Abstract
Objectives
Tissue macrophages induce and perpetuate pro-inflammatory responses, thereby promoting metabolic and cardiovascular disease. Lipoprotein lipase (LpL), the rate-limiting enzyme in blood triglyceride (TG) catabolism, is expressed by macrophages in atherosclerotic plaques. We questioned whether LpL, which is also expressed in the bone marrow (BM), affects circulating white blood cells (WBCs) and BM proliferation, and modulates macrophage retention within the artery.
Approach and Results
We characterized blood and tissue leukocytes and inflammatory molecules in transgenic LpL-knockout mice rescued from lethal hypertriglyceridemia within 18 h of life by muscle-specific LpL expression (MCKL0 mice). LpL-deficient mice had ~40% reduction in blood WBC, neutrophils, and total and inflammatory monocytes (Ly6C/Ghi). LpL deficiency also significantly decreased expression of BM macrophage-associated markers (F4/80 and TNF-α), master transcription factors (PU.1 and C/EBPα) and colony-stimulating factors (CSFs) and their receptors (CSF-Rs), which are required for monocyte and monocyte precursor proliferation and differentiation. As a result, differentiation of macrophages from BM-derived monocyte progenitors and monocytes was decreased in MCKL0 mice. Further, while LpL deficiency was associated with reduced BM uptake and accumulation of TG-rich particles and M-CSF-M-CSF-R binding, TG lipolysis products (e.g., linoleic acid) stimulated expression of M-CSF and M-CSF-R in BM-derived macrophage precursor cells. Arterial macrophage numbers decreased after heparin-mediated LpL cell dissociation and by genetic knockout of arterial LpL. Reconstitution of LpL-expressing BM replenished aortic macrophage density.
Conclusions
LpL regulates peripheral leukocyte levels and affects BM monocyte progenitor differentiation and aortic macrophage accumulation.
Keywords: bone marrow, colony-stimulating factors, hematopoiesis, leukocytosis, lipoprotein lipase, macrophages, monocytes, white blood cells
SUBJECT CODES: Atherosclerosis Inflammation Lipids and Cholesterol Vascular Biology
INTRODUCTION
Although inflammation primarily serves to ameliorate injury or infection, tissue macrophage-mediated inflammatory responses promote atherosclerotic cardiovascular disease (CVD) and metabolic disorders. Accumulation of lipoproteins, especially LDL, in the aortic wall leads to an inflammatory cascade that attracts blood leukocytes and traps monocytes in the subendothelial space 1. Infiltrated monocytes can differentiate to macrophages that phagocytose accumulated LDL and become foam cells, which contribute to fatty streaks that precede atherosclerotic plaque formation 2. High white blood cell (WBC) levels thus directly accelerate atherosclerotic CVD 3, 4 and are also associated with obesity and type 2 diabetes, which also increase risk of CVD 5–7. Further, hypercholesterolemia in mice promotes blood leukocytosis, increases in inflammatory monocyte (Ly6Chi) levels, and macrophage infiltration to enhance progression of atherosclerotic lesions 8.
Tissue macrophages robustly express lipoprotein lipase (LpL), the rate-limiting enzyme in circulating triglyceride (TG) metabolism. Macrophages within atherosclerotic lesions are a major site of LpL expression 9, 10. Loss-of-function studies show that macrophage-derived LpL increases atherosclerosis 11–13, suggesting a direct role of LpL levels in mediating atherosclerotic lesion development. In our previous studies, aortic LpL levels in non-hypercholesterolemic mice positively correlated with aortic wall macrophage levels 14, 15. Several mechanisms by which LpL may promote atherosclerosis have been proposed: a) LpL acts as a non-enzymatic bridging molecule that anchors atherogenic lipoproteins to the extracellular matrix in order to promote lipoprotein retention 16–18; b) LpL hydrolyzes lipoproteins to produce pro-atherogenic, cholesterol-rich LDL 1, 19; c) LpL-mediated lipolysis products serve as energy substrates (fatty acids, FAs) that increase macrophage inflammatory responses 20; d) LpL functions as a monocyte adhesion molecule 21–23; and e) LpL induces pro-inflammatory TNF-α expression and production by macrophages 24. Atherogenic actions of LpL might thus lead to a “vicious cycle” whereby elevated macrophage levels increase LpL secretion, which promotes additional macrophage accumulation in the aortic wall. Recent genetic studies in humans have associated heterozygous LpL deficiency with early-onset coronary heart disease 25, an effect that might reflect hypertriglyceridemia, increased postprandial lipemia, and low HDL cholesterol levels. The systemic and local effects of LpL on atherogenesis are still not fully defined.
We hypothesized that expression of LpL by WBCs and their precursors alters the circulating levels of monocytes as well as their entry into the aortic wall to promote atherosclerosis. We first tested this by examining whether LpL expression affects circulating WBC numbers and bone marrow (BM) myelopoiesis. Here we show that LpL deficiency was associated with reduced circulating WBC and monocyte levels, reduced BM colony-stimulating factor (CSF) and CSF-receptor (CSF-R) expression, and fewer macrophages in the aorta of high fat-fed mice.
METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
LpL deficiency alters blood leukocyte profiling
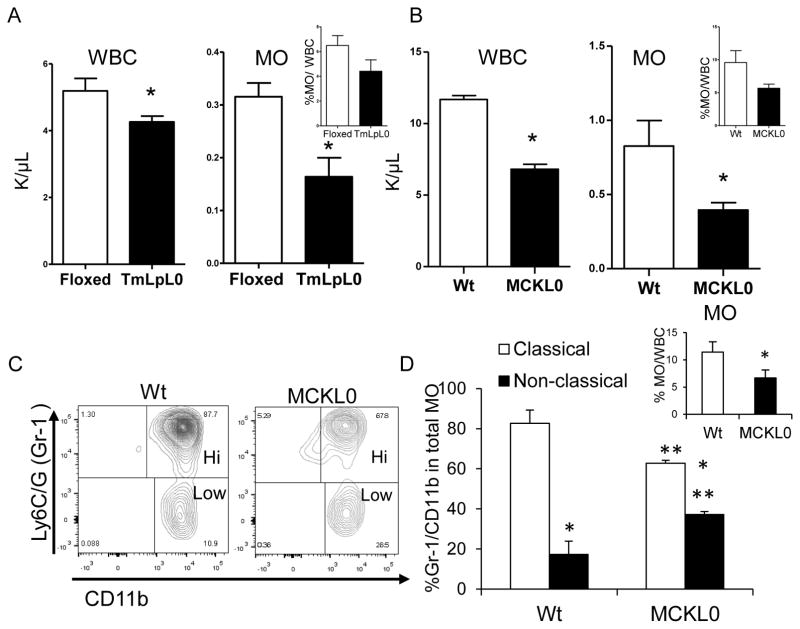
To assess whether LpL expression modulates circulating WBC levels and subtypes, LpL-deficient mice (TmLpL0) were generated by crossing floxed LpL mice with β-actin-driven tamoxifen-inducible-Cre transgenic mice 26. On a chow diet, total WBCs, monocytes and percent monocytes/WBC (inset) were reduced in TmLpL0 mice compared to control floxed mice (Figure 1A). Because TmLpL0 mice are hypertriglyceridemic (~400 mg/dL, n=10), we questioned whether the alteration in blood leukocyte levels was caused by elevated blood TG levels rather than by LpL deficiency. Using an LpL deletion mouse model that expresses LpL only in skeletal muscle (to prevent neonatal death) and maintains normal cholesterol and TG levels 27, denoted as MCKL0 mice 28, we determined that this effect is indeed attributable to LpL deficiency (Figure 1B). The reduction in the percentages of monocytes/WBC (inset) in MCKL0 mice is consistent and comparable with percent monocytes/WBC in TMLpL0 mice, 40±0.62% vs. 36±1.81%, respectively (inset in Figures 1A and 1B). Other major leukocyte subsets, i.e., neutrophils and lymphocytes, were reduced by 30% and 60%, respectively (n=12–15, p<0.05). We also observed a marked shift in the ratio of Ly6C/Ghi (Gr-1hi) to Gr-1lo monocytes that resemble human inflammatory classical and anti-inflammatory non-classical monocytes, respectively, by flow cytometry. Compared to wild type (Wt) mice, blood monocytes in MCKL0 mice had a significantly greater non-classical to classical ratio (Figures 1C and 1D) comprising a smaller percentage of total WBCs (Figure 1D, inset). Both Wt and MCKL0 mice showed no signs of active inflammatory responses.
