Abstract
Self-assembly is a promising route for micro- and nano-fabrication with potential to revolutionise many areas of technology, including personalised medicine. Here we demonstrate that external control of the swimming speed of microswimmers can be used to self assemble reconfigurable designer structures in situ. We implement such ‘smart templated active self assembly’ in a fluid environment by using spatially patterned light fields to control photon-powered strains of motile Escherichia coli bacteria. The physics and biology governing the sharpness and formation speed of patterns is investigated using a bespoke strain designed to respond quickly to changes in light intensity. Our protocol provides a distinct paradigm for self-assembly of structures on the 10 μm to mm scale.
The ability to generate microscale patterns and control microswimmers may be useful for engineering smart materials. Here Arlt et al. use genetically modified bacteria with fast response to changes in light intensity to produce light-induced patterns.
Introduction
There are two conceptually distinct ways to construct nano- and micro-structures: ‘top-down’ techniques such as lithography use ‘scalpels’ (chemical etching or electron beams, etc.), whereas microscopic ‘Lego components’ move themselves into position—self assemble—in ‘bottom-up’ techniques1. Both equilibrium phase transitions (e.g., crystallisation) and non-equilibrium processes are exploited for self assembly. In either case, external templates can be used to direct the process, with reconfigurable templates offering programmability2. Self-assembly was originally inspired by viral biology, where the components are biomacromolecules3. Increasingly, synthetic colloidal building blocks are used, with bespoke particle shape, size and interaction, e.g., ‘patchy particles’ with heterogeneous surface chemistry4. Active, or self-propelled, colloids open up further opportunities5, 6.
Such self-propelled micro-swimmers are attracting significant recent attention7 as ‘active matter’8, 9. They violate time-reversal symmetry10 and may be used to transport colloidal ‘cargos’11, 12. For both fundamental physics and applications, external control of swimming, e.g., using particles with light-activated self-propulsion13–16, opens up many new possibilities. For example, light-activated motile bacteria17 can be used to actuate and control micro-machinery18.
The self-assembly of micro-swimmers into clusters of tens of particles has already been demonstrated16, 19, 20. Recent simulations21 suggest that the patterned illumination of light-activated swimmers can be used for the templated self assembly1 of designer structures comprising 103–104 particles. Real-time reconfiguration of the light field would then allow smart templated active self assembly (STASA), which we here implement for the first time using light-controlled motile bacteria17.
An E. coli bacterium swims22 by turning ≈ 7–10 μm long helical flagella attached to the cell body (≈ 2 μm × 1 μm) using membrane-embedded rotary motors powered by a protonmotive force (PMF), which arises from the active pumping of H+ to the extracellular medium23. Unlike all synthetic active colloids to date and most bacteria, E. coli can generate PMF in nutrient-free motility buffer24 by utilising internal resources and oxygen (O2) to produce energetic electron pairs. These release their energy stepwise along an electrochemical potential ladder of respiratory enzymes located in the inner cell membrane, generating a PMF of ≈ − 150 mV. The electron pair ultimately passes to and reduces O2 to water. Thus, with no O2, PMF = 0 and swimming ceases22. If cells under anaerobic conditions can express proteorhodopsin (PR)17, a green-photon-driven proton pump25, then they will swim only when suitably illuminated: these are living analogues of synthetic light-activated microswimmers13–16.
In the following we demonstrate STASA using light-controlled E. coli bacteria. We show that the rapidity with which such bacteria react to changes in light intensity has a major influence on the pattern formation, and we construct a bespoke strain for which this response is fast on the 1 s time scale. We characterise the dynamics of pattern formation and explain the different scaling laws observed for protocols in which the bacteria are either initially stationary or already swimming. Finally, we explore the physics controlling the sharpness and resolution of patterns.
Results
Temporal response of light-controlled E. coli strains
We suspended cells in phosphate motility buffer at optical density OD ≲ 8 (cell-body volume fraction ≈ 1.1%)22 and sealed 2 μl into 20 μm-high, flat capillaries, where cells swim in two dimensions but have enough room to ‘overtake’ each other in all three spatial dimensions. Differential dynamic microscopy (DDM)26 returned an averaged speed μm s−1 and a non-motile fraction β ≈ 20% at OD = 1 under fully oxygenated conditions. (We use ‘non-motile’ to refer to cells that can never swim and ‘stationary’ for non-swimming cells capable of motility when illuminated.)
Motile cells were allowed to swim until O2 was depleted and dropped abruptly to zero after a few minutes22 (see Supplementary Fig. 1). After these cells were left in the dark for ≈ 10 min, green illumination was turned on (510–560 nm, intensity mW cm−2 at the sample). The stationary cells accelerated uniformly before saturating in speed (Fig. 1a). Fitting the data for freshly prepared cells to gives μm s−1, τon = 30 s. can be controlled by the illumination intensity (Fig. 1a, inset), up to ≲ 28 μm s−1. We find that changed over hours as cells aged.
