Abstract
Background
Erythropoiesis stimulating agents (ESAs) are neuroprotective in cell and animal models of preterm birth. Prematurity has been shown to alter neurometabolite levels in children in studies using proton magnetic resonance spectroscopy (1H-MRS). We hypothesized that ESA treatment in premature infants would tend to normalize neurometabolites by 4-6 years of age.
Design/Methods
Children in a longitudinal study of neurodevelopment underwent MRI and 1H-MRS at approximately 4 and 6 years of age. Prematurely born children (500-1250 grams birth weight) received ESAs (erythropoietin or darbepoetin) or placebo during their neonatal hospitalization, and these groups were compared to healthy term controls. 1H-MRS spectra were obtained from the anterior cingulate (gray matter) and frontal lobe white matter, assessing combined N-acetylaspartate and N-acetylaspartylglutamate (tNAA), myo-inositol, choline compounds (Cho), combined creatine and phosphocreatine, and combined glutamate and glutamine.
Results
No significant (p≤0.5) group differences were observed for any metabolite level. Significant age-related increases in white matter tNAA and Cho were observed, as well as a trend for increased gray matter tNAA.
Conclusions
Neither prematurity nor neonatal ESA treatment were associated with differences in brain metabolite levels in the children of this study at a significance level of 0.05. These findings suggest that earlier differences that may have existed had normalized by 4-6 years of age or were too small to be statistically significant in the current sample.
Keywords: Prematurity, brain, erythropoiesis stimulating agents, proton magnetic resonance spectroscopy
Introduction
Neurodevelopmental disabilities such as cerebral palsy, cognitive delays, and learning and attention deficits during school age figure prominently in the outcomes of very low birth weight (VLBW) infants [1]. However, successful neuroprotective interventions have yet to be developed[2] (Back, 2015). Recent studies in animals and humans have shown that erythropoiesis stimulating agents (ESAs) such as erythropoietin (Epo) and darbepoetin (Darbe) have trophic, anti-inflammatory, and anti-apoptotic effects on neurons and oligodendrocytes, suggesting a strong neuroprotective potential of ESAs in the treatment of VLBW infants[3]. Whether ESAs can normalize brain structural or metabolic deficits in VLBW infants, though, remains to be evaluated.
Proton magnetic resonance spectroscopy (1H-MRS) has revealed altered levels of neurochemicals soon after premature birth[4-18] which, in some studies, have been shown to be associated with structural brain abnormalities evident on magnetic resonance imaging (MRI)[5,10,11,18,17]. The variety of 1H-MRS methods applied in these studies (e.g., different pulse sequences, echo times, analysis methods, with or without correction for tissue composition) and regions examined (e.g., cortical gray matter, white matter, cerebellum, thalamus, hippocampus), combined with diverse statistical methods, have made comparing their results challenging. However, generally, the metabolite signals measured are from the neuronal metabolite N-acetylaspartate (NAA), often assessed in combination with its overlapping spectral neighbor N-acetylaspartatylglutamate (NAAG); choline groups, primarily on the membrane lipid metabolites phosphocholine and glycerophosphocholine (Cho); myo-inositol (mI), an osmolyte found primarily in glia; lactate; taurine; and glutamate (Glu) and/or combined Glu and overlapping glutamine (the combination designated as Glx). Most studies assessed differences in the levels of the metabolites either in terms of their ratios with Cr or Cho, with the assumption that the latter do not vary between groups or with time, or as concentrations, estimated using the tissue water signal as a reference.
Few studies have examined metabolic abnormalities in preterm-born children beyond the neonatal period. Bathen et al[19] found no significant metabolic differences in left frontal white matter between preterm-born children, term-born children with low birth weight, and healthy term control subjects who underwent 1H-MRS at about 15 years of age. However, group membership in this study could be identified with high accuracy by neural network and discriminant analysis methods. Gimenez et al[20] found that NAA+NAAG and Cr in the hippocampus of preterm-born adolescents at about 15 years of age were lower than these metabolites in the hippocampus of age-matched term-born adolescents, and that gestational age at birth across both groups correlated with NAA+NAAG, Cr, mI, and hippocampal volume. In a previous study on anterior cingulate and frontal periventricular white matter in preterm-born children and term control subjects evaluated at 18-22 months or 3-4 years [21], we found significant differences in metabolites only in frontal white matter. At 18-22 months NAA+NAAG was lower in preterm-born children, and at 3-4 years preterm Cr and NAA/Cho were lower and Glx/Cr and Cho/Cr were higher in preterm-born children.
To date, no studies have sought to determine whether ESAs improve the neurometabolic outcome of preterm birth, either during the perinatal period or later in development. In the current study, we address the question of long-term metabolic improvement in a cohort of term- and preterm-born children examined at approximately 4 years of age and in a subset of these children returning at approximately 6 years of age, ages at which rapid developmental changes in neurometabolite levels are expected to have largely stabilized[22,13]. We examined 1H-MRS data from single voxels placed in frontal white matter and in the anterior cingulate gyrus, a region that is involved in attention and self-regulation, among other cognitive functions, and is thus a critical structure in neurodevelopment[23]. The preterm-born subjects had previously been randomized to receive Epo, Darbe or placebo within 48 hours of birth as part of a larger study examining cognitive and other neuroimaging measures of neurodevelopment.
