Abstract
Objective
The aim of this investigation is to compare outcomes of patients according to the presence of cancer arising from endometriosis in ovarian clear cell carcinoma (CCC) and endometrioid carcinoma (EC).
Methods
This study retrospectively investigated 224 CCC and EC patients treated in Samsung Medical Center from 2001 to 2015 to identify cancer arising from endometriosis according to Sampson and Scott criteria. Propensity score matching was performed to compare patients arising from endometriosis to patients without endometriosis (ratio 1:1) according to stage, age, lymph node metastasis (LNM), cancer antigen (CA)-125 level, and residual status after debulking surgery.
Results
Forty-five cases arising from endometriosis were compared with 179 cases without endometriosis. CCC and EC arising from endometriosis tended to present with early age (mean, 45.2 vs. 49.2 years; p=0.003), early-stage (stages I and II, 92.7% vs. 62.3%; p<0.001), lower CA-125 level (mean, 307.1 vs. 556.7; p=0.041), higher percentages of no gross residual disease after surgery (87.8% vs.56.8%; p=0.001), and higher percentages of negative LNM (82.9% vs. 59.0%; p=0.008) compared to cases without endometriosis. Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) showed better outcomes for groups with cancer arising from endometriosis (p=0.014 for PFS; and p=0.010 for OS). However, the association with endometriosis was not significant in multivariate analysis. Also, after propensity score matching, survival differences between the 2 groups were not significant.
Conclusion
CCC and EC arising from endometriosis are diagnosed at an earlier age and stage. However, cancer arising from endometriosis was not a significant prognostic factor.
Keywords: Ovarian Neoplasms, Endometriosis, Endometrioid Carcinoma, Clear Cell Carcinoma
Introduction
Endometriosis is an estrogen-dependent chronic benign disease that demonstrates characteristics of ovarian malignancy such as invasive growth, hormone dependency, and recurrence [1]. Although endometriosis remains largely benign, the malignant potential of endometriosis has been suggested with epidemiological, histopathological, and molecular data [2,3]. Previous studies reported an increased risk of ovarian cancer in women with endometriosis, predominantly for clear cell and endometrioid type histology [2,4,5]. In Korean women, age-standardized incidence rates for clear cell carcinoma (CCC) in all ages and endometrioid carcinoma (EC) in young age have increased according to recent data [6]. Increase in the CCC and EC type in Korean women might be explained with increase of endometriosis by westernization of diet and life styles leading to earlier menarche, increased obesity, and decreased childbearing in Korean women [7].
The pathologic findings of malignancy with endometriosis have been demonstrated in the literature since Sampson [8] first described them in 1925. In 1953, Scott [9] added criteria for the morphology of benign and malignant epithelia within endometriosis. Controversy remains regarding the possibility that endometriosis-associated cancers represent a distinct category from the typical histologic type. A number of studies have presented the tendency of patients with endometriosis to be diagnosed at a younger age, with earlier stage and lower grade lesions, and to have better survival outcomes [10,11,12,13]. However, other studies fail to demonstrate survival differences between groups with and without endometriosis [14,15] or the significance of association with endometriosis as an independent prognostic factor [16,17,18].
The present study evaluated the incidence of cancer arising from endometriosis in cases with clear cell and endometrioid type epithelial ovarian cancer (EOC) treated at single institution. We also investigated clinicopathological characteristics and survival outcomes in terms of progression-free survival (PFS) and overall survival (OS) compared to patients with cancer not arising from endometriosis.
