Abstract
The Manila clam Ruditapes philippinarum inhabits the intertidal zone and must therefore tolerate broad fluctuations in water temperature and salinity. Heat shock protein 60 (HSP60) is an evolutionarily conserved, multi-functional protein that plays a significant role in protecting organisms from harmful stress conditions. We cloned the R. philippinarum HSP60 (RpHSP60) gene and analyzed its transcriptional responses to thermal and low-salinity stresses. The complete sequence of RpHSP60 cDNA was 1777 nucleotides, containing a 1728-bp open reading frame encoding a polypeptide of 576-amino acids, with a calculated molecular mass of 61.25 kDa and predicted isoelectric point of 5.08. Comparisons of amino acid sequences and three-dimensional structures of HSP60 revealed that RpHSP60 was highly conserved in the signature HSP60-family domains. RpHSP60 mRNA was detected in all the tested tissues of R. philippinarum, with the highest expression levels in hemocytes. We measured RpHSP60 mRNA levels in the gills under thermal and low-salinity stresses using quantitative real-time reverse transcription-polymerase chain reaction. Following the thermal challenge, RpHSP60 mRNA was significantly upregulated at 6 h, and then progressively downregulated under high-temperature stress (30 °C), while only slight fluctuations were observed under low-temperature stress (−1 °C). Under low-salinity (17 ppt) stress, RpHSP60 mRNA levels were significantly increased at 3, 72, and 96 h (P < 0.05). These results suggest that HSP60 of R. philippinarum may play important roles in responding to high-temperature and low-salinity stresses.
Electronic supplementary material
The online version of this article (10.1007/s12192-017-0796-7) contains supplementary material, which is available to authorized users.
Keywords: Ruditapes philippinarum, HSP60, Temperature stress, Low-salinity stress, Expression profile
Introduction
Heat shock proteins (HSPs) are evolutionarily highly conserved proteins with a range of important physiological functions. These proteins exist in all cells, ranging from bacteria to humans, and are involved in numerous essential cellular functions and immune responses (Ranford et al. 2000; Ranford and Henderson 2002). They can also be induced by denatured proteins produced as a result of environmental-stress conditions, such as heat or cold, osmotic imbalance, toxins, heavy metals, and microbial damage (Ranford et al. 2000; Chen et al. 2007; Zhou et al. 2010; Rhee et al. 2009). These proteins serve as chaperones to repair nuclear proteins, and thus protect cells from the damaging effects of environmental stresses (Bensaude 1990; Lewis et al. 1999). HSPs is classified into families based on their molecular weights, including HSP100, HSP90, HSP70, HSP60, HSP40, and several smaller HSPs (Kregel 2002).
HSP60 proteins are chaperones that are mainly localized in the mitochondria of eukaryotic cells. They consist of two back-to-back rings with a total of 14–18 subunits, forming a protected environment for protein folding (Zhang et al. 2010; Horwich et al. 2006). Three domains of the subunit architecture, the equatorial, intermediate, and apical domains, are preserved in all chaperones (Ditzel et al. 1998). HSP60 has been suggested to aid in folding and maintaining the conformation of 15–30% of all cellular proteins (Ranford et al. 2000). As a typical HSP, HSP60 has been shown to play important roles in cellular processes, such as development, reproduction, thermoprotection, toxic-stress response, and immune defense (Kozlova et al. 1997; Timakov and Zhang 2001). HSP60 genes have been studied in some aquatic invertebrates, including sea anemones (Anemonia viridis) (Choresh et al. 2001; Choresh et al. 2004), white shrimp (Litopenaeus vannamei) (Zhou et al. 2010), crabs (Portunus trituberculatus) (Xu and Qin 2012), and the mud crab (Scylla paramamosain) (Yang et al. 2013). However, the stress response in mollusks has been relatively poorly studied, except the zebra mussel (Dreissena polymorpha) (Clayton et al. 2000), golden apple snail (Pomacea canaliculata) (Xu et al. 2014), Japanese scallop (Mizuhopecten yessoensis) (Fandong et al. 2015), and Pacific oyster (Crassostrea gigas) (Zhu et al. 2016), and information about the role of HSP60 in the stress response in mollusks is therefore limited.
The Manila clam, Ruditapes philippinarum, is a commercial mollusk species native from southern Siberia to China. They are cultured along the shore, where they burrow partially or completely in the sediment. As bivalves inhabiting the intertidal zone, clams are inevitably exposed to temperature fluctuations associated with diurnal/tidal cycles and seasonal changes (Ivanina et al. 2009), as well as to dramatic changes in water salinity as a result of the ebb and flood of the tide, combined with freshwater inputs from rivers or during periods of heavy rain. These fluctuations can cause mass mortality of cultured bivalves (Carregosa et al. 2014; Matozzo et al. 2007; Kim et al. 2001b). A better understanding of the capacities of clams to adapt to temperature and salinity stresses may thus provide new insights for improving health management and disease control in aquaculture clams. Many members of the HSP family have been identified in R. philippinarum, including HSP22, HSP40, HSP70, and HSP90, and have been shown to play important roles in responding to pathogen challenge and toxic stress (Liu et al. 2015; Li et al. 2011; Li et al. 2010). However, reports of HSPs in relation to environmental adaptation in clams are lacking.
The present study aimed to clone and characterize a novel HSP60 complementary DNA (cDNA) from Manila clams (RpHSP60) and to examine its temporal and tissue-specific expression following thermal and low-salinity challenges. These results will further our understanding of the response mechanism of R. philippinarum to temperature stress and hypotonic stimulation.
Material and methods
Animals
About 200 individuals of R. philippinarum (25.5 ± 0.46 mm) were collected from a commercial farm in Dalian and transported to the laboratory. The clams were reared in 250-L flat-bottomed tanks in aerated seawater (salinity 32 ppt, temperature 22 ± 1 °C, no sand was added into tanks to avoid affecting the quality of stress), at a density of 60 animals per tank, for 7 days prior to the experiments.