In a larger sample of this study, subjects who had received Epo or Darbe had significantly higher composite cognitive scores and decreased neurodevelopmental impairment relative to subjects who had received placebo[24,25]. Neuroimaging data from a subset of this sample revealed significantly higher mean fractional anisotropy (FA), a measure of white matter health, in ESA-treated preterm-born female children at 4 years of age relative to untreated preterm-born females, but no other significant group differences in either FA or cortical thickness between groups [26]. Additionally, in the combined sample, significant correlations were found between FA and Full-Scale IQ and Verbal IQ, although there was no significant relationship between Full-Scale IQ and FA among just the preterm children.
In the subset of subjects with adequate 1H-MRS data reported here, we similarly searched for group metabolic differences that would reflect the cognitive differences observed. Hence, we hypothesized that neurometabolites would be altered in placebo-treated children and, furthermore, demonstrate a trend toward normalization in ESA-treated children.
Methods
Eligibility
The subjects were children previously enrolled in a randomized trial of Darbe, Epo, (collectively referred to as the ESA group) or placebo (the placebo group) (NCT# 00334737) performed at the University of New Mexico, the University of Colorado, the University of Utah, and Intermountain Health Care. Briefly, infants were eligible for the initial randomized masked trial if they weighed 500-1,250 grams at birth, were ≤48 hours of age, and were expected to survive the first days of life (as determined by the attending neonatologist). Infants with trisomy 21, 18 or 13, significant congenital anomalies (including known neurologic anomalies), hypertension, seizures, thromboses, hemolytic disease, or already receiving Epo were ineligible for study.
Former preterm children in the RCT surviving to follow up were then enrolled in the BRITE (Brain Imaging and Developmental Follow up of Infants Treated with ESAs) Study in order to continue following development and perform imaging. In addition, children born at term (37-41 weeks gestation) without complications (no prolonged hospitalization, hyperbilirubinemia, sepsis, hypoglycemia or prenatal drug exposure) were enrolled to serve as a healthy control group. All subjects underwent magnetic resonance imaging and 1H-MRS at approximately 4 and 6 years of age. Only the 1H-MRS data acquired at the New Mexico site are presented in this report. The study was approved by the Institutional Review Board at the University of New Mexico. Parental consent was obtained from all participating families in the BRITE Study.
Magnetic Resonance Imaging and Spectroscopy
Scanning was performed at night during natural sleep (all full term children) or with light chloral hydrate sedation (50 mg/kg orally), used for VLBW children who did not fall asleep naturally. Parents remained with the children during the scanning. Once children were asleep, scanning took 45 to 60 minutes to complete. Ear plugs and head phones were placed on children’s ears for noise protection. All magnetic resonance imaging scans were performed on a Siemens 3 T Trio Tim scanner using the standard 12-channel phased array head coils provided with the system. Sagittal T1-weighted anatomical images were obtained with a multi-echo three dimensional Magnetization Prepared Rapid Acquisition Gradient Echo sequence. Single voxel 1H-MRS data were obtained from a frontal gray matter voxel along the anterior cingulate gyrus (AC) and from a frontal white matter (WM) voxel with a point-resolved spectroscopy sequence (PRESS) sequence, with and without water suppression (TR/TE=1500/40ms, 192 averages with water suppression, 16 averages without water suppression). The transmitter frequency was set to 2.3 ppm for the acquisition of the metabolite spectrum and to 4.7 ppm (water resonance) for the acquisition of the water spectrum to reduce the chemical shift artifact. The AC and WM voxels were both 2.5 cm × 1.5 cm × 1 cm (3.75 cm3) in size. The AC voxel was located along the interhemispheric fissure in the anterior cingulate gyrus with the long axis along the fissure and the 1.5-cm dimension parallel to the fissure. The white-matter voxel was located in in a WM region to the left of the AC. Both locations and example spectra from one subject are shown in Figure 1.
Figure 1.
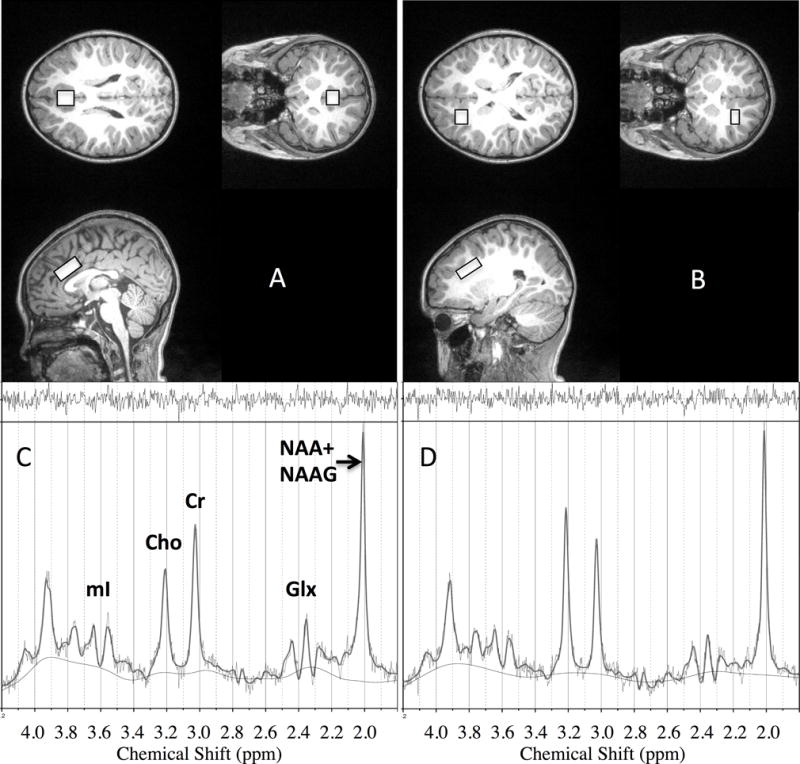
Example of voxel placement (overlaid rectangles) for the anterior cingulate (A) and frontal white matter (B) in one male 4 year-old subject and spectra from these locations (C=anterior cingulate, D=white matter) with LCModel fit overlays. NAA = N-acetylaspartate ; NAAG = N-acetylaspartatylglutamate; Cho = total choline groups; mI = myo-inositol; Glx = glutamate + glutamine.