MATERIALS and Methods
1. Patients and treatment
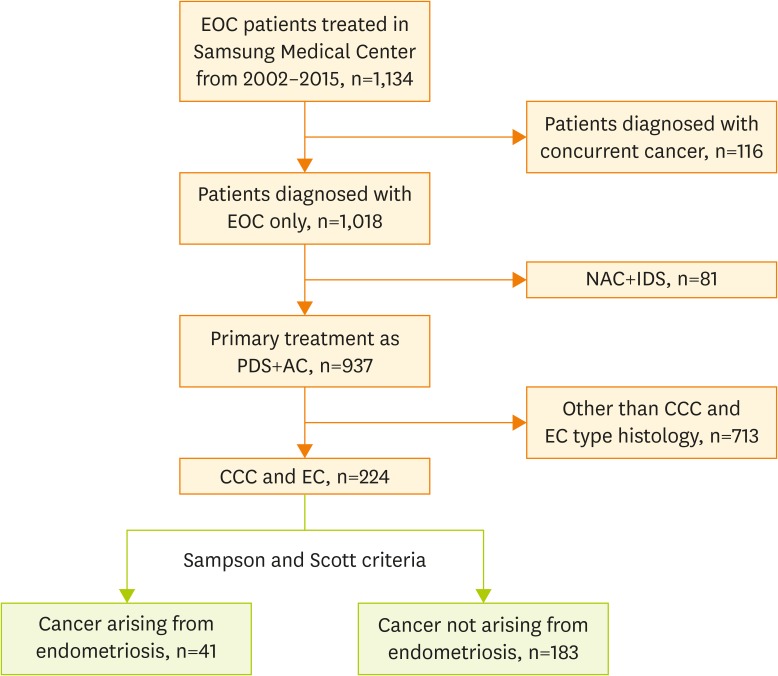
With Institutional Review Board approval (No. 2017-03-082), all patients with primary EOC who were treated at Samsung Medical Center from 2002 to 2015 were reviewed. Data from electronic medical records was retrospectively reviewed. Flowchart of included patients is shown in Fig. 1. In this study, we included primary EOC patients with clear cell and endometrioid type histology among patients who had undergone primary treatment including primary debulking surgery (PDS) and adjuvant chemotherapy (AC). Patients who had concurrent cancer other than EOC were excluded from the study at the beginning of the process. A total of 224 patients were selected for this study. Among these, 41 patients (18.3%) were categorized in the group with cancer arising from endometriosis.
Fig. 1.
Flowchart of included patients.
AC, adjuvant chemotherapy; CCC, clear cell carcinoma; EC, endometrioid carcinoma; EOC, epithelial ovarian cancer; IDS, interval debulking surgery; NAC, neo-adjuvant chemotherapy; PDS, primary debulking surgery.
Bilateral salpingo-oophorectomy, hysterectomy, peritoneal washing, retroperitoneal lymphadenectomy, omentectomy, and tumorectomy of any metastatic lesions were routinely performed for primary surgical treatment. If any abnormalities were suspected, peritoneal biopsies were performed for pathologic confirmation. To define residual disease status after PDS, the largest diameter of residual disease was measured and categorized as follows: no residual disease, 0.1–1 cm residual disease, and >1 cm residual disease. For AC, first cycle combination chemotherapy consisting of taxane/platinum was routinely initiated within 2 weeks of surgery. Chemotherapy was continued every 3 weeks for 6 cycles, but there may have been variations in the number of cycles depending on the patient's situation. OS was defined as the time between diagnosis and either patient death or loss to follow-up. PFS was described as time between diagnosis and patient recurrence/progression or loss to follow-up.
Routinely, tumor tissue specimens were obtained during surgery, and resected tumor sections were fixed in 10% buffered formalin and embedded in paraffin. In all cases, at least one section was obtained for every 1 cm of maximal tumor diameter and processed after formalin fixation. Then the tumor tissue sections were cut into 3- to 4-μm slices and stained with hematoxylin and eosin. Gynecologic pathologists reviewed all surgical pathology slides with confirming that all samples contained more than 80% of the total tumor area with less than 20% of necrosis.
Patients were divided into 2 groups according to detection of cancer arising from ovarian endometriosis or not based on the Sampson and Scott criteria [8,9]: 1) The presence of both benign and neoplastic endometrial tissues in the tumor, 2) histological findings compatible with endometrial origin, 3) the discovery of no other primary tumor sites, and 4) morphologic demonstration of a continuum between benign and malignant epithelium.
