Abstract
Background
Preliminary data suggest that treatment optimization can reverse immunogenicity and regain response in patients with IBD and secondary loss of response (SLR) to anti-TNF therapy due to anti-drug antibodies. However, data regarding the long-term outcome of these patients are scarce.
Aims
We aimed to investigate drug retention in IBD patients of whom infliximab was optimized to overcome immunogenicity and variables associated with drug retention.
Methods
This was a retrospective, multi-center study of consecutive IBD patients with antibodies to infliximab (ATI), based on either proactive or reactive therapeutic drug monitoring, who underwent infliximab optimization (increasing dose, shortening interval, adding an immunomodulator, or combination) to overcome immunogenicity from September 2012 to July 2015; they were followed through December 2015. ATI were analyzed using the drug-tolerant Prometheus homogeneous mobility shift assay. Drug retention was defined as no need for drug discontinuation due to SLR or serious adverse event.
Results
Our cohort consisted of 22 patients (Crohn’s disease, n=15). At the end of follow up [median, (IQR): 17.3 (10.5–32.8) months] 77% (15/22) of patients were still on drug. Univariable Cox proportional hazards regression analysis identified first detectable ATI titer as the only variable associated with drug retention (HR: 0.89; 95%CI: 0.82–0.98, p=0.016). Receiver operating characteristic analysis identified an ATI titer <8.8 U/mL associated with drug retention.
Conclusions
In real-life clinical practice optimization of infliximab therapy can prevent drug discontinuation in approximately 3/4 of patients with ATI, especially in those with low titers. Large prospective studies are needed to confirm these data.
Keywords: Crohn’s disease, ulcerative colitis, therapeutic drug monitoring, antibodies to infliximab
INTRODUCTION
Anti-tumor necrosis factor (TNF) therapy has revolutionized the care of patients with inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC). [1] Nevertheless, up to 20% of patients receiving regular maintenance dosing may develop antibodies to infliximab (ATI) leading to a rapid immune clearance and inadequate drug concentrations and subsequent secondary loss of response (SLR) and treatment failure.2–6 These ATI, typically present in higher concentration, and are commonly defined as persistent. In contrast, transient ATI are typically present in lower concentrations and do not appear to negatively affect the pharmacokinetic profile of infliximab and/or have a significant clinical impact on disease activity. Immunogenicity has also been linked to serious infusion reactions necessitating drug discontinuation.7
As therapeutic options for patients with moderate to severe IBD and SLR to anti-TNF therapy are still limited, several strategies have been applied to regain response before changing to another biologic agent. Such approaches include increasing of dose, shortening of infusion interval and/or the addition of an immunomodulator (IMM).8–12 Preliminary data show that anti-TNF therapy optimization can reduce immunogenicity, increase drug concentration and consequently regain response in patients with SLR to anti-TNF therapy and anti-drug antibodies.13–17 Nevertheless, there are only limited data regarding the long-term efficacy of these strategies.
The primary aim of this study was to investigate drug retention in IBD patients with ATI who subsequently underwent infliximab optimization to overcome immunogenicity. The secondary aim was to determine variables associated with drug retention.
MATERIAL AND METHODS
Study design and population
This was an observational, retrospective, multi-center study [Beth Israel Deaconess Medical Center, Harvard Medical School and University of Pennsylvania, Perelman School of Medicine]. All consecutive IBD patients with detectable ATI, based on either proactive or reactive therapeutic drug monitoring, who underwent infliximab optimization (increasing dose, shortening interval, adding an immunomodulator, or combination) to overcome immunogenicity, from September 2012 to July 2015, were included in the study. Patients underwent either reactive or proactive TDM, as previously described.18 Changes in the infliximab regimen (including treatment discontinuation) were made based on physician global assessment reflecting real-life clinical practice. Patients who withdrew from treatment after the initial detection of antibodies were excluded. The decision of who would be dose escalated and/or receive an IMM versus discontinue was at the physician’s discretion reflecting real-life clinical practice in two large tertiary IBD centers and was typically based on the concentration of ATIs and not any specific clinical or biochemical characteristics of the patients. The median (IQR) ATI concentration of the 18 patients who were withdrawn from treatment after the initial detection of antibodies was higher compared to the optimized group [72.5 (17.7–100) vs. 7.6 (4.8–9.9) U/ml, p<0.001). Demographic and clinical characteristics of the patients were acquired via their electronic medical records. The study was approved by the Institutional Review Boards of each institution.