1H-MRS data processing
The 1H-MRSI data were analyzed with LCModel[27] using tissue water as a concentration reference. The 1H-MRS signals analyzed were from combined N-acetylaspartate and N-acetylaspartyl glutamate (tNAA), choline containing compounds (Cho), combined creatine and phosphocreatine (Cr), myo-inositol (mI), and combined glutamate and glutamine (Glx). We used the LCModel standard deviation of the fit to the peak of interest, related to the Cramer-Rao lower bounds, as a criterion to exclude data of poor quality from the final analysis. If this statistic was greater than 20% for the peak of interest, the data were excluded. Additionally, if the signal-to-noise ratio of the spectrum, evaluated by LCModel as the ratio of the NAA peak height to the root mean square of the residuals to the fit, was less than 10, the data were excluded. The results from LCModel were corrected for cerebrospinal fluid (CSF), WM, and gray matter (GM) content (partial volume effects) as previously reported[28]. The normalized gray matter fraction (fGM/(fGM + fWM) of the AC voxel was generally high (0.94 ± 0.04), while the GM contribution to the WM voxel varied considerably more (0.27 ± 0.14). For this reason, an additional inclusion criterion in the analysis of the WM metabolite data was that the WM composition be greater than 0.5.
Statistical Analysis
In order to account for multiple metabolite comparisons as well as variance due to voxel tissue composition, group comparisons of metabolite levels in each 1H-MRS voxel (AC or WM) at each age (4 and 6 year visits) were made with multivariate analysis of covariance (MANCOVA) followed by univariate analyses on each metabolite using IBM SPSS (version 24.0). Both one-way (treatment group) and two-way (treatment group and sex, with and without a treatment group-by-sex interaction term) MANCOVAs were performed. A small number of outliers in each treatment group data set were identified (3 × interquartile range) and analyses were performed with and without them to test their impact on the results. Longitudinal effects were examined with repeated measures MANOVA. Our statistical power varied slightly across analyses, due mostly to variable numbers of participants with high-quality data. A post hoc power analysis using the G*Power calculator [29] based on the 4 year-old AC sample, the largest in the study, estimated that a medium to large effect size (Cohen’s f 2 ≥ .28) would be needed to observe a significant group effect in that sample with a statistical power of 0.8.
The concentrations of NAA, Cr, mI, and Glu are substantially higher in GM than in WM. Hence, variability in the GM-WM composition of the voxel can be expected to contribute significantly to the total variance in the data and potentially bias group differences. To account for this variance in the analyses of both AC and WM data, the normalized gray matter fraction was entered as a covariate in all MANCOVA models.
Results
Demographic and other background data on participants is provided in Table 1. During the initial 4 year visit, anterior cingulate 1H-MRS datasets of adequate quality were obtained from 52 subjects: 22 term controls (10 male [M]/12 female [F]), 15 preterm placebo (9 M/6 F), and 15 preterm ESA treated (7 M/8 F). Adequate white matter data sets were obtained from 44 subjects: 15 term controls (8 M/7 F), 16 placebo (6 M/10 F), 13 ESA treated (7 M/6 F). During the 6 year follow-up visit, adequate anterior cingulate 1H-MRS datasets were obtained from 41 returning subjects: 12 term controls (6 M/6 F), 12 placebo (6 M/6 F), and 17 ESA treated (10 M/7 F). Adequate white matter data sets were obtained from 30 of these subjects: 8 term (4 M/4F), 10 placebo (5 M/5 F), and 12 ESA treated (4 M/8 F). Because of missing data at each test session, the number of participants with complete, high quality data at both time points was reduced: 35 participants (10 placebo, 13 ESA treated, and 12 term controls) had longitudinal AC data and 26 (10 placebo, 9 ESA treated, 7 term controls had longitudinal WM data.
Table 1.