2. Statistical analysis
Summary statistics were used to describe the data. Medians (range) or means (standard deviation) were used for continuous variables. After the Shapiro-Wilks test confirmed normal distributions, Mann-Whitney U test was used to compare median values and Student's t-test was used to compare mean values. Categorical variables were presented as frequencies (percentages). Fisher's exact test or χ2 test were used to analyze the distribution of characteristics according to association with endometriosis. Survival curve analyses were performed by the Kaplan-Meier method, and comparison was performed using the log-rank test. Cox proportional hazards model was used to perform univariate and multivariate analyses for evaluation of the prognostic significance of association with endometriosis and other clinicopathological features. Multivariate p-values were used to present the significance of each feature. To quantify the correlation between survival time and each independent feature, a 95% confidence interval (CI) was used. All p-values were 2-sided, and p-values less than 0.05 were considered statistically significant. Statistical analyses were performed using R 3.0.3 (R Foundation, Vienna, Austria; http://www.R-project.org).
After the total cohort analyses, propensity score matching was performed to further elucidate patient characteristics. Cases associated with endometriosis were 1:1 matched according to age, International Federation of Gynecology and Obstetrics (FIGO) stage, initial cancer antigen (CA)-125 level, American Society of Anesthesiologist (ASA) performance status, and residual disease status after cytoreductive surgery with the closest propensity patients without endometriosis. Propensity scores were calculated using a multivariable logistic regression model based on factors that demonstrated significant differences between the 2 groups in the total cohort.
Results
1. Patient characteristics and associations with endometriosis
Among EOC patients treated in Samsung Medical Center between 2002 and 2015, 224 patients who were diagnosed with clear cell and endometrioid type histology were investigated. Of 224 patients, 41 patients had cancer arising from endometriosis and 183 patients did not have associated endometriosis based on the Sampson and Scott criteria.
At the time of this analysis, of 224 patients, 80 patients (35.7%) experienced relapse and 58 (25.9%) died after a median observation period of 55 months (range, 3–161 months). The clinical characteristics of patients are listed in Table 1; patients with cancer arising from endometriosis had a lower average age (45.1±7.3 vs. 49.2±10.3 years; p=0.003) and lower initial CA-125 level (307.1±588.4 vs. 556.7±1,056.6 U/mL; p=0.041) than those without endometriosis. Regarding CA-125 level, surgical procedures prior to primary cytoreduction were investigated, and there were no significant differences between 2 groups. Also, no significant differences were found regarding type of cytoreductive surgery, number of AC cycle, and platinum sensitivity. There were significant differences between patients with and without endometriosis for FIGO stage, grade, residual disease status, lymph node metastasis (LNM), and ASA physical status. Patients with cancer arising from endometriosis presented with a higher percentage of early-stage (stages I–II, 92.7% vs. 62.3%; p=0.002), lower grade (grades 1–2, 53.7% vs. 38.8%; p=0.033), no residual disease after PDS (87.8% vs. 56.8%; p=0.001), and negative LNM (82.9% vs. 59.0%; p=0.008) compared to those without endometriosis.
Table 1. Characteristics of study cohorts.