Outcomes and definitions
Drug retention was defined as no need for drug discontinuation due to SLR or a serious adverse event. The study observation time was defined as the time from first detectable ATI until drug discontinuation or the end of follow up (December 2015) following optimization of infliximab therapy for overcoming immunogenicity. Serious infusion reaction was defined as any acute or delayed infusion reaction necessitating infliximab discontinuation.
Infliximab concentration and ATI measurement
Serum infliximab concentrations and ATI were analyzed by the drug-tolerant Prometheus homogeneous mobility shift assay.19 Infliximab concentration of < 1 μg/mL and ATI < 3.1 U/mL were considered as undetectable.
Statistical analysis
Descriptive statistics were provided with median and interquartile range (IQR) for continuous variables, and frequency and percentages for categorical variables. Kaplan-Meier survival analysis (Log-rank test) was used to estimate drug retention. Univariable Cox proportional hazards regression analysis was also performed to determine the variables associated with drug retention. The following variables were examined: gender, age at diagnosis, age at start of infliximab treatment, duration from infliximab initiation until first detectable ATI, IBD subtype, UC extension, CD location and behaviour, perianal fistulizing CD, ileocolonic resection and anti-TNF therapy prior to first detectable ATI, smoking status (ever/never), concomitant IMM, infliximab dosing other than 5 mg/kg every 8 weeks, first detectable ATI titer [either as continuous or as a categorical variable, using a cut-off from receiver-operating characteristic (ROC) analysis], center and type of first intervention. Due to the small sample size a multivariable Cox proportional hazards regression analysis was not performed. A ROC analysis was performed to trace ATI thresholds associated with drug retention. Optimal thresholds were chosen using the Youden index, which maximizes the sum of the sensitivity (SN) and specificity (SP) of the ROC curve. All analyses were performed using SPSS version 23.0 (SPSS, Chicago, IL, USA) and GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
Study Population
The study cohort consisted of 22 patients (CD: n=15, 68%) (Figure 1). The median follow-up was 17.3 (IQR: 10.5–32.8) months. Patient baseline characteristics are shown in Table 1. Half of the patients underwent reactive TDM (n=11), the great majority for SLR (n=10, 91%) and only one patient for drug intolerance (infusion reaction). Half of the patients (n=11) had consecutive ATI measurements after first detectable ATI (range: 2–8). There were 3/11 (27%) patients in the reactive group and 8/11 (73%) patients in the proactive group with serial testing available. The median (range) ATI concentrations between the proactive and the reactive group [7.5 (5.9–8.7) U/ml vs. 7.6 (4.7–24.7) U/ml, respectively] were similar (p=0.743, Mann–Whitney U test).
Figure 1.
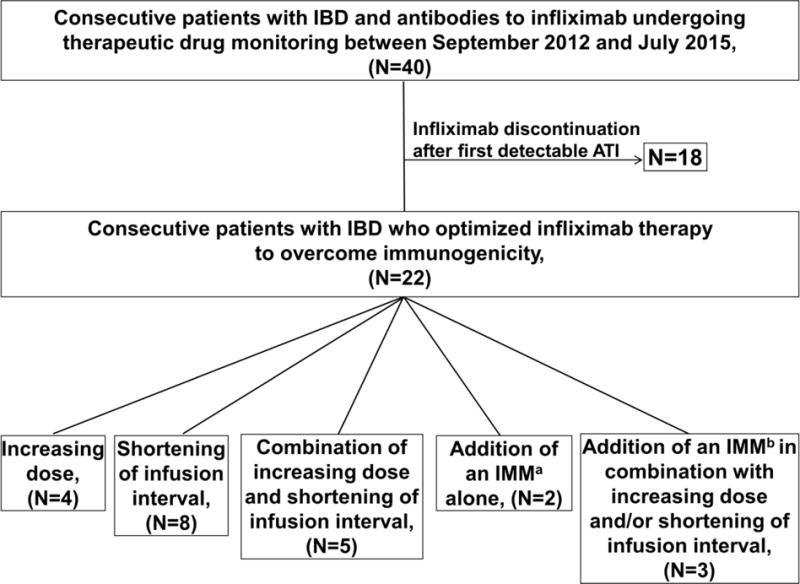
Flow chart of study population.
