Abstract
Endothelia inflammation damage is vital to the development and progression of chronic venous disease. In the present study, we explored the protective effect of melatonin on endothelia apoptosis induced by LPS, particularly focusing on the mitochondrial fission. We demonstrated that human umbilical vein endothelial cells (HUVEC) subjected to LPS for 12 h exhibited a higher apoptotic rate. However, melatonin (1–20 μM) treatment 12 h before LPS had the ability to protect HUVEC cell against LPS-mediated apoptosis in a dose-dependent manner. Furthermore, LPS induced the cytoplasmic calcium overload which was responsible for the upregulation of calcium-dependent xanthine oxidase (XO). Higher XO expression was associated with reactive oxygen species (ROS) overproduction, leading to the Drp1 phosphorylation at the Ser616 site and migration on the surface of mitochondria. Furthermore, phosphorylated Drp1 initiated the mitochondrial fission contributing to the caspase9-dependent mitochondrial apoptosis as evidenced by lower membrane potential, more cyt-c leakage into the nuclear, and higher expression of proapoptotic proteins. However, melatonin treatment could trigger the AMPK pathway, which was followed by the increased SERCA2a expression. Activation of AMPK/SERCA2a by melatonin inhibited the calcium overload, XO-mediated ROS outburst, Drp1-required mitochondrial fission, and final mitochondrial apoptosis. In summary, this study confirmed that LPS induced HUVEC apoptosis through Ca2+-XO-ROS-Drp1-mitochondrial fission axis and that melatonin reduced the apoptosis of HUVEC through activation of the AMPK/SERCA2a pathway.
Keywords: Melatonin, LPS, HUVEC, Mitochondrial fission, Calcium overload, Oxidative stress, AMPK, SERCA2a
Introduction
Chronic venous disease (CVeD) represents the most common prevalent phlebo-pathological conditions (Chi et al. 2014; Pannier and Rabe 2012), which entails significant health and economic burden. CVeD is characterized by morphological and/or functional changes in the lower limb venous system, including spider veins (telangiectasia), asymptomatic varicosities, large painful varicose veins, edema, hyperpigmentation, lipodermatosclerosis, and ulceration of skin (Hamdan 2012). The etiopathology of CVeD includes a series of factors such as genetics, lifestyle, female sex, occupations involving prolonged standing position, obesity, multiple pregnancies, family history of varices, and deep and superficial vein thrombosis (Nicolaides et al. 2008). Among these processes, the excessive inflammation response plays a key role in the development and progression of CVeD (Mannello et al. 2014; Mannello and Raffetto 2011). The inflammation induces the damage of endothelia resulting into the remodeling of the vessel wall (Raffetto 2013). As a consequence of damaged endothelia and vessel wall, hemodynamic disorder and hypertension appear and contribute to the chronic venous stasis, microcirculatory congestion, and capillary failure. Therefore, therapeutic strategies to enhance the resistance of endothelia to the inflammatory damage are vital to retard or intervene against CVeD.
The mitochondria, which are organelles present in all cells of the human body except erythrocytes, play a pivotal role in energy production (Bolanos et al. 2016). In addition to this vital function, the mitochondria are involved in other complex processes such as metabolism, reactive oxygen and nitrogen species production, calcium regulation, cellular proliferation, cell division, and programmed cell death (apoptosis) (Dan Dunn et al. 2015; Gidlof et al. 2016; Osborne et al. 2016; Quijano et al. 2016). Several studies have indicated that inflammation induces endothelia apoptosis via activation of caspase9-dependent mitochondrial apoptosis pathways (Zhang et al. 2011). Furthermore, recent studies have found that mitochondrial dynamics (fission and fusion) is the housekeeper for mitochondrial homeostasis (Gorojod et al. 2015). A shift to excessive mitochondrial fission is the early stage of mitochondrial apoptosis (Ni et al. 2015). Excessive mitochondrial fission promotes the leakage of proapoptotic proteins (such as cytochrome c (cyt-c)) into the cytoplasm launching the mitochondria-related cellular apoptosis (Zhang 2015). These data suggest that mitochondrial fission is the potential target for endothelia survival and venous homeostasis. However, we still did not know the upstream molecular mechanism related to the mitochondrial fission under inflammation stimulation. Several studies have raised that mitochondrial fission is closely dependent on cellular calcium concentration (Huang et al. 2017) because fission is a process of self-contraction. Whether inflammation could regulate the mitochondrial fission via calcium remains unclear. If so, what molecular mechanism links calcium to mitochondrial fission?
Several studies have proven that mitochondrial fission is heavily regulated by dynamin-related protein 1 (Drp1) (Park et al. 2016). Activated Drp1 via phosphorylation has the ability to migrate toward the surface of the mitochondria and locate to the predicted sites of division along mitochondrial tubules (Dan Dunn et al. 2015). Several pathways have been reported to modify the Drp1 activation such as JNK, ERK, and PKA (Michalska et al. 2016). However, little evidence is available for the role of calcium in Drp1 activation. Considering that Drp1 phosphorylation happens at the Ser616 site which is prone to be modified by oxidative stress or reactive oxygen species (ROS) (Flippo and Strack 2017), we therefore guess whether the ROS-dependent Drp1 posttranscription is the bridge connecting calcium with mitochondrial fission.
Melatonin is an endogenous indolamine synthesized by almost all vertebrate organs (Dong et al. 2016). Although it has been found to reduce perinatal hypoxia-ischemia, improve sleep, ameliorate cardiac ischemia/reperfusion injury, inhibit oxidative stress-mediated endothelia cell death, and increase cancer sensitivity to chemotherapy (Lin et al. 2016; Manchester et al. 2015; Mao et al. 2016; Nduhirabandi et al. 2016; Reiter et al. 2016), few literature is available to establish its role in endothelia inflammatory injury. Furthermore, whether melatonin could abate the inflammatory injury via modification of mitochondrial fission remains unknown. In our study, we applied LPS to induce endothelia inflammatory damage and explored the effect and mechanism of melatonin on endothelia protection. Our data demonstrated that LPS evoked the endothelia apoptosis via induction of Drp1-dependent mitochondrial fission. Excessive mitochondrial fission contributed to activation of caspase9-dependent mitochondrial apoptosis. Mechanistically, LPS treatment insulted the calcium overload which increased the xanthine oxidase (XO) expression, a calcium-dependent superoxide-producing enzyme. XO upregulation augmented the ROS production, leading to oxidative stress which was responsible for Drp1 phosphorylation at the Ser616 site and subsequent mitochondrial fission. However, melatonin had the ability to activate the AMPK pathway which increased the expression of SERCA2a, leading to the suppression of calcium overload and subsequent XO-Drp1 activation.
Method
Cell culture
The human umbilical vein endothelial cell (HUVEC) was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The cell line HUVEC was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA) and supplemented with 10% FBS at 37 °C in a humidified atmosphere containing 5% CO2.
The effect of melatonin on HUVEC viability
The HUVEC viability was detected via MTT, trypan blue staining, and TUNEL assay. MTT assay was conducted in 96-well plates. Then, LPS with or without melatonin was applied to treat the cells followed by incubation of the MTT solution (Sigma-Aldrich) at 37 °C for 4 h. Then, the medium was removed, and 100 μl of dimethyl sulfoxide (DMSO) was added to each well. The absorbance was measured at a wavelength of 570 nm. The data are expressed as the ratio of the optical density (OD) value of the treated group to the OD of the control group. As for the trypan blue assay, trypan blue 0.04% (m/v) was used to observe cell death. The number of trypan blue positive cells was calculated by counting at least three random separate fields. TUNEL assay was used to detect the cellular apoptosis according to the manufacturer’s protocol. Cells in which the nucleus was stained were defined as TUNEL positive.