| Characteristics | All patients (n=224) | Without endometriosis (n=183) | Arising from endometriosis (n=41) | p-value | |
|---|---|---|---|---|---|
| Age (yr) | 48.4±9.9 | 49.2±10.3 | 45.1±7.0 | 0.003 | |
| Histology | 0.752 | ||||
| Clear cell | 107 (47.8) | 86 (47.0) | 21 (51.2) | ||
| Endometrioid | 117 (52.2) | 97 (53.0) | 20 (48.8) | ||
| FIGO stage | 0.002 | ||||
| I | 122 (54.5) | 91 (49.7) | 31 (75.6) | ||
| II | 30 (13.4) | 23 (12.6) | 7 (17.1) | ||
| III | 64 (28.6) | 62 (33.9) | 2 (4.9) | ||
| IV | 8 (3.6) | 7 (3.8) | 1 (2.4) | ||
| Grade | 0.033 | ||||
| 1 | 39 (17.4) | 26 (14.2) | 13 (31.7) | ||
| 2 | 54 (24.1) | 45 (24.6) | 9 (22.0) | ||
| 3 | 131 (58.5) | 107 (61.2) | 19 (46.3) | ||
| Initial CA-125 (U/mL) | 510.9±991.3 | 556.7±1,056.6 | 307.1±588.4 | 0.041 | |
| Residual disease status after PDS | 0.001 | ||||
| No residual disease | 140 (62.5) | 104 (56.8) | 36 (87.8) | ||
| 0.1–1 cm residual disease | 60 (26.8) | 56 (30.6) | 4 (9.8) | ||
| >1 cm residual disease | 24 (10.7) | 23 (12.6) | 1 (2.4) | ||
| LNM | 0.008 | ||||
| Negative | 142 (63.4) | 108 (59.0) | 34 (82.9) | ||
| Positive | 21 (9.4) | 21 (11.5) | 0 | ||
| Not done | 61 (27.2) | 54 (29.5) | 7 (17.1) | ||
| ASA physical status | 0.001 | ||||
| I | 114 (50.9) | 94 (51.4) | 20 (48.8) | ||
| II | 61 (27.2) | 56 (30.6) | 5 (12.2) | ||
| III | 6 (2.7) | 6 (3.3) | 0 | ||
| Unknown | 43 (19.2) | 27 (14.8) | 16 (39.0) | ||
| Type of cytoreductive surgery | 0.641 | ||||
| Complete staging | 216 (96.4) | 177 (96.7) | 39 (95.1) | ||
| Fertility saving surgery | 8 (3.6) | 6 (3.3) | 2 (4.9) | ||
| Surgical procedures before cytoreductive surgery | 0.938 | ||||
| None | 196 (87.5) | 160 (87.4) | 36 (87.8) | ||
| Unilateral oophorectomy | 20 (8.9) | 16 (8.7) | 4 (9.8) | ||
| Bilateral oophorectomy | 2 (0.9) | 2 (1.1) | 0 | ||
| Unilateral ovarian cystectomy | 6 (2.7) | 5 (2.7) | 1 (2.4) | ||
| No. of AC cycle | 0.376 | ||||
| 4–6 | 172 (76.8) | 143 (78.1) | 29 (70.7) | ||
| 1–3 | 22 (9.8) | 18 (9.8) | 4 (9.8) | ||
| None | 30 (13.4) | 22 (12.0) | 8 (19.5) | ||
| Platinum sensitivity | 0.545 | ||||
| Resistant | 13 (5.8) | 12 (6.6) | 1 (2.4) | ||
| Sensitive | 176 (78.6) | 144 (78.7) | 32 (78.0) | ||
| Unknown | 35 (15.6) | 27 (14.8) | 8 (19.5) | ||
Data are shown as mean±standard deviation or number (%).
AC, adjuvant chemotherapy; ASA, American Society of Anesthesiologist; CA, cancer antigen; FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; PDS, primary debulking surgery.
2. Survival comparison according to association with endometriosis and multivariate analysis for PFS and OS
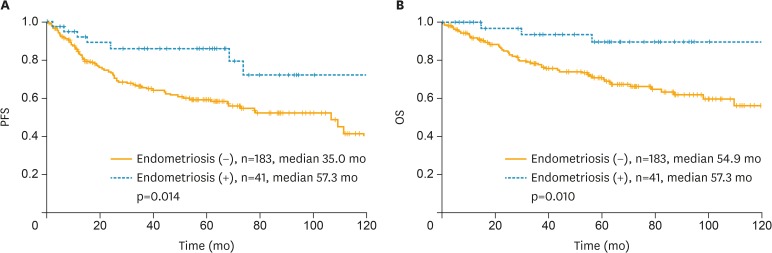
Survival analysis of 224 patients with EC and CCC revealed significant differences in PFS and OS between patients with and without associated endometriosis (Fig. 2). The Kaplan-Meier curve for PFS showed a survival advantage for patients with cancer arising from endometriosis (median, 57.3 vs. 35.0 months; p=0.014; Fig. 2A). Results for OS indicated that patients with cancer arising from endometriosis had better survival outcomes compared to patients without associated endometriosis (median, 57.3 vs. 54.9 months; p=0.010; Fig. 2B).
Fig. 2.
Kaplan-Meier curve for (A) PFS and (B) OS in all patients.
PFS, progression-free survival; OS, overall survival.
In multivariate analysis of clinicopathologic variables in the entire cohort, cancer arising from endometriosis was not a significant prognostic factor for PFS (p=0.347) and OS (p=0.247) (Table 2). Previously known prognostic factors in EOC, such as FIGO stage and residual disease after PDS, remained significant prognostic factors for PFS and OS in this study cohort. Additionally, initial CA-125 level was significant in multivariate analysis for OS.