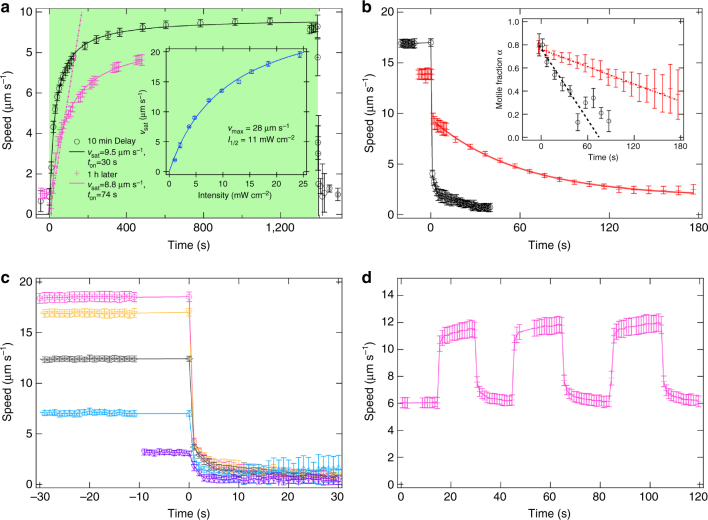
Fig. 1.
Response to light. a Population-averaged speed vs. time for initially stationary E. coli AD10, light on at t = 0 and off at t ≈ 1,400 s (black circles); green shading = illuminated, and the data point just after switch-off are 1 s apart. This sample was measured soon after the cells had run out of O2. One hour later, cells accelerated less rapidly (magenta crosses). Full lines: fits to . The dot-dashed line shows the acceleration (0.05 μm s−2) obtained from ‘box emptying’ experiments starting from stationary cells. Inset: dependence of on incident light intensity (points) fitted to (see Supplementary Note 1). b Strain AD10 with unc-cluster deletion shows a rapid decrease in when illumination ceases (black circles). In a strain without unc-cluster deletion (AD57), a significant fraction of the population (see inset for the motile fraction α = 1 − β, where lines are guides to the eye) continues to swim at reduced speed, resulting in a much slower decay of (red crosses; solid red line: offset exponential fit, giving τ ≈ 60 s). c Average swimming speed of AD10 as green light is switched off (at t = 0): τoff ≈ 1 s independent of the initial swimming speed (at the resolution limit of our technique). d AD10 bacteria adapt their swimming speed within 1 s as the green light is alternated between low and high intensity. In this case τon ≈ τoff. Error bars in all these plots depict SD from the mean speed over a range of q = 0.5–1.5 μm−1
What happens when illumination ceases can be optimised by genetic modification. In the strain we constructed for this work (AD10), in which the unc gene cluster has been deleted (see Methods), has dropped sharply by the time the first data point was taken after the illumination was switched off, so that τoff < 1 s, the sampling period in this part of the experiment (Fig. 1b). It is unclear why a few cells (< 1%) continued to swim, so that never quite reached zero. In a strain without unc deletion (AD57), dropped much slower upon cessation of illumination (Fig. 1b) as seen previously17, 27. The unc cluster codes for F1Fo-ATPase, which is normally powered by the PMF to generate ATP. However, in darkness the unc wild type can use this membrane protein complex in reverse to export protons and so sustains a PMF for some time28; this is not possible with unc deletion29.
In the absence of any source of PMF in darkness, τoff should be controlled by the discharging of the PMF through an effective resistor–capacitor circuit (see Supplementary Note 1 for details). The effective membrane capacitance is C ≳ 10−14 F. The effective resistance, Rtot, combines in parallel the resistance of the membrane, the motors (each of which is Rmot ≲ 1014 Ω) and other transmembrane components, so that Rtot < Rmot is an upper bound, and we expect RtotC < 1 s, as observed. Consistent with this interpretation, τoff is approximately independent of the starting speed of decelerating cells at our experimental time resolution (Fig. 1c).
The fitted ‘on time’ for freshly prepared cells, τon ≈ 30 s (Fig. 1a, black points), is likely controlled by the rate constant30 for stator units to come on and off motors, . In sustained darkness, motors disassemble in PR-bearing E. coli, and full ‘motor resurrection’ upon illumination takes ~ 200 s 27, in agreement with our data (Fig. 1a). Consistent with this, if illumination is lowered and then restored rapidly (i.e., within a time interval ), motors do not have time to disassemble and τon ≈ τoff (Fig. 1d).
