Abstract
Heat shock protein (HSP) 70 is an abundant cytosolic chaperone protein that is deficient in insulin-sensitive tissues in diabetes and unhealthy aging, and is considered a longevity target. It is also protective in neurological disease models. Using HSP70 purified from alfalfa and administered as an intranasal solution, we tested in whether the administration of Hsp70 to diet-induced diabetic mice would improve insulin sensitivity. Both the 10 and 40 μg given three times per week for 26 days significantly improved the response to insulin. The HSP70 was found to pass into the olfactory bulbs within 4–6 hours of a single dose. These results suggest that a relatively inexpensive, plentiful source of HSP70 administered in a simple, non-invasive manner, has therapeutic potential in diabetes.
Keywords: HSP70, Insulin resistance, Obesity, Intranasal administration, Brain
Introduction
Heat shock protein (HSP) 70, a major inducible molecular chaperone, plays a critical role in providing cellular protection against stressful metabolic events in all living organisms (Young 2010). HSP70 and its transcription factor is neuroprotective and deficient in Alzheimer’s disease (AD)(Bobkova et al. 2015; Goetzl et al. 2015), consistent with its role in preventing protein aggregation which is a common pathology to both type 2 diabetes (T2DM) and AD (Akerfelt et al. 2010; Ortega et al. 2012). Intranasal administration is known to allow proteins to enter the brain and potentially the vasculature with systemic distribution (Lochhead and Thorne 2012; Fortuna et al. 2014). This route of administration of various isoforms of HSP70 has been beneficial in mouse models of AD and age-related cognitive impairment (Bobkova et al. 2014, 2015). Recent evidence suggests that even if HSP70 remains extracellular, it can still protect neurons in the brain against the toxic effects of amyloid-ß42, (De Mena et al. 2017).
T2DM is a chronic disease characterized by high circulating glucose and insulin resistance, and has been shown to cause, and be caused by, insufficient intracellular HSP70 levels in animal models and humans (Kavanagh et al. 2012; Hooper et al. 2014; Chichester et al. 2015; Silverstein et al. 2014). Restoration of peripheral tissue HSP70 levels by genetic modulation or pharmacological induction improves glycemic control (Kavanagh et al. 2011, Chung et al. 2008); however, the mechanisms are not fully understood. There is ample evidence that upregulating peripheral tissue HSP70 levels will improve insulin sensitivity; however, the role played by augmenting CNS HSP70 on peripheral tissue insulin sensitivity is unknown, although the brain-body connection of insulin action is well accepted (Ott et al. 2012; Perez et al. 2014). Here, we aimed to demonstrate that (i) intranasally administered alfalfa-derived HSP70 (aHSP70) is taken up specifically to the CNS and (ii) intranasal aHSP70 was able to improve peripheral insulin sensitivity in diet-induced insulin-resistant mice.
Methods
Fluorescent protein labeling
Lyophilized alfalfa-derived inducible form of HSP70 (aHSP70; Alfa Biogene International, The Netherlands, http://www.alfabiogene.de) or serum bovine albumin was reconstituted to 1 mg/ml in deionized water and then reacted with Alexa fluor 546 succinimidyl ester for 1 h at room temperature and then overnight at 4 °C (Invitrogen/Molecular Probes). Separation of labeled protein from unbound fluorophore was achieved by EMD Millipore molecular weight exclusion membrane filters in centrifuge tubes. The labeled protein and free fluorophore solution was added to the top chamber of the filter tube and the tube spun at 3220 g until all but about 50 μl of the solution passed through the filter (the retentate). To remove more of the unreacted fluorophore, 2 ml of buffered saline was then added to the retentate and centrifuged again under the same conditions. This was repeated one more time. The protein concentration in the retentate was measured in a small aliquot using a bicinchoninic acid assay and the final protein concentration adjusted by addition of buffered saline to 4 μg/μl, (57 μM HSP70 and 60 μM albumin).