Immunofluorescence staining
For the immunofluorescence staining, cells were fixed with 4% paraformaldehyde and then permeabilized with 0.3% Triton X-100. The 10% goat serum albumin was used to block the samples for 1 h followed by incubation of primary antibodies. After washed with phosphate-buffered saline (PBS) and incubated with secondary antibody for 45 min at room temperature, samples were analyzed under a fluorescent microscope. DAPI and a mitochondrion-selective Mito-Fluor stain (Molecular Probes, USA) were used to mark the nuclear and mitochondria, respectively. The primary antibodies are as follows: XO (Abcam, #ab222081, USA), SERCA2a (Abcam, #ab2861, USA), and p-Drp1 (Cell Signaling Technology, #4494, USA).
Western blots
Following the appropriate treatment, cultured cells were lysed with RIPA lysis buffer (Beyotime, China) for 30 min and centrifuged for 30 min. Equal amounts of protein were separated and transferred to a polyvinylidene difluoride membrane (Millipore). After being blocked with 5% milk in Tris-buffered saline containing 0.05% Tween20 (TBST) at room temperature for 1 h, the membrane was incubated at 4 °C overnight with the following primary antibodies: pro-caspase3 (1:1000, Cell Signaling Technology, #9662), cleaved caspase3 (1:1000, Cell Signaling Technology, #9664), c-IAP1 (1:2000, Cell Signaling Technology, #7065), Bax (1:2000, Cell Signaling Technology, #5023), caspase9 (1:1000, Abcam, #ab32539), XO (1:1000, #ab109235), Drp1 (1:1000, Cell Signaling Technology, #8570), p-Drp1 (1:1000, Cell Signaling Technology, #4494), SERCA2a (1:1000, Abcam, #ab2861), p-AMPK Thr172 (1:1000, Cell Signaling Technology, #8208), and t-AMPK (1:1000, Cell Signaling Technology, #5831). After being washed with TBST and further incubated with the appropriate secondary antibody at 37 °C for 60 min, the blots were developed with an enhanced chemiluminescence (ECL) reagent.
Mitochondrial membrane potential, mitochondrial ROS, and mitochondrial permeability transition pore opening
The mitochondrial dysfunction was detected via the change of mitochondrial membrane potential (ΔΨm), mitochondrial ROS (mROS), and mitochondrial permeability transition pore (mPTP) opening. The mROS measurement was conducted using a MitoSOX red mitochondrial superoxide indicator (Molecular Probes, USA). The ΔΨm was analyzed using a JC-1 Kit (Beyotime, China). Images were captured using a fluorescence microscope (OLYMPUS DX51; Olympus, Tokyo, Japan) and were analyzed with Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD) to obtain the mean densities of the region of interest, which was normalized to that of the control group. The opening of the mPTP was visualized as a rapid dissipation of tetramethylrhodamine ethyl ester fluorescence. Arbitrary mPTP opening time was determined as the time when tetramethylrhodamine ethyl ester fluorescence intensity decreased by half between initial and residual fluorescence intensity according to a previous study (Rizzo et al. 2016).
Caspase3, caspase9, and SERCA2a activity assay
The activation of caspase3 represents an essential step in the apoptotic process, and caspase9 activity is elevated during mitochondrial apoptosis. Thus, caspase3/9 activity kits were used according to the manufacturer’s protocols. The relative caspase3/9 activity was calculated from the ratio of treated cells to untreated cells. The assays were repeated thrice. The SERCA2a activity was detected according to a previous study (Zhang et al. 2016). Cells after treatment were harvested to isolate the total proteins. The protein concentration of the supernatant was determined using the BCA method. Sample protein concentrations were adjusted to 1000 μg/ml. The proteins were incubated in a 30 °C water bath for 10 min and 20 μl ATP was added. Thirty seconds later, 20 μl of the sample was added. After mixing for 45 s, the OD values were measured.
RNAi assay
To investigate the role of XO in cellular apoptosis, XO was knocked down by transient transfection of small interfering RNA (siRNA). The siRNA targeting XO was purchased from Santa Cruz Biotechnology. For the RNAi knockdown, HUVEC were transfected in 3 ml of serum-free and antibiotic-free DMEM containing 500 μl of Opti-MEM (Invitrogen), 6 μl of Lipofectamine RNAiMAX (Invitrogen, Ireland), and 50 nmol/l of each siRNA. The knockdown of gene expression was assessed by western blots.
Detection of calcium and ROS concentration
Intracellular calcium was measured using the calcium-dependent fluorescent dye Fura-2 as previously described (Zhu et al. 2017). After treatment, the intracellular ROS and superoxide generation were measured with DCFH-DA (Beyotime Institute of Biotechnology, Jiangsu, China) staining as previously described. Then, fluorescence microscopy (Olympus) and flow cytometry were used to examine the calcium and ROS fluorescence. Images were captured using a fluorescence microscope (OLYMPUS DX51; Olympus, Tokyo, Japan) and were analyzed with Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD) to obtain the mean densities of the region of interest, which was normalized to that of the control group.
Reagent treatment
For drug treatment, cultured HUVEC were treated with 10 μg/ml LPS for 12 h to induce cellular death. The melatonin used in the present study was 0–20 μM treatment 12 h before LPS. These particular concentrations of drugs were selected because they were previously reported to be effective cellular experiments in vitro (Zhu et al. 2017). To activate the mitochondrial fission, carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) (5 μM, Selleck Chemicals) was used 2 h before melatonin or LPS treatment. To inhibit the Drp1 activation, its specific inhibitor Mdivi1 (10 μM) was added to the medium for 1 h prior to LPS or melatonin treatment to block Drp1 activation. To block intracellular calcium overload, cell-permeable calcium chelator BAPTA (Sigma, #A1076) was diluted in D-Hanks solution to a final concentration of 50 μM for 30 min before LPS treatment. In contrast, to induce cellular calcium overload, ionomycin, a calcium agonist was used 30 min before melatonin treatment. To identify the role of ROS in cell apoptosis, N-acetyl-l-cysteine (NAC), a cell-permeable antioxidant (10 mM), and exogenous H2O2 (0.2 nM) were introduced as negative and positive control groups, respectively. To suppress and activate the AMPK pathway, compound c (cc, 5 μM, Selleck Chemicals) and AICAR (AI, 200 μM, Selleck Chemicals) were used 2 h before melatonin treatment, respectively.
Statistical analyses
Values are presented as the mean ± SE. The statistical analysis was performed using Statistical Package for Social Science software, version 16 (SPSS, Chicago, IL, USA). ANOVA followed by Tukey’s post hoc test was used to compare data among three or more groups.