Table 2. Multivariate Cox proportional hazards analysis for PFS and OS used to adjust risk associated prognostic clinical features.
| All patients (n=224) | PFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (continuous) | 0.985 (0.962–1.009) | 0.212 | 0.977 (0.951–1.004) | 0.100 | |
| Initial CA-125 level (U/mL) | |||||
| <35 | 1.000 | - | 1.000 | - | |
| ≥35 | 1.920 (0.983–3.752) | 0.056 | 2.834 (1.055–7.611) | 0.039 | |
| FIGO stage | |||||
| I/II | 1.000 | - | 1.000 | - | |
| III/IV | 2.857 (1.634–4.995) | <0.001 | 4.033 (2.007–8.107) | <0.001 | |
| Grade | |||||
| 1 | 1.000 | - | 1.000 | - | |
| 2 | 1.923 (0.758–4.878) | 0.168 | 1.938 (0.545–6.893) | 0.307 | |
| 3 | 2.233 (0.923–5.405) | 0.075 | 2.530 (0.751–8.526) | 0.134 | |
| Residual disease status after PDS | |||||
| No residual disease | 1.000 | - | 1.000 | - | |
| 0.1–1 cm residual disease | 1.996 (1.161–3.432) | 0.012 | 2.743 (1.361–5.527) | 0.005 | |
| >1 cm residual disease | 2.806 (1.422–5.534) | 0.003 | 3.869 (1.731–8.651) | 0.001 | |
| Arising from endometriosis | |||||
| No | 1.000 | - | 1.000 | - | |
| Yes | 0.675 (0.297–1.532) | 0.347 | 0.483 (0.145–1.613) | 0.237 | |
CA, cancer antigen; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; OS, overall survival; PDS, primary debulking surgery; PFS, progression-free survival.
3. Comparison after propensity score matching
To reduce selection bias when comparing 2 groups of patients, propensity score matching was performed with R using the MatchIt package with nearest-neighbor 1-to-1 matching according to age, FIGO stage, CA-125 level, ASA performance status, and residual disease status after PDS. Matching was successful without significant differences between the 2 groups in all matched variables (Table 3). After propensity score matching, there were 82 patients; 41 had cancer arising from endometriosis and 41 had cancer without associated endometriosis. No significant differences were observed between the 2 groups in age, stage, grade, initial serum CA-125 level, residual disease status, LNM, and ASA physical status. Propensity score distribution and histograms of propensity scores before and after matching are shown in Supplementary Figs. 1 and 2.
Table 3. Patient characteristics after propensity score matching.
| Characteristics | Without endometriosis (n=41) | Arising from endometriosis (n=41) | p-value | |
|---|---|---|---|---|
| Age (yr) | 46.7±10.3 | 45.1±7.0 | 0.403 | |
| Histology | 0.752 | |||
| Clear cell | 20 (48.8) | 21 (51.2) | ||
| Endometrioid | 21 (51.2) | 20 (48.8) | ||
| FIGO stage | 0.896 | |||
| I | 29 (70.7) | 31 (75.6) | ||
| II | 7 (17.1) | 7 (17.1) | ||
| III | 3 (7.3) | 2 (4.9) | ||
| IV | 2 (4.9) | 1 (2.4) | ||
| Grade | 0.272 | |||
| 1 | 7 (17.1) | 13 (31.7) | ||
| 2 | 7 (17.1) | 9 (22.0) | ||
| 3 | 27 (65.8) | 19 (46.3) | ||
| Initial CA-125 (U/mL) | 367.7±745.9 | 307.1±588.4 | 0.684 | |
| Residual disease status after PDS | 0.222 | |||
| No residual disease | 40 (97.6) | 36 (87.8) | ||
| 0.1–1 cm residual disease | 1 (2.4) | 4 (9.8) | ||
| >1 cm residual disease | 0 | 1 (2.4) | ||
| LNM | >0.999 | |||
| Negative | 33 (80.5) | 34 (82.9) | ||
| Positive | 0 | 0 | ||
| Not done | 8 (19.5) | 7 (17.1) | ||
| ASA physical status | 0.179 | |||
| I | 15 (36.6) | 20 (48.8) | ||
| II | 12 (29.3) | 5 (12.2) | ||
| III | 1 (2.4) | 0 | ||
| unknown | 13 (31.7) | 16 (39.0) | ||
| Type of cytoreductive surgery | >0.999 | |||
| Complete staging | 40 (97.6) | 39 (95.1) | ||
| Fertility saving surgery | 1 (2.4) | 2 (4.9) | ||
| Surgical procedures before cytoreductive surgery | >0.999 | |||
| None | 35 (85.4) | 36 (87.8) | ||
| Unilateral oophorectomy | 5 (12.2) | 4 (9.8) | ||
| Unilateral ovarian cystectomy | 1 (2.4) | 1 (2.4) | ||
| No. of AC cycle | >0.999 | |||
| 4–6 | 30 (73.2) | 29 (70.7) | ||
| 1–3 | 4 (9.8) | 4 (9.8) | ||
| None | 7 (17.1) | 8 (19.5) | ||
| Platinum sensitivity | >0.999 | |||
| Resistant | 2 (4.9) | 1 (2.4) | ||
| Sensitive | 31 (75.6) | 32 (78.0) | ||
| Unknown | 8 (19.5) | 8 (19.5) | ||
Data are shown as mean±standard deviation or number (%).