Molecular cloning of RpHSP60 cDNA
We identified a 513-bp sequence in our transcriptome database of R. philippinarum (unpublished) with high similarity to the known HSP60 protein sequence. Contiguity of the clone was confirmed by a single PCR using primers (Table 1) on 5′ and 3′ ends of cDNA and sequence analysis. Gene-specific primers (Table 1) were designed based on the sequence, and nested polymerase chain reaction (PCR) was performed using a SMART™ RACE cDNA amplification kit (Clontech, CA, USA) to obtain the 3′ and 5′ ends of RpHSP60, respectively. The 5′ and 3′ RACE PCR products were gel-purified, cloned into the pMD18-T simple vector (TaKaRa, Dalian, China), and sequenced. The full-length gene was assembled using DNAman 5.2 software (Lynnon Biosoft, Quebec, Canada).
Table 1.
Primer names and sequences used in this study
| Primer name | Sequences (5′ → 3′) |
|---|---|
| Contiguity-Hsp60F | GCATCGAATTTCACCACTTGTACG |
| Contiguity-Hsp60R | CTGGAGAGTCATGTAAGGCGGGTA |
| 5′RACE-HSP60-1 | AGTATTCTTACGATTGCCACC |
| 5′RACE-HSP60-2 | TAATGACAAGTGGTTTACGGG |
| 3′RACE-HSP60-1 | CCAAATGTGAGTTTCAGGATG |
| 3′RACE-HSP60-2 | AACCACTTGTCATTATTGCTG |
| RT-HSP60F | CCAAATGTGAGTTTCAGGATG |
| RT-HSP60R | TAATGACAAGTGGTTTACGGG |
| β-actin-F | CTCCCTTGAGAAGAGCTACGA |
| β-actin-R | GATACCAGCAGATTCCATACCC |
Experimental design
To investigate the effect of temperature stress on RpHSP60 gene expression, 120 individuals of R. philippinarum were randomly divided into two groups and placed in separate tanks at −1 °C (low temperature) and 30 °C (high temperature), respectively. Gill samples from five individuals were designated as control (0 h) samples, and samples from five other randomly selected individuals from each group were collected after 3, 6, 12, 24, 48, 72, and 96 h, respectively.
To study the expression profile of RpHSP60 under low-salinity stress, 60 individuals of R. philippinarum were put into a tank containing seawater at 17 ppt salinity at 21 ± 1 °C. Five untreated clams were used as the control samples (0 h), and another five clams were randomly collected at 3, 6, 12, 24, 48, 72, and 96 h, respectively, and gill tissues were dissected and analyzed.
RNA extraction and cDNA synthesis
To determine the expression levels of RpHsp60 in different tissues in R. philippinarum, hemolymph was collected from the posterior adductor muscle sinus using a syringe fitted with a 22-G needle, and hemocytes were harvested immediately by centrifugation (3000×g for 10 min at 4 °C). The animals were then placed on ice and samples of gill, digestive tract, mantle, foot, and muscle tissue were dissected, snap-frozen in liquid nitrogen, and stored at −80 °C for further analysis.
Total RNA from hemocytes and tissues was extracted using an RNA Extraction kit (Tiangen, Beijing, China) and treated with DNase-I to remove contaminating DNA. The quality of the total RNA was detected using a nucleic acid spectrometer (Bio-Rad, Hercules, CA, USA). A sample of 1-μg purified RNA was used to synthesize cDNA using a PrimeScript™ RT reagent kit (TaKaRa, Dalian, China), according to the manufacturer’s instructions.
Bioinformatic analysis
The amino acid sequence of each HSP gene was deduced using ORF Finder in NCBI (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and analyzed using the Expasy stands for Expert Protein Analysis System (http://www.expasy.org). The deduced amino acid sequence of RpHSP60 was aligned with that of other homologous proteins using the Clustal Omega Multiple Alignment program (http://www.ebi.ac.uk/Tools/msa/clustalo/). A phylogenetic tree was constructed using MEGA 7.0 (Sudhir Kumar, Arizona State University, Tempe, AZ, USA) using the neighbor-joining method with 5000 cycles of bootstrapping. The three-dimensional (3D) protein structures of HSP60 from C. gigas, R. philippinarum, and Escherichia coli were generated using the I-TASSER online server (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) and further analyzed with PyMOL version 1.3 molecular graphics software (DeLano 2002).
Expression profile analysis of RpHSP60 by quantitative PCR
All gene-specific primers were designed using Primer premier 5.0 software (Premier Biosoft International, Palo Alto, CA) (Table 1). Real-time reverse transcription (RT)-PCR amplifications were carried out in triplicate on a 96-well rotor in a total volume of 20 μL containing 10 μL 2 × SYBR PremixEx Taq (TaKaRa), 2 μL cDNA template, 0.8 μL of 10 μmol/L of each forward and reverse primer, and 6.4 μL Milli-Q water. The ultra-purified water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). The real-time RT-PCR program was 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s, and 60 °C for 30 s (Light Cycler480 II, Roche Diagnostics, Penzberg, Germany).
Taking β-actin as the internal control, the relative expression levels of RpHSP60 were calculated using the 2-ΔΔCT method. The statistical significance of differences between groups was analyzed by one-way analysis of variance, and paired comparisons were made using Tukey-Kramer test for multiple paired comparisons, using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant at P < 0.05.
Results
Characterization of RpHSP60 cDNA
The complete sequence of RpHSP60 cDNA was 1777 bp long (GenBank accession no. KT987978), containing a 1728-bp open reading frame encoding a polypeptide of 575 amino acids, a 21-bp 5′ untranslated region (UTR), and a 49-bp 3′ UTR with a poly(A) tail (Fig. 1). The calculated molecular mass of RpHSP60 was 61.25 kDa and the predicted isoelectric point was 5.08. Searching the NCBI database and using the patmatmotifes program in EMBOSS identified a 26-amino acid presequence required for import into the mitochondria at the N terminus, typical of mitochondrial HSP60s, the ATP-binding motif, and a conserved GGM repeat at the C-terminal end (Supplementary Fig. 1).
