Abstract
Objective
We explored mechanisms that alter mitochondrial structure and function in pulmonary endothelial cells (PEC) function after hyperoxia.
Approach and Results
Mitochondrial structures of PECs exposed to hyperoxia or normoxia were visualized and mitochondrial fragmentation quantified. Expression of pro-fission or fusion proteins or autophagy-related proteins were assessed by western blot. Mitochondrial oxidative state was determined using mito-roGFP. TMRM estimated mitochondrial polarization in treatment groups. The role of mitochondrially-derived ROS in mt-fragmentation was investigated with mito-TEMPOL, and mitochondrial DNA (mtDNA) damage studied by using ENDO III, a protein that repairs mDNA damage. Drp-1 was over-expressed or silenced to test the role of this protein in cell survival or transwell resistance.
Hyperoxia increased fragmentation of PEC mitochondria in a time-dependent manner through 48 hours of exposure. Hyperoxic PECs exhibited increased phosphorylation of Drp-1 (serine 616), decreases in Mfn1, but increases in OPA-1. Pro-autophagy proteins p62, PINK-1 and LC3B were increased. Returning cells to normoxia for 24 hours reversed the increased mt-fragmentation and changes in expression of pro-fission proteins. Hyperoxia-induced changes in mitochondrial structure and/or cell survival were mitigated by anti-oxidants mito-TEMPOL, Drp-1 silencing or inhibition or protection by the mitochondrial endonuclease ENDO III. Hyperoxia induced oxidation and mitochondrial depolarization and impaired transwell resistance. Decrease in resistance was mitigated by mito-TEMPOL or ENDO-III, and reproduced by over-expression of Drp-1.
Conclusions
Because hyperoxia evoked mt-fragmentation, cell survival and/or transwell resistance are prevented by ENDO III and mito-TEMPOL, and Drp-1 silencing, these data link hyperoxia-induced mt-DNA damage, Drp-1 expression, mt-fragmentation and PEC dysfunction.
Keywords: Reactive oxygen species, oxidation, cell survival
Introduction
The potential of high fractions of oxygen to damage lungs of humans and experimental animals has been recognized for more than 35 years1–3. Acute lung injury (ALI) or Adult Respiratory Distress Syndrome (ARDS) is the leading cause for admission to non-surgical ICUs and complicates the course of more than 25% of patients admitted to ICUs. ARDS is invariably treated with supplemental oxygen to sustain vital organ function, and is life-saving in the short term. However supplemental oxygen is injurious to the lungs, increasing pulmonary microvascular permeability within less than 24 hours2. With movement of fluid into the alveolar spaces, gas exchange is impaired such that ever-higher fractions of inhaled oxygen and higher airway pressures are required to maintain tissue oxygenation. Therefore, interventions which limit hyperoxia-associated pulmonary edema are highly desirable.
Rats exposed to hyperoxia show increased death of pulmonary endothelial cells in a time- and concentration-dependent manner4. A central role for mitochondria in hyperoxic-induced PEC death is supported by previous reports of increased reactive oxygen species (ROS), decreased ATP production, and reduced respiratory chain activity in the lungs of rodents exposed to hyperoxia3. However, the exact mechanisms by which hyperoxic exposure leads to PEC death are not well understood.
One poorly defined connection is how mitochondrial ultrastructure contributes to hyperoxic injury. Mitochondria exist in a dynamic state with frequent mitochondrial fission and fusion in healthy cells5–7. A balance between mitochondrial fission and fusion is paramount to properly functioning mitochondria8, 9. Shifts in endothelial or smooth muscle cell mito-fission contribute to endothelial cell injury in diabetes, atherosclerosis, hypertension and pulmonary artery hypertension. But the effects of hyperoxia on mitochondrial structure in pulmonary endothelial cells (PECs) are not known. Of the 40+ cell types in the lung, endothelial cells are among the most abundant and sensitive to hyperoxic injury, showing decreases in cell numbers as well as morphological changes before other indices of injury including edema, neutrophilic influx or hypoxemia. We examined mitochondrial structure and oxidative state using immunofluorescence and recombinant protein probes along with expression of genes that regulate mitochondrial shape in PECs exposed to hyperoxia. We also investigated the functional implications of hyperoxia on PEC survival, mitochondrial structure and endothelial cell function. We find that hyperoxia induces mitochondrial fragmentation that correlates to both increased fission and decreased fusion protein expression, to mitochondrially derived ROS and trans-endothelial electrical resistance. Our detailed findings of hyperoxia-induced mitochondrial fragmentation in PECs afford potentially novel therapeutic targets.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
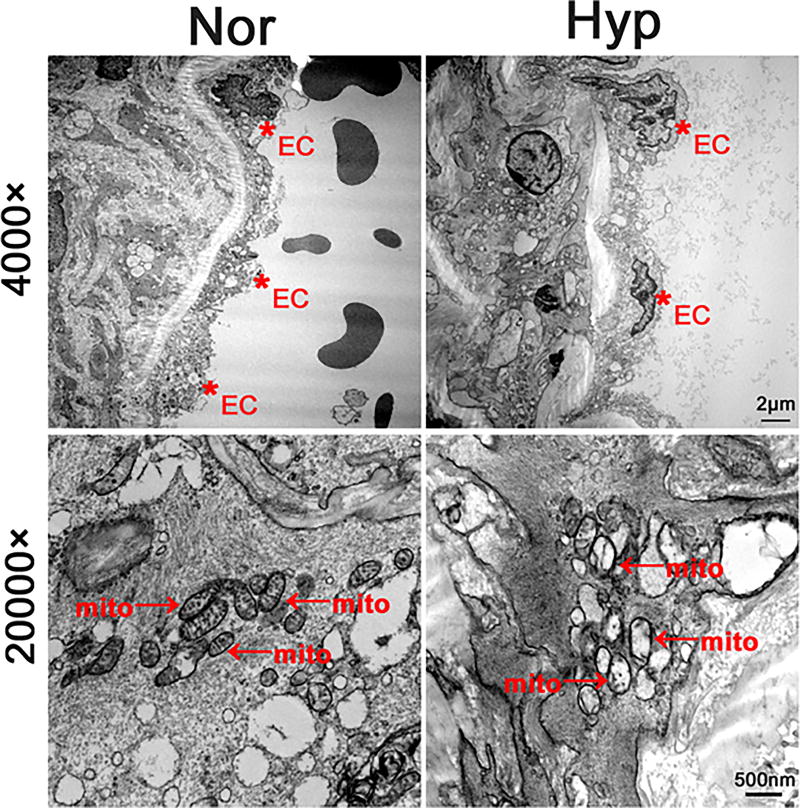
We obtained transmission electron microscopy of endothelial cells in pulmonary arteries from rats treated in vivo to normoxic (21% O2) or hyperoxic (>95% O2) exposures for 48 hours (Figure 1). Hyperoxia caused scattered separation of the endothelium from the basement membrane, but the basement membranes appeared generally intact. Widespread loss in mitochondrial ultrastructure occurred with cristae disrupted and diminished.
Figure 1.
Transmission electron micrographs at two magnifications show structural changes in pulmonary artery endothelial cells of rats exposed for 48 hours to normoxia or hyperoxia in vivo. Lower power studies demonstrated scattered separation of PECs (identified with red asterix and “EC”) from underlying basement membrane in hyperoxia samples, but not normoxic ones. At higher power, the mitochondrial cristae are visible in cells maintained in normoxia, but are disrupted or absent in hyperoxic specimens (see red arrows with “mito” label).
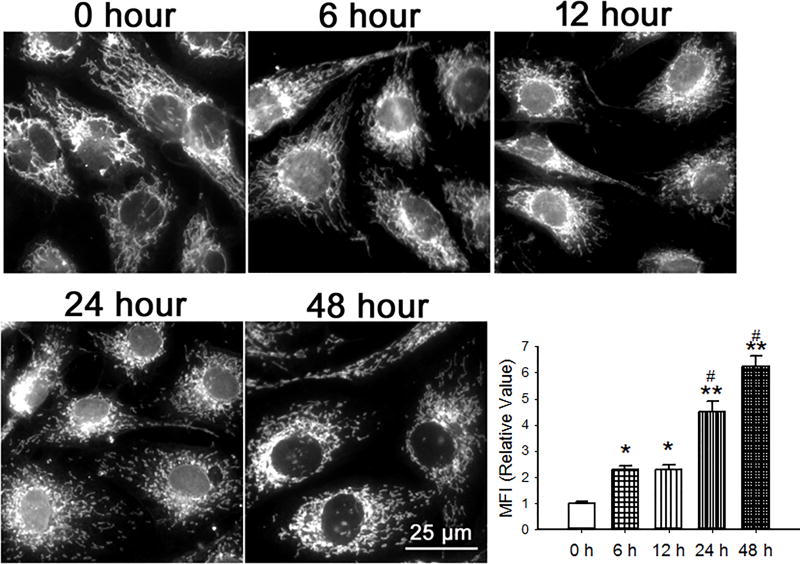
To examine the effect of hyperoxia on the structure of mitochondria in cultured PECs, we used either a fluorescently tagged antibody to the translocase of outer membrane receptor complex (TOM20, Figure 2) or mitochondrially targeted roGFP (data not shown). Mitochondrial fragmentation index (MFI), a quantification of the non-contiguous (fragmented) mitochondria, increased as early as 6 hours after hyperoxia and further over 48 hours.
Figure 2.
PECs were cultured in normoxia or hyperoxia for 6 through 48 hours. Mitochondria were visualized with a fluorescent antibody to the translocase of outer membrane (TOM-20) receptor complex. Representative images as well as a graph of averaged mitochondrial fragmentation indices (n=6 random cells from at least 3 isolations for each time point, 20 total from each time point) are shown. The number of non-contiguous mitochondrial fragments and the number of pixels in the mitochondria were identified by ImageJ. The Mitochondrial Fragmentation Index (MFI) was calculated by dividing the number of non-contiguous fragments by the number of pixels and multiplying the result by 1000. Data were normally distributed with equal variance and compared by ANOVA. *P < 0.05; **P < 0.01, different from normoxia, #P<0.05, different from 6 & 12 hours hyperoxia.