Smart templated active self assembly
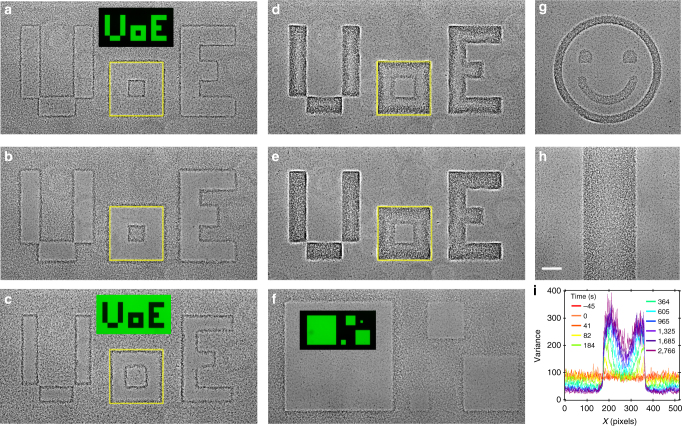
Our STASA protocol uses a digital mirror device to project patterns of illumination onto a uniform field of cells rendered stationary by O2 exhaustion. We first projected a positive mask with bright features on a dark background (Fig. 2a, inset). The width of the features, 90 μm, is comparable to the persistence length of the smooth-swimming E. coli strains used in this work (i.e., the length scale over which cells effectively swims in straight lines)31. Over time, the mask pattern was replicated in the cell population with increasing clarity: cells swim out of illuminated areas, stop within τoff ~ 1 s and accumulate at the edges just outside the boundary of these areas (Fig. 2b and see also Supplementary Movie 1). Next, we abruptly switched to a negative mask with the same pattern (dark pattern against bright background; Fig. 2c, inset). Edge-accumulated cells start swimming, about half of which arrive inside the dark features immediately and stop within τoff, rapidly outlining the inside edge of the masked areas (Fig. 2c). Thereafter, the dark areas progressively filled up: bacteria continually arrive from the outside, which acts as an essentially infinite reservoir of swimming cells (Fig. 2d, e and see also Supplementary Movies 2 and 3). Other patterns can be ‘painted’ using this method (Fig. 2g), their shape can be updated in situ and the patterns can be erased by illuminating them with a uniform light field (see Supplementary Movie 4). In contrast to the recently demonstrated painting of one-off polychromatic patterns using mixed colonies of differently coloured bacteria on solid agar32, our method produces dynamic monochrome patterns that are reconfigurable in real time inside a liquid medium.
Fig. 2.
Smart templated active self-assembly. Positive and negative masks projected onto initially stationary cells: a sample illuminated for 1 min with a positive ‘UoE’ (University of Edinburgh) pattern shown in inset, b same after 9 min of illumination, c 1 min after switching to the negative pattern shown in inset (with green = bright), as well as d 6 min and e 12 min after switching. Yellow square = boundary between light and dark regions of the ‘o’. f Samples illuminated with pattern of squares shown in inset. g Negative smiley pattern. h Negative strip together with i time evolution of the strip’s density profile. Scale bar in h is 100 μm and applies throughout
Dynamics of pattern formation
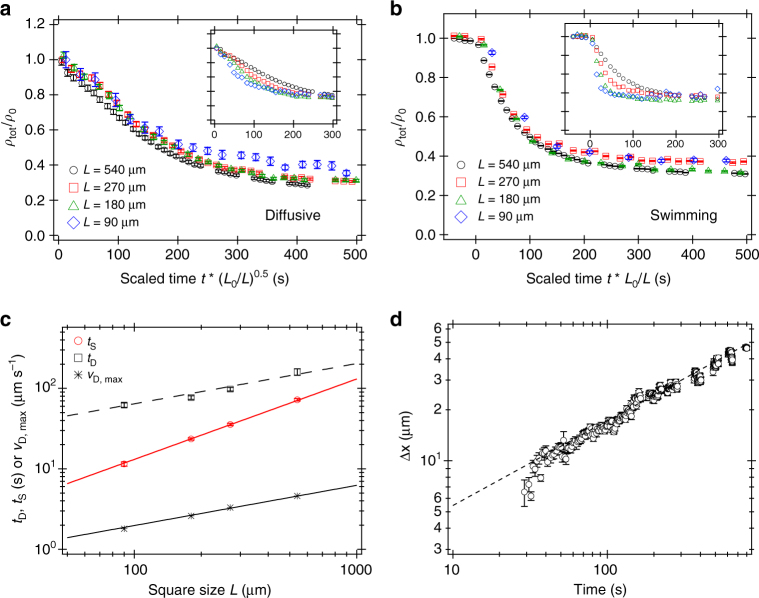
To understand how fast spatial patterns can be established, we experimented with square masks (Fig. 2f) starting from two initial conditions: a (dark) field of stationary cells (, i.e., diffusive) and a field of (illuminated) cells that have reached speed saturation (). We studied the evolution of cell density (see Methods) and the speed inside bright squares against a dark background. Starting with diffusive cells, with for t ≲ τon. The time to empty an L × L square should thus scale as
| 1 |
The measured evolution of the density of 1 h-old cells, ρtot(t), in illuminated squares of different sizes is shown in Fig. 3a, from which we extracted tD, the time for ρtot to drop to 1/e of its decay. As expected, (Fig. 3c). Fitting Eq. (1) gives a = 0.05 μm s−2, which is a reasonable average acceleration over the first ~ 100 s for the magenta data points in Fig. 1 for 1 h-old cells (where the dot-dash line has slope 0.05 μm s−2). Moreover, , the maximum average swimming speed reached during emptying depends on the box size, displaying a L0.5 scaling, which is also consistent with our model (Fig. 3c and Supplementary Note 2).