Administration of Alexa-546-labeled protein to mice and tissue collection
Fourteen 8-week-old C57BL/6 mice were fed a high-fat diet for 1 month to induce obesity and insulin resistance prior to HSP70 administration (calories from fat were 38%, from carbohydrate, 48%, and from protein, 16%; see Silverstein et al. 2014). Mice were randomly assigned to receive 40 μg of intranasal Alexa-546-labeled aHSP70 (n = 5), Alexa-546-labeled albumin (n = 4), or saline (n = 5). Intranasal administration was performed under isoflurane anesthesia with 10 μl of the appropriate solution instilled into one nostril with the mouse placed supine for 1–2 min. Four to 6 hours post-dose, mice were euthanized with rapid brain collection (with olfactory bulbs), the nose, liver, and skeletal muscle (gastrocnemius and quadriceps) from one hind limb. A portion of each tissue was either immersion fixed in 10% neutral buffered formalin or quick-frozen in a dry ice isopentane bath. In the case of the brain, it was bisected along the sagittal midline and so that one half could be fixed and the other frozen.
Tissue sample analyses
All the fixed tissues except the noses were put on a shaker overnight at room temperature and then embedded in optimal cutting temperature compound (OCT) and tissue blocks were stored at – 20 °C until sectioning at 10 or 20 μm for microscopy. Mounted sections were stained with 5% DAPI solution. The noses were rinsed in 70% ethanol and stored at 4 °C until dissection. The nasal epithelium was isolated as described by Dunston et al. (2013) and embedded in OCT for epifluorescence microscopy as described above. Epifluorescence microscopy used an Olympus BX51 microscope with PlanFluor objectives and a rhodamine filter set. Images were captured using an Optronics MicroFire digital camera. To detect specific Alexa fluor 546 fluorescence, first tissue samples from saline-treated mice were examined and the microscope photomultiplier was adjusted so that the nonspecific background fluorescence from these samples was just barely visible in the image. Then samples from mice treated with the fluorescently tagged protein were examined and images were collected only from fields that showed fluorescence well above the background. Three or more fields were examined in specimens from at least three mice. Frozen tissues were assayed for the presence of fluorescent protein by homogenizing the in RIPA buffer (Invitrogen cat. #FNN0011, 100 μl/mg tissue), centrifuged at 10,000 RPM for 10 min and the supernatant assayed for fluorescence in a microtiter plate.
Insulin sensitivity assessments
Twenty-four 8-week-old C57BL/6 mice were fed the high-fat diet for 4 weeks prior to initiation of study. aHSP70 or bovine serum albumin were dissolved in HEPES buffer at 4 μg/μl so that 0, 10, 20, or 40 μg of the protein or control (n = 6/group) was instilled into one nostril of each mouse under brief anesthesia as described above. Mice were treated for 4 weeks with intranasal administrations every 72 h. Fasting intraperitoneal glucose and insulin tolerance tests were performed on days 25 and 32, respectively, using standard methods as described previously (Silverstein et al. 2014). Procedures were performed 24 h after the prior aHSP70 or saline dose. Area under the curves (AUC) was calculated using the trapezoidal method.
Data analysis
One way analysis of variance was used to assess for overall group differences and post-hoc testing was conducted using Tukey’s honestly significant difference (Statistica 64, StatSoft Inc. Tulsa OK). Associations between dose and AUC were assessed by non-parametric methods. Significance was set to be α < 0.05 with trends defined as α ≤ 0.10.