Fig. 1.
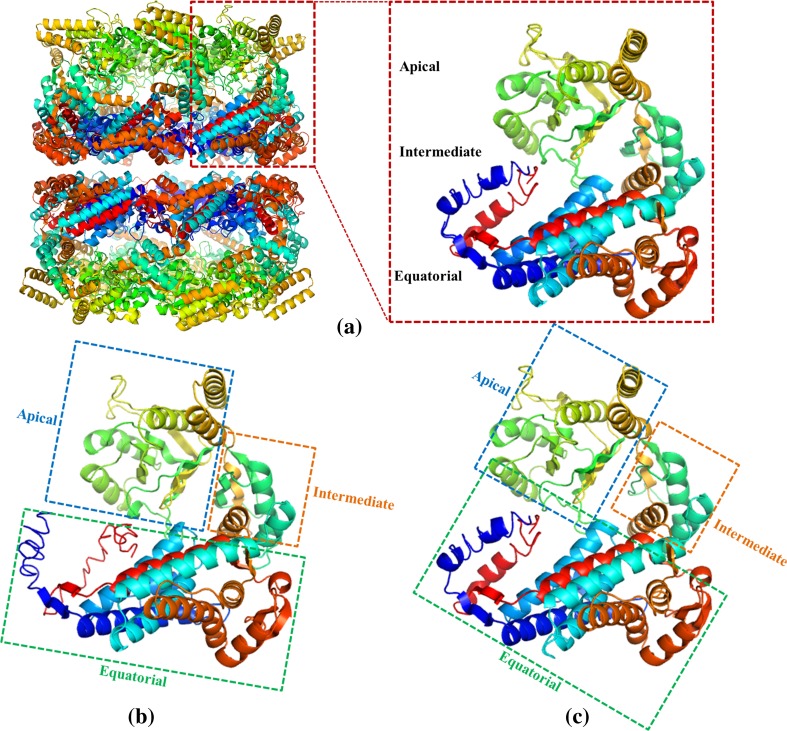
Comparison of monomeric structures of HSP60 proteins from Escherichia coli (a), Crassostrea gigas (b), and Ruditapes philippinarum (c). The oligomeric and monomeric structure of HSP60 from E. coli (PDB ID codes 4aaq and 1kp8A, respectively) are shown in a, and the three domains of the HSP60 monomers from C. gigas and R. philippinarum are boxed with green (equatorial domain), yellow (intermediate domain) and blue (apical domain)
Sequence comparisons and phylogenetic analysis of RpHSP60
The deduced amino acid sequence of RpHSP60 showed a high degree of similarity to other reported HSP60s (Supplementary Fig. 2). RpHSP60 exhibited significant sequence identity of 78.15–81.55% with HSP60 proteins from other mollusks, and 76.06 and 76.58% identity with Rattus norvegicus (CAA38564.1) and Gallus gallus (NP_001012934.1), respectively. Multiple sequence alignment analysis revealed that the functional structure of the deduced HSP60 protein, including an ATP/Mg2+ binding site, hinge regions, and ring oligomerization interface, was also conserved in RpHSP60.
The 3D model of RpHSP60 was modeled employing I-TASSER online platform, using a number of sequences which share 48.5–62.6% sequence identity with the query sequence as templates, including the HSP60 sequences of C. gigas and E. coli. The C-score (confidence score) was used for estimating the quality of the predicted models by I-TASSER (Zhang 2008). The 3D model of RpHSP60 was predicted adopting the same approach. Molecular graphic software (PyMOL) was applied for decorating the retrieved models of HSP60 from C. gigas, R. philippinarum, and E. coli (Fig. 1). The results indicated the three crucial domains including the apical, equatorial, and intermediate of HSP60 were nearly in the same positions, suggesting that they may form similar crystal structure and perform comparable functions (Nisemblat et al. 2015).
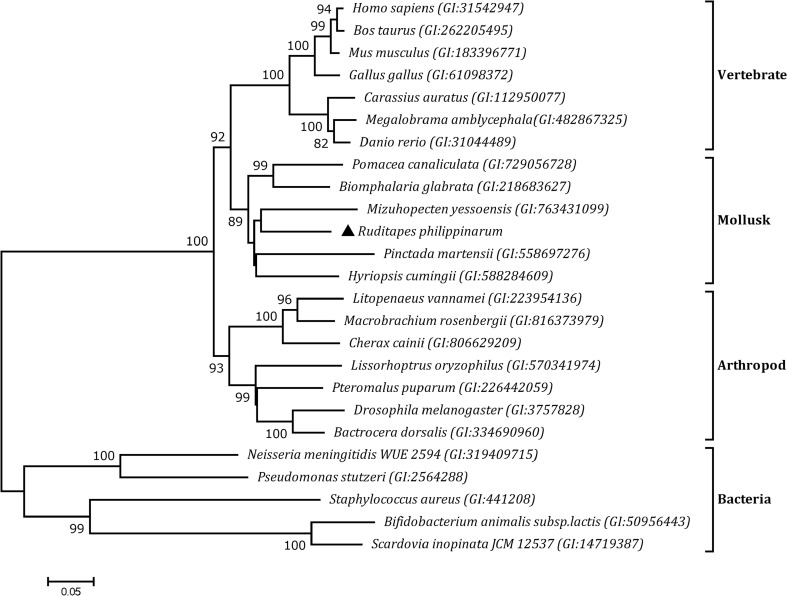
Based on the amino acid sequences of HSPs from other organisms, we generated a phylogenetic tree using the neighbor-joining method and the maximum-likelihood method, and both phylogeny methods produced the same results, but only one representative tree is shown in Fig. 2. HSP60 family members were divided into two sub-groups, one including vertebrates, mollusks and arthropods, and the other comprising bacteria. As expected, RpHSP60 was most closely related to HSP60 sequences from other mollusks.