Figure 1.
LpL deficiency alters blood white cell (WBC) profiling. Total WBCs, blood monocytes (MO) and % MO/WBC (inset) in (A) floxed control and TmLpL0 mice (n=9 or 10) and in (B) Wt and MCKL0 mice (n=12–15) mice. *, p<0.001. (C) Representative flow cytometry plots and (D) quantification of MO subsets. Inset: quantification of blood total MO (CD45+CD115hiGr-1+) by flow cytometry. *, p<0.001, classical vs. non-classical; **, p<0.05, MCKL0 vs. Wt, n=4, mean±SE.
LpL deletion modulates expression of growth factors required for BM hematopoiesis and myelopoiesis
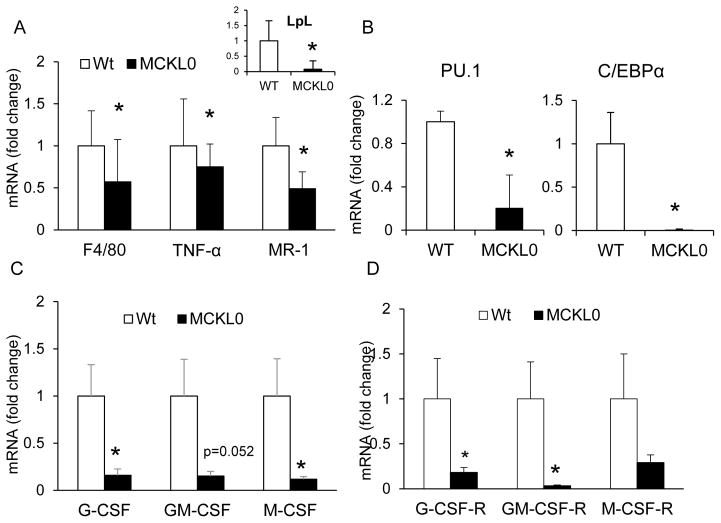
We next studied whether changes in blood leukocyte profiling and decreases in total leukocytes in MCKL0 mice were associated with altered BM myelopoiesis. We observed decreased gene expression of macrophage-associated markers—F4/80, TNF-α, and mannose receptor (MR) —in unsorted BM cell homogenates of MCKL0 mice compared to Wt (Figure 2A). Expression of master regulatory transcription factors required for hematopoietic progenitor cell proliferation and differentiation, including PU.1 (p<0.05) and CEBP/α (p<0.05) (Figure 2B), was also reduced in BM of MCKL0 mice when compared with Wt mice. Reduced expression of PU.1 and C/EBPα may be attributable to the reduction in BM total lineage negative (lin−) stem and progenitor cells in MCKL0 mice when compared to Wt (supplemental Figures I a and I b). However, populations of BM progenitor cells at early hematopoietic branch points including hematopoietic stem and progenitor cells, common myeloid progenitors, and granulocyte-macrophage progenitors were not altered in MCKL0 compared to Wt mice (supplemental Figure I c).
Figure 2.
Loss of LpL reduces bone marrow (BM) expression of growth factors essential for myelopoiesis and differentiation of MO to macrophages. (A) mRNA gene analyses of LpL (inset), macrophage marker (F4/80), macrophage-derived TNF-α and MR, (B) hematopoietic transcription factors (PU.1 and C/EBPα) and (C) G-CSF, GM-CSF and M-CSF and (D) their corresponding receptors in BM of Wt and MCKL0 mice. n=4–6, mean±SE. *, p<0.05.
These modifications in blood leukocyte profiling can also occur at the terminally differentiated stages of hematopoiesis. We thus examined whether LpL deficiency affects expression of growth factors that act on BM progenitor cells at lineage-committed stages, such as monocyte progenitors and monocytes. Strikingly, BM expression of all CSFs, including granulocyte-CSF (G-CSF), granulocyte macrophage-CSF (GM-CSF) and macrophage-CSF (M-CSF) (Figure 2C), as well as their corresponding receptors (G-CSF-R, GM-CSF-R and M-CSF-R, respectively), were substantially decreased in MCKL0 mice (Figure 2D).