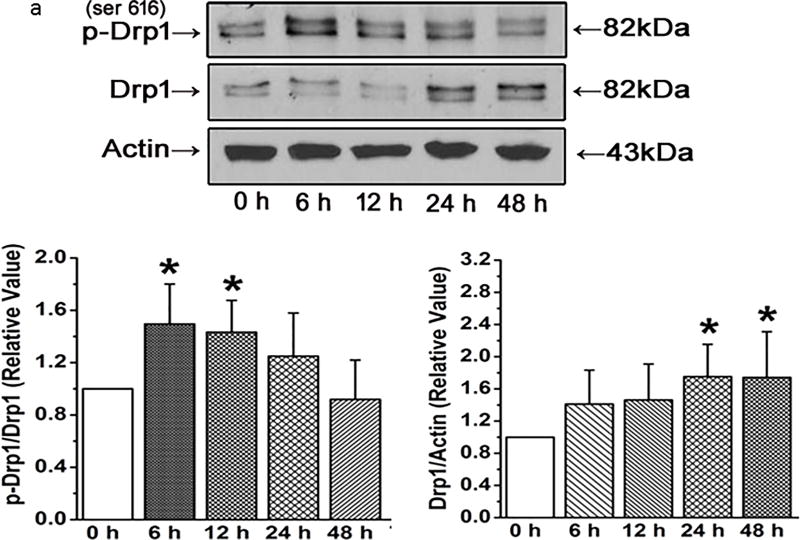
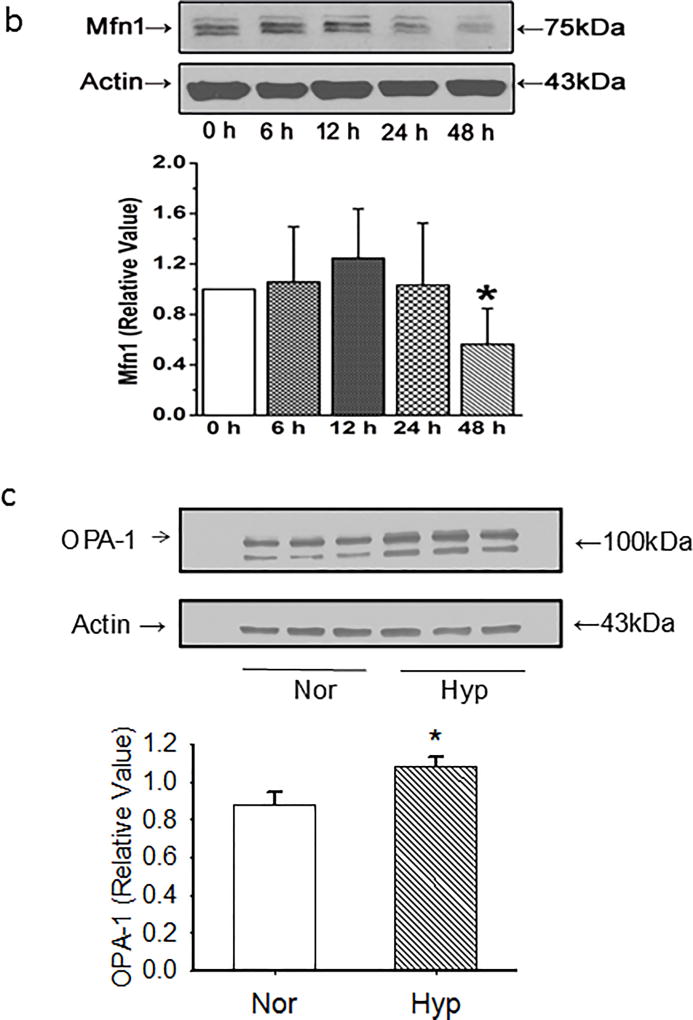
We next assessed time-dependent changes in pro-fission or fusion proteins. PECs exhibit increases in the pro-fission phosphorylated Drp-1 as early as 6 hours after exposure to hyperoxia, increases which are gone by 24 hours (Figure 3a). In contrast, total Drp-1 rises over 48 hours such that the ratio of serine 616 phospho-Drp-1 to Drp-1 is increased 6 through 12 hours after hyperoxia, but decreases thereafter to baseline values. Mfn1 decreases by 48 hours after hyperoxia (Figure 3b). OPA-1, a pro-fusion protein is increased after 48 hours (Figure 3c).
Figure 3.
(a) Representative and averaged data from western blots of PECs exposed in culture to hyperoxia for periods of time as appear on the x axis and probed with a primary antibody for phosphorylated Drp1, total Drp-1 or beta actin. N=6 for each bar in both graphs. Phosphorylated Drp1 relative to total Drp1 was increased at 6 and 12 hours after exposure to hyperoxia, then decreased back to baseline values thereafter. In contrast, total Drp-1 increased after 24 hours relative to beta-actin and remained elevated through 48 hours demonstrating 2 levels of regulation. Data were normally distributed with equal variance and compared by ANOVA. *P < 0.05, different from normoxia controls.
(b) Representative images of western blots and averaged data show Mfn1 density decreased by 48 hours. N=6 endothelial cell isolates for each time point. Data were not normally distributed with equal variance and compared by ANOVA on Ranks. *P < 0.05, different from normoxia controls.
(c). Representative and averaged data show that OPA-1 density relative to that of beta actin is increased in PECs exposed for 48 hours to hyperoxia (n=7 each for normoxia and hyperoxia). Data were normally distributed with equal variance and compared by ANOVA with Dunnett’s. *P < 0.03, different from normoxia.
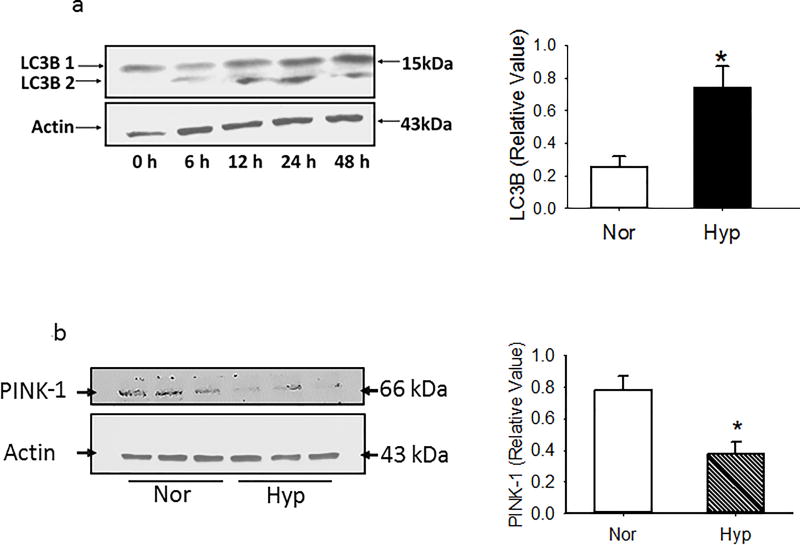
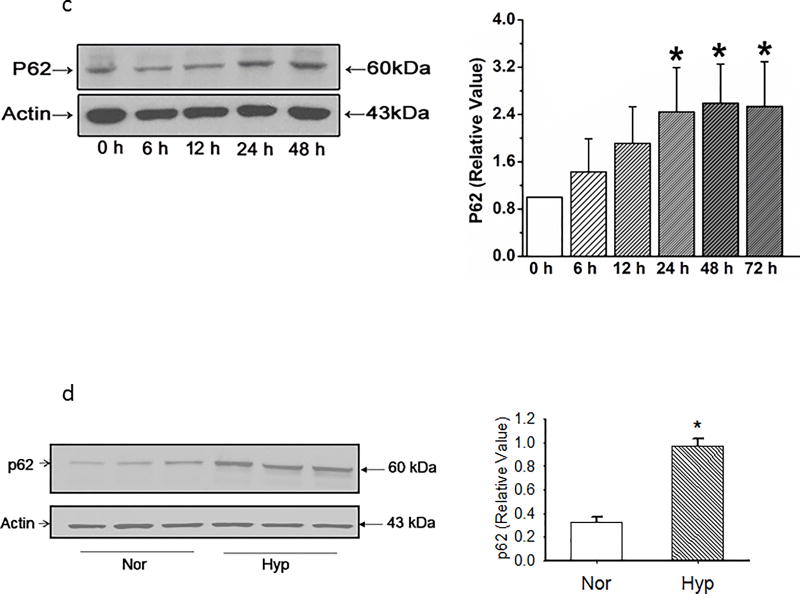
Because hyperoxia is reported to activate apoptosis and autophagy4, 10, we also examined caspase 3/7 activity and expression of LC3B, p62 and PINK-1 in PECs. Apoptosis as quantified by caspase 3/7 activity in PECs maintained in normoxia or hyperoxia was low, but increased by ~1.8 fold (1.2 ± 0.2 vs 2.2 ± 0.1%; n=8; p<0.01) with hyperoxia. LC3B expression increased in PECs exposed to hyperoxia relative to that of counterparts cultured in normoxia (Figure 4a). PTEN induced putative kinase 1 (PINK-1) expression was decreased by hyperoxia, consistent with increased Drp-1 and/or defective parkin localization to damaged mitochondria, and impaired autophagy (Figure 4b)11. Ubiquitin binding protein p62 increased in a time dependent manner in hyperoxic PECs, as well as in whole lung homogenates of rat lungs exposed in vivo to hyperoxia (Figures 4c and 4d).
Figure 4.
(a). Representative and averaged data for normoxia and 48 hours show that LC3B 1/2 density relative to that of beta actin is increased by hyperoxia. N≥4 for each time point in graph. Data were normally distributed with equal variance and compared by t-test. *P < 0.01, different from normoxia.
(b). Representative and averaged data show that PINK-1 density relative to that of beta actin is decreased in PECs exposed for 48 hours to hyperoxia (n=7 each for normoxia and hyperoxia). Data were normally distributed with equal variance and compared by t-test. *P < 0.005, different from normoxia.
(c) Representative western blots and averaged band intensity showing p62 density normalized to beta actin in PECs. N=6 endothelial cell isolates for each time point. P62 density increased after 24 hours and remained elevated through 48 hours. Data were normally distributed with equal variance and compared by ANOVA. *P < 0.05, different from normoxia.
(d). Representative and averaged data show that p62 density relative to that of beta actin is increased in whole lung homogenates by 48 hours exposure of rats in vivo to hyperoxia (n=9 each for normoxia and hyperoxia). Data were normally distributed with equal variance and compared by t-test. *P < 0.001, different from normoxia.
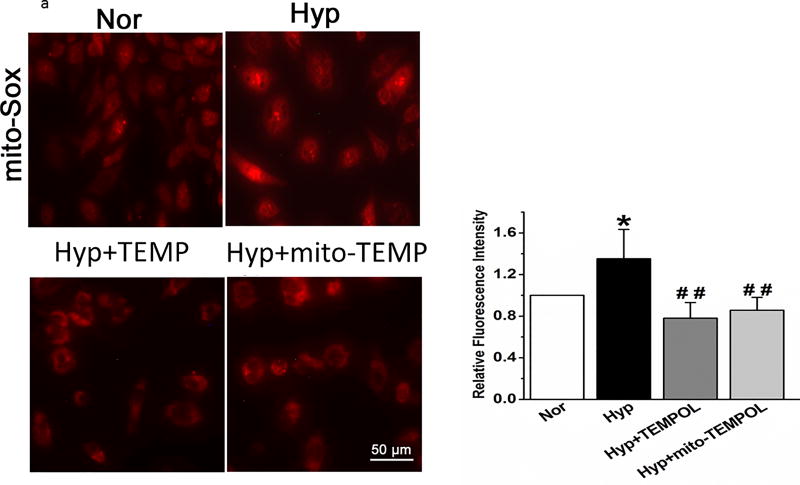
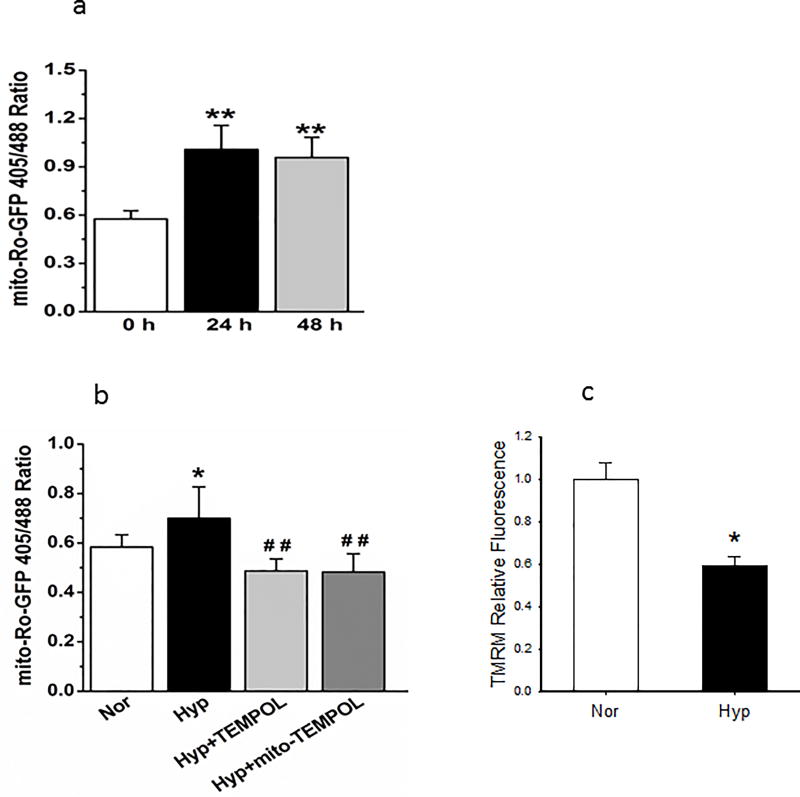
To investigate the role of mitochondrial ROS in enhanced mitochondrial fragmentation, we used mito-SOX and mito-TEMPOL. Mito-SOX fluorescence increased by 48 hours hyperoxia exposure; treatment with mito-TEMPOL or TEMPOL (Figure 5a) blocked the increase in mito-SOX.