Fig. 3.
Characterising the dynamics of pattern formation. Time evolution of normalised bacterial density (as measured by DDM, see Methods) inside squares of different dimensions L (inset, Fig. 2f), starting from a diffusive state and b uniformly swimming state. Inset: data plotted against time; main plots: data collapse when scaling time by in a and L0/L in b (both with L0 = 540 μm). c Scaling of ‘box emptying times’ tD, tS, as well as (the maximum swimming speed reached when starting with diffusive cells) with square size L. d Shift of the peak position over time in the dark stripe (Fig. 2h); dashed line = power law fit of slope 0.5. Error bars in a, b are SE of the q-averages and in c, d the SD estimates returned by the fitting routines
On the other hand, starting with cells all swimming (on average) at , a square should empty in
| 2 |
From the measured ρtot(t) in this case (Fig. 3b), we deduced a ‘box emptying time’ and find (Fig. 3c) the expected scaling, . Fitting Eq. (2) gives μm s−1, consistent with the measured μm s−1. Equations (1) and (2) are also confirmed by the data collapse achieved for ρtot(t) when we scale the time axis by L0.5 and L, respectively (Fig. 3a, b).
Further insight on the time scale for feature formation comes from studying cells moving into a dark area. We projected a 270 μm-wide dark strip onto a field of initially diffusive cells (Fig. 2h). An edge rapidly developed just inside the darkened area, corresponding to twin peaks in the spatial cell density profile (Fig. 2i; as measured by the variance of image intensity, see Methods), which broaden and fill the stripe over time. The squared displacement of this peak shows diffusive dynamics, Δx2 = 2Dt (Fig. 3d) with a fitted D = 0.74 μm2 s−1. The interface between bright and dark areas acts as an absorber of swimmers arriving with an effective diffusive process. Owing to conservation of mass, the diffusive propagation of the peak is thus expected, although quantitative aspects remain to be elucidated.
Pattern sharpness and resolution
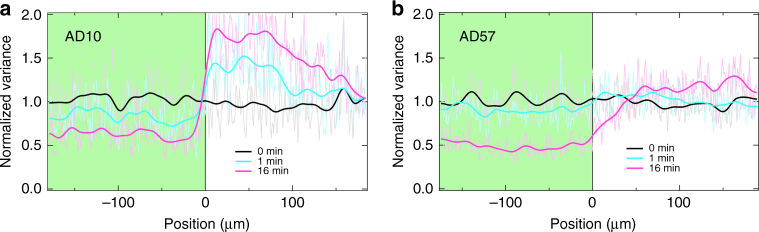
Finally, we seek to understand what controls the sharpness of the self-assembled features using our method. A stark demonstration of the answer comes from projecting a step-function pattern of light–dark illumination on initially uniformly swimming cells (see Supplementary Note 3 for details). Figure 4 shows the resulting cell density profile (from the intensity variance, see Methods) for two strains, AD10, with τstop ≲ 1 s, and AD57, a strain lacking the unc cluster deletion and having τoff > 1 min. In the case of AD10, the illumination pattern is clearly replicated in cell density within 1 min and the steady state shows a sharp feature with half width ≲ 10 μm. For AD57, it takes min to establish a corresponding density pattern, which, in the steady state, is ≳ 50 μm wide. Thus, a short enough τoff, or more specifically a short mean stopping distance , is necessary for the fast establishment of sharp features using our protocol.
Fig. 4.
Effect of stopping distance. Response to a step illumination pattern (left bright, right dark) projected at time t = 0 by two strains starting from initially uniformly swimming cells. Faint lines show the normalised variance (a measure of cell density) profile, whereas bold lines show numerically smoothed profiles. a AD10 with τoff ≲ 1 s and b AD57 with τoff ≳ 1 min (corresponding images in Supplementary Fig. 3)
The resolution achievable with STASA depends on the mean stopping distance of the bacteria, as well as the sharpness and contrast of the projected light template. In our current experimental implementation, the optics projecting the templating pattern is designed to illuminate a large area of the sample at the expense of spatial resolution. This allowed us to reliably construct features of about 10 μm in width (Fig. 5). However, ultimately the stopping distance will prove the practical limit of STASA, which at low swimming speeds is comparable to the size of individual bacteria. The use of more complex grayscale templates and dynamically adjustable patterns will help reaching this limit more rapidly and reliably.