Alterations in M-CSF and M–CSF-R expression relate to LpL-mediated TG metabolism
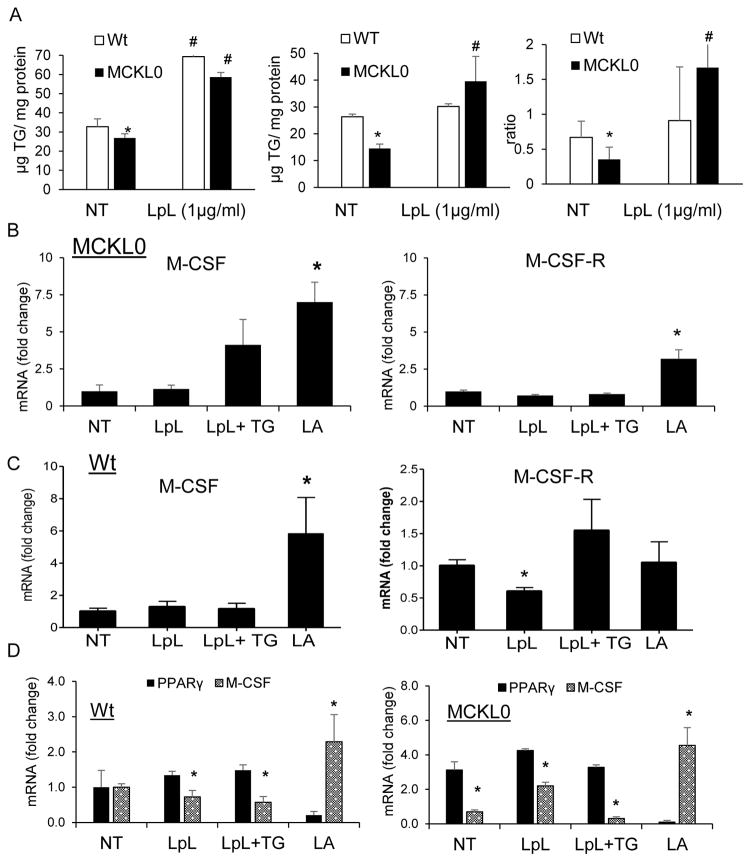
BM expression of LpL is associated with TG-rich lipoprotein catabolism, such as chylomicrons 29. We questioned if LpL-mediated reductions in CSF/CSF-R expression that contribute to altered BM myelopoiesis and WBC levels reflect changes in cellular TG uptake. We speculated that LpL deficiency would reduce cellular TG uptake and metabolism, which would alter the expression of essential growth factors for myeloid cell differentiation and proliferation. To test this, we treated differentiating macrophages derived from Wt and MCKL0 mouse BM with linoleic acid (LA)-rich TG particles (soy oil-based lipid emulsion, Intralipid), a key pathway for delivering FAs to cells, labeled with [3H]cholesteryl ether 30. As previously measured by our laboratory30–32, we have established that TG mass essentially equals TG deposition since basal cellular TG levels are low and cellular TG synthesis is negligible during the 4h incubation (i.e., less than 1% of TG uptake). Our studies examining BM cell TG metabolism show that cellular TG uptake and accumulation were reduced by 22% and 50%, respectively, in the LpL-deficient cells (Figure 3A). The ratio of cellular TG deposition to TG uptake, which represents cellular TG utilization, was reduced by ~40% in BM-derived differentiating macrophages from MCKL0 mice compared to those in Wt mice (Figure 3A).
Figure 3.
LpL deletion affects cellular TG uptake and expression of M-CSF and M-CSF-R in BM-derived macrophages. Effects of LpL on (A) cellular TG uptake, TG deposition and TG utilization (ratio of TG deposition/uptake) in BM-derived differentiating macrophages. Results represent the mean±SE of experiments performed in triplicate (n=6). BM-derived macrophage precursor cells from MCKL0 (B) and Wt (C) mice (n=6) were treated with LpL (1 μg/ml), LpL+TG (100μg/ml) or LpL-mediated lipolysis products (LA, 150 μM in 1% BSA) for 3 h. Non-treated, NT. Changes in M-CSF, M-CSF-R and PPARγ (D) mRNA expression were assayed by RT-PCR. *, p<0.05.
We also examined whether the addition of exogenous LpL can rescue the defective TG uptake and metabolism. Addition of exogenous LpL increased TG uptake in Wt BM-derived macrophage precursor cells that already have adequate expression of LpL. The addition of exogenous LpL to the LpL-deficient cells increased cellular TG uptake, mass and utilization to levels similar to that in Wt cells (Figure 3A).
We next examined whether exogenous LpL or TG-derived metabolites, such as LA, can modulate M-CSF/M-CSF-R expression in BM-derived monocyte or macrophage precursor (BMMP) cells from MCKL0 mice. Differentiating monocytes or monocyte precursors were treated with LpL, LA-rich TG particles (Intralipid) in the presence of LpL and LA. The results show that the addition of exogenous LpL and TG particles had a small effect on M-CSF expression but had no effect on M-CSF-R expression. LA stimulated expression of both M-CSF and M-CSF-R (Figure 3B) (p<0.05) in MCKL0 BM-derived myeloid precursor cells. In Wt BMMP cells, while the addition of exogenous LpL alone had no effect on M-CSF and a small effect on M-CSF-R (p<0.05) expression, addition of TG in the presence of LpL increased M-CSF-R expression (ns) with a lesser effect on M-CSF levels. LA stimulated expression of M-CSF (Figure 3C).
Because of earlier reports on the role of peroxisome proliferator-activated receptor γ (PPARγ) in influencing lipid metabolism and production of M-CSF, we questioned if transcription of M-CSF is regulated by the PPARγ-mediated NF-κB signaling cascade. Our data show a consistent inverse relation between PPARγ and M-CSF in BMMP cells. Expression of M-CSF was downregulated, while PPARγ was upregulated, in the untreated BMMP cells in MCKL0 mice when compared to Wt. Further, when additional exogenous FA was supplied during BMMP differentiation, increases in M-CSF expression were associated with the suppression of PPARγ expression in both Wt and MCKL0 mice (Figure 3D).
LpL deletion attenuates BM-derived monocyte differentiation to macrophages
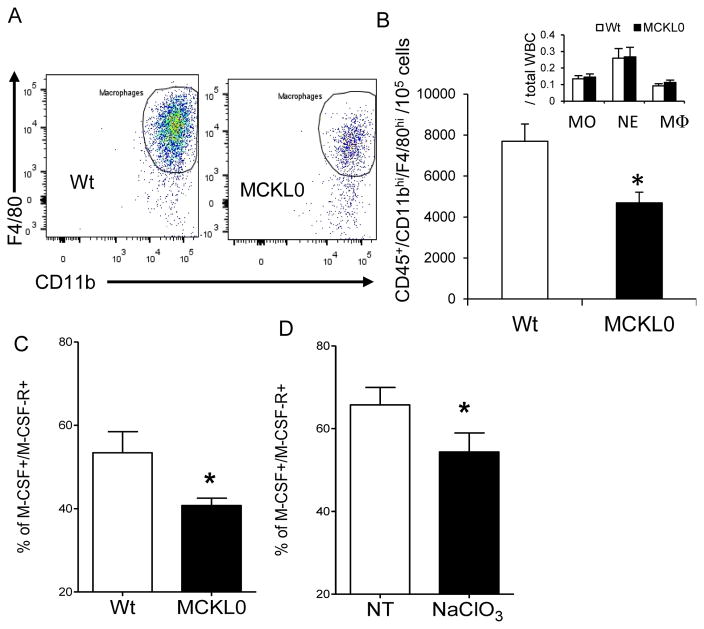
Because our data show that LpL deficiency reduced the expression of growth factors necessary for the differentiation of monocytes or monocyte precursor cells, we sought to directly assess whether the reduced expression of CSFs and CSF-Rs in BM or in local tissues modulates differentiation or proliferation of BM myeloid precursor cells. We isolated BM cells, including monocyte/macrophage precursors, from MCKL0 and Wt mice. There was no significant difference in total proliferated BM cells determined by hemocytometer (70~80 × 106 cells/femur) between Wt and MCKL0 mice. Isolated BM myeloid precursor cells were cultured according to the standard protocol, including the addition of M-CSF to stimulate cell differentiation (See supplemental Methods and Materials). At day 1, similar numbers of monocytes, neutrophils and macrophages adhered to the culture dishes (Figures 4A and 4B inset). At day 7, quantification of harvested mature differentiated macrophages by flow cytometry revealed that monocyte differentiation to macrophages was decreased by ~40% under the MCKL0 background (Figures 4A and 4B). Thus, our findings (Figures 3A–B, 4A–B) suggest that LpL deficiency impairs monocyte/macrophage differentiation by causing reduced M-CSF/M-CSF-R expression and by attenuating cellular responsiveness to M-CSF.