Figure 5.
(a). Representative and averaged values of mito-SOX fluorescence. Hyperoxia-induced increments in fluorescence of mito-SOX were blocked by TEMPOL and mito-TEMPOL (n=6 representative fields for each condition, from 3 separate experiments). Data failed normality tests and were compared by ANOVA on Ranks. *P < 0.05, different from normoxia, ##P < 0.01, different from hyperoxia.
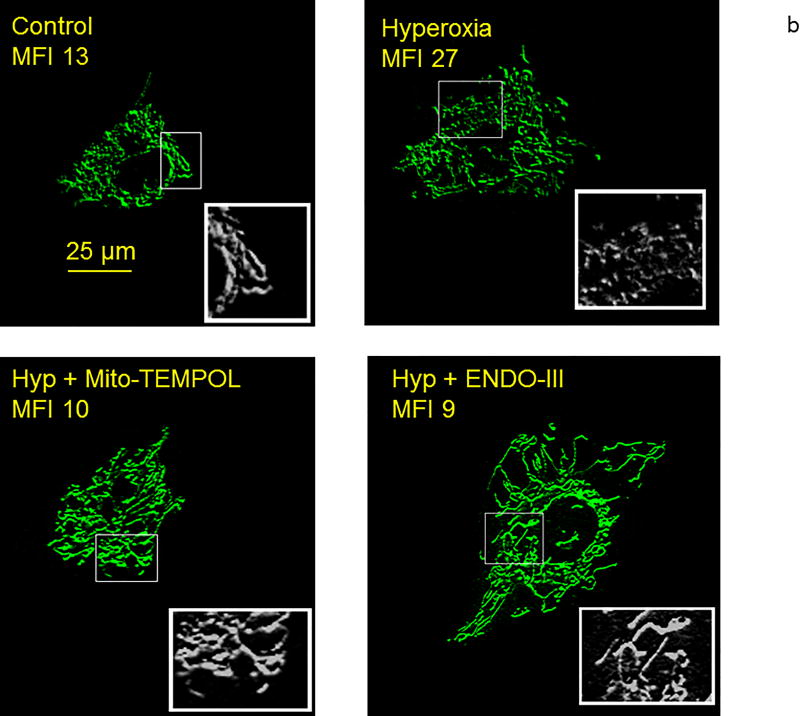
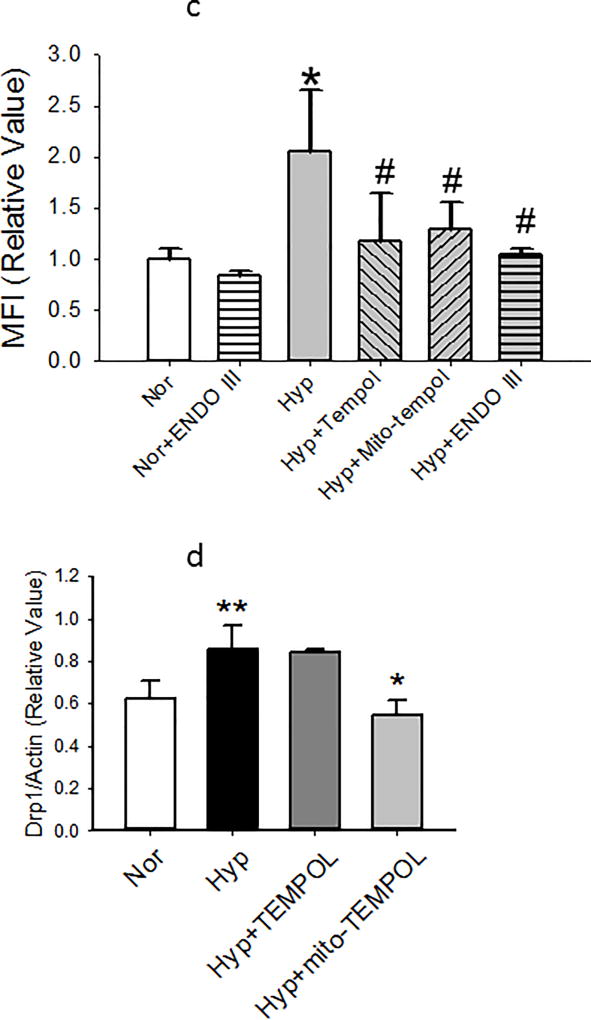
(b). Representative high resolution images of PECS exposed to normoxia (control) hyperoxia for 48 hours, hyperoxia +mito-TEMPOL for 48 hours, or hyperoxia + ENDO-III for 48 hours. Cells were imaged live with Mito-Tracker staining. Insets show grey scale, higher magnification images with ROIs indicated in the full cell images to show increase in fragmentation by hyperoxia and improvement by mito-TEMPOL and ENDO-III. MFIs calculated from whole cell ROIs appear under the legends that identify treatment. These data demonstrate increased mitochondrial fragmentation with hyperoxia, and protection with either mito-TEMPOL or ENDO-III.
(c). “Nor” cells were treated with vehicle for tempol, mito-tempol or ENDO III. MFIs were measured in images of cells exposed to normoxia or hyperoxia 48 hours later. Hyperoxia-induced increases in MFI are blocked by both TEMPOL and mito-TEMPOL as well as 0.1 µg mt-tat-ENDO III (n= 20 cells from each group). Data were normally distributed with equal variance and compared by ANOVA. *P < 0.01, different from normoxia, #P < 0.01, different from hyperoxia.
(d) PECs were exposed to normoxia or hyperoxia for 48 hours. Inclusion of mito-TEMPOL but not TEMPOL(10 µm each) prevented the increase in ratio of total Drp1 to actin. Data were normally distributed with equal variance and compared by ANOVA. ** p<0.01 different from normoxia; *p<0.05 different from hyperoxia and hyperoxia + TEMPOL. N=11,11,4, and 4 endothelial cell isolates.
To identify a connection between mt-ROS, mt-fragmentation and mitochondrial DNA damage, we measured MFIs in cells incubated for 48 hours in normoxia or hyperoxia with tempol, mito-TEMPOL, or ENDO III. Figure 5b shows images of mitochondria in representative cells cultured in normoxia, hyperoxia, hyperoxia with mito-TEMPOL or hyperoxia with ENDO III. Figure 5c shows that both TEMPOL and mito-TEMPOL (10 µm each) diminished hyperoxia-induced increments in MFI. Similarly, the mitochondrial DNA repair protein ENDO-III, blunted hyperoxia-induced increases in MFI. Mito-TEMPOL but not TEMPOL blocked hyperoxia induced increments in Drp-1 expression by 48 hours (Figure 5d).
We next examined time dependent changes in the oxidative state of PECs to correlate these changes to mitochondrial fragmentation. We observed an increase in oxidation (defined by an increase in the ratio of roGFP fluorescence at 405/485) by 24 hours, a shift which was sustained but not further increased by 48 hours (Figure 6a). TEMPOL or mito-TEMPOL prevented the oxidative shift by hyperoxia (Figure 6b). These data tie an increase in PEC oxidation with hyperoxia to mitochondrial ROS, though we cannot entirely exclude the possibility of a non-mitochondrial ROS contribution given the relatively high dose of mito-TEMPOL and similar effects of TMEPOL on this endpoint.
Figure 6.
(a). We studies the oxidative status of cells infected with ro-GFP lentivirus based on the ratio of fluorescence at 405/488 nm. Oxidation was increased by 24 hours and remained elevated through 48 hours (n=5, 5 and 8 representative fields from 3 separate experiments for 0, 24, and 48 hours respectively). Data were not normally distributed and compared by ANOVA on Ranks. **P < 0.01, different from normoxia.
(b). Inclusion of TEMPOL or mito-TEMPOL (10 mM each) in incubation media for 48 hours during hyperoxia prevented the increase in oxidation as quantified by the ro-GFP fluorescence ratio 405/488. N=5 fields/condition, from 3 separate experiments. Data were not normally distributed and compared by ANOVA on Ranks. *P < 0.05; ##P < 0.01, different from hyperoxia.
(c). After incubation for 48 hours in normoxia or hypoxia, PECs were washed with PBS, then loaded with a final concentration of 100 nM TMRM. TMRM fluorescence in images of cells from hyperoxic and normoxic environments were compared in order to assess for relative mitochondrial polarization. N=9 representative fields each from normoxic and hyperoxic cells from 2 separate isolations. Data passed normality and equal variance tests and were compared by ANOVA. *=p<0.001 relative to normoxia.
Because increased mitochondrial fragmentation may be associated with mitochondrial depolarization, we assessed the effect of hyperoxia on mitochondrial polarization in cultured PECs that exhibit mt-fragmentation. Using TMRM (100 nM) we identified decreased uptake of dye consistent with mitochondrial membrane depolarization by 48 hours hyperoxia, a time when these cells exhibit mt-fragmentation (Figure 6c).
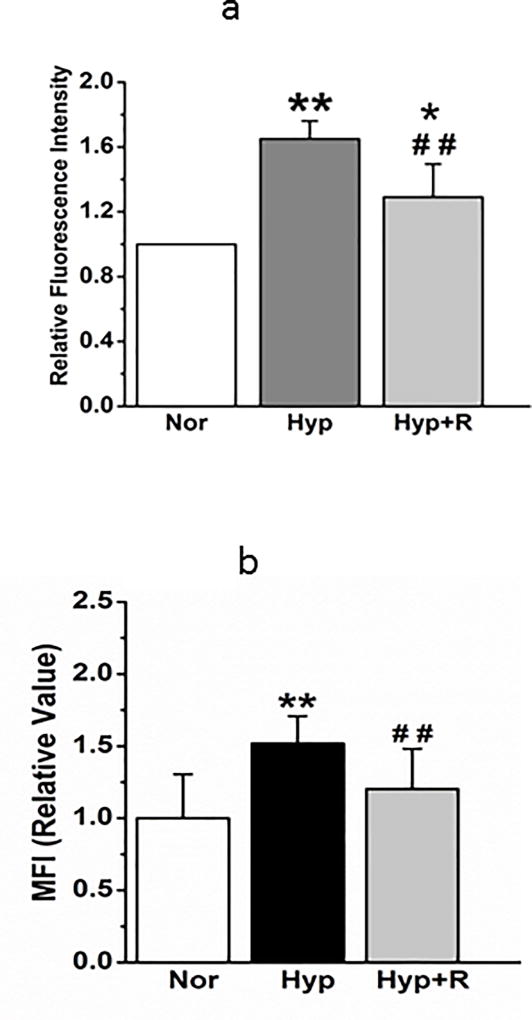
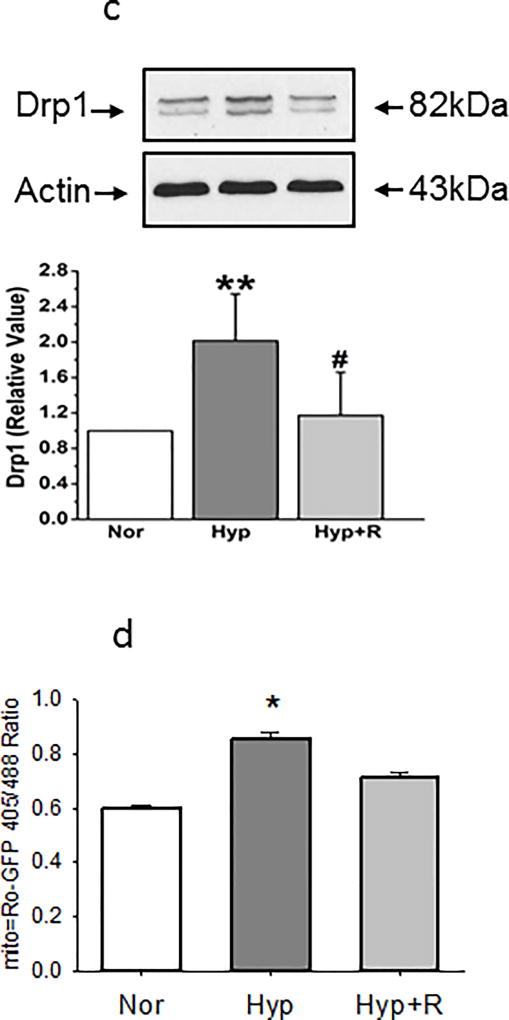
We next examined reversibility of changes in mitochondrial fragmentation, pro-fission and pro-fission/fusion protein expression. Return to normoxia for 24 hours after 48-hour exposure to hyperoxia reversed hyperoxia-induced changes in mito-SOX and MFI (Figures 7a and 7b respectively). Increases in Drp-1 evoked by 48 hour hyperoxia were also reversed by 24 hours return to normoxia, strengthening the association between changes in mitochondrial fragmentation and these proteins (Figures 7c). Oxidative changes in PECs (Figure 7d) were likewise reversed within 24 hours return to normoxia.