Fig. 5.
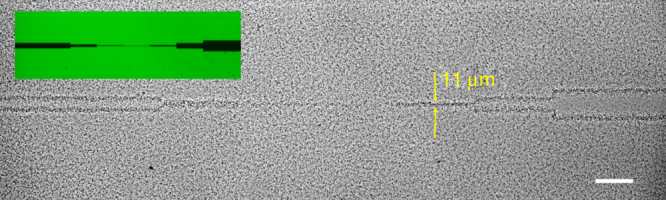
Spatial resolution for AD10 strain. A pattern of dark lines of different width (see inset; nominal line width on the sample from left to right: 45, 22, 6, 3, 11, 45 and 90 μm) is projected onto a dense (OD ≈ 8) sample of AD10. At low swimming speeds (v = 2.7 μm s−1) the 11 μm-wide line still shows up clearly while the density contrast for the thinner lines is very low. Scale bar: 100 μm
Discussion
We have demonstrated how to perform smart templated self assembly using a proteorhodopsin-bearing strain of E. coli with unc cluster deletion dispersed in anaerobic motility buffer. Spatial patterns of light are reproduced sharply as patterns in cell density. The time scale of pattern formation is controlled by the (saturation) swimming speed and the ‘on time’, and the sharpness of the features by the ‘off time’. Once established, the patterns persist for as long as the light template is applied. Uniform light can be used to quickly erase the patterning, whereas removing the light completely will lead to a slow diffusion driven ‘dissolution’ of the pattern.
The successful implementation of this protocol opens up many opportunities. In terms of fundamental active matter physics, it should now be possible to test the fundamental prediction that the product of density and speed is constant in a system with spatially dependent activity33. For practical applications, it should now be possible to demonstrate an ‘all biological’ version21 of the controlled actuation of lithographed micro-devices using light-activated bacteria18: STASA allows the assembly and real-time reconfiguration of the devices themselves using the same bacteria used to actuate the devices. On the other hand, if high cell density is used to trigger the secretion of adhesive biopolymers, and/or bacteria can be turned into microparticles of refractory ceramic-precursor materials such as silica34, then bacterial STASA offers a route for manufacturing bespoke 10–100 μm microstructures35, which remains a challenging length scale for current additive manufacturing methods.
Methods
Bacterial strains
Both AD10 and AD57 are smooth-swimming mutants, which were constructed using our laboratory stock of E.coli AB115736. For AD10, all of the genes present within the operon encoding the ATP synthase complex (atpI, atpB, atpE, atpF, atpH, atpA, atpG, atpD, atpC) were deleted in a single step by allelic replacement using a recombinant pTOF24 vector37 containing 400 bp homology arms flanking the deletion on each side. This resulted in the Δunc strain AD10 with a deletion of 7504 bp (corresponding to position 3,915,552–3,923,056 of the E.coli K12 MG1655 chromosome). P1 transduction was used to delete the cheY gene in both this strain and the parental strain (to generate AD57) using JW1871 (BW25113 cheY) as donor38. Diagnostic PCR reactions were performed to confirm genotypes. Both strains were transformed with plasmid pBAD-His C (a kind gift from Jan Liphardt, UC Berkley) encoding the SAR86 γ-proteobacterial photorhodopsin variant27.
Sample preparation
Overnight cultures were grown aerobically in 10 ml Luria–Bertani broth using an orbital shaker at 30 °C and 200 r.p.m. A fresh culture was inoculated as 1 : 100 dilution of overnight-grown cells in 35 ml tryptone broth and grown for 4 h, by which point AD10 has reached an optical density of OD600 ≈ 0.2 (whereas AD57 reached OD ≈ 0.35). The production of PR was induced by adding arabinose to a concentration of 1 mM, as well as the necessary cofactor all-trans-retinal to 10 μM in the growth medium. Cells were incubated under the same conditions for a further hour to allow protein expression to take place and then transferred to motility buffer (MB, pH 7.0, 6.2 mM K2HPO4, 3.8 mM KH2PO4, 67 mM NaCl and 0.1 mM EDTA). Cells were re-suspended in MB after a single filtration (0.45 μm HATF filter, Millipore), yielding high-concentration stock solutions (OD ≈ 8–10), which were then diluted with MB as required.
Suspensions were loaded into 2 μl sample chambers (SC-20-01-08-B, Leja, NL, ≈ 6 mm × 10 mm × 20 μm), giving a quasi-two-dimensional environment for E. coli swimming. The chamber was sealed using vaseline to stop flow and oxygen supply, and placed onto a microscope for video recording. E. coli initially swims under endogenous metabolism, which depletes the oxygen within the sample cells22. Once oxygen is depleted, the cells stop swimming unless illuminated by green light.