Results
Melatonin alleviates LPS-induced cellular death
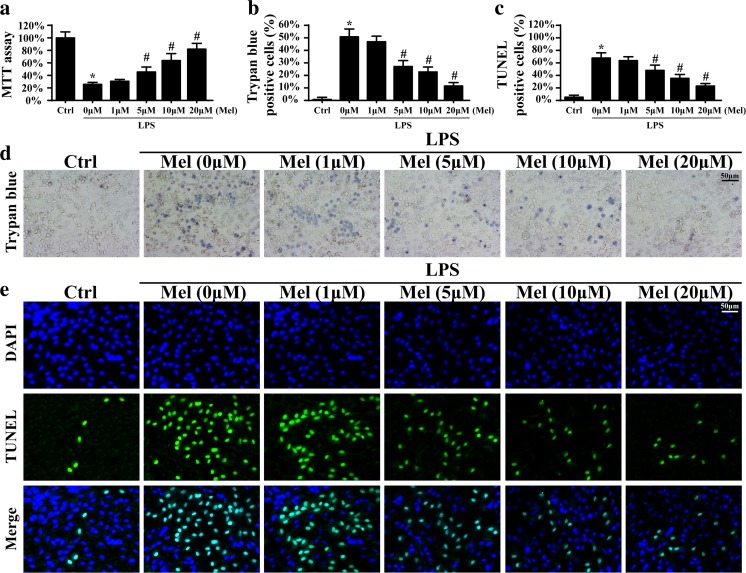
To explore the role of melatonin played in the LPS-mediated HUVEC damage, the MTT assay was used. Compared to the control group, LPS reduced the cellular viability evidenced by lower OD value (Fig. 1a). However, melatonin could reverse cellular viability in the presence of LPS in a dose-dependent manner. Furthermore, trypan blue staining was used to observe the protective effect of melatonin on HUVEC death. Compared to the control group, LPS elevated the numbers of trypan blue-positive cells (Fig. 1b, d). However, co-treatment with melatonin abated the ratio of trypan blue+ cells, suggesting that melatonin inhibited LPS-induced cellular death. Meanwhile, the results in the TUNEL assay were also in accordance with such findings (Fig. 1c, e). Altogether, these data indicated that melatonin could reduce HUVEC death induced by LPS. Because the minimum antiapoptotic effect of melatonin was 5 μm/l, therefore, 5 μm/l of melatonin was used for the following experiments.
Fig. 1.
The effects of melatonin on LPS-induced cellular death. a The cellular viability was detected via MTT assay. Melatonin had the ability to protect cellular activity. b–d The trypan blue staining was used to observe cellular death under LPS treatment in the presence or absence of melatonin. Melatonin could reduce the number of trypan blue positive cells when compared to LPS treatment. c–e TUNEL assay was conducted to observe cell death with melatonin treatment. *P < 0.05 vs Ctrl group, # P < 0.05 vs LPS group. LPS: lipopolysaccharide (10 μg/ml for 12 h treatment); Ctrl: control group
Melatonin suppresses caspase9-dependent mitochondrial apoptosis via inhibiting mitochondrial fission
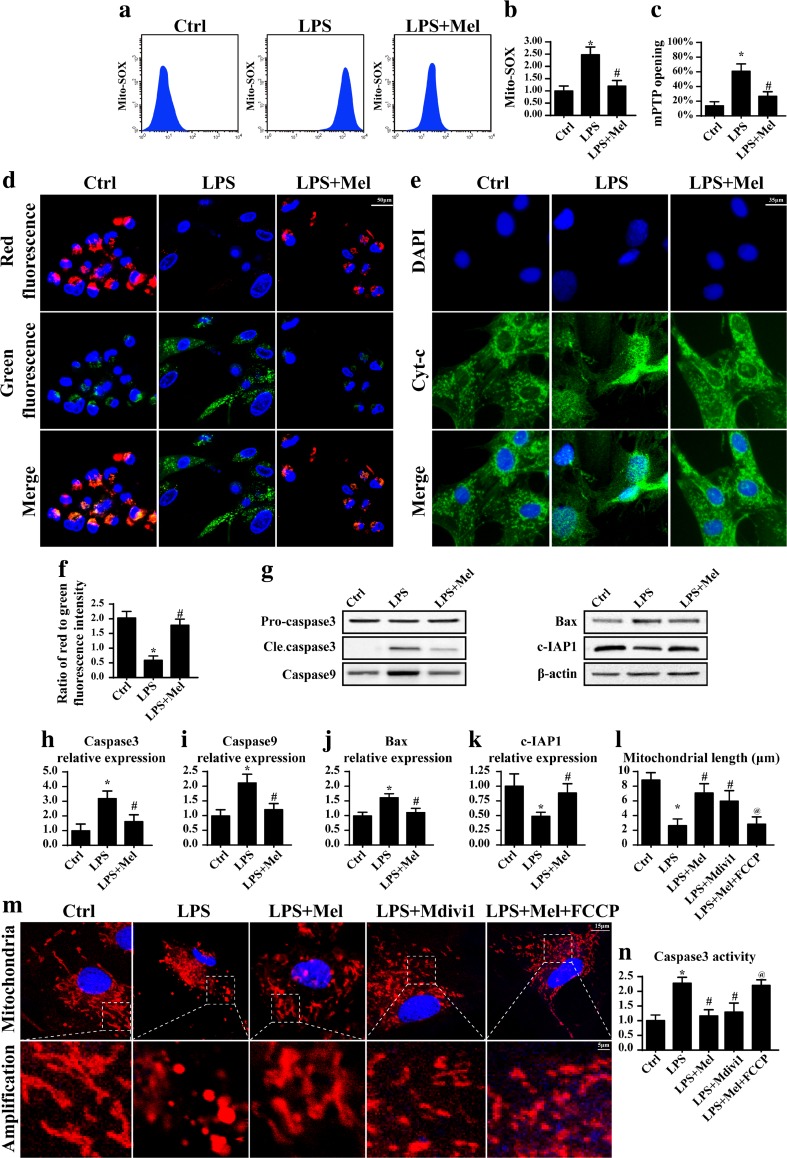
To investigate the mechanism by which melatonin reduced LPS-induced HUVEC apoptosis, we focused on the mitochondria-related apoptosis. Because the mitochondria is not only the center for energy production, it also transmits the proapoptosis signal and directs cells to programmed cellular death. The mitochondrial apoptosis is triggered due to excessive oxidative stress that attacks its membrane structure, leading to mPTP opening and proton gradient collapse (Li et al. 2016b). Therefore, we firstly used flow cytometry to detect ROS content in mitochondria. Compared to the control group, LPS significantly elevated the mROS, indicative of excessive mitochondrial oxidative stress (Fig. 2a, b). However, melatonin alleviated the outburst of mROS, suggesting the regulatory effect of melatonin on mitochondrial oxidative stress in the presence of LPS. Moreover, as a consequence of mitochondrial oxidative damage, the mitochondria extensively opens the mPTP, leading to ΔΨm dissipation. As shown in Fig. 2c, the mPTP opening ratio was low in the control group but obviously increased in response to the LPS treatment. Besides, the ΔΨm displayed more red fluorescence in the control group but exhibited more green fluorescence in the setting of LPS treatment (Fig. 2d, f). Furthermore, application of melatonin in the presence of LPS could reduce the mPTP opening rate as well as reverse the ΔΨm. The opened mPTP would bridge the cytoplasm and mitochondria, increasing the likelihood of mitochondria-contained proapoptotic factor (such as cyt-c) leakage into the cytoplasm where it initiates the mitochondrial apoptosis (Mukherjee et al. 2015). As shown in Fig. 2e, LPS could trigger the cyt-c diffusion from the mitochondria into the cytoplasm and even into the nuclear, which was reversed by melatonin treatment. As a consequence of cyt-c leakage, more apoptotic proteins appeared (Fig. 2g–k). However, this tendency was blocked by melatonin. Altogether, these data indicated that melatonin had the ability to suppress caspase9-dependent mitochondrial apoptosis under LPS stimulation.