Descriptive statistics stratified by group.
| Placebo | ESA | Term Controls | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Test Age Scan 1 (years) | 3.78 | 0.22 | 4.08 | 0.42 | 3.79 | 0.19 |
| Test Age Scan 2 (years) | 6.10 | 0.33 | 6.05 | 0.26 | 6.07 | 0.27 |
| Gestational Age (weeks) | 29.24 | 1.19 | 27.27 | 1.49 | 38.99 | 1.38 |
| Maternal Age (years) | 27.24 | 5.70 | 28.75 | 6.65 | 29.29 | 7.60 |
| Maternal Education* | 4.76 | 1.30 | 4.81 | 1.28 | 5.33 | 1.39 |
| Anglo/Hispanic | 8/7 | 8/7 | 9/13 | |||
Maternal Education: 1=less than high school; 2=some high school; 3=high school degree; 4=some college; 5=college degree; 6=graduate degree
In order to maximize use of all data, we first conducted analyses of the visit 1 data (4 years), using one MANCOVA for the AC data and one for the frontal WM data. Preliminary analyses showed no relationships between age and any metabolite levels within either age group; hence, this variable was not included in the analyses described below. In each MANCOVA, sex and group (placebo, treated, term) were fixed factors, while the relevant gray matter fraction was entered as a covariate. For visit 1 AC data, no significant multivariate effects of group were observed (F(10, 84) = .862, p = .57, partial eta squared = .09). Similarly, for visit 2 AC data (6 years), no significant multivariate effects of group were observed (F(10, 64) = .823, p = .608, partial eta squared = .114). For visit 1 WM data, we again noted no significant multivariate effects of group, (F(10, 74) = .570, p = .833, partial eta squared = .072). And for visit 2 WM data, no significant multivariate effects of group were noted (F(10, 48) = .820, p = .611, partial eta squared = .146).
For participants with high quality data sets on both occasions, we conducted two multivariate repeated measures analyses, one for AC and one for WM data. Age represented the repeated measures factor, sex and group were fixed factors, and voxel gray matter fractions on both occasions were included as covariates. For the AC, there was no significant multivariate effect of group, F(10, 46) = 1.244, p = .290, partial eta squared = .213. Nor was any significant interaction observed between group and any other factor in the model. For the frontal WM analysis, there was again no significant multivariate effect of group, F(10, 30) = .664, p = .748, partial eta squared = .181. Further, no significant interaction was observed between group and any other factor in the model.
Finally, we investigated possible age-related changes in metabolites across the entire sample using paired t-tests. Of the 10 metabolites examined, five each from GM and WM, two were found to significantly differ with age. WM tNAA increased from 12.721 (SD = .932) to 13.485 (SD = 1.058), t(25) = 3.449, p = .002). Also, WM Cho increased from 3.192 (SD + .408) to 3.396 (SD = .490), t(25) = 2.277, p = .032. A trend was noted for increased AC tNAA, from 14.187 (SD = .889) to 14.574 (SD = .830), t(33) = 1.870, p = .07.
Discussion
During the perinatal period the fetal brain is increasing in size, shape and complexity: and normal brain development is especially vulnerable to interruption by hypoxia-ischemia and associated oxidative stress, inflammation, and excitotoxicity[3,30]. Although the transition from fetal to early postnatal life is the period of greatest vulnerability, the infant remains at risk for brain injury throughout the period of oligodendrocyte development, approximately 33 weeks post-menstrual age. Treatment with neuroprotective agents during this period have the greatest chance for improving long term neurodevelopmental outcomes in preterm infants at highest risk for neurodevelopmental impairment. ESAs such as Epo have been shown to ameliorate neural injury in animal models of neonatal ischemia[31-34] and improve neurodevelopmental outcome in premature human neonates administered these agents[3,24,25]. The neurocognitive outcomes we reported at 2 years in this cohort [25] remained significantly better at 4 and 6 years of age in children who as preterm infants received ESAs during their initial hospital stay (Table 3) [25,35]. At 4 years of age, full scale IQ was 91.1 ±17.5 in ESA recipients compared with 79.2 ±18.5 in placebo recipients (p = 0.036), and performance IQ was 93.0±17.0 in ESA recipients compared with 79.5±19.5 in placebo recipients (p=0.018).
Table 3.
Summary of neurocognitive outcomes at 4 and 6 years of age.
| ESA | Placebo | Term | ESA vs Placebo | ESA vs Term | |
|---|---|---|---|---|---|
| 4 year visit: subjects — no. | 39 | 14 | 24 | ||
| Full-scale IQ (FSIQ): | 91.5 (18.1) | 79.1 (18.5) | 102.6 (12.8) | 0.034 | 0.011 |
| Performance IQ (PIQ) | 93.0 (17.0) | 79.1 (19.6) | 103.9 (10.6) | 0.015 | 0.006 |
| Verbal IQ (VIQ) | 91.7 (18.3) | 82.9 (16.9) | 100.8 (15.2) | NS | NS |
| ESA | Placebo | Term | |||
| 6 year visit: subjects — no. | 44 | 17 | 21 | ||
|
| |||||
| Full-scale IQ (FSIQ): | 94.1 (18.0) | 83.1 (17.3) | 100.4 (13.5) | 0.039 | NS |
| Performance IQ (PIQ) | 97.3 (15.8) | 83.7 (15.2) | 101.6 (14.1) | 0.004 | NS |
| Verbal IQ (VIQ) | 92.1 (17.7) | 86.0 (16.8) | 98.3 (15.2) | NS | NS |
NS: not significant
A number of studies have established evidence of metabolic abnormalities in the brains of preterm infants within weeks of birth. In an early 1H-MRS study, NAA/Cr was found to be lower in the cerebellum of preterm neonates relative to its level in term neonates[4]. Examining only preterm neonates, Robertson et al[5] found that lactate/Cr and mI/Cr were higher in posterior periventricular white matter in infants with white matter abnormalities on MRI. In a cross sectional study comparing term to preterm neonates within a few weeks of birth, Kreis et al[6] examined correlations between gestational age and several metabolite concentrations, including NAA, Cho, Cr, mI, lactate, and glutamate. No significant differences were found between groups with respect to any metabolite in the cortical gray, thalamic, or white matter regions examined. Similarly, Roelants-van Rijn et al[7] measured NAA/Cho, mI/Cho, lactate/Cho and Glx/Cho in basal ganglia and periventricular white matter in preterm and term neonates and found associations with gestational age and NAA/Cho (positive) and mI/Cho (negative), but no significant differences between the preterm and term groups.