Figure 4.
LpL deficiency reduces differentiation of BM-derived monocytes to macrophages and association of M-CSF and M-CSF-R. (A) Representative images, flow cytometry plots and (B) quantitation of macrophages differentiated from BM precursor cells at day 7 (inset, plated MO, neutrophils-NE and macrophages-MΦ at day 1) in Wt and MCKL0 mice. *, p<0.05 vs. Wt, n=6. (C) Cellular association of M-CSF and M-CSF-R was examined by flow cytometry in BM-derived MΦ precursor cells in Wt and MCKL0 mice. *, p<0.05. n=6. (D) Effects of sodium chlorate (NaClO3) on cellular association of M-CSF and M-CSF. *, p<0.05. n=9, mean±SE.
LpL promotes binding of M-CSF to its receptor
We next sought to examine the potential mechanisms underlying the effects of LpL on modulating BM-derived macrophage precursor cellular responsiveness to M-CSF. Mammalian M-CSF is expressed as three subtypes – soluble glycoprotein, cell membrane-associated glycoprotein and matrix-anchored proteoglycan (PG) – that differentially stimulate target cells expressing M-CSF-R. It has been suggested that the density of monocyte-differentiated macrophages at the particular sites is controlled by local synthesis of the membrane-associated M-CSF and/or selective sequestration of the secreted PG form of M-CSF 33. When we assessed this effect in vivo by sorting differentiating BM myeloid precursor cells in MCKL0 and Wt mice, we observed fewer M-CSF+/M-CSF-R+ cell populations in MCKL0 mice compared to Wt (Figure 4C). Potential interacting factors that may affect M-CSF and M-CSF-R cellular interaction were examined. These include heparin, a LpL cell dissociator, THL, a LpL lipolytic activity inhibitor, and sodium chlorate (NaClO3), a proteoglycan synthesis inhibitor. Our data show that THL has no effects on M-CSF and M-CSF-R interaction, suggesting that the interaction of M-CSF and M-CSF-R is independent of LpL enzymatic activity (data not shown) and addition of heparin had no effects on cell association of M-CSF and M-CSF-R in BM (data not shown). On the other hand, cellular association of M-CSF and M-CSF-R was significantly reduced by NaClO3 (Figure 4D), which supports the hypothesis that LpL plays a role in bridging BM M-CSF proteoglycan to cells to stimulate the maturation of monocyte precursor or monocytes. We thus propose that LpL modulates BM myelopoiesis by influencing M-CSF interaction with its receptor, which triggers a downstream signaling cascade to stimulate monocyte differentiation to macrophages.
Aortic macrophage levels correspond to LpL expression
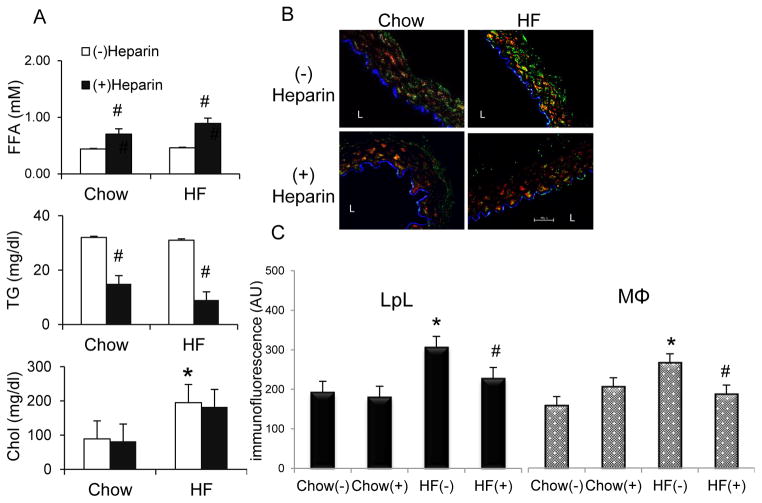
LpL actions described above led us to investigate in vivo the effects of LpL deletion on monocyte homing and macrophage accumulation in the aortic wall. For these effects, we proposed that LpL would need to be present at the surface of cells. To test this hypothesis, we utilized heparin to dissociate LpL from cell surfaces. We examined whether LpL affects aortic monocyte or macrophage levels through its cell-surface localization in the aortic lumen modulated by heparin. Wt mice were fed a chow or high saturated fat (HF) diet for 12 weeks in order to increase arterial LpL levels as previously described34. Injection of heparin resulted in increased plasma free FA (FFA) levels (Figure 5A) and decreased plasma TG levels in both chow and HF feeding groups (Figure 5A). Plasma cholesterol levels were increased by HF diet but were not affected by heparin (Figure 5A). Aorta sections were stained with specific fluorescent markers for LpL, macrophages, and endothelial cells (ECs). Consistent with our previous reports 14, 34, aortic immunofluorescence of ECs and smooth muscle cells was not altered by heparin treatment or HF-feeding, suggesting that these cells remained intact in the artery and their proliferation is not altered by heparin or HF diet (supplemental Figure II). On the other hand, HF-fed mice exhibited increased LpL and macrophage immunofluorescence in the aorta when compared with chow-fed mice (Figure 5B), with a major fraction of aortic LpL co-localizing with macrophages as indicated by yellow fluorescence in the merged images. Heparin treatment reduced this co-localization, especially in HF-fed mice (Figure 5C), indicating that a significant portion of macrophage-associated LpL is extracellular. Heparin treatment also led to a significant decrease in aortic macrophage number in HF-fed mice (Figure 5C), likely attributable to reduced LpL at cell surfaces, which would decrease macrophage levels in the aorta. This effect was rapid, manifesting by 10 min after heparin treatment, indicating that heparin treatment causes a rapid egress of these cells from the arterial wall.
Figure 5.
Effects of HF diets and heparin on plasma lipid profiles and arterial cell numbers. (A) At the end of chow or HF feeding, C57BL/6 mice were injected with saline (open bars) or heparin (filled bars) 10 min before sacrifice. Blood samples were assayed for (A) FFA, TG, and cholesterol (Chol). The results are expressed as mean±SE (n=10). (B) Representative confocal images and (C) quantitation of immunofluorescence (arbitrary units, AU) of aortic EC (blue), LpL (green), and macrophages (MΦs, red) in C57BL/6 mice fed chow or HF diets with or without heparin injection. L=lumen. Magnification is 40x. Scale bar: 50μm. n=3–4. #, (−) vs. (+) heparin; *, HF (−) vs. chow (−), p<0.05.