Figure 7.
(a). Mito-SOX fluorescence, which is increased 48 hours after hyperoxia, then decreases after 24 hours in normoxia (n=6 representative fields of cells for each condition, from 3 separate experiments). Data passed normality and equal variance tests and were compared by ANOVA. *P < 0.05, different from normoxia; **P < 0.01, different from normoxia, ##P < 0.01, different from hyperoxia.
(b) Averaged MFI show that increased mitochondrial fragmentation is resolved after 24 hours back in normoxia (N=18, 11, and 17 cells from 3 experiments). Data passed normality and equal variance tests and were compared by ANOVA. **P < 0.01, different from normoxia, ##P < 0.01, different from hyperoxia.
(c). Representative and averaged data show that Drp-1 density relative to that of beta actin is increased by 48 hours hyperoxia, but recovered to baseline within 24 hours in normoxia (n=11 for each condition). Data were not normally distributed and compared by ANOVA on Ranks. **P < 0.01, different from normoxia, #P < 0.05; #P < 0.01, different from hyperoxia.
(d). Oxidative status of cells infected with roGFP based on the ratio of fluorescence at 405/488 nm is increased by 48 hours hyperoxia (Hyp) and partially recovered after 24 hours back in normoxia (Hyp + R). N=5–8 fields/condition, from 3 separate experiments. Data failed equal variance test and were compared by ANOVA on Ranks. *=p<0.01 relative to normoxia.
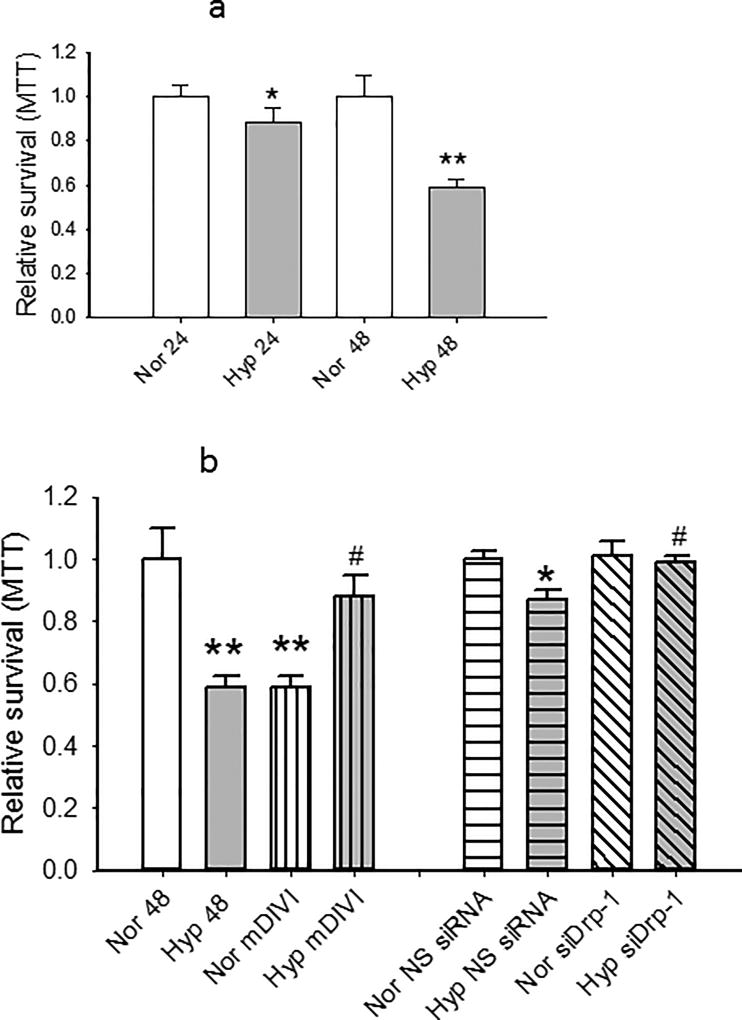
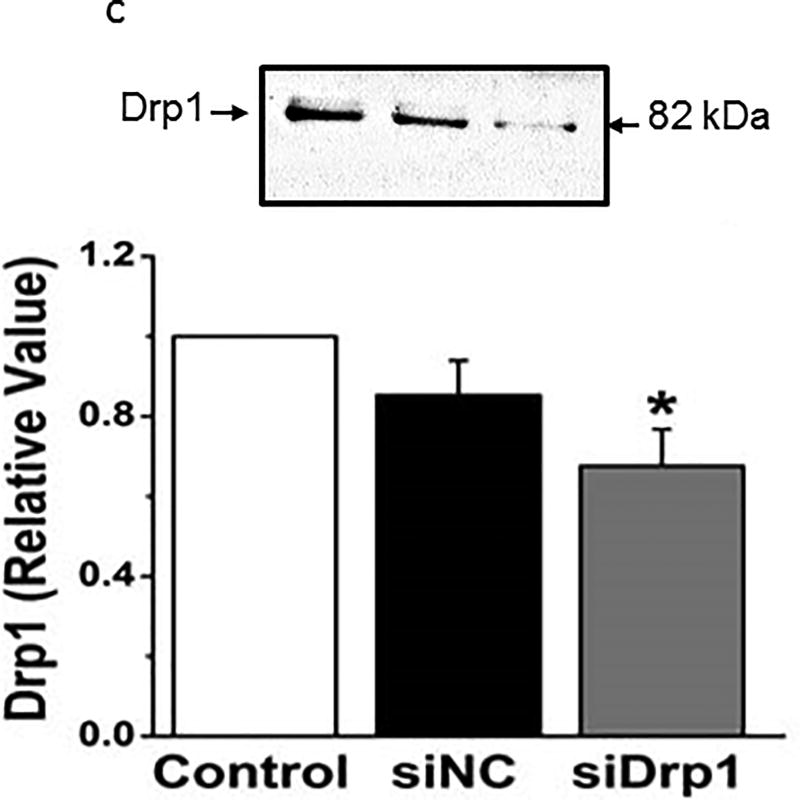
To test the role of Drp-1 activation on the survival of cells, we measured the effect of hyperoxia, the Drp-1 inhibitor mDIVI, or siRNA to Drp-1 on incorporation of MTT. MTT incorporation was decreased by 24 hours hyperoxia, and more densely so by 48 hours (Figure 8a). mDIVI (1 µM) decreased MTT incorporation in normoxic PECs, but increased it in hyperoxic cells (Figure 8b), consistent with the interpretation that activation of Drp-1 and pro-fission evoked by hyperoxia impair mitochondrial function and cell survival. siRNA to Drp-1 had no effect on MTT incorporation in normoxic cells, consistent with low expression of this protein in healthy cells. However, MTT incorporation in hyperoxic cells was enhanced by Drp-1 siRNA (and not non-sense siRNA). Together these data suggest hyperoxia evoked increases in Drp-1 decrease cell survival. Figure 8c shows that siRNA to Drp-1 decreased Drp-1 protein expression to ~65% that of control cells.
Figure 8.
(a). MTT was measured in PECs exposed to hyperoxia or normoxia for 24 or 48 hours (n=7 sets for 24 hours and n=8 sets for 48 hours each). Values were normalized to concomitant normoxia controls. Hyperoxia decreased MTT incorporation by 24 hours, and the decrease was greater by 48 hours.
(b). In cells treated for 48 hours with the Dpr-1 inhibitor mDIVI (I µM final concentration), MTT uptake decreased under normoxic conditions, but increased MTT uptake after 48 hours hyperoxia. To further test the role of Drp-1 in cell survival, we treated rat PECs with non-sense (NS) siRNA or siRNA directed against Drp-1. These data are normalized to normoxic NS siRNA treated cells. Drp-1 directed siRNA protected from hyperoxia linked decrease in MTT. Together, these data show improved survival by limiting expression of pro-fission Drp-1 in hyperoxia treated cells (n=6 sets except n=4 for siRNA experiments. MTT data for figures 8a and 8b were normally distributed with equal variance and compared by ANOVA. #p<0.01 relative to hyperoxia); *p<0.05 relative to normoxia; **p<0.01 relative to normoxia.
(c). These data show that rat PECs treated with non-sense control siRNA and lipofectamine exhibited a modest and insignificant decrease in Drp-1 protein expression over that of parallel control cells (~15%). In contrast, the decrease in Drp-1 expression with siRNA directed to Drp-1 was to ~65% that of control cells. N=4 groups of cells for each group. Data were normally distributed with equal variance and compared by ANOVA followed by Dunnett’s. *=p<0.05.
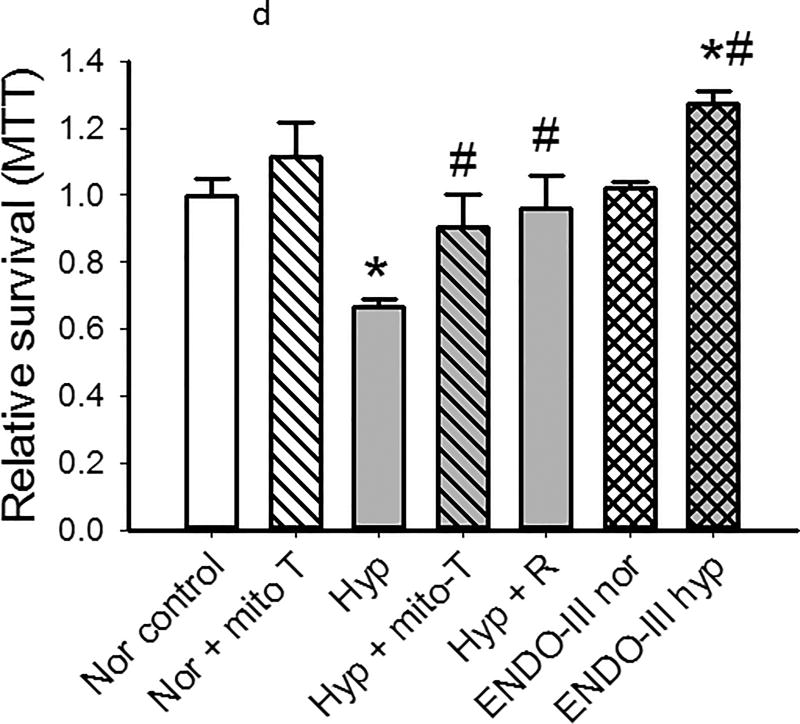
(d). All data represent MTT in cells studied after 48 hours in normoxia or hyperoxia with vehicle or drugs/conditions per the x-axis. Mito-TEMPOL did not change viability of cells in normoxia, but increased survival of cells in hyperoxia (n= 6 for each condition). Return of PECs to normoxia for 24 hours after 48 hours in hyperoxia results in recovery of MTT to baseline normoxia values (Hyper + R). mt-tat-ENDO III (0.1µg/ml) which repairs mtDNA damage, has no effect on MTT in control (normoxic) cells, but affords protection from hyperoxia such that viability is increased over that of cells cultured in normoxia. (n= 6 for each condition). Data were normally distributed with equal variance and compared by ANOVA. *P < 0.05, different from normoxia, # P < 0.05, different from hyperoxia.