Fig. 2.
Neighbor-joining phylogenetic tree of RpHSP60 protein sequences and other selected species. The RpHSP60 is marked by the black triangle. Aligned sequences were bootstrapped 5000 times. The GenBank accession numbers for amino acid sequences of all the HSP60 are shown next to each species
Tissue-specific expression of RpHSP60 mRNA
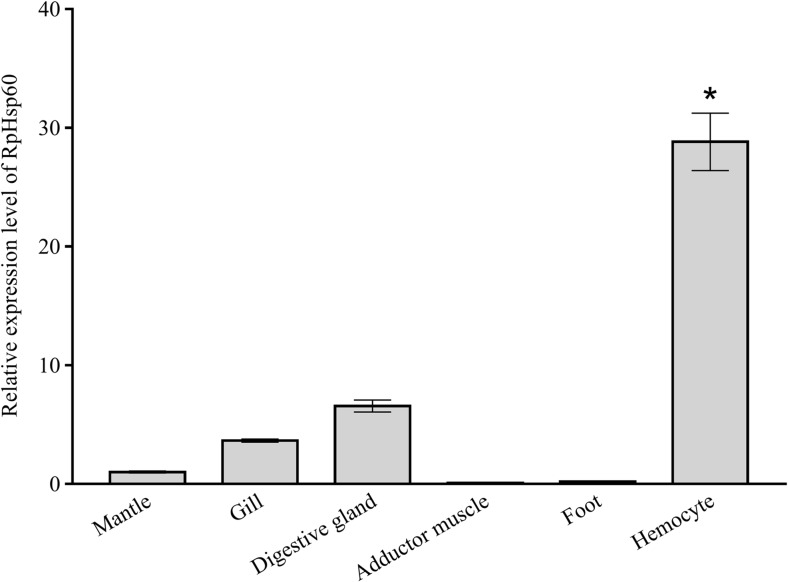
The results of real-time RT-PCR revealed that RpHSP60 was expressed in all the tested tissues, including the mantle, gill, digestive gland, adductor muscle, foot, and hemocyte. The highest expression levels were detected in hemocyte (P < 0.05) (Fig. 3).
Fig. 3.
The tissue distribution of RpHSP60 mRNA in mantle, gill, digestive gland, adductor muscle, foot, and hemocyte by qRT-PCR using β-actin as a reference gene. The values are shown as mean ± standard deviation (n = 4; *significantly different from mantle, p < 0.05)
Expression analysis of RpHSP60 mRNA under temperature stress
Under high-temperature stress, RpHSP60 mRNA expression dropped slightly at 3 h, increased to reach a peak at 6 h (P < 0.05), and was then downregulated gradually to normal levels (Fig. 4a). In contrast, only slight fluctuations in RpHSP60 mRNA levels were observed under low-temperature conditions (Fig. 4b).
Fig. 4.
Temporal expression profile of RpHSP60 in gills under high-temperature stress (a) and low-temperature conditions (b). Relative fold change in expression was compared to that of 0 h at different time points. Vertical bars represent the mean ± S.D. (n = 4) and significant differences are indicated with lowercase letters at P < 0.05
Expression analysis of RpHSP60 mRNA under low-salinity stress
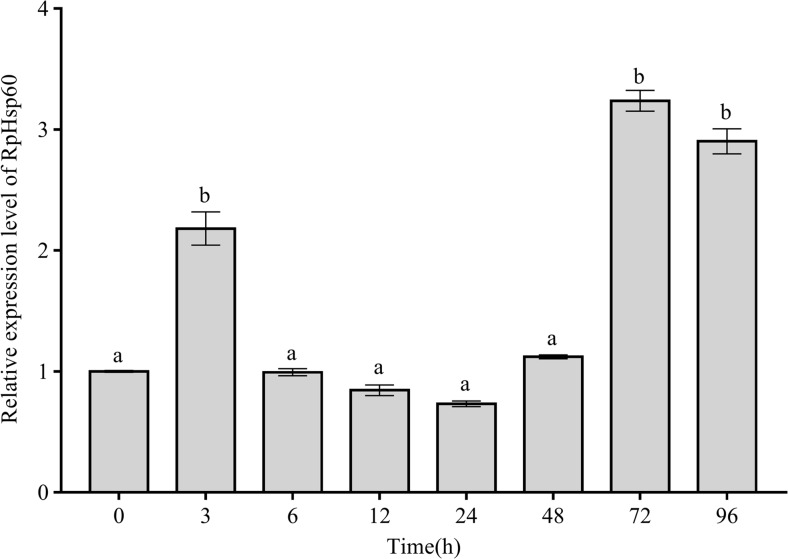
Under conditions of low salinity, RpHSP60 mRNA expression levels increased to peaks at 3, 72, and 96 h of 2.18-, 3.23-, and 2.90-fold that in the pretreatment group, respectively, and fluctuated within a narrow range at other time points (Fig. 5) (P < 0.05).
Fig. 5.
Temporal expression profile of RpHSP60 in gills under low-salinity stress. Relative fold change in expression was compared to that of 0 h at different time points. Vertical bars represent the mean ± S.D. (n = 4) and significant differences are indicated with lowercase letters at P < 0.05
Discussion
In this study, we cloned the full-length cDNA of HSP60 from R. philippinarum. Multiple-sequence-alignment analysis showed that the conserved sequences and characteristic motifs, including HSP60 family signatures, ATP-binding sites, and GGM repeats at the C-terminal end, were highly conserved in RpHSP60 (Brocchieri and Karlin 2000; Tsugeki et al. 1992). The typical structural and functional domains of Hsp60 proteins, including the apical, equatorial, and intermediate domains, were also present in RpHSP60, as demonstrated by 3D-structure comparisons (Pereira et al. 2012). Construction of a phylogenetic tree showed that RpHSP60 clustered with sequences from other mollusks. Molecular-feature analysis, multiple sequence alignment, 3D-structure comparisons, and phylogenetic analysis confirmed RpHSP60 as a new member of the HSP60 family. The high degree of similarity in the crucial domains suggests that RpHSP60 might share similar regulatory functions with other known HSP60s.