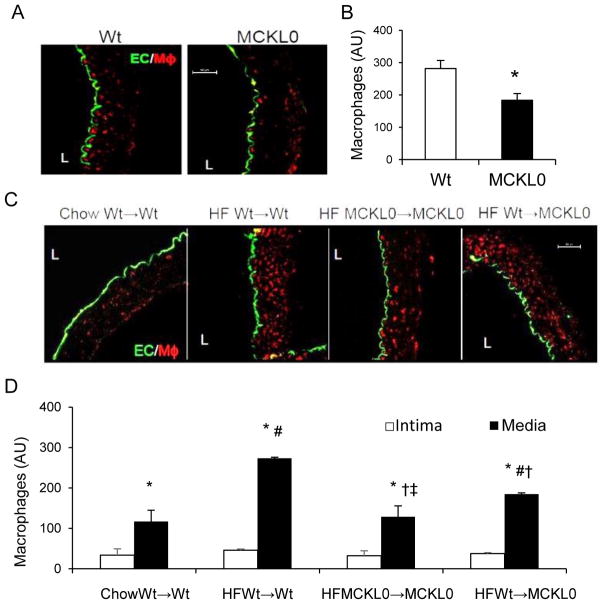
To confirm that these changes in aortic macrophage levels were due to the presence of aortic LpL, we examined aortic macrophage density and distribution in MCKL0 mice. Levels of aortic macrophage immunofluorescence were visually low in both chow-fed Wt and MCKL0 mice (Figure 6A), however, quantified macrophage levels were significantly reduced in MCKL0 mice when compared with Wt mice (Figure 6B). We next examined the role of LpL expressed specifically by macrophages in influencing aortic macrophage levels by comparing the number of aortic macrophages in MCKL0 mice transplanted with MCKL0 BM producing LpL-deficient macrophages (MCKL0→MCKL0) or Wt mouse BM producing LpL-expressing macrophages (Wt→MCKL0), as well as control Wt mice transplanted with Wt BM (Wt→Wt; control). Four weeks after transplantation, mice were placed on HF diets for 8 weeks. Although HF diets can increase MCKL0 mouse plasma cholesterol levels, their plasma TG levels remained in the normal range 27. In the aorta, as expected, HF diets increased macrophage levels in Wt mice transplanted with Wt BM (HF Wt→Wt) when compared with the chow-fed group (Chow Wt→Wt) mice. Aortic LpL deficiency (HF MCKL0→MCKL0) reduced macrophage accumulation by >40% when compared with HF Wt→Wt. Reconstitution of Wt BM into MCKL0 mice (HF Wt→MCKL0) significantly increased aortic macrophages (p<0.05), and the levels of LpL and macrophages were comparable to those in Wt mice (Figure 6C). Quantification of macrophages in different aortic layers show that LpL deletion led to reduced macrophage counts primarily in the aortic media (Figure 6D). Aortic F4/80, CD/68, and chemoattractant MCP-1 mRNA levels were decreased in MCKL0→MCKL0 mice compared to Wt→MCKL0 (Supplemental Table I).
Figure 6.
Loss of LpL reduces macrophage levels in the arterial wall. (A) Representative confocal images and (B) quantification of macrophage (MΦ) immunofluorescence of Wt and MCKL0 mouse artery sections stained for EC and MΦ (n=4). *, p<0.05, vs. Wt. (C) Representative confocal images and (D) immunofluorescence (arbitrary units, AU) of MΦs in specific aortic layers quantitated for chow-fed Wt→Wt, HF Wt→Wt, MCKL0→MCKL0 and Wt→MCKL0 mouse artery sections stained for EC and MΦ (n=4–5). Scale bar: 50 μm.*, vs. intima; #, vs. chow Wt→Wt; †, vs. HF Wt→Wt; ‡, MCKL0→MCKL0 vs. Wt→MCKL0, p<0.05.
Therefore, our data in the aorta indicates that effects of LpL on blood monocyte levels and properties can contribute to modulations in immune cell responses in an important tissue involved in CVD, the artery.
DISCUSSION
The role of LpL in vascular disease is unclear, as macrophage-specific LpL deficiency reduces atherosclerotic CVD in animal models but partial LpL deficiency in humans increases CVD risk 11, 13, 25, 35. These data suggest that the role of macrophage-LpL in vascular pathology is cell-specific. Indeed, we found that loss of LpL reduced the response of BM-derived macrophages to M-CSF. We went on to show that this is due to changes in both expression of M-CSF and in M-CSF interaction with monocyte and macrophage precursor cells. Aside from its action as a lipolytic enzyme, LpL has a number of anchoring functions that increase lipoprotein association with extracellular matrix 17 and attachment of cells to endothelial surfaces 21. We now provide evidence for a new and unique action of LpL that captures and promotes M-CSF activities. In addition, while investigating the effects of loss of LpL in arteries with heparin, we discovered that heparin also reduces arterial macrophage content, likely by inhibiting LpL-mediated macrophage retention in the aortic wall.
Although LpL is highly expressed in BM, few studies have assessed the relationship between LpL and BM hematopoiesis or blood leukocyte parameters. Our findings indicate that transgenic (MCKL0) LpL loss-of-function mouse models are associated with substantial reductions in blood leukocytes and, specifically, monocytes. We found that LpL deficiency led to reduced expression of master regulatory transcription factors (PU.1 and C/EBPα) and growth factors (CSFs and CSF-Rs) critical for BM myeloid cell proliferation and differentiation. Although the total numbers of lineage negative progenitors cells were reduced in MCKL0 mice (supplemental Figure I b), the reduced myeloid cell differentiation was only observed at the later stage of hematopoiesis—monocyte progenitors to monocytes or monocytes to macrophages. Further studies are needed to investigate whether LpL deficiency also alters BM myeloid cell proliferation.
We suggest that the observed decreases in blood monocytes and tissue macrophages in MCKL0 mice result from lower levels of CSFs, such as M-CSF, which catalyze the differentiation of both monocyte progenitors and monocytes. M-CSF is produced in a variety of tissues with expression levels and functions depending on local microenvironmental stimuli. We suggest that the density of macrophages in particular tissues is controlled by local synthesis of membrane-associated M-CSF and/or selective sequestration of the secreted PG M-CSF. For example, the Stanley group has reported that a supplement of recombinant human M-CSF restored macrophage density in some tissues, such as liver or spleen, in CSF-deficient osteopetrotic mice but was unable to completely correct phenotypic defects33. Transgenic expression of membrane-bound CSF alone in these mice restored macrophage numbers in some tissues but did not rescue the loss of overall BM hematopoiesis, implicating the requirement of circulating M-CSF and/or PG M-CSF for BM hematopoiesis. In our MCKL0 mouse model, isolated BM had reduced M-CSF expression levels, presumably representative of membrane-bound M-CSF. When MCKL0-derived BM monocytes were stimulated with exogenous M-CSF (equivalent to secreted M-CSF), these cells differentiated to macrophages at a substantially slower rate than those isolated from Wt mice (Figures 4A and 4B), suggesting impaired M-CSF action in these LpL-deficient BM-derived monocytes. In fact, while LpL deficiency reduced BM M-CSF protein levels (ns), plasma M-CSF levels were similar between WT and MCKL0 mice (supplemental Figure III). However, this lack of response may also be attributable to the reduced expression of M-CSF-R in both plasma (~ 46% reduction, p<0.05) and BM cells (Figure 3B). Our preliminary studies show that the addition of exogenous LpL has little or no effects on BMMP cell differentiation (data not shown).