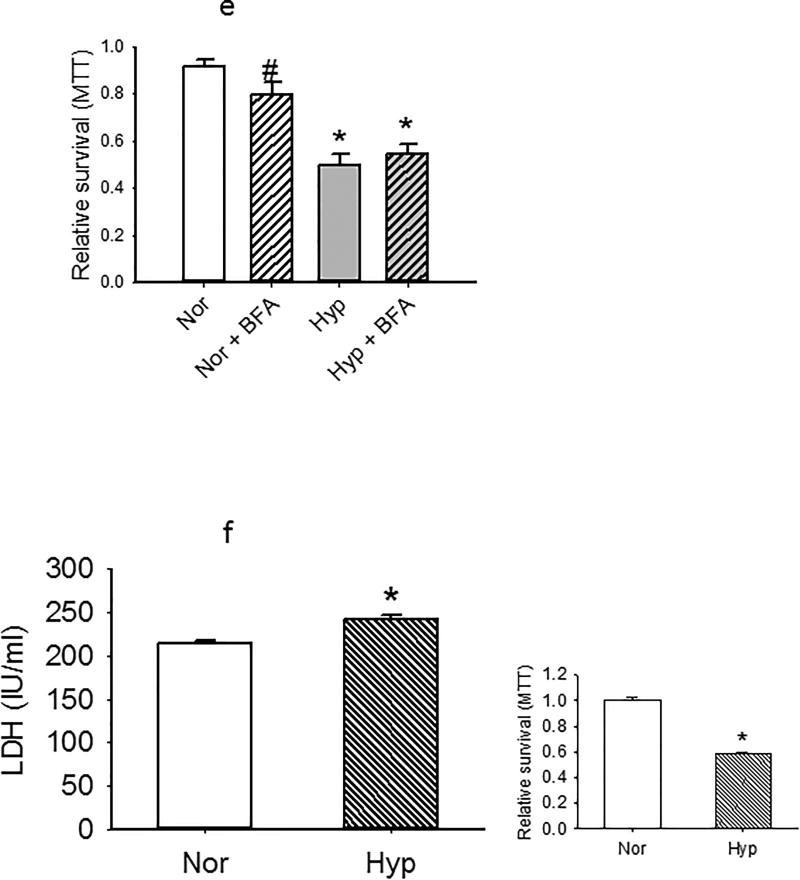
(e). MTT was measured in PECs exposed to hyperoxia or normoxia for 48 hours (n=6 each) in cells treated with vehicle or the autophagy inhibitor bafilomycin (10 nM final concentration). Under normoxic conditions, bafilomycin decreased MTT uptake modestly. In contrast, no change in MTT uptake was observed in hyperoxic cells treated with BFA. Data were normally distributed with equal variance and compared by ANOVA. #p<0.05 relative to normoxia. *p<0.01 relative to normoxia or normoxia +BFA.
(f). LDH is increased modestly (to 114% normoxia) in PECs exposed for 48 hours to hyperoxia relative to normoxia (n=6 each for normoxia and hyperoxia *P < 0.003, different from normoxia). MTT assays in the same subset of cells was performed and is shown to the right. The latter data are consistent with MTT assays for other groups of hyperoxic cells. Both sets of data were normally distributed with equal variance and compared by t-tests.
To investigate a correlation between cell survival, mitochondrial ROS and mt-DNA damage, we utilized mito-TEMPOL and ENDO III, a peptide which contains a mitochondrial localization sequence and endonuclease III to facilitate repair of mitochondrial DNA12. Mito-TEMPOL restored MTT incorporation in hyperoxic cells to that of their normoxic counterparts. Mitochondrially-targeted ENDO III peptide had no effect on MTT under normoxia (Figure 8d), but cell survival in hyperoxic PECs treated with ENDO III was greater than that of vehicle controls. These data directly link inhibition of mitochondrial ROS and mitochondrial DNA repair to cell survival in hyperoxia.
We tested the role of autophagy in survival/mitochondrial function assays (MTT) of cells exposed to normoxia or hyperoxia for 48 hours using the autophagy inhibitor bafilomycin (BFA). BFA decreased MTT uptake in normoxia PECs, but had no effect on that of hyperoxic cells (Figure 8e).
To assess the viability/injury of cells in an assay independent of MTT incorporation (which is most closely linked to mitochondrial function), we measured LDH release. Figure 8f shows that LDH is increased modestly but significantly (to 114% normoxia values) in PECs exposed to hyperoxia for 48 hours, these numbers in cells with MTT incorporation reduced to less than 60% normoxia values.
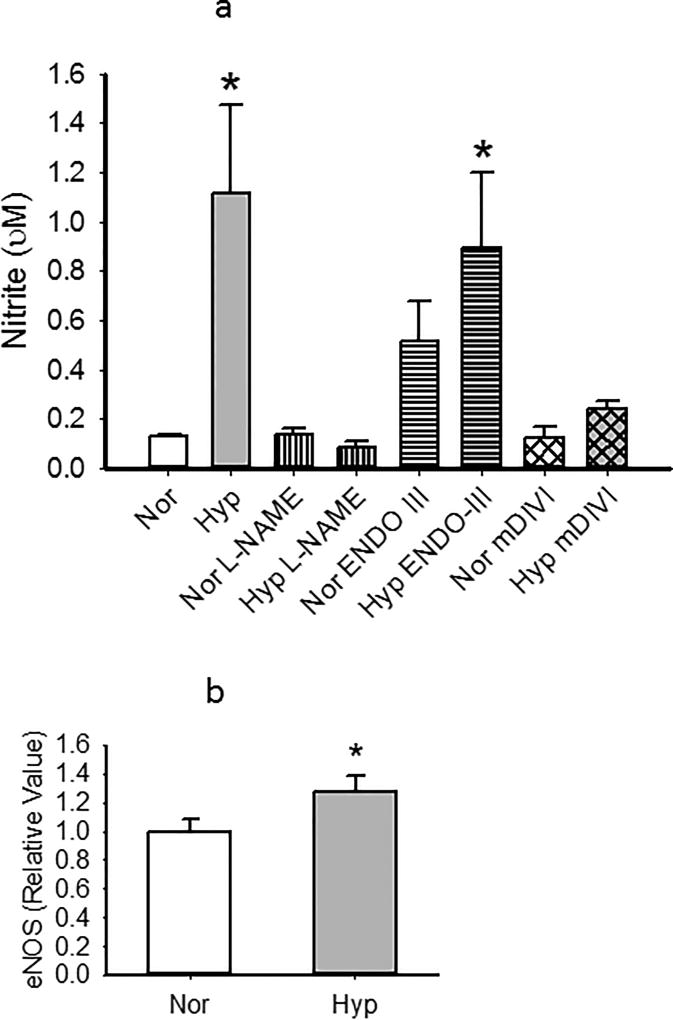
Our subsequent series of experiments were focused on the functional status of PECs under conditions which evoked hyperoxia-induced changes in mitochondrial structure. First we quantified NO production as reflected by nitrite measurements in the media of cells exposed to normoxia or hyperoxia for 48 hours13. Nitrites were low in control cells, and increased by hyperoxia in a manner which was nearly completely inhibited by NOS inhibitor L-NAME. ENDO-III tended to increase nitrites in normoxic PECs, and increased NO release over that of normoxic cells treated with this peptide in hyperoxic PECs (Figure 9a). NO levels in hyperoxic cells treated with mDIVI were not different from those of normoxia values. Consistent with hyperoxic increases in NO formation, eNOS expression was increased (Figure 9b).
Figure 9.
(a). We quantified NO production as reflected by nitrite in the media of PECs exposed to normoxia or hyperoxia for 48 hours. Nitrites were low in control cells, and significantly increased by hyperoxia in a manner which was nearly completely inhibited by NOS inhibitor L-NAME. ENDO-III increased nitrites in hyperoxic PECs over that of normoxic counterparts, whereas mDIVI blocked hyperoxia induced increases in NO. n=4–5 for each group. Data were not normally distributed with normal variance and were compared by ANOVA on Ranks. *p<0.05 relative to normoxia. #p<0.05 vs Hyper.
(b) Consistent with hyperoxic increases in NO formation, eNOS expression was increased in PECs by hyperoxia (n=7 each normoxia and hyperoxia). *p<0.05 relative to normoxia. Data were normally distributed with equal variance and compared by t-test.
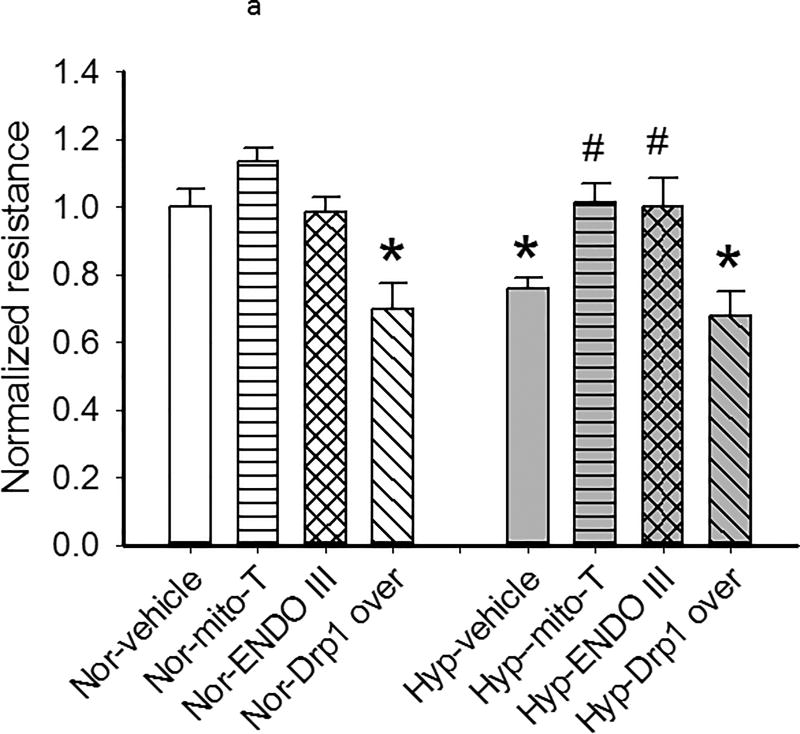
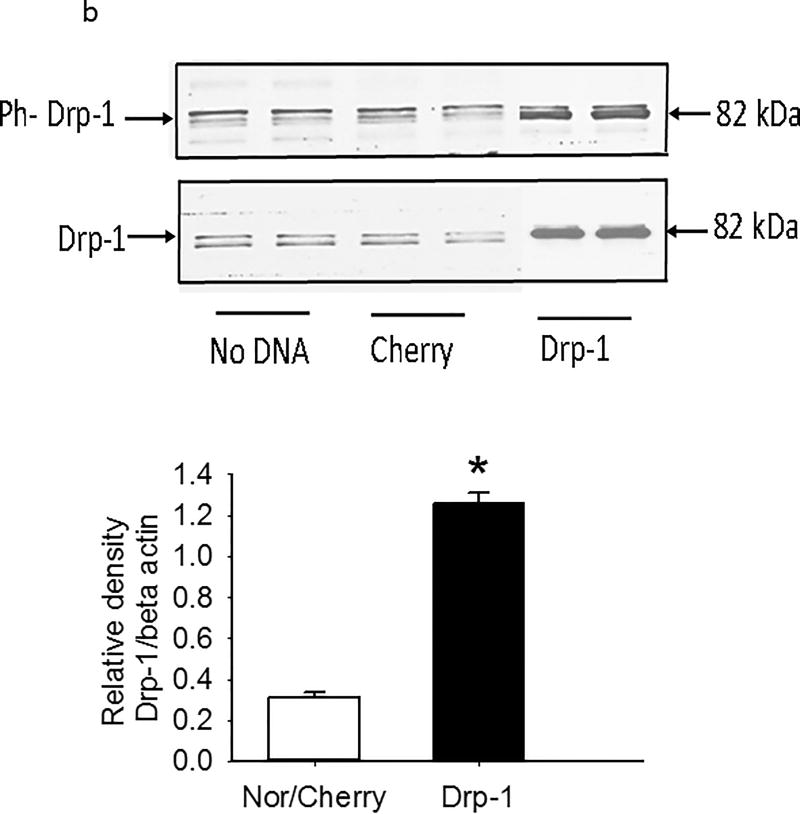
We measured transwell resistance in PEC monolayers exposed in culture to normoxia or hyperoxia for 48 hours. Monolayer resistance was decreased by hyperoxia, but not different from normoxia in hyperoxic PEC monolayers cultured with mito-TEMPOL or ENDO-III in the media, data which link mtROS and mtDNA damage to barrier function in PECs (Figure 10a). Over-expression of Drp-1 diminished TEER in normoxic monolayers, but had no further effect on monolayer resistance in hyperoxic cells (Figure 10a), consistent with a deleterious effect of Drp-1 on TEER. Figure 10b shows that Drp-1 expression was increased more than 4-fold in our PECs transfected with Drp-1.