Optical setup
The samples were observed using a Nikon TE2000 inverted microscope with a phase-contrast objective (PF 10×/0.3). Time series of ≈ 40 s movies were recorded at 100 frames per second using a fast CMOS camera (MC 1362, Mikrotron). A long-pass filter (RG630, Schott Glass) was introduced into the bright-field light path to ensure that the light required for imaging did not activate PR. The light controlling the swimming behaviour of the bacteria was provided by an LED light source (Sola SE II, Lumencor) filtered to a green wavelength range (510–560 nm) to overlap with the absorption peak of PR17. This light was reflected off a digital mirror device (DLP6500, Texas Instruments), which was imaged onto the microscope sample plane using demagnifying relay optics (see Supplementary Fig. 4 for a schematic of the complete optical setup). The light was coupled into the transmission path by introducing a dichroic mirror in between the long working distance phase-contrast condenser and the sample. This ‘trans’ geometry allowed us to uniformly illuminate a circular area of ≈ 2.9 mm in diameter, larger than the objective’s field of view. As the digital mirror chip is conjugate with the sample plane, any pattern applied to it is directly imaged onto the sample, with the edge length of a digital mirror ‘pixel’ corresponding to 2.7 μm. Computer control of the pattern displayed on the digital mirror device gave us precise spatial and temporal control of the illumination pattern. For the work presented here, we restricted ourselves to binary patterns and simply toggled between a few selected patterns, although the setup is capable of projecting dynamic grayscale patterns.
Motility and density measurements
The motility of the samples was quantified using DDM, a fast high-throughput technique for measuring the dynamics of particle suspensions such as colloids39 or swimming microorganisms22, 26, 40. For swimming bacteria, DDM measures motility parameters averaged over ~ 104 cells from ≈ 30 s-long movies via the differential image correlation function , i.e., the power spectrum of the difference between pairs of images delayed by time τ. Under appropriate imaging conditions and for isotropic motion, is related to the intermediate scattering function f(q, τ), which is the qth Fourier component of the density temporal autocorrelation function, via
| 3 |
Here, B(q) relates to the background noise and A(q) is the signal amplitude. Fitting f(q, τ) to a suitable swimming model of E. coli, we obtain the average, , and width, σ, of the speed distribution P(v), the fraction of non-motile bacteria β and their diffusion coefficient D40. Provided the system is dilute enough such that its structure factor S(q) ≈ 1, A(q) is proportional to the particle density41, 42. Thus, in two otherwise identical samples with different cell densities ρ1,2,
| 4 |
We verified this using bacteria suspensions of different cell concentrations (measured by optical density), Supplementary Fig. 5.
However, the spatial resolution of this method of density measurement is very limited. In order to quantify the dynamics of the edge formation we needed to extract density profiles (Fig. 2i) from images such as the band shown in Fig. 2h. We found that neither the intensity along a line nor the average of such line profiles bears much resemblance to the cell density variation visible to direct inspection (see Supplementary Fig. 6). This is related to the nonlinearities at high phase shifts and artifacts inherent to phase contrast imaging43.
Interestingly, the single line profile (Supplementary Fig. 6b) shows that the intensity fluctuates more in the high-density region. Indeed, the averaged horizontal variance profile (Supplementary Fig. 6d) shows qualitative agreement with the visually observed density profile of the band, suggesting that the variance (with background subtracted) could be used to quantify local density. Supplementary Fig. 5 shows that, indeed, densities of uniform samples deduced from variance measurements agree quantitatively with densities deduced from DDM using Eq. (4) and from optical density measurements. At least within the low density limit, this scaling of intensity variance with cell density is not surprising: a (dark) cell on a uniform (light) background will lower the mean intensity slightly and introduce a characteristic amount of variance relative to this background. As more cells are added, they initially increase the variance by the same characteristic amount (as the mean background hardly changes), leading to the observed proportionality. For phase-contrast imaging, a phase-dark object is often surrounded by a lighter halo, so that the net effect of cells on mean intensity is rather small while at the same time leading to a significant characteristic variance per object.
Data availability
The research data presented in this publication is available on the Edinburgh DataShare repository44.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
The work is funded by an EPSRC programme grant (EP/J007404/1) and a ERC Advanced Grant (ADG-PHYSAPS). We thank M.E. Cates for discussions of active self assembly and J. Walter for discussions on strain design.