ATI: antibodies to infliximab; IBD: inflammatory bowel disease; IMM: immunomodulator.
aThiopurines (n=1), methotrexate (n=1). bThiopurines (n=2), methotrexate (n=1).
Table 1.
Baseline characteristics of the study cohort.
| Patient characteristics | N=22 |
|---|---|
|
| |
| Male, (%) | 12 (54) |
|
| |
| Age at diagnosis, median (IQR), years | 25 (20–36) |
|
| |
| Age at start of infliximab, median (IQR), years | 41 (28–47) |
|
| |
| Duration from infliximab initiation until first detectable ATI, median (IQR), months | 19 (9–42) |
|
| |
| CD, (%) | 15 (68) |
|
| |
| CD locationa, (%) | |
| L1 (ileal) | 3/15 (20) |
| L2 (colonic) | 3/15 (20) |
| L3 (ileocolonic) | 8/15 (53) |
| L4 (upper GI disease) | 1/15 (7) |
|
| |
| CD behaviora, (%) | |
| B1 (non-stricturing, non-penetrating) | 7/15 (47) |
| B2 (stricturing) | 5/15 (33) |
| B3 (penetrating) | 3/15 (20) |
|
| |
| Perianal fistulising CD, (%) | 1/15 (7) |
|
| |
| Ileocolonic resection prior to first detectable ATI, (%) | 3/15 (20) |
|
| |
| UC extenta, (%) | |
| E1 (proctitis) | 1/7 (14) |
| E2 (left-sided colitis) | 3/7 (43) |
| E3 (pancolitis) | 3/7 (43) |
|
| |
| Smoking ever, (%) | 4 (18) |
|
| |
| Infliximab dosing other than 5mg/Kg q8 week at first detectable ATI, (%) | 6 (27) |
|
| |
| Reactive TDM, (%) | 11 (50) |
|
| |
| Anti-TNF naive, (%) | 18 (82) |
|
| |
| Concomitant IMM at first detectable ATI, (%) | 1 (5)b |
Montreal classification;
thiopurine.
IBD: inflammatory bowel disease; CD: Crohn’s disease; UC: ulcerative colitis; CI: confidence interval; TDM: therapeutic drug monitoring; IMM: immunomodulators; TNF: tumor necrosis factor; ATI: antibodies to infliximab; IQR: interquartile range; GI: gastrointestinal.
Drug retention
Drug retention was achieved overall in 17/22 (77%) patients (reactive TDM: 7/11, 64%; proactive TDM: 10/11, 91%) with a range of first detectable ATI titer of 3.6 to 24.7 U/mL. In the subset of patients who had reactive testing (n=11), all patients with drug persistence (n=7) achieved clinical response, defined as a marked decrease or disappearance of symptoms based on physicians’ global assessment, 20 after infliximab intensification for overcoming ATI. The five patients who discontinued infliximab had either SLR (n=2, with a first detectable ATI titer of 8.8 and 35 U/mL) or a serious infusion reaction (acute: n=2, with a first detectable ATI titer of 5.8 and 7.2 U/ml and delayed: n=1, with a first detectable ATI titer of 29.5 U/mL). The 1st-year cumulative probability of drug discontinuation was 23% [standard error (SE): 0.09] (Figure 2). Regarding patients with drug retention and consecutive serum samples available (n=9), in the great majority of them (n=8, 89%) ATI disappeared, based on the first available ATI evaluation after treatment optimization.
Figure 2.