Results
Uptake of fluorescently labeled aHSP70
Five tissues (nasal epithelium, olfactory bulb, brain, gastrocnemius, and liver) were examined. Positive fluorescence was found only in the nasal epithelium and olfactory bulb. Fluorescent aHSP70 well above background was observed in many parts of the nasal epithelium. Its distribution was variable, with some portions of the epithelium showing high levels of fluorescence as illustrated in Fig. 1b and c, whereas other areas showed lower levels or none (Fig. 1b to the right of the arrows), which was likely a result of the variable distribution of the administered solution. The fluorescent aHSP70 was observed on the ciliated, luminal surfaces of the cells and to fill some of the columnar cells completely, indicative of it being taken up into the cytoplasm (Fig. 1b and c, arrows). No such fluorescence was observed in saline-treated mice. In the brain, scattered, small granular accumulations of the protein were observed in the olfactory bulb, primarily in the region of the olfactory glomeruli (Fig. 1e and f, arrows). The location of the HSP70 usually coincided with the periglomerular neuron cell bodies, but it was not possible to determine if these granular accumulations of HSP70 were within cell bodies of the periglomerular neurons, in glial cells or both. No fluorescent HSP70 was detected in other brain regions, nor was background fluorescence detected in saline-treated mice (Fig. 1h and i). Fluorescently labeled albumin also appeared to be taken up into nasal epithelial cells and the olfactory bulb in a manner similar to aHSP70 (not shown), confirming that this administration route is not specific for HSP70 uptake, as demonstrated with delivery of insulin by this method (Renner et al. 2012).
Fig. 1.

Examples of the distribution of fluorescently labeled aHSP70 uptake. a A low magnification horizontal section through the entire nasal epithelia structures stained with neutral rad provided for orientation purposes (MOE main olfactory epithelium, Gland nasal glands, RE respiratory epithelium (from Dunston et al. 2013). b Epifluorescence image of a portion of the main olfactory epithelium in a section through the nasal epithelium collected about 6 h after administration showing uptake of Alexa fluor 546-labeled aHsp70. The arrows indicate epithelial cells that are filled with the fluorescent HSP70. c Overlay of the fluorescent aHSP70 and DAPI nuclear fluorescence. d DIC image of the same region of the main olfactory epithelium shown in b and c. The scale bar in d applies to all micrographs. e Arrows indicate granular accumulation of HSP70 in cells in the olfactory bulb of a mouse that was administered the protein 4–6 h before the tissue was collected. f Overlay of the image from e onto the image of the same field showing DAPI fluorescent nuclei, to show that the HSP70 colocalized with periglomerular cells in the olfactory bulb. g DIC image of the same region of the olfactory bulb as shown in e and f. h, i, and j are images from a similar region of the olfactory bulb from a control, saline-treated mouse captured under the same exposure and magnification as for the aHSP70-treated mouse confirming the absence of such fluorescence in mice that did not receive the labeled aHSP70. The images are representative examples of multiple tissue sections of nasal epithelia from four Alexa-aHSP70-treated mice, the brains of three Alexa-aHSP70-treated mice, and three mice each for the control, saline-treated nasal epithelia, and brain