Fig. 2.
Melatonin counteracted caspase9-dependent mitochondrial apoptosis via abating mitochondrial fission. a, b The change of cellular mitochondrial ROS (mROS). Melatonin alleviated the cellular oxidative stress in the presence of LPS. c The change of mitochondrial mPTP opening. Melatonin suppressed the mPTP opening ratio. d The change of mitochondrial membrane potential. The red fluorescence indicated the normal mitochondria with high membrane potential. The green fluorescence was the marker of damaged mitochondria with reduced membrane potential. e The leakage of cyt-c from mitochondria into cytoplasm. f The quantitative expression of mitochondrial membrane potential. g The change of proteins related to the caspase9-dependent mitochondrial apoptosis pathways. h–k The quantitative expression of cleaved caspase3, caspase9, Bax, and c-IAP1. l–m The mitochondrial fission was observed via immunofluorescence. More shorter mitochondria appeared in response to LPS treatment. However, melatonin treatment could reverse the mitochondrial length. Mdivi1 and FCCP were the inhibitor and activator of mitochondrial fission, respectively. n Activation of mitochondrial fission could augment the caspase3 activity in the melatonin treatment. *P < 0.05 vs Ctrl group, # P < 0.05 vs LPS group, @ P < 0.05 vs LPS + melatonin group
To explain the reason by which melatonin blocked the mitochondrial apoptosis, we focused on the mitochondrial fission. As shown in Fig. 2m, compared to the control group, LPS treatment caused more fragmented mitochondria or mitochondrial debris. The length of the mitochondria was also shorter when compared to the control group (Fig. 2l), indicative of mitochondrial fission. However, melatonin treatment had the ability to reduce the mitochondrial fission and reverse the mitochondrial morphology and/or length (Fig. 2l, m). To demonstrate whether mitochondrial fission was responsible for the LPS-induced mitochondrial apoptosis, we used the inhibitor (Mdivi1) of mitochondrial fission under LPS and applied the activator (FCCP) of fission under melatonin. By inhibition of mitochondrial fission, the proapoptotic effect of LPS was suppressed as evidenced by reduced caspase3 activity (Fig. 2n). In contrast, activation of mitochondrial fission via FCCP caused the upregulation of caspase3 activity under melatonin treatment. These data indicated that LPS induced HUVEC inflammatory injury via activation of caspase9-dependent mitochondrial apoptosis which was evoked by mitochondrial fission.
LPS activates mitochondrial fission via the XO-ROS-Drp1 axis
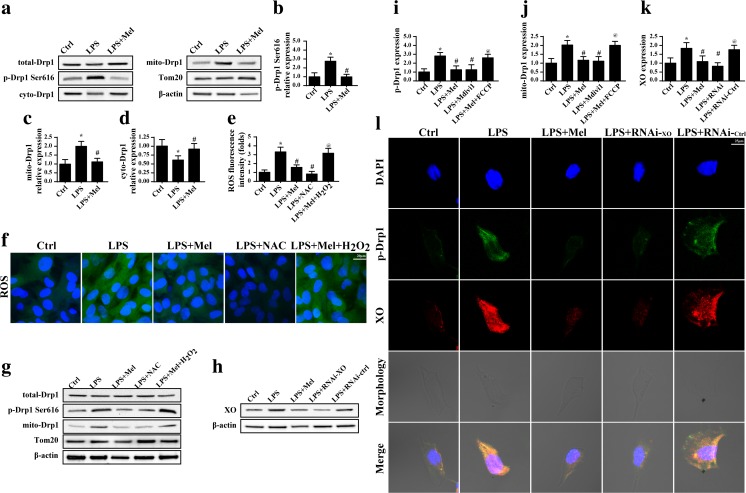
Considering that Drp1 is the executive protein for mitochondrial fission, its activation via phosphorylation is vital for successive mitochondrial fission (Wu et al. 2016). To explain the mechanism by which LPS regulated mitochondrial fission, we focused on Drp1 activation. Firstly, as shown in Fig. 3a–d, LPS induced the phosphorylation of Drp1 at Ser616, leading to Drp1 migration to the mitochondria which was accompanied by lower content of cytoplasm Drp1. These data indicated that LPS had the ability to trigger mitochondrial fission via regulation of Drp1 phosphorylation. In contrast, melatonin treatment could reverse Drp1 phosphorylation and mitochondrial migration (Fig. 3a–d), reconfirming the inhibitory effect of melatonin on mitochondrial fission. Furthermore, several studies have found that Drp1 activation is dependent on the ROS, ERK, and JNK (Santa-Gonzalez et al. 2016; Ursini et al. 2016). Given the fact that LPS had the ability to trigger oxidative stress in endothelia according to previous studies (Li et al. 2016a), we therefore guessed whether ROS was involved in LPS-mediated Drp1 activation. Firstly, ROS staining illustrated the promotive effect of LPS on oxidative stress which was reduced by melatonin (Fig. 3e, f). Furthermore, through loss and gain of function of ROS, we demonstrated that Drp1 phosphorylation was in a ROS-dependent manner. We applied the exogenous H2O2 as the ROS analogue in the melatonin-treated endothelia, and the results indicated that exogenous H2O2 not only elevated the ROS content (Fig. 3e, f) but also increased Drp1 phosphorylation (Fig. 3g–j). In contrast, using NAC in LPS-treated endothelia, a kind of antioxidant to remove ROS, had the ability to partly alleviate ROS production (Fig. 3e, f) and further blocked the Drp1 phosphorylation and mitochondrial translocation (Fig. 3g–j). Based on these results, we concluded that LPS triggered ROS overproduction which was responsible for Drp1 activation via phosphorylated modification. However, melatonin had the ability to repress the ROS content and, therefore, inhibited the Drp1 phosphorylated activation.
Fig. 3.
Mitochondrial fission was signaled by XO-ROS-Drp1 pathways in the setting of LPS treatment. a–d The change of mitochondrial fission protein Drp1. LPS phosphorylated Drp1 at Ser616 and induced Drp1 migration to the mitochondria. e, f The ROS intensity indicated LPS evoked cellular oxidative stress, while melatonin could suppress such changes. NAC and H2O2 were the ROS neutralizer and inducer, respectively. g, i, j Suppression of cellular oxidative via NAC could alleviate the Drp1 phosphorylation and mitochondrial migration. However, induction of oxidative stress could cancel the inhibitory effect of melatonin on Drp1 activation. h, k XO expression was increased under LPS treatment. The siRNA was used to knockdown its expression. l Knockdown of XO expression not only knocked down the expression of XO but also alleviated the p-Drp1 expression. *P < 0.05 vs Ctrl group, # P < 0.05 vs LPS group, @ P < 0.05 vs LPS + melatonin group
To further figure out the reason by which LPS induced the ROS outburst, we focused on XO which is abundant in endothelia (Jarasch et al. 1981) when compared to other cells with vigorous metabolism such as muscle cells, liver cells, and cardiomyocytes (Wang et al. 2016). XO produces excessive ROS via ADP metabolism. XO employs ADP and oxygen as the electron carrier to generate the superoxide (Peleli et al. 2016). Considering that XO is particularly rich in endothelia, we therefore guessed whether LPS triggered ROS outburst via XO. Firstly, we detected the expression of XO in response to LPS treatment. Expectedly, XO expression was increased in the setting of LPS-mediated inflammatory response (Fig. 3h, k). However, melatonin had the ability to inhibit XO upregulation. To provide solid evidence for the role of XO and subsequent Drp1 phosphorylation, we co-stained the XO and p-Drp1 in endothelia under LPS with or without melatonin treatment. As shown in Fig. 3l, higher XO expression was paralleled to the p-Drp1 content. However, siRNA against XO not only abated XO expression but also alleviated p-Drp1, suggesting that XO was the upstream regulator of Drp1 activation. Similarly, melatonin suppressed XO and, therefore, inhibited p-Drp1 expression. Altogether, these data indicated that LPS increased XO expression which evoked ROS overproduction leading to Drp1 phosphorylation and that melatonin inhibited Drp1 activation via blockade of XO-ROS pathways.