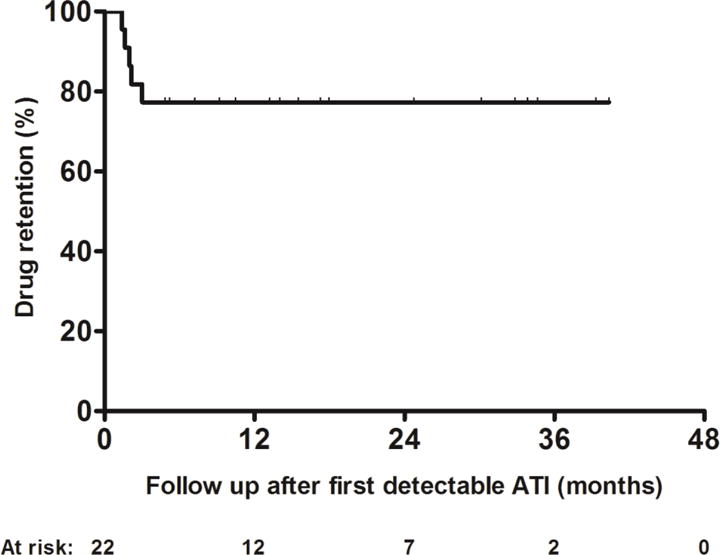
Kaplan–Meier curve for drug retention after first detectable ATI.
ATI: antibodies to infliximab.
Variables associated with drug retention
Univariable Cox regression analysis identified first detectable ATI titer [hazard ratio (HR): 0.89; 95% confidence interval (CI): 0.82–0.98; p=0.016, Table 2) as the only variable associated with drug retention. Type of first intervention to overcome immunogenicity was not associated with drug retention. A ROC analysis of first detectable ATI titer identified a threshold of <8.8 U/mL to be associated with drug retention (SN: 0.60; SP: 0.82, Figure 3A). This threshold remained the same (SN: 0.87; SP: 0.82, Figure 3B) based on ROC analysis including also the 18 patients that were excluded, as they were withdrawn from treatment after the initial detection of antibodies (Figure 1). Kaplan-Meier analysis showed a significantly higher cumulative probability for drug retention in patients with a first detectable ATI titer <8.8 U/mL, compared with patients with higher titer ATI (Figure 4). At the end of follow up, drug retention was achieved in 14 out of 16 (87.5%) patients with a first detectable ATI titer <8.8 U/mL compared to only 3 out of 6 (50%) patients with a first detectable ATI titer ≥8.8 U/mL.
Table 2.
Variables associated with drug retention.
| Variables | p | HR | 95%CI |
|---|---|---|---|
| Gender | 0.241 | ||
| CD | 0.587 | ||
| CD location | 0.697 | ||
| CD behaviour | 0.212 | ||
| Age at diagnosis | 0.477 | ||
| Age at start of infliximab | 0.537 | ||
| Duration from infliximab initiation until first detectable ATI | 0.777 | ||
| UC extension | 0.552 | ||
| Perianal fistulising disease | 1.000 | ||
| Ileocolonic resection prior to first detectable ATI | 0.623 | ||
| Concomitant IMM at first detectable ATI | 0.735 | ||
| Anti-TNF naive | 0.164 | ||
| Smoking ever | 0.485 | ||
| Infliximab dosing other than 5mg/Kg q8w at first detectable ATI | 0.440 | ||
| Type of first intervention after first detectable ATI | 0.408 | ||
| Titer of first detectable ATI | 0.016 | 0.89 | 0.82–0.98 |
| Reactive TDM | 0.151 | ||
| Center | 0.431 | ||
| Infliximab concentration at first detectable ATI | 0.847 | ||
| Undetectable infliximab concentration at first detectable ATI | 0.718 |
IBD: inflammatory bowel disease; CD: Crohn’s disease; UC: ulcerative colitis; HR: hazard ratio; CI: confidence interval; TDM: therapeutic drug monitoring; IMM: immunomodulators; TNF: tumor necrosis factor; ATI: antibodies to infliximab; w: week.
Figure 3.