Transcriptional control of M-CSF-R has been extensively studied 36. Master regulators of hematopoiesis, such as PU.1, C/EBPα and NFκB, control M-CSF-R expression. Inflammatory stimuli, such as endotoxin LPS, also induce M-CSF-R expression. On the other hand, transcriptional control of M-CSF is still not understood. Our data show that, although exogenous LpL slightly increased M-CSF expression in MCKL0 BM myeloid precursor cells, there were no effects on M-CSF-R expression. Can increased cellular FA uptake stimulate M-CSF or M-CSF-R expression in vivo? It has been shown that peripheral monocyte overexpression of scavenger receptor type A (SR-A) and CD36, which enhance FA uptake, positively correlated with serum M-CSF levels in humans 37. Therefore, when BM-derived myeloid precursor cells were treated with FAs, LA increased M-CSF expression. Thus, we suggest that LpL is important for M-CSF expression and required for M-CSF cellular interactions that cooperatively regulate monocyte differentiation to macrophages. On the other hand, the PPARs, which modulate lipid metabolism, cellular proliferation and inflammatory responses, are widely expressed in many tissues, including in monocytes. Some FAs are direct ligands of PPARs 38 and important regulators of the expression of different PPARs 39. Among the PPAR family, PPARγ is a negative regulator of M-CSF expression and production through transrepression of NFκB 40. PPARγ overexpression and treatment with its ligand, pioglitazone, decreased M-CSF gene expression and protein levels 40. Our data show a consistent inverse relation between PPARγ and M-CSF in BMMP cells; expression of M-CSF was downregulated, while PPARγ was upregulated in MCKL0 mice. The addition of exogenous LpL and FA during BMMP differentiation increased PPARγ expression, which linked to a decrease in M-CSF expression in both Wt and MCKL0 mice. Our results are consistent with our earlier report 27 that MCKL0 mice had greater increases in expression of all PPARs in adipose tissues. It has been postulated that increased PPAR expression is induced by FAs through de novo lipogenesis 27, 41.
Because there are several positively charged binding motifs in LpL, we hypothesize that LpL anchors M-CSF by binding to the negatively charged residues on M-CSF. Studies from the Chait group show that macrophage-secreted M-CSF contains PG domains that can bind lipoproteins, such as LDL 42. Acetylation or copper oxidation, which instill a net positive charge upon LDL, resulted in a loss of M-CSF binding 42. Based on the known ability of LpL to bind PGs or LDL, we propose that LpL sequesters secreted circulating M-CSF at the cell surface and directs it to the M-CSF-R. In fact, our data on assessing whether exogenous LpL affects binding of M-CSF to its receptor by immunobinding assays show that M-CSF association with M-CSF-R decreased with increasing amounts of LpL in a solid-phase assay (supplemental Figure IV). In the absence of LpL, cellular association of M-CSF and M-CSF-R was significantly reduced. We have examined potential interacting factors that may affect M-CSF and M-CSF-R cellular interaction. The results indicate that M-CSF and M-CSF-R interaction is independent of LpL enzymatic activity. Consistent with the negative results from the immunobinding assay (data not shown), the addition of heparin has little or no effects on cell association of M-CSF and M-CSF-R in BM. On the other hand, cellular association of M-CSF and M-CSF-R was significantly reduced by NaClO3, which supports our hypothesis that LpL plays a role in bridging BM M-CSF proteoglycan to cells to catalyze the maturation of monocyte precursor or monocytes. We have previously reported that LpL bound to cell-surface PGs also bound LDL and facilitated LDL delivery to the LDL receptor 43. Thus, similar to lipoproteins with anchoring functions, dimeric LpL might capture circulating M-CSF and increase its proximity to its receptor. Lack of LpL thus impairs the efficacy of M-CSF in stimulating myelopoiesis.
Decreased myelopoiesis induced by selective LpL deletion may be beneficial in some scenarios. The M-CSF/M-CSF-R signaling pathway is activated via the concerted action of other growth factors, such as GM-CSF/GM-CSF-R. While M-CSF expression is generally constant, GM-CSF expression increases in response to inflammatory stimuli 44. Myeloid lineage populations display differential responses to M-CSF and GM-CSF, albeit through unknown mechanisms. Interestingly, therapeutic antagonism of M-CSF and GM-CSF has been shown to ameliorate some inflammatory/autoimmune diseases, such as rheumatoid arthritis 44.
Atherosclerosis-prone disease models, such as apoE- or LDL receptor (LDLR)-knockout (KO) mice, display reduced atherosclerosis if macrophage-derived LpL is deleted. ApoE-KO mice lacking LpL expression in macrophages after cre/loxP gene targeting had fewer atherosclerotic lesions than apoE-KO with LpL-expressing macrophages after a HF, high-cholesterol diet 13. Further, Babaev et al. reported that irradiated LDLR−/− mice transplanted with fetal liver cells from LpL-deficient mice had smaller atherosclerotic lesions when compared to LDLR−/− mice reconstituted with LpL-expressing fetal liver cells 35. In our models, LpL deficiency not only reduced aortic macrophage levels, but also reduced monocyte counts substantially. This makes it difficult to determine whether the reduction of atherosclerotic lesions observed in other studies is due to the lack of local LpL expression in arterial MΦs or due to changes in altered BM and circulating leukocyte profiles.
LpL deficiency in the arterial wall is associated with fewer aortic macrophages even prior to atherosclerotic development 14. Associations between LpL and aortic macrophages were examined through BM transplantation techniques that restore LpL to arterial cells, and the use of heparin, which mediates dissociation of LpL from the cell surface. We expect that heparin would further decrease aortic macrophages in LpL-deficient MCKL0 mice; however, the decreases would be difficult to detect since the levels of aortic macrophage in MCKL0 mice are already low. Heparin has been proposed to have anti-inflammatory effects through its binding of various chemokines and cytokines 45, prevention of pro-inflammatory mediators from interacting with their respective receptors 46, 47, and modulation of TNFα and NFκB activity 48–50. Although the ability of heparin to induce LpL cellular dissociation is well established, the phenomenon of “heparin-releasable macrophages” in an acute setting has not been previously reported. The interaction of LpL with heparin, and likely a number of other molecules, is due to regions of positively charged heparin binding motifs in LpL. Others have reported that heparin interferes with the adhesion of leukocytes to the endothelium through its binding to L- and P-selectin 51. In contrast to other studies, we report an acute effect (~10 min) of heparin on dissociation of arterial macrophages and these effects on macrophage levels are correlated with macrophage-associated heparin-releasable LpL. Further, we have established the close relation between LpL and macrophages in atherogenesis in LDLR−/− mice in our earlier reports 15. We speculate that the heparin would also affect arterial macrophage abundance in LDLR−/− mice. Nonetheless, the effects of heparin are complex in modulating function and activity of a number of proteins that are important in regulating inflammatory responses acutely and chronically. This is one limitation of the current studies.