AC, adjuvant chemotherapy; ASA, American Society of Anesthesiologist; CA, cancer antigen; FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; PDS, primary debulking surgery.
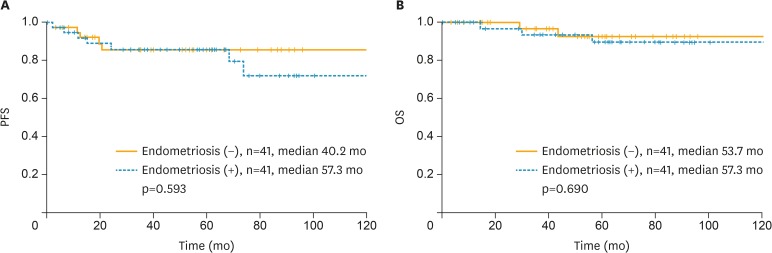
After propensity score matching, survival differences between the 2 groups were not significant. Kaplan-Meier curves for PFS and OS are shown in Fig. 3, and p-values were 0.593 and 0.690, respectively.
Fig. 3.
Kaplan-Meier curve for (A) PFS and (B) OS in patients after propensity score matching.
PFS, progression-free survival; OS, overall survival.
Discussion
This study investigated patients with CCC and EC of the ovary and cancer arising from endometriosis. Patients with cancer arising from endometriosis presented with a lower average age, higher percentage of early-stage, lower grade, and no residual disease after PDS compared to those without endometriosis. PFS and OS indicated better survival outcomes for cancer arising from endometriosis. However, in multivariate analysis for PFS and OS, cancer arising from endometriosis was not statistically significant as an independent prognostic factor, and the results of propensity score matching showed that survival differences between the 2 groups were not significant, suggesting endometriosis as a possible precursor of EOC but not a factor that exacerbates cancer after its onset.
In previous studies, endometriosis-associated EOC cases were typically associated with early-stage, low-grade disease that may be related to better survival results [2,10,11,12,13]. However, the association with endometriosis remains controversial as an independent prognostic factor after calibrating related clinical situations. In a study by Bounous et al. [17], endometriosis-associated cancer presented with early-stage, and OS was significantly longer in EOC patients associated with endometriosis. After stratification by stage, the survival advantages of endometriosis-associated cancer patients disappeared. Conversely, a recent study showed cancer arising from endometriosis as an independent prognostic factor for PFS and OS. Furthermore, prognostic nomograms with endometriosis as a significant factor were provided [13]. In the current study, patients with cancer arising from endometriosis showed longer PFS and OS than another patient group. After propensity score matching, differences in survival outcomes between the 2 groups were not significant, and cancer arising from endometriosis was not an independent significant factor in multivariate analysis. This study is a continuation of these previous studies and was intended to provide additional information about cancer arising from endometriosis in EOC patients.