In a more recent study, Bluml et al[13] showed that the trajectories of NAA and Cho levels in a large group of term and preterm neonates differed significantly in parietal white matter over a cross-sectional sample with gestational ages from 270 to 380 days. No significant differences in any metabolite examined (NAA, Cr, Cho, glutamate, taurine and mI) were found in gray matter, and the white matter differences approached zero at the end of the gestational age range, suggesting that metabolite levels in preterm born infants normalize relatively soon after birth. Koob et al[17] found lower levels of Cr, taurine, and Glx in the centrum semiovale of preterm neonates relative to those in term neonates examined at 40 weeks of gestational age. along with evidence of white matter pathology with diffusion tensor imaging (DTI). Infants with white matter pathology evident on clinical MRI images (e.g., atrophy, hemorrhage, ventriculomegaly) were excluded in this study. However, studies by Wisnowski el al. have shown that punctate white matter lesions evident on MRI in preterm neonates were associated with greater glutamine and lower NAA in white matter, while more diffuse white matter abnormalities were associated with lactate and lower mI[11,16]. A subsequent analysis of data from this sample revealed that lower NAA and higher lactate and Cho in white matter were associated with both lower white matter lesion load and thalamic volume[18]. Similarly, Card et al[10], in a study on preterm infants shortly after birth and then at a term-equivalent number of weeks, observed that white matter abnormalities were associated with lower NAA/Cr in the basal ganglia, and that Clinical Risk for Babies II and Apgar scores were associated with higher lactate/Cr or lactate/Cho in this region. In a large voxel in frontal gray and white matter, Kwon et al[15] found lower glutamate and gamma-aminobutyric acid (GABA) in preterm neonates relative to these metabolites in term neonates, and that GABA was more strongly correlated with a fMRI measure of resting state connectivity in preterm neonates.
Studies have also examined the relationship between metabolite levels soon after preterm birth and cognitive performance at some later age. Augustine et al[8] measured NAA/Cho within a few weeks of birth in both the thalamus and basal ganglia and found no significant correlations between this ratio and Bayley cognitive scores at 18 to 24 months of age. However, Van Kooij et al[9] measured cerebellum volume and metabolite levels after birth in preterm neonates and found that both volume and NAA/Cho correlated with Bayley scores at 24 months of age, though the lack of a term-born control group in this study prohibits inferences as to whether these correlations are related to prematurity. Similarly, Kendall et al[14] reported that higher Cho/Cr and lower NAA/Cho in the periventricular white matter of a cohort of preterm infants measured shortly after birth predicted impaired motor outcome at the age of 1 year, with no comparison to term infants. Bapat et al[12] found lower NAA/Cho in subventricular white matter and frontal cortex of preterm infants relative to these ratios in term infants, and that NAA/Cho in subventricular white matter and frontal cortex and NAA/mI in subventricular white matter correlated with Bayley mental scores at 18-22 months corrected for age in both groups.
Based on the most consistent outcomes from the studies on neonates, though widely ranging in methodology, one might conclude that 1) the primary regions of metabolic abnormalities during the perinatal period of premature birth are in white matter and 2) these abnormalities are associated with evidence of white matter structural abnormalities evident on MRI.
Only a few 1H-MRS studies have shown evidence of long term metabolic deficits in preterm-born children[19,21,20]. In spite of the very important implications of these findings, in terms of the possible mechanisms that might underlie lifelong deficits in learning and cognition in preterm-born children, there have been no reports on the replication of these studies in larger, independent cohorts.
To our knowledge, the present study is the first to report the long-term neurometabolic effects of ESA treatment in preterm-born children. Though significant age-related effects and a pattern of lower mean tNAA, Cho, and mI in the placebo-treated group relative to the ESA group and term group were observed at both 4 and 6 years, no metabolite difference among treatment groups was significant in multivariate, univariate or repeated measures analyses of the data at the 0.05 level of significance. Given our sample sizes and statistical model, we had adequate power to observe only medium to large statistically significant effect sizes across the central comparison of placebo and ESA treated children. Thus, we can not rule out small effects of prematurity or treatment on neurometabolites, which may still be clinically meaningful. Aggregating results of similar studies through meta-analysis may help detect smaller effect sizes.
