced HDL Functionality in small HDL species produced upon remodeling of HDL by reconstituted HDL, CSL112: Effects on cholesterol efflux, anti-inflammatory and antioxidative activity. Circ Res. 2016;119:751–63. doi: 10.1161/CIRCRESAHA.116.308685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Steeg WA, Holme I, Boekholdt SM, et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol. 2008;51:634–642. doi: 10.1016/j.jacc.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 56.Wilkins JT, Ning H, Stone NJ, Criqui MH, Zhao L, Greenland P, Lloyd-Jones DM. Coronary heart disease risk asssociated with high levels of HDL cholesterol. J Am Heart Assoc. 2014;3:e000519. doi: 10.1161/JAHA.113.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akinkuolie AO, Paynter NP, Padmanabhan L, Mora S. High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circulation Cardiovascular Quality and Outcomes. 2014;7:55–63. doi: 10.1161/CIRCOUTCOMES.113.000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (Multi-ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, McGuire DK, Rohatgi A. Relation of black race between high density lipoprotein cholesterol content, lipoprotein particles, and coronary events (from the Dallas Heart Study) Am J Cardiol. 2015;115:890–894. doi: 10.1016/j.amjcard.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ference BA, Kastelein JJ, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, Packard CJ, Laufs U, Brook RD, Oliver-Williams C, Butterworth AS, Danesh J, Smith GD, Catapano AL, Sabatine MS. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318:947–956. doi: 10.1001/jama.2017.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng C, Furtado J, Khoo C, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense LDL phenotype. Circulation. 2010;121:1722–34. doi: 10.1161/CIRCULATIONAHA.109.875807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendivil C, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of VLDL and LDL containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30:239–45. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sacks FM. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr Opin Lipidol. 2015;26:56–63. doi: 10.1097/MOL.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodis HN, Mack WJ, Azen SP, Alaupovic P, Pogoda JM, LaBree L, Hemphill LC, Kramsch DM, Blankenhorn DH. Triglyceride- and cholesterol-rich lipoproteins have a differential effect on mild/moderate and severe lesion progression as assessed by quantitative coronary angiography in a controlled trial of lovastatin. Circulation. 1994;90:42–49. doi: 10.1161/01.cir.90.1.42. [DOI] [PubMed] [Google Scholar]
- 67.Mendivil CO, Rimm EB, Furtado J, Chiuve SE, Sacks FM. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124:2065–72. doi: 10.1161/CIRCULATIONAHA.111.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asztalos BF, Cupples LA, Demissie S, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 69.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parish S, Offer A, Clarke R, et al. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–2478. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 71.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 72.Joshi PH, Toth PP, Lirette ST, et al. Association of high-density lipoprotein subclasses and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. European Journal of Preventive Cardiology. 2016;23:41–49. doi: 10.1177/2047487314543890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams PT, Feldman DE. Prospective study of coronary heart disease vs HDL2, HDL3, and other lipoproteins in Gofman’s Livermore Cohort. Atherosclerosis. 2011;214:196–202. doi: 10.1016/j.atherosclerosis.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guey LT, Pullinger CR, Ishida BY, et al. Relation of Increased Prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am J Cardiol. 2011;108:360–366. doi: 10.1016/j.amjcard.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 75.Asztalos BF, Collins D, Cupples LA, et al. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol. 2005;25:2185–2191. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 76.Asztalos BF, Collins D, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism. 2008;57:77–83. doi: 10.1016/j.metabol.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9:5239–5249. doi: 10.1021/pr100520x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–550. doi: 10.1093/eurheartj/eht571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White J, Swerdlow DI, Preiss D, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiology. 2016;1:692–699. doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li XM, Tang WH, Mosior MK, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ritsch A, Scharnagl H, Marz W. HDL cholesterol efflux capacity and cardiovascular events. Letter to Editor N Engl J Med. 2015;372:19. doi: 10.1056/NEJMc1503139. [DOI] [PubMed] [Google Scholar]
- 85.Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes & Endocrinology. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol efflux capacity, HDL particle number, and incident cardiovascular events An analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) Circulation. 2017;135:2494–2504. doi: 10.1161/CIRCULATIONAHA.116.025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–9. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr Opin Lipidol. 2000;11:253–60. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Wang N, Silver DL, Costet P, Tall AR. Specific binding of apoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABCA1. J Biol Chem. 2000;275:33053–8. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 90.Remaley AT, Stonik JA, Demosky SJ, Neufeld EB, Bocharov AV, Vishnyakova TG, et al. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280:818–23. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 91.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem. 2005;280:30150–7. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 93.Bates SR, Rothblat GH. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974;360:38–55. doi: 10.1016/0005-2760(74)90178-7. [DOI] [PubMed] [Google Scholar]
- 94.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, et al. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–62. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 95.Riwanto M, Rohrer L, Roschitzki B, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 96.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- 97.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovasc Res. 2014;103:372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 98.Shao B, Tang C, Sinha A, et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Y, Didonato JA, Levison BS, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med. 2014;20:193–203. doi: 10.1038/nm.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koch M, Furtado JD, Jiang GZ, Gray BE, Cai T, Sacks FM, Tjoenneland A, Overvad K, Jensen MK. Associations of anthropometry and lifestyle factors with HDL subspecies according to apolipoprotein C-III. J Lipid Res. 2017;58:1196–1203. doi: 10.1194/jlr.P073288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aroner SA, Yang M, Li J, Furtado JD, Sacks FM, Tjoenneland A, Overvad K, Cai T, Jensen MK. Apolipoprotein C-III and high-density lipoprotein subspecies defined by apolipoprotein C-III in relation to diabetes risk. Am J Epidemiol. 2017;186:736–744. doi: 10.1093/aje/kwx143. [DOI] [PubMed] [Google Scholar]
- 102.Denimal D, Monier S, Brindisi MC, Petit JM, Bouillet B, Nguyen A, Demizieux L, Sinoneau I, de Barros JPP, Verges B, Duvillard L. Impairment of the ability of HDL from patients with metabolic syndrome but without diabetes mellitus to activate eNOS Correction by S1P enrichment. Arterioscler Thromb Vasc Biol. 2017;37:804–811. doi: 10.1161/ATVBAHA.117.309287. [DOI] [PubMed] [Google Scholar]
- 103.Ruiz M, Frej C, Holmer A, Guo LJ, Tran S, Dahlback B. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphage receptor 1. Arterioscler Thromb Vasc Biol. 2017;37:118–129. doi: 10.1161/ATVBAHA.116.308435. [DOI] [PubMed] [Google Scholar]