Author contributions
J.A., V.A.M., W.C.K.P. and T.P. designed the research. A.D. designed, constructed and verified all the strains. J.A. and V.A.M. performed the experiments. J.A., V.A.M. and W.C.K.P. analysed the data. All authors contributed to the interpretation and discussion of results, and W.C.K.P., J.A. and V.A.M. wrote the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03161-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y, Shah AA, Solomon MJ. Spatially and temporally reconfigurable assembly of colloidal crystals. Nat. Commun. 2014;5:1–8. doi: 10.1038/ncomms4676. [DOI] [PubMed] [Google Scholar]
- 3.Caspar DLD, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol. 1962;27:1–24. doi: 10.1101/SQB.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 4.Romano F, Sciortino F. Colloidal self assembly: patchy from the bottom up. Nat. Mater. 2011;10:171–173. doi: 10.1038/nmat2975. [DOI] [PubMed] [Google Scholar]
- 5.Pinçe E, et al. Disorder-mediated crowd control in an active matter system. Nat. Commun. 2016;7:10907. doi: 10.1038/ncomms10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takatori SC, De Dier R, Vermant J, Brady JF. Acoustic trapping of active matter. Nat. Commun. 2016;7:10694. doi: 10.1038/ncomms10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon WCK. From Clarkia to Escherichia and Janus: the physics of natural and synthetic active colloids. In: Bechinger C, Sciortino F, Ziherl P, editors. Physics of Complex Colloids. Bologna: Società Italiana di Fisica; 2013. pp. 317–386. [Google Scholar]
- 8.Ramaswamy S. The mechanics and statistics of active matter. Annu. Rev. Condens. Matter Phys. 2010;1:323–345. doi: 10.1146/annurev-conmatphys-070909-104101. [DOI] [Google Scholar]
- 9.Marchetti MC. Hydrodynamics of soft active matter. Rev. Mod. Phys. 2014;85:1143. doi: 10.1103/RevModPhys.85.1143. [DOI] [Google Scholar]
- 10.Cates ME. Diffusive transport without detailed balance in motile bacteria: does microbiology need statistical physics? Rep. Prog. Phys. 2012;75:042601. doi: 10.1088/0034-4885/75/4/042601. [DOI] [PubMed] [Google Scholar]
- 11.Sundararajan S, Lammert PE, Zudans AW, Crespi VH, Sen A. Catalytic motors for transport of colloidal cargo. Nano. Lett. 2008;8:1271–1276. doi: 10.1021/nl072275j. [DOI] [PubMed] [Google Scholar]
- 12.Koumakis N, Leopore A, Maggi C, Di Leonardo R. Targeted delivery of colloids by swimming bacteria. Nat. Commun. 2013;4:2588. doi: 10.1038/ncomms3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozano C, ten Hagen B, Löwen H, Bechinger C. Phototaxis of synthetic microswimmers in optical landscapes. Nat. Commun. 2016;7:12828. doi: 10.1038/ncomms12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Wu X, Qin H, Zhao Z, Liu H. Light-driven and light-guided microswimmers. Adv. Func. Mat. 2016;26:3164–3171. doi: 10.1002/adfm.201505378. [DOI] [Google Scholar]
- 15.Sen A, Ibele M, Hong Y, Velegol D. Chemo and phototactic nano/microbots. Faraday Discuss. 2009;143:15. doi: 10.1039/b900971j. [DOI] [PubMed] [Google Scholar]
- 16.Palacci J, Sacanna S, Steinberg AP, Pine DJ, Chaikin PM. Living crystals of light-activated colloidal surfers. Science. 2013;339:936–940. doi: 10.1126/science.1230020. [DOI] [PubMed] [Google Scholar]
- 17.Walter JM, Greenfield D, Bustamante C, Liphardt J. Light-powering Escherichia coli with proteorhodopsin. Proc. Natl Acad. Sci. USA. 2007;104:2408–2412. doi: 10.1073/pnas.0611035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vizsnyiczai G, et al. Light controlled 3D micromotors powered by bacteria. Nat. Commun. 2017;8:15974. doi: 10.1038/ncomms15974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz-Linek J, et al. Phase separation and rotor self-assembly in active particle suspensions. Proc. Natl Acad. Sci. USA. 2012;109:4052–4057. doi: 10.1073/pnas.1116334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theurkauff I, Cottin-Bizonne C, Palacci J, Ybert C, Bocquet L. Dynamic clustering in active colloidal suspensions with chemical signaling. Phys. Rev. Lett. 2012;108:268303. doi: 10.1103/PhysRevLett.108.268303. [DOI] [PubMed] [Google Scholar]
- 21.Stenhammar, J., Wittkowski, R., Marenduzzo, D. & Cates, M. E. Light-induced self-assembly of active rectification devices. Sci. Adv. 2, e1501850 (2016). [DOI] [PMC free article] [PubMed]