In this study, patients with cancer arising from endometriosis were diagnosed at an early-stage, which were consistent result with previous literatures [2,18,19,20]. The reason for diagnosis of early-stage could be due to the signs and symptoms related endometriosis (such as pelvic pain and adnexal mass) or concurrent presences of endometrial lesions (polyps, hyperplasia) that lead to frequent follow-ups. The prevalence of endometriosis in EOC is reported as range from 3.4% to 52.6% [21]. Also, in previous Korean multicenter study with CCC with endometriosis, 43.1% patients had CCC arising from endometriosis, which was higher than 18.3% in our study [22]. Different prevalence may be due to the different definitions used in studies for classifying the association with endometriosis. A number of criteria have been used to define EOC with endometriosis. The Van Gorp classification provides broad ranging criteria that emphasize identification of endometriosis alone or at any transition point within the surgical specimen coexisting with EOC [3]. The Sampson and Scott criteria apply strict histologic conditions for diagnosis of cancer arising from endometriosis based on demonstration of malignant transformation in the endometriosis glands leading to EOC [18,23]. This study applied the Sampson and Scott criteria for analysis.
Efforts to identify whether the EOC arising from endometriosis is clinically different entity are still ongoing. According to model suggested for EOC pathogenesis, type I tumors which consist of endometrioid, clear cell, mucinous and low-grade serous carcinoma, are presented with an indolent clinical behavior, are confined to the ovary, and, are relatively genetically stable [24]. Moreover, type I tumors exhibit a shared lineage with the corresponding pre-malignant lesion such as borderline tumors and endometriosis. Type I tumors are known to carry mutation of Kirsten rat sarcoma virus (KRAS), phosphatase and tensin homolog (PTEN), AT-rich interactive domain 1A (ARID1A) genes [3]. In contrast, type II tumors include high-grade serous and undifferentiated carcinoma, have a very aggressive clinical behavior, are usually advanced stage at presentation, often harbor p53 gene mutations and are genetically unstable. EOC arising from endometriosis seems to show more similar characteristics to type I. According to previous studies, ARID1A mutations and loss of brahma-related gene (BRG)-associated factor 250a (BAF250a) expression were evident in ovarian CCC, especially in tumor and contiguous atypical endometriosis but not in distant endometriotic lesions, which may be considered as early occurrence for malignant change of endometriosis [25]. Also, atypical endometriosis and endometriosis-related cancers share similar molecular alterations, such as PTEN mutations, ARID1A mutations and up-regulation of hepatocyte nuclear factor-1-beta (HNF-1β), indicating mechanism of malignant change of endometriosis [26]. However, the clinical significance of loss of ARID1A in cancer arising from endometriosis has not been fully understood, and needs to be further elucidated.
This study has limitations due to its retrospective nature, limited number of patients from a single institution, and lack of detailed medical histories or treatment data after recurrence, which is related to survival outcomes. Also, we analyzed cancer associated with endometriosis group which include both CCC and EC, as we could not obtain a large number of data due to relatively low prevalence of EC and CCC. A larger number of cohort studies will help to obtain definite results in the future. Further analysis of the etiology for the early-stage and early age of patients with cancer arising from endometriosis is necessary.
Our data showed that patients with cancer arising from endometriosis were younger and had early-stage and low-grade disease compared to those without endometriosis. This may be related to the trend of better survival outcomes. However, in multivariate analysis for PFS and OS, cancer arising from endometriosis was not a significant prognostic factor, and survival differences between matched groups were not significant after propensity score matching.
Footnotes
Funding: This research was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (1520100), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2016R1A2B3006644), the National Research Foundation of Korea(NRF) Grant funded by the Korean Government(MSIP) (2016R1A5A2945889), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute(KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3418).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: B.D.S., L.J.W.
- Formal analysis: P.E.S., C.C.H.
- Funding acquisition: L.J.W.
- Investigation: P.E.S., C.C.H.
- Methodology: P.E.S.
- Supervision: L.J.W.
- Writing - original draft: P.E.S.
- Writing - review & editing: K.T.J., C.C.H., K.B.G., L.J.W.
Supplementary Materials
Distribution of propensity scores.
Histograms of propensity scores before and after matching.