The current study differed from our previous study of preterm- and term-born children[21], in which significant metabolite differences in WM were observed, not only in terms of the subject ages (18-22 months and 3-4 years vs. 4 years and 6 years) but in the statistical analysis applied. Due to a high number of outliers in the former study, we used a non-parametric method for group comparisons (Mann-Whitney-Wilcoxon) which precluded adjusting the metabolite levels for tissue GM-WM composition, a source of variance that proved to be consistently significant in the various analyses on the current data. The variability in GM composition was particularly high at the periventricular WM voxel location. Our present findings underscore the need to account for GM-WM composition when analyzing 1H-MRS data from brain tissue. Metabolite levels can vary substantially between GM and WM and if there are group differences in tissue composition at a voxel location, failing to control for this variance could introduce a systematic bias in the results. Furthermore, it is worth noting that only a limited number of brain metabolites can be measured relatively reliably by in vivo 1H-MRS, with a variability that is typically of the order of 5-10% in cooperative adult subjects[26]. Given these caveats, along with the inconsistent results of the small number of studies on the long-term neurometabolic effects of prematurity, further studies are needed to probe the underlying causes of the persistent cognitive deficits of prematurity. Additionally, in view of the more consistently significant findings of 1H-MRS studies performed soon after preterm birth, it may be worthwhile to examine the effects of ESA treatment on brain metabolism during this early period of life. Such studies, for example, could explore the possibility that ESA treatment causes early neurometabolic differences that resolve over time but are part of processes leading to enduring benefits in brain function such as improved cortical connectivity.
In conclusion, no significant differences in metabolite levels were observed at either 4 or 6 years of age between preterm-born children treated shortly after birth with ESAs, preterm-born children who received a placebo after birth, and term-born children. With the caveat that the study was not adequately powered to observe statistically significant small effect sizes, these results suggest that earlier neurometabolite differences between preterm and full term infants that may have existed, such as those observed in other studies, have resolved by 4 years of age and, therefore, the beneficial effect of ESA treatment on neural development in this age range is achieved through mechanisms not involving the normalization of persistent deficits in these metabolites. Future carefully conducted and adequately powered studies are needed to characterize the effect of ESA treatment on neonatal neurometabolism and the mechanisms underlying long term benefits of ESAs on neurodevelopment.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Table 2.
Mean group metabolite concentrations (milimoles/kg tissue water), standard deviations (SD), differences between group means (Diff), and the 95% confidence intervals of these differences (CI(±)). tNAA = N-acetylaspartate + N-acetylaspartatylglutamate; Cho = total choline groups; mI = myo-inositol; Glx = glutamate + glutamine.
| 4 year visit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Treated | Control | Mean Difference 95% Confidence Interval | |||||||||
| AC | n=15 | n=15 | n=22 | Treat-Placebo | Contr-Placebo | Contr-Treat | ||||||
| Mean | SD | Mean | SD | Mean | SD | Diff | CI (±) | Diff | CI (±) | Diff | CI (±) | |
| tNAA | 14.03 | 0.90 | 14.35 | 0.99 | 14.22 | 0.96 | 0.32 | 0.68 | 0.19 | 0.61 | −0.13 | 0.64 |
| Cr | 12.09 | 1.17 | 12.29 | 0.91 | 12.00 | 0.96 | 0.21 | 0.75 | −0.08 | 0.72 | −0.29 | 0.61 |
| Cho | 2.67 | 0.32 | 2.77 | 0.34 | 2.81 | 0.31 | 0.10 | 0.24 | 0.14 | 0.21 | 0.04 | 0.22 |
| mI | 10.00 | 1.57 | 10.51 | 1.14 | 10.30 | 1.02 | 0.50 | 0.98 | 0.29 | 0.90 | −0.21 | 0.72 |
| Glx | 18.02 | 2.24 | 19.41 | 2.58 | 17.86 | 1.39 | 1.40 | 1.73 | −0.16 | 1.28 | −1.55 | 1.43 |
| WM | n=16 | n=13 | n=15 | |||||||||
| tNAA | 12.99 | 0.91 | 12.79 | 1.30 | 12.84 | 1.74 | −0.20 | 0.83 | −0.15 | 0.99 | 0.05 | 1.13 |
| Cr | 9.89 | 0.89 | 9.51 | 1.02 | 9.50 | 1.49 | −0.38 | 0.71 | −0.38 | 0.87 | −0.01 | 0.94 |
| Cho | 3.31 | 0.37 | 3.16 | 0.55 | 3.32 | 0.57 | −0.15 | 0.35 | 0.01 | 0.34 | 0.16 | 0.42 |
| mI | 7.57 | 1.05 | 7.41 | 0.86 | 7.19 | 1.08 | −0.16 | 0.69 | −0.38 | 0.75 | −0.22 | 0.72 |
| Glx | 13.18 | 1.89 | 12.78 | 1.52 | 13.26 | 2.44 | −0.40 | 1.24 | 0.08 | 1.54 | 0.48 | 1.49 |
| 6 year visit | ||||||||||||
| Placebo | Treated | Control | Mean Difference 95% Confidence Interval | |||||||||
| AC | n=12 | n=17 | n=12 | Treat-Placebo | Contr-Placebo | Contr-Treat | ||||||
| Mean | SD | Mean | SD | Mean | SD | Diff | CI (±) | Diff | CI (±) | Diff | CI (±) | |
| tNAA | 14.42 | 0.94 | 14.74 | 0.70 | 14.66 | 0.69 | 0.32 | 0.63 | 0.25 | 0.66 | −0.07 | 0.51 |