XO-Drp1 upregulation is attributed to the LPS-induced calcium overload
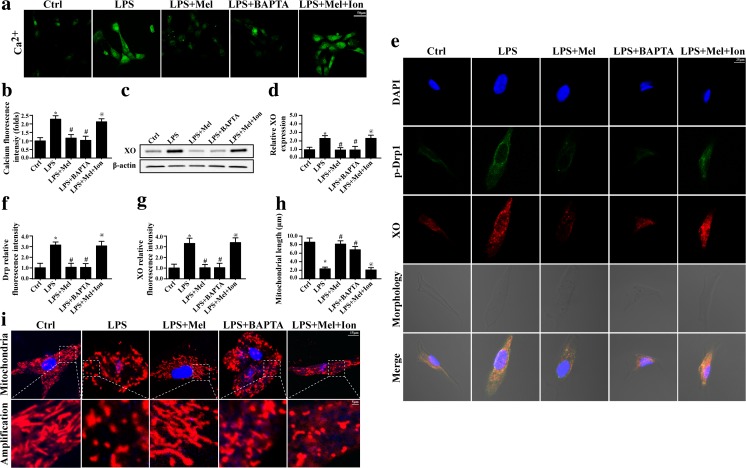
XO is a calcium concentration-dependent, superoxide-producing enzyme that is localized to the endoplasmic reticulum (ER) (Sheikh-Ali et al. 2010). Previous studies have found that XO expression could be regulated by cellular calcium (Zhang et al. 2016). Therefore, we guessed whether LPS induced the XO upregulation via calcium overload. Firstly, we demonstrated that LPS had the ability to increase calcium concentration in endothelia (Fig. 4a, b). However, this tendency was blocked by melatonin. Furthermore, to illustrate the role of calcium in XO expression, the calcium chelator and calcium agonist were used. BAPTA, a calcium chelator, had the ability to reduce the intracellular calcium level (Fig. 4a, b) and further abolished XO expression (Fig. 4c, d). In contrast, in melatonin-treated cells, ionomycin, a calcium agonist, not only re-increased the intracellular calcium overload (Fig. 4a, b) but also reintroduced XO expression (Fig. 4c, d). These data displayed that calcium overload signaled XO upregulation. Furthermore, to observe whether calcium had the role in Drp1 activation, immunofluorescence was used. As shown in Fig. 4e–g, removal of excessive calcium in LPS-treated endothelia, the XO and p-Drp1 expression was decreased. In contrast, in melatonin-treated endothelia, activation of calcium overload increased XO and p-Drp1 expression. Furthermore, the change of mitochondrial morphology was also in accordance with such results (Fig. 4h, i). Altogether, these data suggested that calcium was the upstream signal for the activation of XO-ROS-Drp1-mitochondrial fission, whereas melatonin had the ability to alleviate the calcium overload and therefore inactivated the XO-ROS-Drp1-mitochodnrial fission pathways.
Fig. 4.
XO-Drp1 axis was regulated by calcium overload under LPS treatment. a, b The change of intracellular calcium. BAPTA and ionomycin (Ion) were the calcium chelator and inducer, respectively. c, d The XO expression was regulated by the intracellular concentration. e–g The calcium overload could elevate the p-Drp1 expression. h, i The mitochondrial fission was modulated by the calcium overload. *P < 0.05 vs Ctrl group, # P < 0.05 vs LPS group, @ P < 0.05 vs LPS + melatonin group
Melatonin suppresses Ca2+-XO-ROS-Drp1 pathway via activation of AMPK/SERCA2a
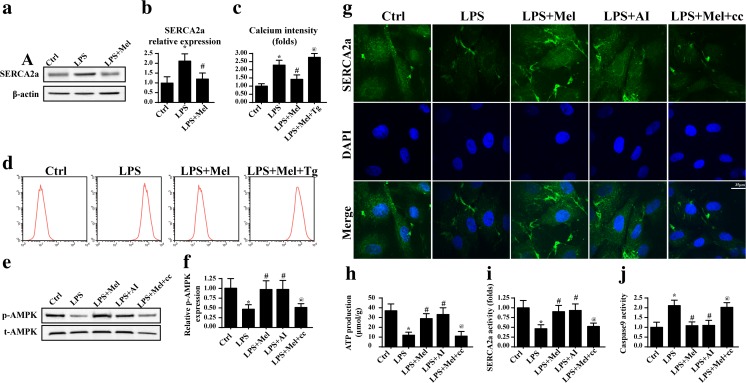
Finally, to explain the reason by which melatonin abated calcium, we focused on the SERCA2a expression. As the most important calcium reuptake channel in the ER, SERCA2a contributes 92% of the released Ca2+ reabsorption to the ER (Marks 2013), which is the keeper of intracellular calcium homeostasis. As shown in Fig. 5a, b, LPS treatment led to a drop in the SERCA2a expression which was reversed by melatonin, suggesting the possible role of SERCA2a in calcium overload under LPS treatment. To establish the relationship between SERCA2a and calcium overload, its inhibitor was used. In the melatonin-treated endothelia, the SERCA2a blocker thapsigargin (Tg) elevated the calcium concentrations as evidenced by flow cytometry (Fig. 5c, d), suggesting that the beneficial effect of melatonin on calcium homeostasis was attributed to SERCA2a. Next, to further explore the reason by which melatonin modified the SERCA2a, the AMPK pathway was considered. Previous studies have indicated that melatonin could activate the AMPK pathway to protect the cardiac microvascular against ischemia reperfusion (Zhou et al. 2017). Thus, we asked whether the AMPK pathway was also the molecular signal underlying melatonin-mediated endothelial protection via targeting to SERCA2a. Firstly, we demonstrated that the AMPK pathway was activated in response to melatonin as evidenced by increased p-AMPK expression (Fig. 5e, f). Notably, the AMPK pathway inhibitor compound c (cc) had the ability to abolish the role of melatonin on p-AMPK. Furthermore, blockade of AMPK by cc also suppressed the melatonin-mediated SERCA2a upregulation (Fig. 5g), suggesting that melatonin regulated SERCA2a expression via the AMPK pathway. Apart from SERCA2a expression, its activity is vital for calcium recycling to the ER. Because SERCA2a, a calcium-ATPase, is an ATP-driven protein that inversely transports calcium back to the ER, its activity is determined by the intracellular ATP content. In our study, we found that LPS-treated cells had a lower content of ATP whereas melatonin sustained ATP level in an AMPK-dependent manner (Fig. 5h). Besides, the results of enzyme activity assay of SERCA2a were in parallel with the above changes (Fig. 5i), suggesting that the AMPK pathway regulated the SERCA2a activity. Finally, to explain whether SERCA2a and AMPK were involved in LPS-mediated mitochondrial apoptosis, caspase9 activity was assessed. After blockade of SERCA2a or AMPK, the caspase9 activity was increased (Fig. 5j). Altogether, these data indicated that melatonin activated AMPK/SERCA2a against calcium overload and subsequent mitochondrial apoptosis.