References
- 1.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 2.Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer. 2014;110:1878–1890. doi: 10.1038/bjc.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol. 2004;18:349–371. doi: 10.1016/j.bpobgyn.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somigliana E, Vigano' P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331–341. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Kim SI, Lim MC, Lim J, Won YJ, Seo SS, Kang S, et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J Gynecol Oncol. 2016;27:e5. doi: 10.3802/jgo.2016.27.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- 9.Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283–289. [PubMed] [Google Scholar]
- 10.Orezzoli JP, Russell AH, Oliva E, Del Carmen MG, Eichhorn J, Fuller AF. Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol. 2008;110:336–344. doi: 10.1016/j.ygyno.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Boyraz G, Selcuk I, Yazıcıoğlu A, Tuncer ZS. Ovarian carcinoma associated with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2013;170:211–213. doi: 10.1016/j.ejogrb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Qiu L, Lang JH, Shen K, Yang JX, Huang HF, et al. Clinical analysis of ovarian epithelial carcinoma with coexisting pelvic endometriosis. Am J Obstet Gynecol. 2013;208:413.e1–413.e5. doi: 10.1016/j.ajog.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Tao X, Zhou J, Lu Y, Wang Z, Liu H, et al. Improved clinical outcomes of patients with ovarian carcinoma arising in endometriosis. Oncotarget. 2017;8:5843–5852. doi: 10.18632/oncotarget.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangili G, Bergamini A, Taccagni G, Gentile C, Panina P, Viganò P, et al. Unraveling the two entities of endometrioid ovarian cancer: a single center clinical experience. Gynecol Oncol. 2012;126:403–407. doi: 10.1016/j.ygyno.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Noli S, Cipriani S, Scarfone G, Villa A, Grossi E, Monti E, et al. Long term survival of ovarian endometriosis associated clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer. 2013;23:244–248. doi: 10.1097/IGC.0b013e31827aa0bb. [DOI] [PubMed] [Google Scholar]
- 16.Cuff J, Longacre TA. Endometriosis does not confer improved prognosis in ovarian carcinoma of uniform cell type. Am J Surg Pathol. 2012;36:688–695. doi: 10.1097/PAS.0b013e31824b6eed. [DOI] [PubMed] [Google Scholar]
- 17.Bounous VE, Ferrero A, Fuso L, Ravarino N, Ceccaroni M, Menato G, et al. Endometriosis-associated ovarian cancer: a distinct clinical entity? Anticancer Res. 2016;36:3445–3449. [PubMed] [Google Scholar]
- 18.Kumar S, Munkarah A, Arabi H, Bandyopadhyay S, Semaan A, Hayek K, et al. Prognostic analysis of ovarian cancer associated with endometriosis. Am J Obstet Gynecol. 2011;204:63.e1–63.e7. doi: 10.1016/j.ajog.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Erzen M, Rakar S, Klancnik B, Syrjänen K. Endometriosis-associated ovarian carcinoma (EAOC): an entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol Oncol. 2001;83:100–108. doi: 10.1006/gyno.2001.6382. [DOI] [PubMed] [Google Scholar]
- 20.McMeekin DS, Burger RA, Manetta A, DiSaia P, Berman ML. Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. Gynecol Oncol. 1995;59:81–86. doi: 10.1006/gyno.1995.1271. [DOI] [PubMed] [Google Scholar]
- 21.Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The relation between endometriosis and ovarian cancer - a review. Acta Obstet Gynecol Scand. 2014;93:20–31. doi: 10.1111/aogs.12255. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Kim MA, Lee M, Suh DH, Kim K, No JH, et al. Effect of endometriosis on the prognosis of ovarian clear cell carcinoma: a two-center cohort study and meta-analysis. Ann Surg Oncol. 2015;22:2738–2745. doi: 10.1245/s10434-014-4319-9. [DOI] [PubMed] [Google Scholar]
- 23.Scarfone G, Bergamini A, Noli S, Villa A, Cipriani S, Taccagni G, et al. Characteristics of clear cell ovarian cancer arising from endometriosis: a two center cohort study. Gynecol Oncol. 2014;133:480–484. doi: 10.1016/j.ygyno.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadducci A, Lanfredini N, Tana R. Novel insights on the malignant transformation of endometriosis into ovarian carcinoma. Gynecol Endocrinol. 2014;30:612–617. doi: 10.3109/09513590.2014.926325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of propensity scores.
Histograms of propensity scores before and after matching.