Insulin sensitivity
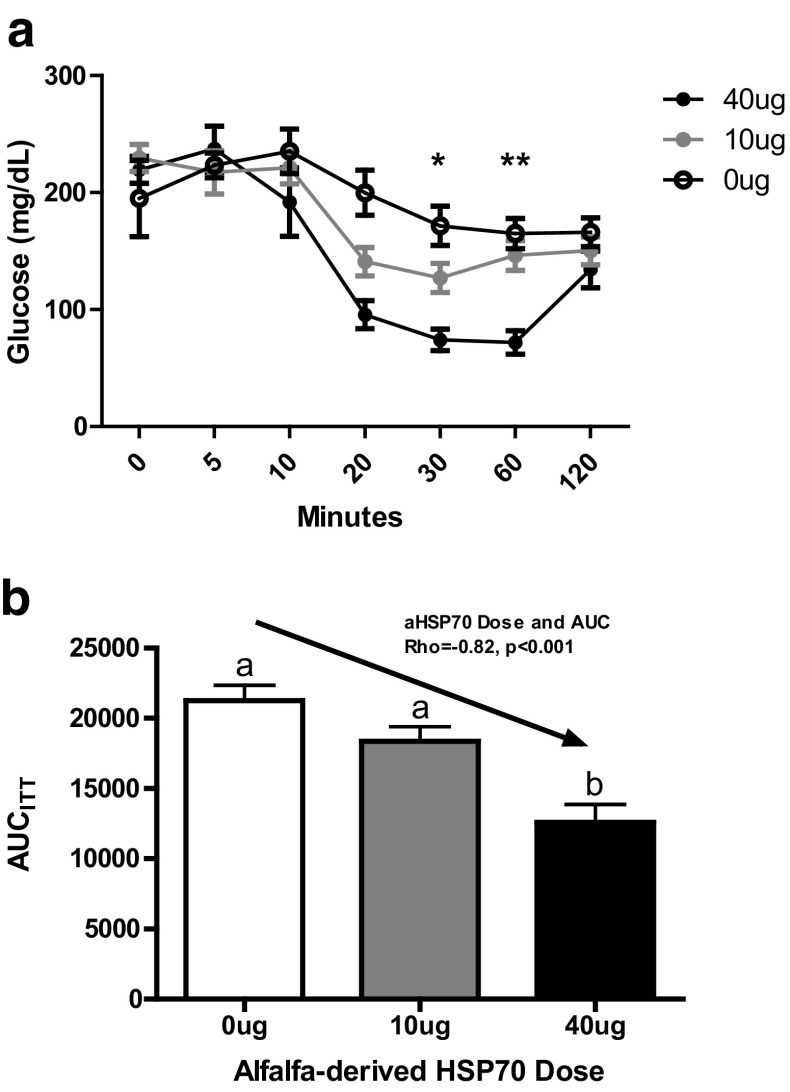
A dose of 40 μg aHSP70 significantly improved insulin sensitivity, and 10 μg trended towards improvement such that a strong linear dose response in insulin sensitivity was observed (Fig. 2a and b). Insulin sensitivity was 14% better and 40% better with 10 and 40 μg aHSP70 doses, respectively. Glucose tolerance was unchanged with aHSP70 administration (Table 1). There were lower non-fasting glucose and insulin in the aHSP70-treated groups, but these did not attain statistical significance (Table 1), likely because of the small sample size and short duration of the study. Body composition parameters did not differ by treatment (Table 1).
Fig. 2.
Effects of aHSP70 on insulin sensitivity. a Blood glucose decreases in response to an ip injection of insulin showed increased sensitivity following intranasal administration of 10 or 40 μg of aHSP70 three times per week for 24 days. Repeated measures ANOVA showed that the time x group interaction was significant (p < 0.001) and that at 30 and 60 min, the 40 μg group was significantly lower than the 0 μg control group (*p < 0.05; **p < 0.001). Means ± SEM of six mice per group. b Insulin level reductions were significantly correlated with aHSP70 dose
Table 1.
Glycemic-related outcome measures after 4 weeks of intranasal aHSP70 delivery in high-fat-fed mice (n = 8/group; mean ± SEM shown in parentheses)
| Group | Body weight (g) | Epididymal fat (g) | Non-fasted glucose (mg/dL) | Fasting glucose (mg/dL) | Non-fasted insulin (ng/mL) | Fasting insulin (ng/mL) | AUC (GTT) | HbA1c (%) |
|---|---|---|---|---|---|---|---|---|
| 0 μg | 29.2 (0.84) | 2.18 (0.47) | 216 (31.7) | 219 (14) | 4.08 (2.41) | 2.06 (0.88) | 17,992 (1350) | 2.81 (0.07) |
| 10 μg | 29.2 (1.48) | 3.71 (0.58) | 185 (9.99) | 230 (11) | 0.85 (0.10) | 0.86 (0.08) | 18,359 (1055) | 2.71 (0.04) |
| 40 μg | 29.5 (1.05) | 2.58 (0.60) | 176 (11.6) | 223 (12) | 1.38 (0.65) | 0.97 (0.30) | 21,269 (1091) | 2.74 (0.08) |
| p value | 0.99 | 0.56 | 0.31 | 0.73 | 0.11 | 0.44 | 0.33 | 0.19 |
Other measures of aHSP70 uptake and effects on abnormal glucose metabolism
We examined several other parameters related to glucose metabolism in control and aHSP70-treated mice, including body and epididymal fat pad weight, and HbA1c (Table 1). No significant changes were detected, although HbA1c was on average 0.1% lower with aHSP70 treatment, consistent with insulin sensitivity improvements. The study duration was not long enough for HbA1c to fully turnover and so reductions are likely to be greater with more time. The attempt to extract fluorescently labeled aHSP70 from frozen, homogenized brain and other tissues and measure it via a microtiter plate assay was unsuccessful, possibly because the labeled protein was too dilute.