The distribution RpHSP60 gene expression could provide useful information for analyzing its biological function in mollusks. We detected constitutive RpHSP60 mRNA expression in all the tested tissues. Constitutive expression of HSP60 has also been reported in several taxa of marine invertebrates, including the mud crab S. paramamosain and P. trituberculatus (Yang et al. 2013; Xu and Qin 2012), white shrimp L. vannamei (Zhou et al. 2010), sea anemones A. viridis (Choresh et al. 2001), Mediterranean sponges Tetilla sp (Choresh et al. 2004), and scallops M. yessoensis (Fandong et al. 2015). Particularly high expression levels of RpHSP60 were found in hemocytes, which play an important role in the response to bacterial challenge and environmental stress. This was in accord with the results in S. paramamosain (Yang et al. 2013), indicating that RpHSP60 was required to maintain cell homeostasis in R. philippinarum. In addition, some studies show that hemocyte of mollusca may be one of the important tissue induces new synthesis of the heat shock protein to cope with stress (Cellura et al. 2006; Gao et al. 2007; Farcy et al. 2007; Wang et al. 2009; Gao et al. 2008).
HSPs are known to be involved in preventing protein denaturation and in processing proteins denatured as a result of oxidative damage in response to thermal stress (Kregel 2002). Reactive oxygen species (ROS) in the mitochondria of the Antarctic bivalve Laternula elliptica were significantly increased under heat stress (Heise et al. 2003), while ROS production in hemocytes was increased following acute temperature elevation in eastern oysters, Crassostrea virginica, and in Chlamys farreri (Hégaret et al. 2003; Chen et al. 2007). In the intertidal mud clam Mya arenaria, mitochondrial function was reported to be temperature dependent and to correlate significantly with the production of ROS (Abele et al. 2002). High-temperature stress and the concomitant increase in ROS production may lead to an accumulation of denatured proteins in the cells, thus triggering HSP expression. HSP60 is thought to play a key role in the resistance to oxidative stress (Lee et al. 2008). A Hsp60 gene was induced by higher temperatures in the mud crab S. paramamosain (Yang et al. 2013). In our study, RpHSP60 expression in the gill of R. philippinarum were increased after 6 hours of high temperature stress. Similarly, elevated HSP60 expression in the gills to increase their resistance to heat stress have been reported in rainbow trout O. mykiss and mussel Mytilus edulis (Shi et al. 2015; Sanders et al. 1992). This suggests that RpHSP60 may be involved in the adaptive response to heat stress by maintaining correct protein folding (Oksala et al. 2014). In contrast, cold treatment had little effect on RpHSP60 expression in the current study, in accordance with a previous study of HSP60 expression levels in the gills of P. canaliculata (Xu et al. 2014). This may be because low temperature reduced ROS-production rates in R. philippinarum, in line with the general perception of the effects of body temperature on biochemical and enzymatically catalyzed cellular reactions (Abele and Puntarulo 2004). However, low temperature resulted in decreased expression of one HSP60 family member in C. gigas and increased expression of another, with maximal effects at 7 days (Zhu et al. 2016). The apparent differences in the responses of HSP60 to low-temperature stress may be associated with their different locations in cells or differences between specific organs (Lee et al. 2008; Eder et al. 2009).
Salinity stress is considered to affect the folding and transformation of proteins, and HSPs are thought to play important roles in the refolding of stress-denatured proteins in organisms subjected to osmotic stress (Smurov et al. 2007; Xu and Qin 2012). In bivalves, the gills are in direct contact with the external environment and are thus assumed to be sensitive to environmental changes (Freas and Grollman 1980; Powell et al. 1982; Raftopoulou and Dimitriadis 2011; Gliński and Jarosz 1996). In our study, RpHSP60 expression levels in R. philippinarum gills peaked at 3, 72, and 96 h during low-salinity stress (17 ppt), compared to the HSP60 gene in P. trituberculatus, which demonstrated a similar expression pattern after low-salinity challenge (Xu and Qin 2012). Notably, the increases in HSP60 expression in both R. philippinarum and P. trituberculatus were not continuous, and the changes may reflect changes in osmolarity of the body fluid in mollusks. Bivalves are often subject to frequent and rapid changes in the osmolarity of their external medium, and utilize their shell-closing mechanism to help them withstand sudden osmotic stress, and free intracellular amino acids to regulate the osmolarity of the intracellular medium (Powell et al. 1982; Hoyaux et al. 1976; Carregosa et al. 2014), resulting in discontinuous osmotic-pressure changes in the body fluid. In the gastropod Littorina littorea, the osmolarities of both the perivisceral fluid and the blood decreased rapidly from 0 to 5 h and 136–150 h after direct transfer from pure sea water to 40% sea water (Hoyaux et al. 1976). These results suggest that HSP60 may be involved in adaptive responses to low salinity and indicate a possible positive relationship between RpHSP60 induction and rapid changes in salinity. Further studies are needed to confirm this speculation.
Conclusion
In this study, we identified a HSP60 gene in the Manila clam R. philippinarum, with constitutive expression in various tissues. The dramatic upregulation of RpHSP60 expression following exposure to high temperature and low salinity suggests that it participates in essential survival responses to environmental stresses in mollusks.