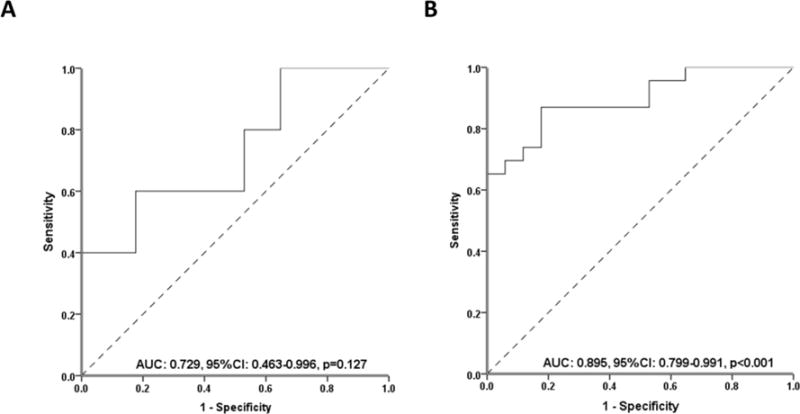
Receiver operator curve analysis for first detectable ATI titer stratifying patients with or without drug retention regarding either only the 22 patients who optimized infliximab to overcome immunogenicity (A) or including also the 18 patients (Figure 1) that were excluded, as they were withdrawn from treatment after the initial detection of antibodies (B).
ATI: antibodies to infliximab; AUC: area under the curve; CI: confidence intervals.
Figure 4.
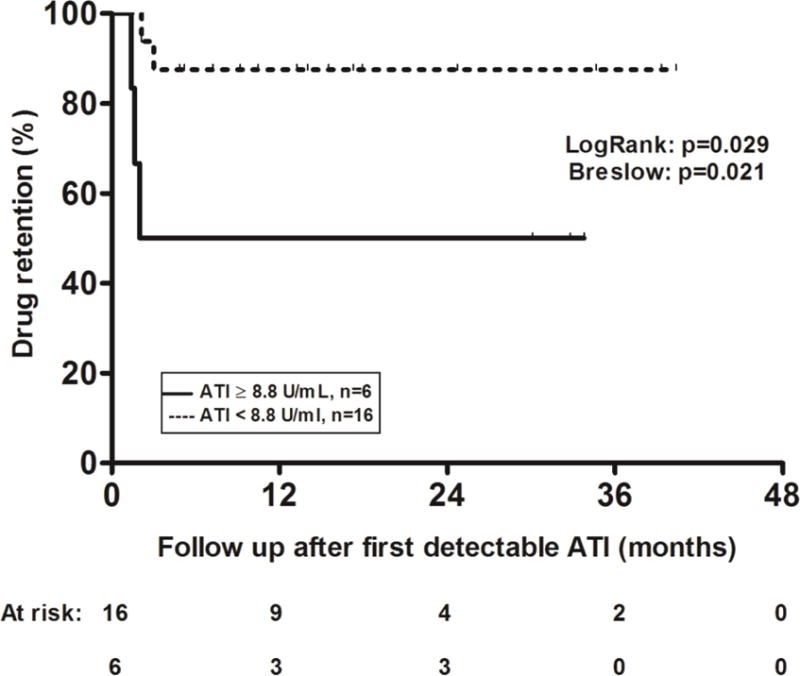
Kaplan–Meier curves for drug retention in patients with first detectable ATI <8.8 U/ml (dotted line) compared to those with ATI ≥8.8 U/ml (solid line).
ATI: antibodies to infliximab.
DISCUSSION
This retrospective, multi-center study demonstrates that when infliximab optimization to overcome ATI is performed, long-term drug retention can be achieved overall in approximately 3/4 of patients. Drug retention was numerically higher in patients undergoing proactive TDM compared to patients undergoing reactive TDM (91% vs. 64%). Consequently, ATI that are found during routine proactive TDM may be different to overcome compared to ATI found in the setting of reactive TDM.