- 22.Schwarz-Linek J, et al. Escherichia coli as a model active colloid: a practical introduction. Colloids Surf. B. 2016;137:2–16. doi: 10.1016/j.colsurfb.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 23.Manson MD, Tedesco P, Berg HC, Harold FM, van der Drift C. A proton motive force drives bacterial flagella. Proc. Natl Acad. Sci. USA. 1977;74:3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler J, Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J. Gen. Microbiol. 1967;46:175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- 25.Béjà O. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science. 2000;289:1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 26.Wilson MJ, et al. Differential dynamic microscopy of bacterial motility. Phys. Rev. Lett. 2011;106:018101. doi: 10.1103/PhysRevLett.106.018101. [DOI] [PubMed] [Google Scholar]
- 27.Tipping MJ, Steel BC, Delalez NJ, Berry RM, Armitage JP. Quantification of flagellar motor stator dynamics through in vivo proton-motive force control. Mol. Microbiol. 2013;87:338–347. doi: 10.1111/mmi.12098. [DOI] [PubMed] [Google Scholar]
- 28.Keis S, Stocker A, Dimroth P, Cook GM. Inhibition of ATP hydrolysis by thermoalkaliphilic F1Fo-ATP synthase is controlled by the C terminus of the ε subunit. J. Bact. 2006;188:3796–3804. doi: 10.1128/JB.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanazawa H, Tamura F, Mabuchi K, Miki T, Futai M. Organization of unc gene-cluster of Echerichia coli coding for proton-translocating ATPase of oxidative-phorphorylation. Proc. Natl Acad. Sci. USA. 1980;77:7005–7009. doi: 10.1073/pnas.77.12.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leake MC, et al. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature. 2006;443:355–358. doi: 10.1038/nature05135. [DOI] [PubMed] [Google Scholar]
- 31.Wu M, Roberts JW, Kim S, Koch DL, DeLisa MP. Collective bacterial dynamics revealed using a three-dimensional population-scale defocused particle tracking technique. Appl. Environ. Microbiol. 2006;72:4987–4994. doi: 10.1128/AEM.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Rodriguez J, Moser F, Song M, Voigt CA. Engineering RGB color vision into Escherichia coli. Nat. Chem. Biol. 2017;13:706–708. doi: 10.1038/nchembio.2390. [DOI] [PubMed] [Google Scholar]
- 33.Tailleur J, Cates ME. Sedimentation, trapping and rectification of dilute bacteria. Europhys. Lett. 2009;86:60002. doi: 10.1209/0295-5075/86/60002. [DOI] [Google Scholar]
- 34.Nomura T, Morimoto Y, Ishikawa M, Tokumoto H, Konishi Y. Synthesis of hollow silica microparticles from bacterial templates. Adv. Powder Technol. 2010;21:8–12. doi: 10.1016/j.apt.2009.07.005. [DOI] [Google Scholar]
- 35.Piotter V, et al. Powder injection moulding of metallic and ceramic micro parts. Microsyst. Technol. 2011;17:251–263. doi: 10.1007/s00542-011-1274-2. [DOI] [Google Scholar]
- 36.DeWitt SK, Adelberg EA. The occurrence of a genetic transposition in a strain of Escherichia coli. Genetics. 1962;47:577–685. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merlin C, McAteer S, Masters M. Tools for characterization of Escherichia coli genes of unknown function. J. Bacteriol. 2002;184:4573–4581. doi: 10.1128/JB.184.16.4573-4581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2:1–11. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerbino R, Trappe V. Differential dynamic microscopy: Probing wave vector dependent dynamics with a microscope. Phys. Rev. Lett. 2008;100:188102. doi: 10.1103/PhysRevLett.100.188102. [DOI] [PubMed] [Google Scholar]
- 40.Martinez VA, et al. Differential dynamic microscopy: a high-throughput method for characterizing the motility of microorganisms. Biophys. J. 2012;103:1637–1647. doi: 10.1016/j.bpj.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reufer M, Martinez VA, Schurtenberger P, Poon WCK. Differential dynamic microscopy for anisotropic colloidal dynamics. Langmuir. 2012;28:4618–4624. doi: 10.1021/la204904a. [DOI] [PubMed] [Google Scholar]
- 42.Lu PJ, et al. Characterizing concentrated, multiply scattering, and actively driven fluorescent systems with confocal differential dynamic microscopy. Phys. Rev. Lett. 2012;108:218103. doi: 10.1103/PhysRevLett.108.218103. [DOI] [PubMed] [Google Scholar]
- 43.Pluta, M. Advanced Light Microscopy, vol. 2 Specialized Methods (Elsevier, 1989).
- 44.Arlt, J., Martinez, V. A., Dawson, A., Pilizota, T. & Poon, W. C. K. Painting with bacteria: smart templated self assembly using motile bacteria (dataset). Edinburgh DataShare 10.7488/ds/2263 (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The research data presented in this publication is available on the Edinburgh DataShare repository44.