Electronic supplementary material
The cDNA sequence and the deduced amino acid sequence of RpHSP60. The start codon ATG and the termination codon are boxed. A 26-amino acid presequence at the N terminus required for import into the mitochondria is underlined. Characteristic mitochondrial HSP60 signature motif is double underlined, the ATP-binding motif is marked with wavy underlines, and a typical GGM repeat motif at the C terminus is indicated in light gray. (GIF 260 kb)
Multiple alignment of RpHSP60 with other known HSP60 members. The species and GenBank accession numbers used for the multiple alignments were as follows: Biomphalaria glabrata(P_001298236.1), Aplysia californica (XP_005097387.1), Pomacea canaliculata (AIZ03411.1), Crassostrea gigas (XP_011456445.1 and EKC31862.1), Pinctada martensii (AHA85006.1), Mizuhopecten yessoensis (AJQ21379.1), Hyriopsis cumingii (AHK22785.1), G. gallus (NP_001012934.1), R. norvegicus (CAA38564.1). Identical and similar residues are shaded with black and gray, respectively. Deletions are indicated by dashes. ATP/ADP binding segments are boxed with black, Mg2+ binding segment is boxed with gray. Substrate-binding sites is marked with double-lined. Hinge regions site and polypeptide binding site are marked with black diamond and round, respectively. (GIF 553 kb)
Acknowledgments
This research is supported by the National High Technology Research and Development Program of China (863 Program No. 2012AA10A410-2) and the Research Foundation of Education Bureau of Liaoning Province, China (No. L2014276)
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12192-017-0796-7) contains supplementary material, which is available to authorized users.
References
- Abele D, Puntarulo S. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A:Mol Integr Physiol. 2004;138(4):405–415. doi: 10.1016/j.cbpb.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Abele D, Heise K, Pörtner H-O, Puntarulo S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J Exp Biol. 2002;205(13):1831–1841. doi: 10.1242/jeb.205.13.1831. [DOI] [PubMed] [Google Scholar]
- Bensaude O. Protein denaturation during heat shock and related stress. In: Bensaude O, editor. Stress proteins. Berlin Heidelberg: Springer; 1990. pp. 89–99. [Google Scholar]
- Brocchieri L, Karlin S. Conservation among HSP60 sequences in relation to structure, function, and evolution. Protein Sci. 2000;9(03):476–486. doi: 10.1110/ps.9.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carregosa V, Figueira E, Gil AM, Pereira S, Pinto J, Soares AM, Freitas R. Tolerance of Venerupis philippinarum to salinity: osmotic and metabolic aspects. Comp Biochem Physiol A:Mol Integr Physiol. 2014;171:36–43. doi: 10.1016/j.cbpa.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Cellura C, Toubiana M, Parrinello N, Roch P. HSP70 gene expression in Mytilus galloprovincialis hemocytes is triggered by moderate heat shock and Vibrio anguillarum, but not by V-splendidus or Micrococcus lysodeikticus. Dev Comp Immunol. 2006;30(11):984–997. doi: 10.1016/j.dci.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Chen M, Yang H, Delaporte M, Zhao S. Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture. 2007;271(1):479–487. doi: 10.1016/j.aquaculture.2007.04.051. [DOI] [Google Scholar]
- Choresh O, Ron E, Loya Y. The 60-kDa heat shock protein (HSP60) of the sea anemone Anemonia viridis: a potential early warning system for environmental changes. Mar Biotechnol. 2001;3(5):501–508. doi: 10.1007/s10126-001-0007-4. [DOI] [PubMed] [Google Scholar]
- Choresh O, Loya Y, Müller WE, Wiedenmann J, Azem A. The mitochondrial 60-kDa heat shock protein in marine invertebrates: biochemical purification and molecular characterization. Cell Stress Chaperones. 2004;9(1):38–48. doi: 10.1379/469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton ME, Steinmann R, Fent K. Different expression patterns of heat shock proteins hsp 60 and hsp 70 in zebra mussels (Dreissena polymorpha) exposed to copper and tributyltin. Aquat Toxicol. 2000;47(3):213–226. doi: 10.1016/S0166-445X(99)00022-3. [DOI] [Google Scholar]
- DeLano WL. The PyMOL molecular graphics system. San Carlos: DeLano Scientific LLC; 2002. [Google Scholar]
- Ditzel L, Löwe J, Stock D, Stetter K-O, Huber H, Huber R, Steinbacher S. Crystal structure of the thermosome, the archaeal chaperonin and homolog of CCT. Cell. 1998;93(1):125–138. doi: 10.1016/S0092-8674(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Eder KJ, Leutenegger CM, Köhler H-R, Werner I. Effects of neurotoxic insecticides on heat-shock proteins and cytokine transcription in Chinook salmon (Oncorhynchus tshawytscha) Ecotox and Environ Safe. 2009;72(1):182–190. doi: 10.1016/j.ecoenv.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Fandong K, Jingyun S, Xiangbo B, Xianggang G, Chongbo H, Weidong L. Gene cloning and expression characterization of gene for a heat shock protein 60 from Japanese scallop Mizuhopecten yessoensis. Biotechnol Bull. 2015;31(10):157–164. [Google Scholar]
- Farcy E, Serpentini A, Fievet B, Lebel JM. Identification of cDNAs encoding HSP70 and HSP90 in the abalone Haliotis tuberculata: transcriptional induction in response to thermal stress in hemocyte primary culture. Comp Biochem Physiol B-Biochem Mol Biol. 2007;146(4):540–550. doi: 10.1016/j.cbpb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Freas W, Grollman S. Ionic and osmotic influence on prostaglandin release from the gill tissue of a marine bivalve, Modiolus demissus. J Exp Biol. 1980;84(1):169–185. doi: 10.1242/jeb.84.1.169. [DOI] [PubMed] [Google Scholar]