In summary, LpL deficiency decreased circulating levels of total leukocytes, neutrophils, and total and inflammatory monocytes. These effects were partly mediated through CSFs and CSF-Rs, resulting in significant decreases in blood monocytes and BM-derived monocyte differentiation to macrophages. Loss of LpL in the arterial wall was also associated with reduced aortic macrophages in non-atherosclerotic mice. LpL can now be recognized as not only a key player in directing arterial lipid deposition, but also as a potential regulator of blood and peripheral inflammatory responses.
Supplementary Material
HIGHLIGHTS.
Loss of LpL reduces circulating WBC and monocyte levels.
Loss of LpL decreases bone marrow expression of master regulators includes PU.1, C/EBPα and colony-stimulating factors and their receptors that are essential for myeloid cell differentiation.
LpL deficiency markedly reduces aortic macrophage counts. Reconstitution of LpL-expressing bone marrow cells was able to replenish aortic macrophage density.
We propose that LpL, in addition to being an important molecule for triglyceride metabolism and anchoring of arterial LDL and other lipoproteins, also contributes to regulation of blood monocyte levels and monocyte differentiation to macrophages.
Our findings provide novel insights into the regulation of inflammatory responses and may have therapeutic applications for a number of metabolic and blood cell diseases.
Acknowledgments
We thank Dr. Andre Bensadoun for providing LpL antibodies for analyses included in this paper.
SOURCES OF FUNDING
This work was supported by National Institutes of Health grant HL40404 (R.J.D.), T32 DK007647/ HL007343 (C.L.C.), HL45095 and DK095684 (I.J.G.).
ABBREVIATIONS
- BM
bone marrow
- CSF
colony-stimulating factor
- LpL
lipoprotein lipase
- WBC
white blood cell
Footnotes
DISCLOSURE
None.
References
- 1.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 2.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman DL, Mogelesky TC, Liptak BF, Gerrity RG. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991;11:985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- 4.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twig G, Afek A, Shamiss A, Derazne E, Tzur D, Gordon B, Tirosh A. White blood cell count and the risk for coronary artery disease in young adults. PLoS One. 2012;7:e47183. doi: 10.1371/journal.pone.0047183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Goldberg IJ, Steinberg D, Witztum JL. Macrophages and smooth muscle cells express lipoprotein lipase in human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:10143–10147. doi: 10.1073/pnas.88.22.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien KD, Gordon D, Deeb S, Ferguson M, Chait A. Lipoprotein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest. 1992;89:1544–1550. doi: 10.1172/JCI115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–1705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Eck M, Zimmermann R, Groot PH, Zechner R, Van Berkel TJ. Role of macrophage-derived lipoprotein lipase in lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:E53–62. doi: 10.1161/01.atv.20.9.e53. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Yagyu H, Tazoe F, Nagashima S, Ohshiro T, Okada K, Osuga J, Goldberg IJ, Ishibashi S. Macrophage lipoprotein lipase modulates the development of atherosclerosis but not adiposity. J Lipid Res. 2013;54:1124–1134. doi: 10.1194/jlr.M035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CL, Seo T, Du CB, Accili D, Deckelbaum RJ. N-3 fatty acids decrease arterial low-density lipoprotein cholesterol delivery and lipoprotein lipase levels in insulin-resistant mice. Arterioscler Thromb Vasc Biol. 2010;30:2510–2517. doi: 10.1161/ATVBAHA.110.215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CL, Torrejon C, Jung UJ, Graf K, Deckelbaum RJ. Incremental replacement of saturated fats by n-3 fatty acids in high-fat, high-cholesterol diets reduces elevated plasma lipid levels and arterial lipoprotein lipase, macrophages and atherosclerosis in ldlr−/− mice. Atherosclerosis. 2014;234:401–409. doi: 10.1016/j.atherosclerosis.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafsson M, Levin M, Skalen K, Perman J, Friden V, Jirholt P, Olofsson SO, Fazio S, Linton MF, Semenkovich CF, Olivecrona G, Boren J. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: Evidence for a role of lipoprotein lipase. Circ Res. 2007;101:777–783. doi: 10.1161/CIRCRESAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 17.Saxena U, Klein MG, Vanni TM, Goldberg IJ. Lipoprotein lipase increases low density lipoprotein retention by subendothelial cell matrix. J Clin Invest. 1992;89:373–380. doi: 10.1172/JCI115595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutledge JC, Woo MM, Rezai AA, Curtiss LK, Goldberg IJ. Lipoprotein lipase increases lipoprotein binding to the artery wall and increases endothelial layer permeability by formation of lipolysis products. Circ Res. 1997;80:819–828. doi: 10.1161/01.res.80.6.819. [DOI] [PubMed] [Google Scholar]
- 19.Corey JE, Zilversmit DB. Effect of cholesterol feeding on arterial lipolytic activity in the rabbit. Atherosclerosis. 1977;27:201–212. doi: 10.1016/0021-9150(77)90057-0. [DOI] [PubMed] [Google Scholar]
- 20.Yin B, Loike JD, Kako Y, Weinstock PH, Breslow JL, Silverstein SC, Goldberg IJ. Lipoprotein lipase regulates fc receptor-mediated phagocytosis by macrophages maintained in glucose-deficient medium. J Clin Invest. 1997;100:649–657. doi: 10.1172/JCI119576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamputu JC, Desfaits AC, Renier G. Lipoprotein lipase enhances human monocyte adhesion to aortic endothelial cells. J Lipid Res. 1997;38:1722–1729. [PubMed] [Google Scholar]
- 22.Obunike JC, Paka S, Pillarisetti S, Goldberg IJ. Lipoprotein lipase can function as a monocyte adhesion protein. Arterioscler Thromb Vasc Biol. 1997;17:1414–1420. doi: 10.1161/01.atv.17.7.1414. [DOI] [PubMed] [Google Scholar]
- 23.Fisher RM, Benhizia F, Schreiber R, Makoveichuk E, Putt W, Al-Haideri M, Deckelbaum RJ, Olivecrona G, Humphries SE, Talmud PJ. Enhanced bridging function and augmented monocyte adhesion by lipoprotein lipase n9: Insights into increased risk of coronary artery disease in n9 carriers. Atherosclerosis. 2003;166:243–251. doi: 10.1016/s0021-9150(02)00337-4. [DOI] [PubMed] [Google Scholar]
- 24.Mamputu JC, Renier G. Differentiation of human monocytes to monocyte-derived macrophages is associated with increased lipoprotein lipase-induced tumor necrosis factor-alpha expression and production: A process involving cell surface proteoglycans and protein kinase c. Arterioscler Thromb Vasc Biol. 1999;19:1405–1411. doi: 10.1161/01.atv.19.6.1405. [DOI] [PubMed] [Google Scholar]