Discussion
Our study demonstrated two novel outcomes: (1) alfalfa-derived HSP70 was similar enough in structure and activity to the mammalian form to improve glucose metabolism and (2) it was taken up into the brain just a few hours after intranasal administration. The rationale for this study arose from consensus that diabetes correlates with impairment of the HSP response. This deficit occurs in part because of diabetes-associated increases in glucose synthase kinase-3 (GSK-3) activity, which, in turn, leads to abnormal phosphorylation of heat shock factor 1 (Hsf1). Phosphorylated Hsf1 is less efficient in binding to the heat shock transcription element so that normal stress-induced transcriptional activity is deficient (reviewed in Hooper et al. 2014).
aHSP70 was taken up into the nasal epithelium and could be found in the glomerular region of the olfactory bulb, which includes the axons of the nasal epithelial olfactory receptor cells, the dendrites of mitral cells, and periglomerular glia. Uptake was not specific for HSP70, as a similar pattern was seen with fluorescently tagged albumin (not shown). Albumin uptake after intranasal administration has been shown to reach all parts of the brain via transcytosis (Falcone et al. 2014), so the same mechanism may have contributed to aHSP70 uptake. However, the cell membrane penetrating portion of the HSP70 molecule probably enhanced its uptake compared to albumin (Komarova et al. 2015). No tagged aHSP70 was detected in skeletal muscle or liver using epifluorescence microscopy and immunoblotting (data not shown). It is possible that our techniques were not sufficiently sensitive to detect systemically distributed aHSP70 as intranasally administered insulin in mice show that less than 1% of the dose gets into the brain and peripheral tissues (Kamei and Takeda-Morishita 2015). We suspect that the uptake of aHSP70 may be greater than other proteins because of its membrane-penetrating property referred to above. In fact, Bobkova et al. (2014) found that intranasally administered, fluorescently tagged human recombinant HSP70 was detected within the olfactory bulbs and other brain regions of mice and had a granular appearance within cells like that seen here. Furthermore, they showed it reduced both the structural signs of neurodegeneration and its behavioral manifestations. More detailed studies of the pharmacokinetics and distribution will be needed to better document aHSP70 uptake process following intranasal administration and to determine how insulin sensitivity was improved. We suggest two possibilities. The improvement may have resulted from the action of aHSP70 on the brain only or from a combination of that effect plus additional effects on peripheral tissues of aHSP70 levels below what we could detect. The possibility that action on the brain only could still improve peripheral glucose metabolism is supported by Sukhov et al. (2016), who showed that intranasal insulin in rats with type 2 diabetes at doses lower than those given systemically still improved systemic glucose metabolism. They suggest that normalized signaling in the hypothalamus was responsible for the systemic improvement.
Our results corroborate those of Henstridge et al. (2014) who showed that transgenic mice overexpressing HSP72 were protected against the glucose intolerance effects of a high-fat diet. Furthermore, the observations justify continuing work on this simple, non-invasive method of HSP70 delivery using alfalfa as the source of the protein. A detailed study of the dose response and pharmacokinetics will be needed to determine the effective and safe range of doses of intranasal aHSP70. It will also be important to monitor the effects of such extracellularly supplied HSP70 on the immune system, since other work showed that it can have either anti- or pro-inflammatory activity depending on the timing and context of delivery (van Eden et al. 2012; Garcia et al. 2013; Calderwood et al. 2016).