- Gao Q, Song LS, Ni DJ, Wu LT, Zhang H, Chang YQ. cDNA cloning and mRNA expression of heat shock protein 90 gene in the haemocytes of Zhikong scallop Chlamys farreri. Comp Biochem Physiol B-Biochem Mol Biol. 2007;147(4):704–715. doi: 10.1016/j.cbpb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhao JM, Song LS, Qiu LM, Yu YD, Zhang H, Ni DJ. Molecular cloning, characterization and expression of heat shock protein 90 gene in the haemocytes of bay scallop Argopecten irradians. Fish Shellfish Immun. 2008;24(4):379–385. doi: 10.1016/j.fsi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Gliński Z, Jarosz J. Molluscan immune defenses. Arch Immunol Ther Ex. 1996;45(2–3):149–155. [PubMed] [Google Scholar]
- Hégaret H, Wikfors GH, Soudant P. Flow cytometric analysis of haemocytes from eastern oysters, Crassostrea virginica, subjected to a sudden temperature elevation: II. Haemocyte functions: aggregation, viability, phagocytosis, and respiratory burst. J Exp Mar Biol Ecol. 2003;293(2):249–265. doi: 10.1016/S0022-0981(03)00235-1. [DOI] [Google Scholar]
- Heise K, Puntarulo S, Pörtner H-O, Abele D. Production of reactive oxygen species by isolated mitochondria of the Antarctic bivalve Laternulaelliptica (King and Broderip) under heat stress. Comp Biochem Physiol Part C: Toxicol Pharmacol. 2003;134(1):79–90. doi: 10.1016/S1096-4959(02)00231-2. [DOI] [PubMed] [Google Scholar]
- Horwich AL, Farr GW, Fenton WA. GroEL-GroES-mediated protein folding. Chem Rev. 2006;106(5):1917–1930. doi: 10.1021/cr040435v. [DOI] [PubMed] [Google Scholar]
- Hoyaux J, Gilles R, Jeuniaux C. Osmoregulation in molluscs of the intertidal zone. Comp Biochem Physiol A:Mol Integr Physiol. 1976;53(4):361–365. doi: 10.1016/S0300-9629(76)80157-0. [DOI] [PubMed] [Google Scholar]
- Ivanina A, Taylor C, Sokolova I. Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin) Aquat Toxicol. 2009;91(3):245–254. doi: 10.1016/j.aquatox.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Kim W, Huh H, Huh S-H, Lee T. Effects of salinity on endogenous rhythm of the Manila clam, Ruditapes philippinarum (Bivalvia: Veneridae) Mar Biol. 2001;138(1):157–162. doi: 10.1007/s002270000430. [DOI] [PubMed] [Google Scholar]
- Kozlova T, Perezgasga L, Reynaud E, Zurita M. The Drosophila melanogaster homologue of the hsp60 gene is encoded by the essential locus l (1) 10Ac and is differentially expressed during fly development. Dev Genes Evol. 1997;207(4):253–263. doi: 10.1007/s004270050113. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. Journal of Appl Physiol. 2002;92(5):2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Lee YH, Lee JC, Moon HJ, Jung JE, Sharma M, Park BH, Yi HK, Jhee EC. Differential effect of oxidative stress on the apoptosis of early and late passage human diploid fibroblasts: implication of heat shock protein 60. Cell Biochem Func. 2008;26(4):502–508. doi: 10.1002/cbf.1473. [DOI] [PubMed] [Google Scholar]
- Lewis S, Handy RD, Cordi B, Billinghurst Z, Depledge MH. Stress proteins (HSP’s): methods of detection and their use as an environmental biomarker. Ecotoxicology. 1999;8(5):351–368. doi: 10.1023/A:1008982421299. [DOI] [Google Scholar]
- Li C, Wang L, Ning X, Chen A, Zhang L, Qin S, Wu H, Zhao J. Identification of two small heat shock proteins with different response profile to cadmium and pathogen stresses in Venerupis philippinarum. Cell Stress Chaperones. 2010;15(6):897–904. doi: 10.1007/s12192-010-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li L, Liu F, Ning X, Chen A, Zhang L, Wu H, Zhao J. Alternation of Venerupis philippinarum Hsp40 gene expression in response to pathogen challenge and heavy metal exposure. Fish Shellfish Immun. 2011;30(1):447–450. doi: 10.1016/j.fsi.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Liu T, Pan L, Cai Y, Miao J. Molecular cloning and sequence analysis of heat shock proteins 70 (HSP70) and 90 (HSP90) and their expression analysis when exposed to benzo (a) pyrene in the clam Ruditapes philippinarum. Gene. 2015;555(2):108–118. doi: 10.1016/j.gene.2014.10.051. [DOI] [PubMed] [Google Scholar]
- Matozzo V, Monari M, Foschi J, Serrazanetti GP, Cattani O, Marin MG. Effects of salinity on the clam Chamelea gallina. Part I: alterations in immune responses. Mar Biol. 2007;151(3):1051–1058. doi: 10.1007/s00227-006-0543-6. [DOI] [Google Scholar]
- Nisemblat S, Yaniv O, Parnas A, Frolow F, Azem A. Crystal structure of the human mitochondrial chaperonin symmetrical football complex. P Natl Acad Sci. 2015;112(19):6044–6049. doi: 10.1073/pnas.1411718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksala NKJ, Ekmekci FG, Ozsoy E, Kirankaya S, Kokkola T, Emecen G, Lappalainen J, Kaarniranta K, Atalay M. Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Bio. 2014;3:25–28. doi: 10.1016/j.redox.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JH, Ralston CY, Douglas NR, Kumar R, Lopez T, McAndrew RP, Knee KM, King JA, Frydman J, Adams PD. Mechanism of nucleotide sensing in group II chaperonins. The EMBO J. 2012;31(3):731–740. doi: 10.1038/emboj.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EN, Kasschau M, Chen E, Koenig M, Pecon J. Changes in the free amino acid pool during environmental stress in the gill tissue of the oyster, Crassostrea virginica. Comp Biochem Physiol A Physiol. 1982;71(4):591–598. doi: 10.1016/0300-9629(82)90208-0. [DOI] [Google Scholar]