- 25.Khera AV, Won HH, Peloso GM, O’Dushlaine C, Liu D, Stitziel NO, Natarajan P, Nomura A, Emdin CA, Gupta N, Borecki IB, Asselta R, Duga S, Merlini PA, Correa A, Kessler T, Wilson JG, Bown MJ, Hall AS, Braund PS, Carey DJ, Murray MF, Kirchner HL, Leader JB, Lavage DR, Manus JN, Hartzel DN, Samani NJ, Schunkert H, Marrugat J, Elosua R, McPherson R, Farrall M, Watkins H, Lander ES, Rader DJ, Danesh J, Ardissino D, Gabriel S, Willer C, Abecasis GR, Saleheen D, Dewey FE, Kathiresan S Myocardial Infarction Genetics Consortium DSGCEC, Global Lipids Genetics C. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA. 2017;317:937–946. doi: 10.1001/jama.2017.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and vldl-derived lipids enter the heart through different pathways: In vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285:37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Arcos I, Hiyama Y, Drosatos K, Bharadwaj KG, Hu Y, Son NH, O’Byrne SM, Chang CL, Deckelbaum RJ, Takahashi M, Westerterp M, Obunike JC, Jiang H, Yagyu H, Blaner WS, Goldberg IJ. Adipose-specific lipoprotein lipase deficiency more profoundly affects brown than white fat biology. J Biol Chem. 2013;288:14046–14058. doi: 10.1074/jbc.M113.469270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levak-Frank S, Radner H, Walsh A, Stollberger R, Knipping G, Hoefler G, Sattler W, Weinstock PH, Breslow JL, Zechner R. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J Clin Invest. 1995;96:976–986. doi: 10.1172/JCI118145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain MM, Goldberg IJ, Weisgraber KH, Mahley RW, Innerarity TL. Uptake of chylomicrons by the liver, but not by the bone marrow, is modulated by lipoprotein lipase activity. Arterioscler Thromb Vasc Biol. 1997;17:1407–1413. doi: 10.1161/01.atv.17.7.1407. [DOI] [PubMed] [Google Scholar]
- 30.Schwiegelshohn B, Presley JF, Gorecki M, Vogel T, Carpentier YA, Maxfield FR, Deckelbaum RJ. Effects of apoprotein e on intracellular metabolism of model triglyceride-rich particles are distinct from effects on cell particle uptake. J Biol Chem. 1995;270:1761–1769. doi: 10.1074/jbc.270.4.1761. [DOI] [PubMed] [Google Scholar]
- 31.Ton MN, Chang C, Carpentier YA, Deckelbaum RJ. In vivo and in vitro properties of an intravenous lipid emulsion containing only medium chain and fish oil triglycerides. Clin Nutr. 2005;24:492–501. doi: 10.1016/j.clnu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Ho YY, Al-Haideri M, Mazzone T, Vogel T, Presley JF, Sturley SL, Deckelbaum RJ. Endogenously expressed apolipoprotein e has different effects on cell lipid metabolism as compared to exogenous apolipoprotein e carried on triglyceride-rich particles. Biochemistry. 2000;39:4746–4754. doi: 10.1021/bi992294a. [DOI] [PubMed] [Google Scholar]
- 33.Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (csf-1) function in csf-1-deficient csf1op/csf1op mice by transgenic expression of cell surface csf-1. Blood. 2004;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- 34.Chang CL, Seo T, Matsuzaki M, Worgall TS, Deckelbaum RJ. N-3 fatty acids reduce arterial ldl-cholesterol delivery and arterial lipoprotein lipase levels and lipase distribution. Arterioscler Thromb Vasc Biol. 2009;29:555–561. doi: 10.1161/ATVBAHA.108.182287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babaev VR, Patel MB, Semenkovich CF, Fazio S, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J Biol Chem. 2000;275:26293–26299. doi: 10.1074/jbc.M002423200. [DOI] [PubMed] [Google Scholar]
- 36.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 37.Nishida M, Ando M, Iwamoto Y, Tsuchiya K, Nitta K. New insight into atherosclerosis in hemodialysis patients: Overexpression of scavenger receptor and macrophage colony-stimulating factor genes. Nephron Extra. 2016;6:22–30. doi: 10.1159/000448486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. Ppar-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 39.Jung UJ, Torrejon C, Chang CL, Hamai H, Worgall TS, Deckelbaum RJ. Fatty acids regulate endothelial lipase and inflammatory markers in macrophages and in mouse aorta: A role for ppargamma. Arterioscler Thromb Vasc Biol. 2012;32:2929–2937. doi: 10.1161/ATVBAHA.112.300188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonfield TL, Thomassen MJ, Farver CF, Abraham S, Koloze MT, Zhang X, Mosser DM, Culver DA. Peroxisome proliferator-activated receptor-gamma regulates the expression of alveolar macrophage macrophage colony-stimulating factor. J Immunol. 2008;181:235–242. doi: 10.4049/jimmunol.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates pparalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Chang MY, Olin KL, Tsoi C, Wight TN, Chait A. Human monocyte-derived macrophages secrete two forms of proteoglycan-macrophage colony-stimulating factor that differ in their ability to bind low density lipoproteins. J Biol Chem. 1998;273:15985–15992. doi: 10.1074/jbc.273.26.15985. [DOI] [PubMed] [Google Scholar]
- 43.Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J Clin Invest. 1992;90:1504–1512. doi: 10.1172/JCI116018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 45.Cox JH, Dean RA, Roberts CR, Overall CM. Matrix metalloproteinase processing of cxcl11/i-tac results in loss of chemoattractant activity and altered glycosaminoglycan binding. J Biol Chem. 2008;283:19389–19399. doi: 10.1074/jbc.M800266200. [DOI] [PubMed] [Google Scholar]
- 46.Tyrrell DJ, Horne AP, Holme KR, Preuss JM, Page CP. Heparin in inflammation: Potential therapeutic applications beyond anticoagulation. Adv Pharmacol. 1999;46:151–208. doi: 10.1016/s1054-3589(08)60471-8. [DOI] [PubMed] [Google Scholar]
- 47.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Lee JH, Lee J, Seo GH, Kim CH, Ahn YS. Heparin inhibits nf-kappab activation and increases cell death in cerebral endothelial cells after oxygen-glucose deprivation. J Mol Neurosci. 2007;32:145–154. doi: 10.1007/s12031-007-0026-3. [DOI] [PubMed] [Google Scholar]
- 50.Spratte J, Meyer zu Schwabedissen H, Endlich N, Zygmunt M, Fluhr H. Heparin inhibits tnf-alpha signaling in human endometrial stromal cells by interaction with nf-kappab. Mol Hum Reprod. 2013;19:227–236. doi: 10.1093/molehr/gas060. [DOI] [PubMed] [Google Scholar]
- 51.Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind l- and p-selectin and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.