The potential for aHSP70 to serve as a treatment for T2DM and AD is great (Emery and Dobrowsky, 2016). That source may have advantages over recombinant human protein preparations because it can be produced in large quantities at lower cost and is categorized as a derivative of a natural food product by regulatory agencies. The preparation of aHSP70 used in this study was a mixture of both constitutive and inducible forms of this protein (Alfa Biogene International pers. comm.), which may make it more effective than either form alone (Deane and Brown 2016). Human studies (Goetzl et al., 2015; Gancheva et al. 2015; Di Domenico et al. 2010) support the translational relevance of the HSP70 potential and CNS-mediated effects on metabolism. Work to date has focused on insulin, but the results of intranasal administration on cognition are mixed in rodent models (Maimaiti et al. 2016; Anderson et al. 2017). Systemic upregulation of HSP70 improves cognition in a rat AD model (Zare et al. 2015), which combined with the human data documenting CNS HSP70 deficiencies positions local aHSP70 delivery by the intranasal method as a strategy worth pursuing for both AD and T2DM.
Acknowledgements
We would like to thank Alfa Biogene International (http://www.alfabiogene.de, Baarn, The Netherlands) for providing discounted alfalfa HSP70. This work was funded by grants from the Innovation and Entrepreneurship Initiative of Wake Forest School of Medicine to MT, NIH-T35 OD010946 to KK, and R25 HL092618 to Ann Tallant, Dept. of Surgery, Hypertension and Vascular Research Center, Wake Forest Univ., School of Medicine.
References
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Frazier HN, Maimaiti S, Bakshi VV, Majeed ZR, Brewer LD, et al. Impact of single or repeated dose intranasal zinc-free insulin in young and aged F344 rats on cognition, signaling, and brain metabolism. J Gerontol A Biol Sci Med Sci. 2017;72(2):189–197. doi: 10.1093/gerona/glw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobkova NV, Garbuz DG, Nesterova I, Medvinskaya N, Samokhin A, Alexandrova I, et al. Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer’s disease. J Alzheimers Dis. 2014;38(2):425–435. doi: 10.3233/JAD-130779. [DOI] [PubMed] [Google Scholar]
- Bobkova NV, Evgen’ev M, Garbuz DG, Kulikov AM, Morozov A, Samokhin A, et al. Exogenous HSP70 delays senescence and improves cognitive function in aging mice. Proc Natl Acad Sci. 2015;112(52):16006–16011. doi: 10.1073/pnas.1516131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Gong J, Murshid A. Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol. 2016;7:1–10. doi: 10.3389/fimmu.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichester L, Wylie AT, Craft S, Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci. 2015;70(2):155–162. doi: 10.1093/gerona/glu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen A-KK, Henstridge DC, Holmes AG, Chan MHS, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mena L, Chhangani D, Fernandez-Funez P, Rincon-Limas DE (2017) secHsp70 as a tool to approach amyloid-β42 and other extracellular amyloids. Fly (Austin). 1–6. doi:10.1080/19336934.2017.1291104 [DOI] [PMC free article] [PubMed]
- Deane CAS, Brown IR. Components of a mammalian protein disaggregation/refolding machine are targeted to nuclear speckles following thermal stress in differentiated human neuronal cells. Cell Stress Chaperones. 2016;22(2):1–10. doi: 10.1007/s12192-016-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunston D, Ashby S, Krosnowski K, Ogura T, Lin W (2013) An effective manual deboning method to prepare intact mouse nasal tissue with preserved anatomical organization. J Vis Exp 10(78). doi:10.3791/50538 [DOI] [PMC free article] [PubMed]