Fig. 5.
Melatonin activated the AMPK/SERCA2a pathway to suppress Ca2+-XO-ROS-Drp1 damage signals. a, b The change of SERCA2a. LPS suppressed the expression of SERCA2a. c, d Inhibition of SERCA2a could alleviate the protective effect of melatonin on calcium overload. e, f Melatonin had the ability to activate the AMPK pathway. g Inhibition of AMPK pathway abated the promotive effect of melatonin on SERCA2a expression. However, activation of AMPK under LPS treatment could partly reverse the SERCA2a content. h The AMPK pathway was involved in ATP production. i Apart from SERCA2a expression, AMPK was also activated by melatonin and contributed to the SERCA2a activity. j Inhibition of AMPK augmented the cellular apoptosis despite melatonin treatment. Compound c: cc, AICAR:AI. *P < 0.05 vs Ctrl group, # P < 0.05 vs LPS group, @ P < 0.05 vs LPS + melatonin group
Discussion
In the present study, we found that (1) melatonin had the ability to decrease HUVEC apoptosis induced by LPS; (2) LPS treatment abated SERCA2a expression, contributing to the calcium overload; (3) excessive calcium overload activated the calcium-dependent XO, leading to ROS overproduction; (4) ROS induced oxidative stress which triggered Drp1 phosphorylation and subsequent mitochondrial fission; (5) mitochondrial fission launched the caspase9-dependent mitochondrial apoptosis; (6) melatonin could activate the AMPK pathway which increased the SERCA2a expression; and (7) higher SERCA2a was associated with the calcium balance and mitochondrial apoptosis inhibition. To the best of our knowledge, this is the first study to describe the molecular signal of melatonin as the adjuvant to alleviate inflammation-mediated endothelial damage involving caspase9-dependent mitochondrial apoptosis, Drp1-required mitochondrial fission, XO-related oxidative stress, and SERCA2a-involved calcium homeostasis and AMPK pathway.
CVeD is a debilitating condition that affects millions of individuals worldwide (Bergan 2007). The condition can result in varicose veins or advance to severe skin changes and venous ulceration. The fundamental basis for CVeD is inflammation within the venous circulation and that it is subjected to increased hydrostatic pressure resulting in increased ambulatory venous pressure (Mannello and Raffetto 2011; Raffetto and Khalil 2008). The inflammation involves leukocytes, in particular, macrophages and monocytes, inflammatory modulators and chemokines, cytokine expression, growth factors, metalloproteinase (MMP) activity, and many regulatory pathways that perpetuate inflammation (Sandor 2004; Simka 2010). Therefore, a strategy to regulate the inflammation response is vital to retard or intervene against CVeD progression and development. Here, we applied LPS in endothelia to mimic the inflammatory response in venous. We found that LPS treatment significantly induced endothelia death. Furthermore, we demonstrated that mitochondrial fission was the injury signal directing endothelia apoptosis under LPS treatment. LPS induced mitochondrial fission as evidenced by mitochondrial fragmentations and debris, leading to the mitochondrial potential collapse, excessive mPTP opening, and proapoptotic cyt-c leakage into the cytoplasm. These data firstly link the mitochondrial fission to the inflammation-mediated endothelia apoptosis. However, whether mitochondrial fission contributes to the development and progression of CVeD needs more clinical evidence. Our data in the present study provided the possible role of mitochondrial fission in inflammatory response.
Notably, mitochondrial fission is regulated by the Drp1 (Hall et al. 2014). It is a large GTPase that controls mitochondrial membrane tubulation and fission in mammalian cells. Drp1 forms oligomers and is recruited to the outer mitochondrial membrane to mediate fission in a GTP-dependent manner (Cho et al. 2010). And Drp1 phosphorylation is the prerequisite for its mitochondrial translocation and subsequent fission (Jahani-Asl and Slack 2007). In our study, we found that the upstream signal for Drp1 phosphorylation was ROS. Excessive ROS induced the posttranscription modification of Drp1 at the Ser616 site. Moreover, ROS also elevated the expression of mitochondrially located Drp1, suggestive of the activated Drp1. In fact, ample evidence has explored the regulator for Drp1 such as ERK, JNK, and ROS (Michalska et al. 2016; Xu et al. 2016). Our study further confirmed that ROS had the ability to regulate the phosphorylation of Drp1. Meanwhile, our study also illustrated the regulatory effect of cellular oxidative stress on mitochondrial homeostasis. In previous studies, the majority of studies argued that the mitochondria is the source of ROS and, therefore, is responsible for the cellular damage via induction of oxidative stress (Byon et al. 2016; Du et al. 2016). However, according to the present findings, ROS has the ability to modify the mitochondria itself via mitochondrial fission. This information hints that the mitochondria is not only the origin of ROS but also the target of attack by ROS.
Furthermore, we demonstrated that the ROS outburst and Drp1 phosphorylation were owing to XO upregulation and calcium overload. It is now appreciated that there are various sources of ROS in the endothelia, including the mitochondria, NAD(P)H oxidase, XO, and autoxidation of diverse substances (Du et al. 2016). The relatively low abundance of mitochondria in endothelial cells has suggested that they are unlikely to contribute significantly to ROS production (Joshi et al. 2015). Notably, previous studies have confirmed that XO is primarily located in the luminal surface of the endothelium of many organs (Jarasch et al. 1981). This information indicates that ROS overproduction and oxidative stress in response to LPS-treated endothelia may be attributed to XO. Indeed, in our study, we found that LPS augmented the XO expression. These data provide us an overlooked detail that XO is the potential target to intervene against oxidative stress. More importantly, XO-derived ROS can regulate the mitochondrial homeostasis which adds more information about the upstream molecular of mitochondrial fission. However, more evidence is needed to support our notion.
To explain the reason by which LPS regulated the XO, we focused on calcium due to the fact that XO is a calcium concentration-dependent, superoxide-producing enzyme that is localized to the ER (Sheikh-Ali et al. 2010). We demonstrated that XO expression was regulated by the calcium level, which was similar to a previous study (Zhang et al. 2016). Notably, in the present study, we did not explain the detailed mechanism by which calcium overload elevated the XO expression. We think that the CaMKII may be involved in this process. Previous studies have found that XO expression could be upregulated via CaMKII (Bodhinathan et al. 2010). However, whether CaMKII is also associated with the LPS-mediated XO upregulation requires more studies to investigate.