These results are in line with previous studies showing that treatment optimization can reverse immunogenicity and regain response in 48–77% of patients with SLR to anti-TNF therapy due to anti-drug antibodies15–17. In a multi-center study, the introduction of an IMM restored infliximab trough concentration within the therapeutic range and induced clinical remission in 10 out of 18 (55%) patients with CD with a SLR to infliximab monotherapy due to ATI.15 Moreover, two previous studies demonstrated that the addition of an IMM reversed immunogenicity and regained clinical response in 48% (11/23) and 77% (13/17) of IBD patients with anti-drug antibodies and SLR to adalimumab and either infliximab (n=8) or adalimumab (n=9), respectively.16, 17 Finally, Dreesen et al.20 in a recent retrospective cohort study, investigated outcomes, pharmacokinetics and immunogenicity of treatment intensification strategies in 103 patients with CD who lost clinical response to infliximab. They showed that an ATI concentration threshold below than 282 ng/mL eq. just before treatment intensification measured with a drug tolerant ELISA was associated with a higher clinical response rate (45 of 64; 70%) compared to an ATI concentration above the 282 ng/mL eq. threshold (11 of 24; 46%) (P = 0.034).20
We found that an ATI titer less than 8.8 U/ml was associated with drug retention; thus, an ATI titer of <8.8 U/ml may be sufficiently low enough to overcome immunogenicity. Although decisions should be made on a case-by-case basis, this cut-off may help physicians determine whether to attempt infliximab optimization following first detectable ATI. This threshold is similar to one reported in a previous study showing that in patients who lose response to infliximab and have ATI <9.1 U/ml dose optimization is a valid therapeutic option.3 No association was found between type of first intervention to overcome immunogenicity and drug retention suggesting that increasing the dose, shortening the interval, adding an IMM, or combination may be equally efficacious for preventing drug discontinuation due to treatment failure; however, the number of patients undergoing some of these therapeutic maneuvers was small, and thus firm conclusions cannot be made.
The strengths of this study include the long follow-up period, representation of real-life clinical practice at two large referral IBD centers, and the use of a drug tolerant assay for evaluating ATI. However, the clinical utility of using a drug tolerant anti-drug antibody assay for infliximab is not yet clearly understood. Recently Van Stappen et al.21 evaluated the clinical relevance of ATI measured using a drug-tolerant ELISA in a post hoc analysis of the Trough Concentration Adapted Infliximab Treatment (TAXIT) randomized controlled trial. The drug-tolerant compared to the drug-sensitive ELISA increased the immunogenicity detection rate from 21% to 63% at screening, from 0% to 51% after optimization and from 3% to 42% at the end of TAXIT, respectively. They demonstrated that upon dose intensification, low concentration ATI (≤ 217 ng/ml eq), not detectable using a drug-sensitive assay, disappear in more than half of the patients over time and are clinically non-relevant.21 In contrast, high concentration ATI (≥ 799 ng/ml eq) which are typically also detected in a drug-sensitive assay, persist over time and necessitate a higher cumulative dose and drug cost.21 Limitations of our study include the retrospective nature of the design and potential for residual confounding and selection bias, the small sample size and the lack of standardized sampling.
In conclusion, this large, real-life, multi-center, retrospective study demonstrates that long-term drug retention can be achieved in 3/4 of the patients with ATI in whom dose optimization is attempted to overcome immunogenicity. Successful drug retention appears more easily achieved when ATI are less than 8.8 U/ml. Prospective studies are needed to confirm the efficacy of this therapeutic strategy and to identify the ideal candidates and best methods to overcome immunogenicity before switching to other biologic agents.
Acknowledgments
K.P.: Ruth L. Kirschstein NRSA Institutional Research Training Grant (5T32DK007760-18).
A.S.C: received consultancy fees from AbbVie, Janssen, Takeda, Ferring, Samsung, Miraca and Pfizer; M.T.O: received consultancy fees from Janssen, AbbVie, UCB, Takeda, Pfizer, Merck, and Lycera, and received research grant support from UCB;
Footnotes
Author’s contributions K.P.: study concept and design, data acquisition, analysis and interpretation, statistical analysis and manuscript writing; R.V.: data acquisition and manuscript critical revision, A.S.C., M.T.O.: study concept and design, data interpretation and manuscript critical revision. All the authors reviewed and approved the final manuscript.