| Cr | 12.26 | 1.29 | 12.47 | 0.84 | 12.30 | 0.80 | 0.22 | 0.83 | 0.05 | 0.86 | −0.17 | 0.60 |
| Cho | 2.77 | 0.32 | 2.82 | 0.28 | 2.94 | 0.30 | 0.05 | 0.23 | 0.17 | 0.25 | 0.12 | 0.21 |
| mI | 10.20 | 1.55 | 10.74 | 1.39 | 10.89 | 1.27 | 0.53 | 1.10 | 0.68 | 1.14 | 0.15 | 0.98 |
| Glx | 19.87 | 4.26 | 19.89 | 1.64 | 18.84 | 1.60 | 0.02 | 2.53 | −1.03 | 2.57 | −1.05 | 1.19 |
| WM | n=10 | n=12 | n=8 | |||||||||
| tNAA | 13.32 | 0.89 | 13.59 | 1.00 | 13.93 | 0.76 | 0.27 | 0.79 | 0.61 | 0.76 | 0.34 | 0.77 |
| Cr | 9.28 | 0.60 | 9.87 | 0.63 | 9.55 | 0.82 | 0.59 | 0.51 | 0.28 | 0.68 | −0.31 | 0.67 |
| Cho | 3.31 | 0.35 | 3.15 | 0.39 | 3.33 | 0.42 | −0.15 | 0.31 | 0.03 | 0.36 | 0.18 | 0.36 |
| mI | 7.22 | 1.21 | 8.05 | 0.90 | 7.85 | 1.55 | 0.84 | 0.91 | 0.63 | 1.31 | −0.20 | 1.19 |
| Glx | 13.47 | 1.52 | 13.22 | 2.39 | 12.93 | 1.34 | −0.26 | 1.64 | −0.54 | 1.32 | −0.29 | 1.64 |
Acknowledgments
The authors wish to thank research coordinators and bedside nurses involved in the original randomized study. We also wish to thank Sarah Peceny and Sean Gonzales for subject coordination, Cathy Smith and Diana South for performing the scans, Joy Van Meter and the subject monitors who assisted during late night scanning. We are also indebted to the parents of our subjects who provide inspiration and motivation for this work, and finally, we need to acknowledge all US taxpayers who provide funding to support the National Institutes of Health and this study.
Funding Source: Supported by grants from the NIH NICHD (R01-HD059856), the Thrasher Research Fund, and the University of New Mexico Clinical and Translational Science Center (UL1 TR001449)
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Back SA. Brain Injury in the Preterm Infant: New Horizons for Pathogenesis and Prevention. Pediatr Neurol. 2015;53(3):185–192. doi: 10.1016/j.pediatrneurol.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messier AM, Ohls RK. Neuroprotective effects of erythropoiesis-stimulating agents in term and preterm neonates. Curr Opin Pediatr. 2014;26(2):139–145. doi: 10.1097/MOP.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N. Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr Res. 1991;30(6):574–578. doi: 10.1203/00006450-199112000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Robertson NJ, Kuint J, Counsell TJ, Rutherford TA, Coutts A, Cox IJ, Edwards AD. Characterization of cerebral white matter damage in preterm infants using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2000;20(10):1446–1456. doi: 10.1097/00004647-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48(6):949–958. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 7.Roelants-van Rijn AM, van der Grond J, Stigter RH, de Vries LS, Groenendaal F. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr Res. 2004;56(2):285–290. doi: 10.1203/01.PDR.0000132751.09067.3F. [DOI] [PubMed] [Google Scholar]
- 8.Augustine EM, Spielman DM, Barnes PD, Sutcliffe TL, Dermon JD, Mirmiran M, Clayton DB, Ariagno RL. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatol. 2008;28(9):611–618. doi: 10.1038/jp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Kooij BJ, Benders MJ, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54(3):260–266. doi: 10.1111/j.1469-8749.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 10.Card D, Nossin-Manor R, Moore AM, Raybaud C, Sled JG, Taylor MJ. Brain metabolite concentrations are associated with illness severity scores and white matter abnormalities in very preterm infants. Pediatr Res. 2013;74(1):75–81. doi: 10.1038/pr.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wisnowski JL, Bluml S, Paquette L, Zelinski E, Nelson MD, Jr, Painter MJ, Damasio H, Gilles F, Panigrahy A. Altered glutamatergic metabolism associated with punctate white matter lesions in preterm infants. PLoS One. 2013;8(2):e56880. doi: 10.1371/journal.pone.0056880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bapat R, Narayana PA, Zhou Y, Parikh NA. Magnetic resonance spectroscopy at term-equivalent age in extremely preterm infants: association with cognitive and language development. Pediatr Neurol. 2014;51(1):53–59. doi: 10.1016/j.pediatrneurol.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluml S, Wisnowski JL, Nelson MD, Jr, Paquette L, Panigrahy A. Metabolic maturation of white matter is altered in preterm infants. PLoS One. 2014;9(1):e85829. doi: 10.1371/journal.pone.0085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendall GS, Melbourne A, Johnson S, Price D, Bainbridge A, Gunny R, Huertas-Ceballos A, Cady EB, Ourselin S, Marlow N, Robertson NJ. White matter NAA/Cho and Cho/Cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology. 2014;271(1):230–238. doi: 10.1148/radiol.13122679. [DOI] [PubMed] [Google Scholar]