- van Eden W, Spiering R, Broere F, van der Zee R. A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones. 2012;17:281–292. doi: 10.1007/s12192-011-0311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery SM, Dobrowsky RT. Promoting neuronal tolerance of diabetic stress: modulating molecular chaperones. Int Rev Neurobiol. 2016;127:181–210. doi: 10.1016/bs.irn.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone JA, Salameh TS, Yi X, Cordy BJ, Mortell WG, Kabanov AV, Banks WA. Intranasal administration as a route for drug delivery to the brain: evidence for a unique pathway for albumin. J Pharmacol Exp Ther. 2014;351(1):54–60. doi: 10.1124/jpet.114.216705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna A, Alves G, Serralheiro A, Sousa J, Falcão A. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur J Pharm Biopharm. 2014;88(1):8–27. doi: 10.1016/j.ejpb.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Gancheva S, Koliaki C, Bierwagen A, Nowotny P, Heni M, Fritsche A, et al. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes. 2015;64(6):1966–1975. doi: 10.2337/db14-0892. [DOI] [PubMed] [Google Scholar]
- Garcia JJ, Hinchado MD, Bote ME, Ortega E. Effects of habitual exercise on the eHsp72-induced release of inflammatory cytokines by macrophages from obese zucker rats. Int J Sports Med. 2013;34:559–564. doi: 10.1055/s-0032-1327650. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol. 2015;2(7):769–773. doi: 10.1002/acn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63(6):1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Balogh G, Rivas E, Kavanagh K, Vigh L. The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones. 2014;19(4):54–60. doi: 10.1007/s12192-014-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N, Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Control Release. 2015;197:105–110. doi: 10.1016/j.jconrel.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300(5):E894–E901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Wylie AT, Chavanne TJ, Jorgensen MJ, Voruganti VS, Comuzzie AG, et al. Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J Gerontol A Biol Sci Med Sci. 2012;67(10):1014–1021. doi: 10.1093/gerona/gls008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova EY, Meshalkina DA, Aksenov ND, Guzhova IV. The discovery of HSP70 domain with cell-penetrating activity. Cell Stress Chaperones. 2015;20:343–254. doi: 10.1007/s12192-014-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Maimaiti S, Anderson KL, DeMoll C, Brewer LD, Rauh BA, Gant JC, et al. Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J Gerontol A Biol Sci Med Sci. 2016;71(1):30–39. doi: 10.1093/gerona/glu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega E, Bote ME, Besedovsky HO, del Rey A. Hsp72, inflammation, and aging: causes, consequences, and perspectives. Ann N Y Acad Sci. 2012;1261(1):64–71. doi: 10.1111/j.1749-6632.2012.06619.x. [DOI] [PubMed] [Google Scholar]
- Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. 2012;14(3):214–221. doi: 10.1111/j.1463-1326.2011.01490.x. [DOI] [PubMed] [Google Scholar]
- Perez FP, Bose D, Maloney B, Nho K, Shah K, Lahiri DK. Late-onset Alzheimer’s disease, heating up and foxed by several proteins: pathomolecular effects of the aging process. J Alzheimers Dis. 2014;40(1):1–17. doi: 10.3233/JAD-131544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner DB, Svitak AL, Gallus NJ, Ericson ME, Frey WH, Hanson LR. Intranasal delivery of insulin via the olfactory nerve pathway. J Pharm Pharmacol. 2012;64(12):1709–1714. doi: 10.1111/j.2042-7158.2012.01555.x. [DOI] [PubMed] [Google Scholar]
- Silverstein MG, Ordanes D, Wylie AT, Files DC, Milligan C, Presley TD, Kavanagh K. Inducing muscle heat shock protein 70 improves insulin sensitivity and muscular performance in aged mice. J Gerontol A Biol Sci Med Sci. 2014;70(7):800–808. doi: 10.1093/gerona/glu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhov IB, Derkach KV, Chistyakova OV, Bondareva VM, Shpakov AO. Functional state of hypothalamic signaling systems in rats with type 2 diabetes mellitus treated with intranasal insulin. J Evol Biochem Physiol. 2016;52(3):204–216. doi: 10.1134/S0022093016030030. [DOI] [PubMed] [Google Scholar]
- Young JC. Mechanisms of the HSP70 chaperone system. Biochem Cell Biol. 2010;88(2):291–300. doi: 10.1139/O09-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare N, Motamedi F, Digaleh H, Khodagholi F, Maghsoudi N. Collaboration of geldanamycin-activated P70S6K and Hsp70 against beta-amyloid-induced hippocampal apoptosis: an approach to long-term memory and learning. Cell Stress Chaperones. 2015;20(2):309–319. doi: 10.1007/s12192-014-0550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]