Figure 10.
(a). We measured transwell resistance in PEC monolayers exposed for 48 hours to normoxia or hyperoxia and treated with mito-Tempol (10 µM) ENDO-III (0.1 µg/ml), or with over-expression of Drp-1. Resistance was normalized to that of normoxia with vehicle or, in the case of Drp-1 over-expression, to monolayers treated with plasmids to over-express Cherry (to visually identify transfection). Resistance is decreased by hyperoxia and by over-expression of Drp-1 in normoxia. Hyperoxic monolayers treated with mito-TEMPOL or ENDO-III are protected from decreases in monolayer resistance. Over-expression of Drp-1 in hyperoxic monolayers does not change resistance over that of hyperoxia alone. Data passed normality and equal variance tests and were compared by ANOVA. *=p<0.05 relative to normoxia. #=p<0.05 relative to hyperoxia vehicle. N=8 transwells per condition.
(b). Western blots of PEC lysates were probed with primary antibodies for Drp-1 or phospho-Drp-1 48 hours after transfection with no DNA, Cherry (control which permits visualization of cells over-expressing plasmid DNA) or Drp-1. Representative images are shown above. A graph demonstrating average increase in Drp-1 blot density is shown below (n=4). Transfection increases Drp-1 protein expression by more than four fold. Data were normally distributed with equal variance and compared by t-test. *p<0.05.
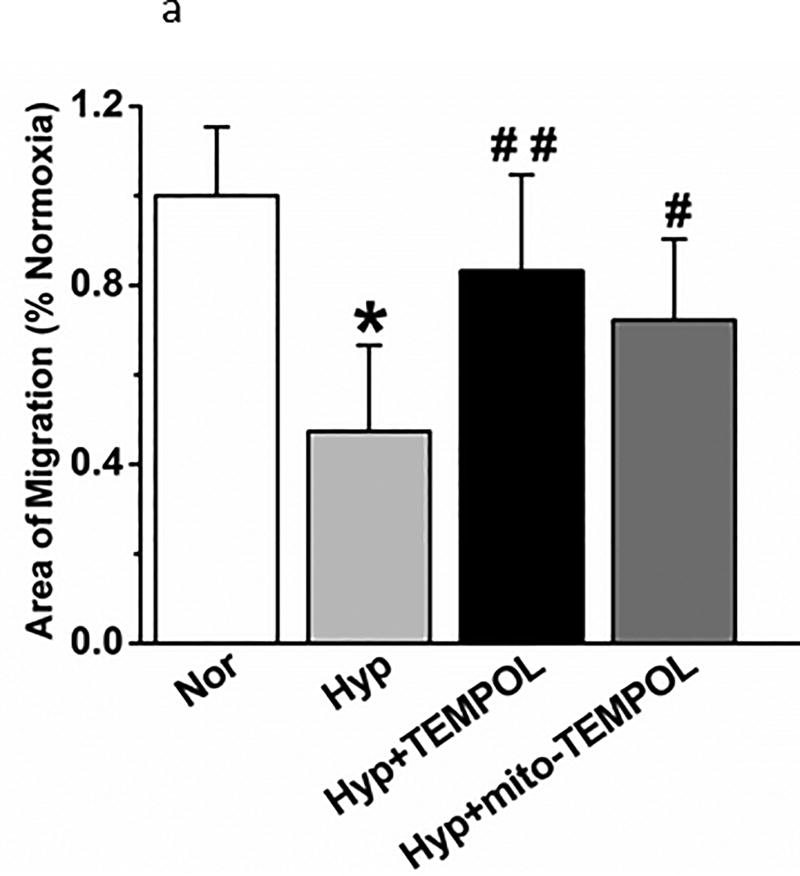
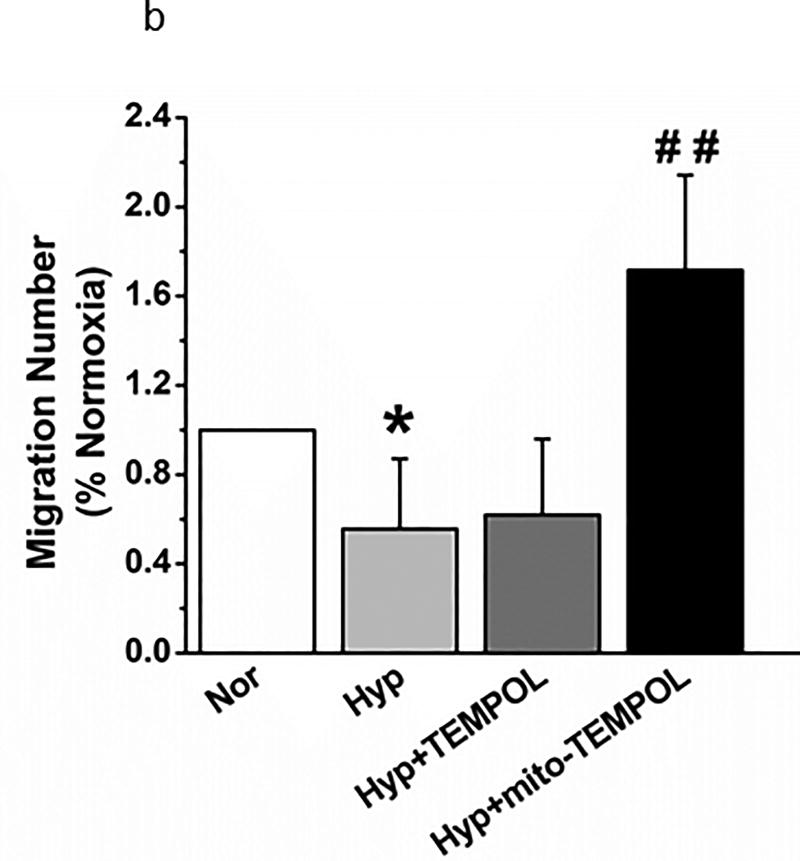
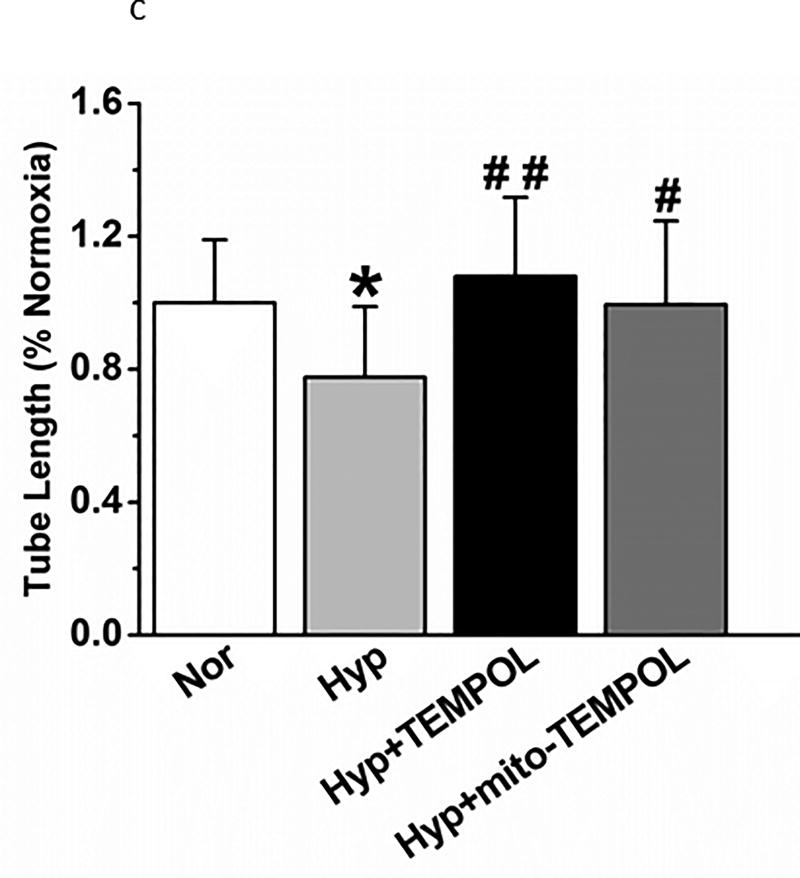
Finally, we performed three additional functional tests of PEC function after hyperoxia which relate to recovery from acute lung injury. A scratch test (measures the capacity of endothelial cells to repair a gap) identified delayed migration by hyperoxia (Figure 11a) which is reversed by TEMPOL or mito-TEMPOL in the media. Transwell migration of PECs (index of the capacity of endothelial cells for tissue invasion) is decreased by hyperoxia (Figure 11b) which is reversed by mito-TEMPOL but not TEMPOL. Networks in a 3-dimensional matrix are needed for angiogenesis (Figure 11c). Hyperoxia decreased the length of network formation of PECs in matrigel. Either mito-TEMPOL or TEMPOL protected against hyperoxia-evoked decreases in PEC network formation.
Figure 11.
(a). Hyperoxia decreased migration of cells into a path cleared by a scratch relative to counterparts maintained in normoxia. TEMPOL or mito-TEMPOL partially restored diminished migration of PECs in hyperoxia by 48 hours. Data were not normally distributed and were compared by ANOVA on Ranks. *P < 0.05, different from normoxia, #P < 0.05 different from hyperoxia; ##P < 0.01, different from hyperoxia. n=12 experiments for each condition and time.
(b). Transwell migration of PECs was compared in normoxia and hyperoxic conditions with 4 separate isolates of endothelial cells. 48 hours hyperoxia decreased migration. Mito-TEMPOL but not TEMPOL restored migration to supra-normoxia values. (n=29,29,23, and 22 wells respectively for each of the above 4 groups from 4 separate experiments. Data were normally distributed with equal variance and compared by ANOVA followed by Dunnett’s. *P < 0.05, different from normoxia, ##P < 0.01, different from hyperoxia.
(c). Network formation of PECs in a 3-dimensional matrix is decreased by 24 hours hyperoxia. Both TEMPOL and mito-TEMPOL restore network formation. (n=17–21 fields/condition, from 4 separate experiments). Data were normally distributed with equal variance and compared by ANOVA followed by Dunnett’s. *P < 0.05, different from normoxia; #P < 0.05 and ##P < 0.01 different from hyperoxia.