- 15.Kwon SH, Scheinost D, Lacadie C, Benjamin J, Myers EH, Qiu M, Schneider KC, Rothman DL, Constable RT, Ment LR. GABA, resting-state connectivity and the developing brain. Neonatology. 2014;106(2):149–155. doi: 10.1159/000362433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisnowski JL, Schmithorst VJ, Rosser T, Paquette L, Nelson MD, Haynes RL, Painter MJ, Bluml S, Panigrahy A. Magnetic resonance spectroscopy markers of axons and astrogliosis in relation to specific features of white matter injury in preterm infants. Neuroradiology. 2014;56(9):771–779. doi: 10.1007/s00234-014-1380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koob M, Viola A, Le Fur Y, Viout P, Ratiney H, Confort-Gouny S, Cozzone PJ, Girard N. Creatine, Glutamine plus Glutamate, and Macromolecules Are Decreased in the Central White Matter of Premature Neonates around Term. PLoS One. 2016;11(8):e0160990. doi: 10.1371/journal.pone.0160990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisnowski JL, Ceschin RC, Choi SY, Schmithorst VJ, Painter MJ, Nelson MD, Bluml S, Panigrahy A. Reduced thalamic volume in preterm infants is associated with abnormal white matter metabolism independent of injury. Neuroradiology. 2015;57(5):515–525. doi: 10.1007/s00234-015-1495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bathen TF, Sjobakk TE, Skranes J, Brubakk AM, Vik T, Martinussen M, Myhr GE, Gribbestad IS, Axelson D. Cerebral metabolite differences in adolescents with low birth weight: assessment with in vivo proton MR spectroscopy. Pediatr Radiol. 2006;36(8):802–809. doi: 10.1007/s00247-006-0159-5. [DOI] [PubMed] [Google Scholar]
- 20.Gimenez M, Soria-Pastor S, Junque C, Caldu X, Narberhaus A, Botet F, Bargallo N, Falcon C, Mercader JM. Proton magnetic resonance spectroscopy reveals medial temporal metabolic abnormalities in adolescents with history of preterm birth. Pediatr Res. 2008;64(5):572–577. doi: 10.1203/PDR.0b013e3181841eab. [DOI] [PubMed] [Google Scholar]
- 21.Phillips JP, Ruhl D, Montague E, Gasparovic C, Caprihan A, Ohls RK, Schrader R, Lowe JR. Anterior cingulate and frontal lobe white matter spectroscopy in early childhood of former very LBW premature infants. Pediatr Res. 2011;69(3):224–229. doi: 10.1203/PDR.0b013e3182091d52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bluml S, Wisnowski JL, Nelson MD, Jr, Paquette L, Gilles FH, Kinney HC, Panigrahy A. Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex. 2013;23(12):2944–2955. doi: 10.1093/cercor/bhs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7(4):391–395. doi: 10.3758/CABN.7.4.39. [DOI] [PubMed] [Google Scholar]
- 24.Ohls RK, Kamath-Rayne BD, Christensen RD, Wiedmeier SE, Rosenberg A, Fuller J, Lacy CB, Roohi M, Lambert DK, Burnett JJ, Pruckler B, Peceny H, Cannon DC, Lowe JR. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133(6):1023–1030. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohls RK, Cannon DC, Phillips J, Caprihan A, Patel S, Winter S, Steffen M, Yeo RA, Campbell R, Wiedmeier S, Baker S, Gonzales S, Lowe J. Preschool Assessment of Preterm Infants Treated With Darbepoetin and Erythropoietin. Pediatrics. 2016;137(3):e20153859. doi: 10.1542/peds.2015-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips J, Yeo RA, Caprihan A, Cannon DC, Patel S, Winter S, Steffen M, Campbell R, Wiedmeier S, Baker S, Gonzales S, Lowe J, Ohls RK. Neuroimaging in former preterm children who received erythropoiesis stimulating agents. Pediatr Res. 2017;82(4):685–690. doi: 10.1038/pr.2017.130. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 28.Gasparovic C, Bedrick EJ, Mayer AR, Yeo RA, Chen H, Damaraju E, Calhoun VD, Jung RE. Test-retest reliability and reproducibility of short-echo-time spectroscopic imaging of human brain at 3T. Magn Reson Med. 2011;66(2):324–332. doi: 10.1002/mrm.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell JR, Yellowhair TR, Oppong AY, Camacho JE, Lowe JR, Jantzie LL, Ohls RK. Cognitive development in preterm infants: multi-faceted deficits reflect vulnerability of rigorous neurodevelopmental pathways. Minerva Pediatr. 2017 doi: 10.23736/S0026-4946.17.04905-2. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001;276(42):39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- 32.Fan X, van Bel F, van der Kooij MA, Heijnen CJ, Groenendaal F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr Res. 2013;73(1):18–23. doi: 10.1038/pr.2012.139. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez FF, Larpthaveesarp A, McQuillen P, Derugin N, Wendland M, Spadafora R, Ferriero DM. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke. 2013;44(3):753–758. doi: 10.1161/STROKEAHA.111.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jantzie LL, Getsy PM, Firl DJ, Wilson CG, Miller RH, Robinson S. Erythropoietin attenuates loss of potassium chloride co-transporters following prenatal brain injury. Mol Cell Neurosci. 2014;61:152–162. doi: 10.1016/j.mcn.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe JR, Rieger RE, Moss NC, Yeo RA, Winter S, Patel S, Phillips J, Campbell R, Baker S, Gonzales S, Ohls RK. Impact of Erythropoiesis-Stimulating Agents on Behavioral Measures in Children Born Preterm. J Pediatr. 2017 May;184:75–80.e1. doi: 10.1016/j.jpeds.2017.01.020. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