In the present study, we used melatonin to intervene the LPS-mediated endothelia apoptosis. The circadian hormone melatonin is primarily synthesized and secreted by the pineal gland depending on the daylight and darkness cycle with a peak in the night even in nocturnal animals (Agorastos and Linthorst 2016). Several studies have found that melatonin protects cells against stress-mediated damage via antiapoptotic and antioxidant effects. Melatonin enhances the resistance of small intestinal microvasculature to systemic inflammation induced by LPS via regulation of inflammation response (Lansink et al. 2017). Moreover, it also protects against blood-brain barrier damage by inhibiting the TLR4 signaling pathway after LPS treatment in neonatal rats (Hu et al. 2017). Besides, melatonin also modulates the inflammation response through endoplasmic reticulum stress and autophagy (Carloni et al. 2016). Furthermore, it also protects the endothelial barrier function under inflammation via blocking p38 MAPK activation (Chu et al. 2016). These data indicate that melatonin has a beneficial effect on the endothelia under an inflammatory environment.
In our study, we demonstrated that melatonin increased the survival of endothelia via inhibition of mitochondrial fission for the first time, which enriches the protective mechanism of melatonin on inflammation-mediated endothelia damage. Notably, the dosage of melatonin used in our experiment is higher than the physiologic concentration in the human body. There are two reasons for this discrepancy. Firstly, melatonin is regularly released from the pineal gland every night. However, in the present study, melatonin was applied only one time. With the time going, the melatonin concentration in the medium declined gradually although we did not measure its concentration. Secondly, we had to emphasize that cellular apoptosis would not happen in the physiologic condition. More importantly, in the in vitro experiment, LPS induced acute cellular damage. Therefore, we had to increase the treatment concentration of melatonin. Furthermore, we determined that the AMPK/SERCA2a pathways were the defensive system enhanced by melatonin against LPS-induced cellular mitochondrial apoptosis. AMPK is the sensor of cellular energy metabolism and several lines of evidence have explained the relationship between melatonin and AMPK activation (Kang et al. 2016; Yang et al. 2016; Zhou et al. 2017). In our study, melatonin increased AMPK activity which was accompanied by the upregulation of SERCA2a. As the most important calcium reuptake channel in ER, SERCA2a contributes 92% of the released Ca2+ reabsorption to the ER, which is the keeper of intracellular calcium homeostasis (Marks 2013). Apart from its expression, melatonin also reversed its activity via AMPK. Because SERCA2a, a calcium-ATPase, is an ATP-driven protein that inversely transports calcium back to the ER, its activity is determined by the intracellular ATP content. The activated AMPK pathway under melatonin treatment significantly enhanced the cellular ATP generation and therefore contributed to the increase in SERCA2a activity. However, inhibition of AMPK or SERCA2a partly caused the rebound of calcium overload, suggesting that AMPK was a defender activated by melatonin to counteract the Ca2+-XO-ROS-Drp1 pathway. Thereby, these findings provide a possible solution and means to intervene against endothelia apoptosis induced by inflammation.
Collectively, the results of our report illustrated the important role of mitochondrial fission in the LPS-mediated HUVEC apoptosis. Mitochondrial fission was signaled by the Ca2+-XO-ROS-Drp1 axis and contributed to the caspase9-dependent mitochondrial apoptosis. Our data provided evidence for the relationship between caspase9-dependent mitochondrial apoptosis, Drp1-required mitochondrial fission, XO-related oxidative stress, and SERCA2a-involved calcium homeostasis, which offered a potential target to prevent LPS-induced endothelia damage. Meanwhile, we also introduced melatonin to reduce endothelia inflammatory damage. Melatonin activated the AMPK/SERCA2a pathway to partly block the Ca2+-XO-ROS-Drp1-mitochondrial fission pathways, enhancing the resistance of endothelia to LPS-mediated apoptosis. Thus, the use of melatonin is a practice and efficient adjuvant for the treatment of endothelia inflammation in CVeD. However, more insights should be obtained to provide ample evidence for clinical applications.
Funding information
This study was supported by grants from the National Natural Science Foundation of China (No. 811126497). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Jiasen Cui, Zeng Li contributed equally to this study.
References
- Agorastos A, Linthorst AC. Potential pleiotropic beneficial effects of adjuvant melatonergic treatment in posttraumatic stress disorder. J Pineal Res. 2016;61:3–26. doi: 10.1111/jpi.12330. [DOI] [PubMed] [Google Scholar]
- Bergan J. Molecular mechanisms in chronic venous insufficiency. Ann Vasc Surg. 2007;21:260–266. doi: 10.1016/j.avsg.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP, Cadenas E, Duchen MR, Hampton MB, Mann GE, Murphy MP. Introduction to special issue on mitochondrial redox signaling in health and disease. Free Radic Biol Med. 2016;100:1–4. doi: 10.1016/j.freeradbiomed.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–253. doi: 10.1016/j.redox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, et al. Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J Pineal Res. 2016;61:370–380. doi: 10.1111/jpi.12354. [DOI] [PubMed] [Google Scholar]
- Chi YW, Schul M, Gibson K, Rosenblatt M, Kabnick L, Jaff M. Chronic venous disorder registry: a new perspective. Phlebology. 2014;29:415–427. doi: 10.1177/0268355513484143. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci. 2010;67:3435–3447. doi: 10.1007/s00018-010-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LY, Wang YF, Cheng HH, Kuo CC, Wu KK. Endothelium-derived 5-methoxytryptophan protects endothelial barrier function by blocking p38 MAPK activation. PLoS One. 2016;11:e0152166. doi: 10.1371/journal.pone.0152166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, et al. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J Pineal Res. 2016;60:253–262. doi: 10.1111/jpi.12300. [DOI] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148–156. doi: 10.1016/j.redox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippo KH, Strack S. Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci. 2017;130:671–681. doi: 10.1242/jcs.171017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlof O et al (2016) Ischemic preconditioning confers epigenetic repression of Mtor and induction of autophagy through G9a-dependent H3K9 dimethylation. J Am Heart Assoc 5. 10.1161/JAHA.116.004076 [DOI] [PMC free article] [PubMed]
- Gorojod RM, Alaimo A, Porte Alcon S, Pomilio C, Saravia F, Kotler ML. The autophagic-lysosomal pathway determines the fate of glial cells under manganese-induced oxidative stress conditions. Free Radic Biol Med. 2015;87:237–251. doi: 10.1016/j.freeradbiomed.2015.06.034. [DOI] [PubMed] [Google Scholar]
- Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan A. Management of varicose veins and venous insufficiency. JAMA. 2012;308:2612–2621. doi: 10.1001/jama.2012.111352. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. Melatonin protects against blood-brain barrier damage by inhibiting the TLR4/NF-kappaB signaling pathway after LPS treatment in neonatal rats. Oncotarget. 2017;8:31638–31654. doi: 10.18632/oncotarget.15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, et al. Mitochondrial fission forms a positive feedback loop with cytosolic calcium signaling pathway to promote autophagy in hepatocellular carcinoma cells. Cancer Lett. 2017;403:108–118. doi: 10.1016/j.canlet.2017.05.034. [DOI] [PubMed] [Google Scholar]