Discussion
We demonstrate for the first time that PECs exposed to hyperoxia exhibit an increase in mitochondrial fragmentation. Changes occur within 6 hours of hyperoxia exposure and are mitigated by mito-TEMPOL or TEMPOL and are reversed within 24 hours of return to normoxia. Increases in phosphorylation of Drp-1, followed by an increase in total Drp-1 expression, as well as decreases in Mfn1 were observed, changes which promote mitochondrial fragmentation in other injury systems. On the other hand, hyperoxia also increases OPA-1 expression, an increase which we postulate may serve as a compensatory mechanism to limit mitochondrial fragmentation.
Hyperoxia-induced mt-fragmentation correlates with diminished MTT (an index of cell survival or mitochondrial function) and elevated LDH, but also increased NO synthesis and eNOS expression. MTT incorporation improves with mito-TEMPOL, the Drp-1 inhibitor m-DIVI or siRNA to Drp-1, and the mitochondrial endonuclease ENDO III, linking increased mitochondrial ROS, Drp-1 activation and mt-DNA damage respectively to changes in cell viability after hyperoxia. Mito-TEMPOL also prevents increases in Drp-1 expression, supporting a role for mt-ROS in shifting the balance to expression of mitochondrial fission proteins. Finally increases in p62 and LC3B expression, and decreases in PINK-1 suggest activation of autophagy/mitophagy in this injury model.
Mitochondrial redox states monitored by roGFP support increased oxidation, consistent with the increase in mitochondrial ROS (Figure 6b) and mitochondrial membrane depolarization (Figure 6c). Direct repair of mitochondrial DNA damage during hyperoxia, by addition of ENDO III, also improves cell survival as well diminishes mt-fragmentation. Hyperoxic changes in mitochondrial structure are accompanied not only by diminished cell survival, but also by disruption of PEC function, including diminished monolayer resistance, gap closing, cell migration and network formation in a 3-dimensional matrix. These functional changes are also prevented by mito-TEMPOL supporting cause and effect for mitochondrial ROS-driven mitochondrial fission, and PEC dysfunction, like increased ROS and mitochondrial fragmentation in endothelial cells exposed to hyperglycemia. Transendothelial electrical resistance (TEER) is diminished by Drp-1 over-expression in normoxia, consistent with a deleterious effect of Drp-1 on endothelial junctions, and protected by ENDO-III in hyperoxic lungs. Our observations support a relationship between mitochondrial DNA injury and barrier integrity in rodent lungs, as has been suggested in glucose-glucose oxidase perfused isolated lungs14, ischemia-reperfusion injury15 or pseudomonas sepsis16. Efficacy of ENDO III is also consistent with the enhanced sensitivity of mitochondrial DNA to oxidative injury relative to nuclear DNA17. Our graphic abstract schematically summarizes a proposed pathway through which hyperoxia initiates mitochondrial fragmentation as well as cellular dysfunction in PECs based on our data.
The endothelium is an important target of lungs exposed to hyperoxia1. Ahmad et al18 reported increases in the number of detached or necrotic cultured human lung microvascular endothelial cells exposed to hyperoxia versus normoxia, as well as mitochondrial cristae which appeared swollen. Others reported decreased DNA and protein synthesis with diminished fluidity of the plasma membrane also resulting in decreased survival19, 20. More importantly survival was decreased in response to hyperoxia in endothelial cells exposed in vivo18, 21. We have identified a time- and concentration-dependent increase in PEC death in the lungs of rats exposed in vivo to hyperoxia4, 22. Our new data support increased caspase 3/7 activity in PECs to hyperoxia in culture.
Augmented ROS production in the lungs of rodents exposed to hyperoxia have been well documented3, 23. NADPH oxidase 2 and 4 (NOX2 and NOX4) expression are increased in human lung microvascular endothelial cells exposed to hyperoxia, and contribute to hyperoxia-evoked ROS by multiple pathways24–26. ATP and activities of complex I and II are decreased in the lungs of hyperoxia exposed rats27–29. We now show that PECs exposed to hyperoxia exhibit increased Drp-1 activation, decreased mfn1 expression and enhanced mt-fragmentation. Hyperoxia-enhanced mito-SOX fluorescence is blocked by mito-TEMPOL, as is enhanced mt-fragmentation. These data connect mtROS to enhanced mt-fragmentation and other downstream changes. There is precedent for fragmentation of endothelial cell mitochondria with increased ROS stress in hyperglycemia which is also linked to vascular dysfunction via mitochondrial fragmentation. Mitochondrial fission is observed along with increased ROS production and endothelial cell apoptosis under diabetic conditions30, 31.
Mitochondrial networks are dynamic in healthy cells, shifting between a fragmented and tubular continuum by active fusion and division (fission)5, 6, 8, 9. Activation of Drp-1 in pulmonary vascular smooth muscle cells is associated with enhanced mitochondrial fragmentation in experimental animal models and samples from patients with idiopathic pulmonary arterial hypertension or diabetes5, 32,33. Increases in mitochondrial fragmentation and shift in proteins controlling mitochondrial dynamics in smooth muscle cells are linked to a pro-proliferative state in PAH. Our observation that mt- TEMPOL improves cell survival and decreases mt-fragmentation in PECs suggests that interventions which decrease mt-fragmentation in PECs exposed to hyperoxia could be protective to lung function. This interpretation is supported by improved MTT incorporation in hyperoxic cells treated with the Drp-1 inhibitor mDIVI or siRNA to Drp-1 (Figure 8a).
Mitofusion proteins (Mfn1 and 2) are dynamin-related GTPases34–36 which promote mitochondrial fusion. Optic Atrophy 1 (OPA1) is a dynamin-related GTPase which mediates fusion of inner mitochondrial membranes in mammalian cells34, 36. Our data show decreases in Mfn1 but increases in OPA-1 expression in hyperoxia-exposed PECs in culture. Decreases in Mfn1 should shift the balance of fusion/fission to more fission. Ryan and Archer32 identified decreased expression of Mfn2 in pulmonary vascular smooth muscle cells from humans and rodents with PAH. Wang37 observed that elongated mitochondria were more resistant to ROS-induced damage and mitophagy compared with fragmented mitochondria, supporting the position that mitochondrial morphology has an important role in regulating ROS and cell survival. Increases in OPA-1 would counter increased mitochondrial fragmentation, thus may represent a compensatory mechanism of cells stressed by hyperoxia. Genetic manipulation of Drp-1 expression in PECs support a role for at least this protein in cell survival and TEER in hyperoxic cells.
Mitochondria can be marked for degradation through autophagy, which is an important mechanism to recover from injury. However, impaired mitophagy is observed in vascular injury secondary to oxidative stress5, 32,38 and is believed to be central to the pro-proliferative state of pulmonary vascular smooth muscle cells in human pulmonary hypertension. Sequestosome-1 or ubiquitin binding protein p62 can directly interact with poly- and mono-ubiquitin and microtubule associated light chain marker 3 (LC3)39. p62 is recruited to ubiquitinated mitochondria39–41 and LC3 subfamily proteins participate in autophagosomal membrane elongation42. A subset of outer mitochondrial membrane proteins including mfn-1 and -2 are ubiquitinated in a Parkin-dependent manner35, 40. p62 can be recruited to the ubiquitinated mitochondria in Parkin-positive cells39–41. PINK1 kinase activity is needed for efficient Parkin recruitment to impaired mitochondria40. It is postulated to protect cells from stress-induced mitochondrial dysfunction by inducing autophagy. Our data support an increase in P62 and LC3B, but diminished PINK-1 in PECs exposed to hyperoxia in culture. P62 is also increased in whole lung homogenates of rats exposed in vivo to hyperoxia, suggesting activation of autophagy in vivo as well as in cultured cells. A decrease in PINK-1 in hyperoxic PECs raises the possibility of inefficient autophagy under this condition and has been reported with increases in Drp-1 activation or decreases in Mfn-111. The fact that the autophagy inhibitor bafilomycin does not improve MTT incorporation in hyperoxia suggests that autophagy is either not involved in hyperoxic injury or it is dysfunctional, as it is in diseases such as aging, ischemia-reperfusion, or pulmonary hypertension38. Definitive studies to evaluate autophagy flux43 and its relationship to mt-fragmentation in hyperoxia are needed.
Cytosolic, surface, endoplasmic reticulum, and mitochondrially-targeted roGFP recombinant viral constructs have been successfully used to identify oxidoreductive states in subcellular locations including in lung cells44. Our data demonstrate mitochondrial oxidation of cells maintained in hyperoxia for 48 hours in a manner that is partially reversible after 24 hours return to normoxia, and is prevented by mito-TEMPOL. These data are consistent with our observations of increased oxidation of rat lungs exposed in vivo to hyperoxia45. Our data in intact PECs are consistent with these observations, and to our knowledge are the first to show hyperoxia-induced oxidative shifts in PECs with mt-fragmentation. Supporting oxidative injury and failed reparative mechanisms, we observed loss of mitochondrial membrane potential (Figure 3f).
We also observe degraded function of hyperoxic-exposed cells that correlate to structural changes, including diminished survival, diminished transwell resistance, transwell migration, scratch healing, and tube formation. Migration, wound healing and tube formation are necessary for resolution of lung injury. Transwell migration of endothelial cells tests the response of endothelial cells to invade non-perfused tissue in response to angiogenic factors and the scratch test identifies the capacity of endothelium to recover a denuded (gap) area. Tubulogenesis in a three-dimensional (3-D) matrix mimics some of the steps in angiogenic remodeling causing alignment of endothelial cells into networks. Tube formation is diminished by 3 days of hyperoxia in murine fetal lung endothelial cells in a VEGF and hepatocyte growth factor dependent manner46. Our study also documents diminished network formation which was partially reversible by mito-TEMPOL. In contrast, Pendyala et al24 reported enhanced wound healing and tube formation in human pulmonary artery endothelial cells after 16 hours of exposure to hyperoxia relative to normoxia, raising the possibility of differences in functional effects based on time after hyperoxia. Our observations of diminished wound healing and transwell migration of PECs after 48 hours hyperoxia in a manner which is reversed by mito-TEMPOL is consistent with diminished cell survival and mitochondrial fragmentation in this time frame and in our injury model. Together, these data suggest that mitochondrial fragmentation correlates to degraded reparative function in cultured PECs.
Despite hyperoxia-evoked decreases in PEC function, data support maintained viability of these cells. NO synthesis was significantly increased by hyperoxia, consistent with enhanced expression of eNOS and iNOS as well as cyclic GMP activity in rat pups exposed in vivo to hyperoxia47. Our cultured PECs also exhibit increased eNOS expression consistent with enhanced NO synthesis. Changes in all endpoints we tested were largely reversible after 24 hours return to normoxia. Limited increases in LDH and apoptosis (similar scale to what we reported in vivo48), as well as reversibility and increased NO support hyperoxia-linked mitochondrial dysfunction that occurs in a setting not characterized by large scale cell death. Of interest, mtDNA injury does not appear to be related to NO synthesis, as ENDO-III does not protect from this endpoint, whereas the Drp-1 inhibitor mDIVI does stop hyperoxia evoked increases NO.
In conclusion, we have observed hyperoxia-induced mitochondrial fragmentation and diminished survival in PECs which is mechanistically linked to mitochondrial ROS and mt-DNA damage in that they are blocked by treatment with mito-TEMPOL or ENDO III. Increased mitochondrial fragmentation is associated with functional consequences as well as shifts in mt-fission and mt-fusion proteins in endothelial cells exposed to hyperoxia. Genetic manipulation studies support a role for Drp-1 over-expression on hyperoxic injury. These proteins could represent targets for therapy in patients requiring hyperoxia if future studies confirm their capacity to modify functional outcomes.
Supplementary Material
Highlights.
This study quantifies for the first-time mitochondrial fragmentation in pulmonary endothelial cells exposed to hyperoxia.