- Raftopoulou E, Dimitriadis V. Comparative study of the accumulation and detoxification of Cu (essential metal) and Hg (nonessential metal) in the digestive gland and gills of mussels Mytilus galloprovincialis, using analytical and histochemical techniques. Chemosphere. 2011;83(8):1155–1165. doi: 10.1016/j.chemosphere.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Ranford J, Henderson B. Chaperonins in disease: mechanisms, models, and treatments. J Clin Pathol. 2002;55(4):209. doi: 10.1136/mp.55.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranford JC, Coates AR, Henderson B. Chaperonins are cell-signalling proteins: the unfolding biology of molecular chaperones. Expert Rev Mol Med. 2000;2(08):1–17. doi: 10.1017/S1462399400002015. [DOI] [PubMed] [Google Scholar]
- Rhee J-S, Raisuddin S, Lee K-W, Seo JS, Ki J-S, Kim I-C, Park HG, Lee J-S. Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp Biochem Physiol C: Toxicol Pharmacol. 2009;149(1):104–112. doi: 10.1016/j.cbpc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Sanders BM, Pascoe VM, Nakagawa PA, Martin LS. Persistence of the heat-shock response over time in a common Mytilus mussel. Mol Mar Biol Biotechnol. 1992;1(2):147–154. [Google Scholar]
- Shi HN, Liu Z, Zhang JP, Kang YJ, Wang JF, Huang JQ, Wang WM. Effect of heat stress on heat-shock protein (Hsp60) mRNA expression in rainbow trout Oncorhynchus mykiss. Gene Mol Res. 2015;14(2):5280–5286. doi: 10.4238/2015.May.18.20. [DOI] [PubMed] [Google Scholar]
- Smurov A, Podlipaeva YI, Goodkov A. Heat shock protein of the Hsp70 family in the euryhaline cilate Paramecium nephridiatum and its role in adaptation to salinity changes. Cell Tissue Biol. 2007;1(3):244–247. doi: 10.1134/S1990519X07030066. [DOI] [Google Scholar]
- Timakov B, Zhang P. The hsp60B gene of Drosophila melanogaster is essential for the spermatid individualization process. Cell Stress and Chaperones. 2001;6(1):71–77. doi: 10.1379/1466-1268(2001)006<0071:THGODM>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugeki R, Mori H, Nishimura M. Purification, cDNA cloning and Northern-blot analysis of mitochondrial chaperonin 60 from pumpkin cotyledons. Eur J Biochem. 1992;209(1):453–458. doi: 10.1111/j.1432-1033.1992.tb17309.x. [DOI] [PubMed] [Google Scholar]
- Wang ZL, Wu ZH, Jian JC, Lu YS. Cloning and expression of heat shock protein 70 gene in the haemocytes of pearl oyster (Pinctada fucata, Gould 1850) responding to bacterial challenge. Fish Shellfish Immun. 2009;26(4):639–645. doi: 10.1016/j.fsi.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Xu Q, Qin Y. Molecular cloning of heat shock protein 60 (PtHSP60) from Portunus trituberculatus and its expression response to salinity stress. Cell Stress Chaperones. 2012;17(5):589–601. doi: 10.1007/s12192-012-0334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zheng G, Dong S, Liu G, Yu X. Molecular cloning, characterization and expression analysis of HSP60, HSP70 and HSP90 in the golden apple snail, Pomacea canaliculata. Fish Shellfish Immun. 2014;41(2):643–653. doi: 10.1016/j.fsi.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ye H, Huang H, Li S, Zeng X, Gong J, Huang X. Characterization and expression of SpHsp60 in hemocytes after challenge to bacterial, osmotic and thermal stress from the mud crab Scylla paramamosain. Fish Shellfish Immun. 2013;35(4):1185–1191. doi: 10.1016/j.fsi.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC bioinformatics. 2008;9(1):1. doi: 10.1186/1471-2105-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Baker ML, Schröder GF, Douglas NR, Reissmann S, Jakana J, Dougherty M, Fu CJ, Levitt M, Ludtke SJ. Mechanism of folding chamber closure in a group II chaperonin. Nature. 2010;463(7279):379–383. doi: 10.1038/nature08701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang W-N, He W-Y, Zheng Y, Wang L, Xin Y, Liu Y, Wang A-L. Expression of HSP60 and HSP70 in white shrimp, Litopenaeus vannamei in response to bacterial challenge. J Invertebr Pathol. 2010;103(3):170–178. doi: 10.1016/j.jip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhang L, Li L, Que H, Zhang G. Expression characterization of stress genes under high and low temperature stresses in the Pacific oyster, Crassostrea gigas. Mar Biotechnol. 2016;18(2):1–13. doi: 10.1007/s10126-015-9678-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The cDNA sequence and the deduced amino acid sequence of RpHSP60. The start codon ATG and the termination codon are boxed. A 26-amino acid presequence at the N terminus required for import into the mitochondria is underlined. Characteristic mitochondrial HSP60 signature motif is double underlined, the ATP-binding motif is marked with wavy underlines, and a typical GGM repeat motif at the C terminus is indicated in light gray. (GIF 260 kb)
Multiple alignment of RpHSP60 with other known HSP60 members. The species and GenBank accession numbers used for the multiple alignments were as follows: Biomphalaria glabrata(P_001298236.1), Aplysia californica (XP_005097387.1), Pomacea canaliculata (AIZ03411.1), Crassostrea gigas (XP_011456445.1 and EKC31862.1), Pinctada martensii (AHA85006.1), Mizuhopecten yessoensis (AJQ21379.1), Hyriopsis cumingii (AHK22785.1), G. gallus (NP_001012934.1), R. norvegicus (CAA38564.1). Identical and similar residues are shaded with black and gray, respectively. Deletions are indicated by dashes. ATP/ADP binding segments are boxed with black, Mg2+ binding segment is boxed with gray. Substrate-binding sites is marked with double-lined. Hinge regions site and polypeptide binding site are marked with black diamond and round, respectively. (GIF 553 kb)