Conflict of interest: The remaining authors disclose no conflicts of interest.
Ethical standards All procedures performed in the study were in accordance with the International Standard of Good Clinical Practice procedures and with the principles of the Declaration of Helsinki (1964) and relevant amendments. The protocol was approved by all institutional review boards and ethics committees at participating sites.
References
- 1.Miligkos M, Papamichael K, Casteele NV, et al. Efficacy and safety profile of anti-tumor necrosis factor-α versus anti-integrin agents for the treatment of Crohn’s Disease: A network meta-analysis of indirect comparisons. Clin Ther. 2016;38:1342–1358.e6. doi: 10.1016/j.clinthera.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Papamichael K, Cheifetz AS. Use of anti-TNF drug concentrations to optimise patient management. Frontline Gastroenterol. 2016;7:289–300. doi: 10.1136/flgastro-2016-100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962–971. doi: 10.1038/ajg.2013.12. [DOI] [PubMed] [Google Scholar]
- 4.Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis. 2012;18:1628–1233. doi: 10.1002/ibd.21919. [DOI] [PubMed] [Google Scholar]
- 5.Vande Casteele N, Gils A. Pharmacokinetics of anti-TNF monoclonal antibodies in inflammatory bowel disease: Adding value to current practice. J Clin Pharmacol. 2015;55(Suppl 3):S39–50. doi: 10.1002/jcph.374. [DOI] [PubMed] [Google Scholar]
- 6.Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539–1545. doi: 10.1136/gutjnl-2014-307883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab concentrations in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40–47. doi: 10.1038/ajg.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanai H, Lichtenstein L, Assa A, et al. Concentrations of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13:522–530. doi: 10.1016/j.cgh.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Papamichael K, Karatzas P, Mantzaris GJ. Addition of an immunomodulator as a rescue therapy for loss of response to adalimumab dose escalation in patients with Crohn’s disease. J Crohns Colitis. 2015;9:589–590. doi: 10.1093/ecco-jcc/jjv062. [DOI] [PubMed] [Google Scholar]
- 10.Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026–2033. doi: 10.1002/ibd.22902. [DOI] [PubMed] [Google Scholar]
- 11.Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1250–1256. doi: 10.1038/ajg.2014.146. [DOI] [PubMed] [Google Scholar]
- 12.Billiet T, Cleynen I, Ballet V, et al. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: a 20-year single centre experience. Aliment Pharmacol Ther. 2016;44:673–683. doi: 10.1111/apt.13754. [DOI] [PubMed] [Google Scholar]
- 13.Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: A Practical Overview. Drugs. 2017;77:363–377. doi: 10.1007/s40265-017-0693-5. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:444–4447. doi: 10.1016/j.cgh.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Peyrin-Biroulet L, Salleron J, Filippi J, et al. Anti-TNF Monotherapy for Crohn’s Disease: a 13-year Multicentre Experience. J Crohns Colitis. 2016;10:516–524. doi: 10.1093/ecco-jcc/jjw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strik AS, van den Brink GR, Ponsioen C, et al. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1128–1134. doi: 10.1111/apt.13994. [DOI] [PubMed] [Google Scholar]
- 17.Ungar B, Kopylov U, Engel T, et al. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther. 2017;45:276–282. doi: 10.1111/apt.13862. [DOI] [PubMed] [Google Scholar]
- 18.Papamichael K, Chachu KA, Vajravelu R, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017 Mar 29; doi: 10.1016/j.cgh.2017.03.031. pii: S1542–3565(17)30385-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab concentrations in patient serum. J Immunol Methods. 2012;382:177–188. doi: 10.1016/j.jim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Dreesen E, Van Stappen T, Ballet V, et al. Anti-infliximab antibody concentrations can guide on treatment intensification in patients with Crohn’s disease who lose clinical response. Aliment Pharmacol Ther. 2017 Dec 11; doi: 10.1111/apt.14452. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2017 Apr 27; doi: 10.1136/gutjnl-2016-313071. pii: gutjnl-2016-313071. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