We demonstrate altered expression of several pro-fission/fusion proteins in hyperoxia, and link enhanced expression of Drp-1 to increased mitochondrial fragmentation, to PEC survival, and to monolayer integrity using gene silencing and over-expression techniques.
Hyperoxia-associated mitochondrial fragmentation is prevented by mito-TEMPOL and the mitochondrial endonuclease ENDO III, linking this structural change to mitochondrial ROS and mitochondrial DNA damage.
Mitochondrial fragmentation in hyperoxia is observed with mitochondrial depolarization and an increase in PEC oxidation.
The above changes after 48 hour hyperoxia are reversible within 24 hours return to normoxia.
Acknowledgments
Electron micrography was performed at the Electron Micrography Core by Dr. Clive Wells. Nitrite levels were measured in the Redox Biology Program under direction of Dr. Neil Hogg. Assistance in transwell resistance measurements was provided by Cathy Paddock in Peter Newman’s lab at the Blood Research Institute. Excellent technical assistance was provided by Ying Gao, Dr. Feng Gao, Carlos Marquez Barrientos, and Jayashree Narayanan. Mitochondrially targeted endonuclease proteins were a generous gift from Dr. Glenn Wilson and Ker Ferguson with Exscien Corporation.
Sources of Funding: NIH/NIHLBI- HL116530, HL1202209, HL 129209, HL128240, VA Merit Review Award 1I01BX001681, NIH/NIAID - AI 101898, AI107305; NIH/NIGMS GM067180, NIH/OD018306, and R01-HL128240, CTSI 8UL1TR000055, the Alvin and Marion Birnschein Foundation, Department of Radiation Oncology, Medical College of Wisconsin.
Abbreviations
- PEC
Pulmonary endothelial cell
- Mfn1
mitofusion protein 1
- Drp-1
dynamin related protein 1
- mDIVI
mitochondrial division mitophagy inhibitor
- LC3B
Microtubule-associated protein 1A/1B-light chain 3
- p62
LC3 adapter binding protein SQSTM1/p62
- ROS
reactive oxygen species
- mito-SOX
mitochondrially targeted superoxide indicator
- ENDO III
mt-tat-endonuclease III
- TMRM
Tetramethylrhodamine methyl ester
- TEER
Trans-endothelial electrical resistance
Footnotes
Disclosures: none.
References
- 1.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Davis WB, Rennard SI, Bitterman PB, Crystal RG. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983;309:878–883. doi: 10.1056/NEJM198310133091502. [DOI] [PubMed] [Google Scholar]
- 3.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 4.Audi SH, Jacobs ER, Zhao M, Roerig DL, Haworth ST, Clough AV. In vivo detection of hyperoxia-induced pulmonary endothelial cell death using (99m)tc-duramycin. Nucl Med Biol. 2015;42:46–52. doi: 10.1016/j.nucmedbio.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer SL. Mitochondrial fission and fusion in human diseases. N Engl J Med. 2014;370:1074. doi: 10.1056/NEJMc1316254. [DOI] [PubMed] [Google Scholar]
- 6.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112:1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp WW, Archer SL. Mitochondrial dynamics in cardiovascular disease: Fission and fusion foretell form and function. J Mol Med (Berl) 2015;93:225–228. doi: 10.1007/s00109-015-1258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan DC. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, Luo YX, Chen HZ, Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol. 2014;5:175. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka A, Jin Y, Lee SJ, Zhang M, Kim HP, Stolz DB, Ryter SW, Choi AM. Hyperoxia-induced lc3b interacts with the fas apoptotic pathway in epithelial cell death. Am J Respir Cell Mol Biol. 2012;46:507–514. doi: 10.1165/rcmb.2009-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The pink1/parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarini G, Kiyooka T, Ohanyan V, Pung YF, Marzilli M, Chen YR, Chen CL, Kang PT, Hardwick JP, Kolz CL, Yin L, Wilson GL, Shokolenko I, Dobson JG, Jr, Fenton R, Chilian WM. Impaired coronary metabolic dilation in the metabolic syndrome is linked to mitochondrial dysfunction and mitochondrial DNA damage. Basic Res Cardiol. 2016;111:29. doi: 10.1007/s00395-016-0547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 14.Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN. Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am J Physiol Lung Cell Mol Physiol. 2011;301:L892–898. doi: 10.1152/ajplung.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan YB, Mulekar S, Gorodnya O, Weyant MJ, Zamora MR, Simmons JD, Machuka T, Gillespie MN. Pharmacologic protection of mitochondrial DNA integrity may afford a new strategy for suppressing lung ischemia-reperfusion injury. Ann Am Thorac Soc. 2017;14:S210–S215. doi: 10.1513/AnnalsATS.201706-438MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YL, Obiako B, Gorodnya OM, Ruchko MV, Kuck JL, Pastukh VM, Wilson GL, Simmons JD, Gillespie MN. Mitochondrial DNA damage initiates acute lung injury and multi-organ system failure evoked in rats by intra-tracheal pseudomonas aeruginosa. Shock. 2017;48:54–60. doi: 10.1097/SHK.0000000000000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1300–1308. doi: 10.1152/ajplung.2001.280.6.L1300. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad S, Ahmad A, White CW. Purinergic signaling and kinase activation for survival in pulmonary oxidative stress and disease. Free Radic Biol Med. 2006;41:29–40. doi: 10.1016/j.freeradbiomed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Block ER, Patel JM, Angelides KJ, Sheridan NP, Garg LC. Hyperoxia reduces plasma membrane fluidity: A mechanism for endothelial cell dysfunction. J Appl Physiol (1985) 1986;60:826–835. doi: 10.1152/jappl.1986.60.3.826. [DOI] [PubMed] [Google Scholar]
- 20.Junod AF, Clement A, Jornot L, Petersen H. Differential effects of hyperoxia and hydrogen peroxide on thymidine kinase and adenosine kinase activities of cultured endothelial cells. Biochim Biophys Acta. 1985;847:20–24. doi: 10.1016/0167-4889(85)90147-8. [DOI] [PubMed] [Google Scholar]
- 21.Wright CJ, Agboke F, Chen F, La P, Yang G, Dennery PA. No inhibits hyperoxia-induced nf-kappab activation in neonatal pulmonary microvascular endothelial cells. Pediatric research. 2010;68:484–489. doi: 10.1203/PDR.0b013e3181f917b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clough AV, Audi SH, Haworth ST, Roerig DL. Differential lung uptake of 99mtc-hexamethylpropyleneamine oxime and 99mtc-duramycin in the chronic hyperoxia rat model. J Nucl Med. 2012;53:1984–1991. doi: 10.2967/jnumed.112.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–463. doi: 10.1165/rcmb.2005-0223OC. [DOI] [PubMed] [Google Scholar]
- 24.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of nox4 and nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2009;11:747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendyala S, Moitra J, Kalari S, Kleeberger SR, Zhao Y, Reddy SP, Garcia JG, Natarajan V. Nrf2 regulates hyperoxia-induced nox4 expression in human lung endothelium: Identification of functional antioxidant response elements on the nox4 promoter. Free Radic Biol Med. 2011;50:1749–1759. doi: 10.1016/j.freeradbiomed.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin ii requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 27.Currie WD, Pratt PC, Sanders AP. Hyperoxia and lung metabolism. Chest. 1974;66:19S–21S. doi: 10.1378/chest.66.1_supplement.19s. [DOI] [PubMed] [Google Scholar]
- 28.Merker MP, Audi SH, Lindemer BJ, Krenz GS, Bongard RD. Role of mitochondrial electron transport complex i in coenzyme q1 reduction by intact pulmonary arterial endothelial cells and the effect of hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;293:L809–819. doi: 10.1152/ajplung.00448.2006. [DOI] [PubMed] [Google Scholar]
- 29.Sepehr R, Audi SH, Staniszewski KS, Haworth ST, Jacobs ER, Ranji M. Novel flurometric tool to assess mitochondrial redox state of isolated perfused rat lungs after exposure to hyperoxia. IEEE J Transl Eng Health Med. 2013;1:1500210. doi: 10.1109/JTEHM.2013.2285916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rube DA, van der Bliek AM. Mitochondrial morphology is dynamic and varied. Molecular and Cellular Biochemistry. 2004;256:331–339. doi: 10.1023/b:mcbi.0000009879.01256.f6. [DOI] [PubMed] [Google Scholar]
- 31.Yu H, Sliedregt-Bol K, Overkleeft H, van der Marel GA, van Berkel TJ, Biessen EA. Therapeutic potential of a synthetic peptide inhibitor of nuclear factor of activated t cells as antirestenotic agent. Arterioscler Thromb Vasc Biol. 2006;26:1531–1537. doi: 10.1161/01.ATV.0000225286.30710.af. [DOI] [PubMed] [Google Scholar]
- 32.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: Part i: Metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131:1691–1702. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorn GW, 2nd, Clark CF, Eschenbacher WH, Kang MY, Engelhard JT, Warner SJ, Matkovich SJ, Jowdy CC. Marf and opa1 control mitochondrial and cardiac function in drosophila. Circ Res. 2011;108:12–17. doi: 10.1161/CIRCRESAHA.110.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a pink1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. Ros-induced mitochondrial depolarization initiates park2/parkin-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8:1462–1476. doi: 10.4161/auto.21211. [DOI] [PubMed] [Google Scholar]
- 38.Go KL, Lee S, Zendejas I, Behrns KE, Kim JS. Mitochondrial dysfunction and autophagy in hepatic ischemia/reperfusion injury. Biomed Res Int. 2015;2015:183469. doi: 10.1155/2015/183469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding H, Jiang N, Liu H, Liu X, Liu D, Zhao F, Wen L, Liu S, Ji LL, Zhang Y. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800:250–256. doi: 10.1016/j.bbagen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Geisler S, Holmstrom KM, Treis A, Skujat D, Weber SS, Fiesel FC, Kahle PJ, Springer W. The pink1/parkin-mediated mitophagy is compromised by pd-associated mutations. Autophagy. 2010;6:871–878. doi: 10.4161/auto.6.7.13286. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by parkin and p62/sqstm1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamacher-Brady A, Brady NR. Mitophagy programs: Mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci. 2016;73:775–795. doi: 10.1007/s00018-015-2087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loos B, du Toit A, Hofmeyr JH. Defining and measuring autophagosome flux-concept and reality. Autophagy. 2014;10:2087–2096. doi: 10.4161/15548627.2014.973338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunniff B, Benson K, Stumpff J, Newick K, Held P, Taatjes D, Joseph J, Kalyanaraman B, Heintz NH. Mitochondrial-targeted nitroxides disrupt mitochondrial architecture and inhibit expression of peroxiredoxin 3 and foxm1 in malignant mesothelioma cells. J Cell Physiol. 2013;228:835–845. doi: 10.1002/jcp.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sepehr R, Staniszewski K, Maleki S, Jacobs ER, Audi S, Ranji M. Optical imaging of tissue mitochondrial redox state in intact rat lungs in two models of pulmonary oxidative stress. J Biomed Opt. 2012;17:046010. doi: 10.1117/1.JBO.17.4.046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seedorf G, Metoxen AJ, Rock R, Markham N, Ryan S, Vu T, Abman SH. Hepatocyte growth factor as a downstream mediator of vascular endothelial growth factor-dependent preservation of growth in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1098–1110. doi: 10.1152/ajplung.00423.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potter CF, Kuo NT, Farver CF, McMahon JT, Chang CH, Agani FH, Haxhiu MA, Martin RJ. Effects of hyperoxia on nitric oxide synthase expression, nitric oxide activity, and lung injury in rat pups. Pediatr Res. 1999;45:8–13. doi: 10.1203/00006450-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Audi SH, Clough AV, Haworth ST, Medhora M, Ranji M, Densmore JC, Jacobs ER. 99mtc-hexamethylpropyleneamine oxime imaging for early detection of acute lung injury in rats exposed to hyperoxia or lipopolysaccharide treatment. Shock. 2016;46:420–430. doi: 10.1097/SHK.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.