- Jahani-Asl A, Slack RS. The phosphorylation state of Drp1 determines cell fate. EMBO Rep. 2007;8:912–913. doi: 10.1038/sj.embor.7401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981;25:67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Joshi MS, et al. Role of mitochondrial dysfunction in hyperglycaemia-induced coronary microvascular dysfunction: protective role of resveratrol. Diab Vasc Dis Res. 2015;12:208–216. doi: 10.1177/1479164114565629. [DOI] [PubMed] [Google Scholar]
- Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res. 2016;60:383–393. doi: 10.1111/jpi.12319. [DOI] [PubMed] [Google Scholar]
- Lansink MO, Patyk V, de Groot H, Effenberger-Neidnicht K. Melatonin reduces changes to small intestinal microvasculature during systemic inflammation. J Surg Res. 2017;211:114–125. doi: 10.1016/j.jss.2016.11.055. [DOI] [PubMed] [Google Scholar]
- Li C, et al. Ulinastatin attenuates LPS-induced human endothelial cells oxidative damage through suppressing JNK/c-Jun signaling pathway. Biochem Biophys Res Commun. 2016;474:572–578. doi: 10.1016/j.bbrc.2016.04.104. [DOI] [PubMed] [Google Scholar]
- Li H, et al. The metabolites of glutamine prevent hydroxyl radical-induced apoptosis through inhibiting mitochondria and calcium ion involved pathways in fish erythrocytes. Free Radic Biol Med. 2016;92:126–140. doi: 10.1016/j.freeradbiomed.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Lin C, et al. Melatonin attenuates traumatic brain injury-induced inflammation: a possible role for mitophagy. J Pineal Res. 2016;61:177–186. doi: 10.1111/jpi.12337. [DOI] [PubMed] [Google Scholar]
- Manchester LC, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. 2015;59:403–419. doi: 10.1111/jpi.12267. [DOI] [PubMed] [Google Scholar]
- Mannello F, Raffetto JD. Matrix metalloproteinase activity and glycosaminoglycans in chronic venous disease: the linkage among cell biology, pathology and translational research. Am J Transl Res. 2011;3:149–158. [PMC free article] [PubMed] [Google Scholar]
- Mannello F, Ligi D, Canale M, Raffetto JD. Sulodexide down-regulates the release of cytokines, chemokines, and leukocyte colony stimulating factors from human macrophages: role of glycosaminoglycans in inflammatory pathways of chronic venous disease. Curr Vasc Pharmacol. 2014;12:173–185. doi: 10.2174/1570161111666131126144025. [DOI] [PubMed] [Google Scholar]
- Mao L, et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J Pineal Res. 2016;60:167–177. doi: 10.1111/jpi.12298. [DOI] [PubMed] [Google Scholar]
- Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska B, Duszynski J, Szymanski J. Mechanism of mitochondrial fission—structure and function of Drp1 protein. Postepy Biochem. 2016;62:127–137. [PubMed] [Google Scholar]
- Mukherjee D, et al. Mechanisms of isoproterenol-induced cardiac mitochondrial damage: protective actions of melatonin. J Pineal Res. 2015;58:275–290. doi: 10.1111/jpi.12213. [DOI] [PubMed] [Google Scholar]
- Nduhirabandi F, Lamont K, Albertyn Z, Opie LH, Lecour S. Role of toll-like receptor 4 in melatonin-induced cardioprotection. J Pineal Res. 2016;60:39–47. doi: 10.1111/jpi.12286. [DOI] [PubMed] [Google Scholar]
- Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides AN, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27:1–59. [PubMed] [Google Scholar]
- Osborne B, Bentley NL, Montgomery MK, Turner N. The role of mitochondrial sirtuins in health and disease. Free Radic Biol Med. 2016;100:164–174. doi: 10.1016/j.freeradbiomed.2016.04.197. [DOI] [PubMed] [Google Scholar]
- Pannier F, Rabe E. The relevance of the natural history of varicose veins and refunded care. Phlebology. 2012;27(Suppl 1):23–26. doi: 10.1258/phleb.2012.012s23. [DOI] [PubMed] [Google Scholar]
- Park J, et al. Peroxiredoxin 5 (Prx5) decreases LPS-induced microglial activation through regulation of Ca2+/calcineurin-Drp1-dependent mitochondrial fission. Free Radic Biol Med. 2016;99:392–404. doi: 10.1016/j.freeradbiomed.2016.08.030. [DOI] [PubMed] [Google Scholar]
- Peleli M, et al. Enhanced XOR activity in eNOS-deficient mice: effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radic Biol Med. 2016;99:472–484. doi: 10.1016/j.freeradbiomed.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Quijano C, Trujillo M, Castro L, Trostchansky A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016;8:28–42. doi: 10.1016/j.redox.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffetto JD. Inflammation in chronic venous ulcers. Phlebology. 2013;28(Suppl 1):61–67. doi: 10.1177/0268355513476844. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr Vasc Pharmacol. 2008;6:158–172. doi: 10.2174/157016108784911957. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- Rizzo NR, Hank NC, Zhang J. Detecting presence of cardiovascular disease through mitochondria respiration as depicted through biophotonic emission. Redox Biol. 2016;8:11–17. doi: 10.1016/j.redox.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor T. Pathomechanism of chronic venous insufficiency and leg ulcer. Acta Physiol Hung. 2004;91:131–145. doi: 10.1556/APhysiol.91.2004.2.5. [DOI] [PubMed] [Google Scholar]
- Santa-Gonzalez GA, Gomez-Molina A, Arcos-Burgos M, Meyer JN, Camargo M. Distinctive adaptive response to repeated exposure to hydrogen peroxide associated with upregulation of DNA repair genes and cell cycle arrest. Redox Biol. 2016;9:124–133. doi: 10.1016/j.redox.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh-Ali M, Sultan S, Alamir AR, Haas MJ, Mooradian AD. Hyperglycemia-induced endoplasmic reticulum stress in endothelial cells. Nutrition. 2010;26:1146–1150. doi: 10.1016/j.nut.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Simka M. Cellular and molecular mechanisms of venous leg ulcers development—the “puzzle” theory. Int Angiol. 2010;29:1–19. [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Forman HJ. Redox homeostasis: the golden mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Zhang C, Xing XH. Xanthine dehydrogenase: an old enzyme with new knowledge and prospects. Bioengineered. 2016;7:395–405. doi: 10.1080/21655979.2016.1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Davies KE, Oliver PL. The antioxidant protein Oxr1 influences aspects of mitochondrial morphology. Free Radic Biol Med. 2016;95:255–267. doi: 10.1016/j.freeradbiomed.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, et al. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J Pineal Res. 2016;60:291–302. doi: 10.1111/jpi.12310. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Melatonin reverses flow shear stress-induced injury in bone marrow mesenchymal stem cells via activation of AMP-activated protein kinase signaling. J Pineal Res. 2016;60:228–241. doi: 10.1111/jpi.12306. [DOI] [PubMed] [Google Scholar]
- Zhang J. Teaching the basics of autophagy and mitophagy to redox biologists—mechanisms and experimental approaches. Redox Biol. 2015;4:242–259. doi: 10.1016/j.redox.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Shang D, Zhang Y, Tian Y. Interleukin-22 suppresses the growth of A498 renal cell carcinoma cells via regulation of STAT1 pathway. PLoS One. 2011;6:e20382. doi: 10.1371/journal.pone.0020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 2016;95:278–292. doi: 10.1016/j.freeradbiomed.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Zhou H et al (2017) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63. 10.1111/jpi.12413 [DOI] [PMC free article] [PubMed]
- Zhu H et al (2017) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones. 10.1007/s12192-017-0827-4 [DOI] [PMC free article] [PubMed]