Abstract
Objective
Reports on the repeated administration of medroxyprogesterone acetate (MPA) for intrauterine recurrence after fertility-preserving therapy for atypical endometrial hyperplasia (AEH) and early grade 1 endometrioid carcinoma (G1) are lacking. We aimed to clarify the outcomes of repeated MPA therapy in cases of intrauterine recurrence after fertility-preserving therapy with MPA against AEH/early G1.
Methods
Patients with AEH or stage IA well-differentiated endometrioid carcinoma without myometrial invasion who underwent first-line MPA therapy for primary lesions or intrauterine recurrence were divided into initial treatment and repeated treatment groups (162 and 82 patients, respectively). Oral MPA administration (400−600 mg/day) was continued until pathological tumor disappearance. Data regarding clinicopathological factors, adverse events, and outcomes following the initial and repeated hormonal treatments were extracted from medical records and analyzed.
Results
Complete response rates in the initial and repeated treatment groups were 98.5% and 96.4%, respectively, among patients with AEH, and were 90.7% and 98.1%, respectively, among patients with G1. In the initial treatment group, 5-year recurrence-free survival (RFS) rates were 53.7% and 33.2% among patients with AEH and G1, respectively. In the repeated treatment group, RFS rates were 14.0% and 11.2% among patients with AEH and G1, respectively. Among patients with AEH, the pregnancy rate tended to be lower in the repeated treatment group than in the initial treatment group (11.1% vs. 29.2%; p=0.107), while no significant group difference was observed among patients with G1 (20.8% vs. 22.7%).
Conclusion
Repeated treatment is sufficiently effective for intrauterine recurrence after hormonal therapy for AEH/early G1.
Keywords: Endometrial Neoplasms, Endometrial Hyperplasia, Hormone Replacement Therapy, Fertility Preservation, Intrauterine Recurrence
INTRODUCTION
Endometrial cancer (EC) and atypical endometrial hyperplasia (AEH) are common conditions in menopause, but are relatively rare in women aged <40 years [1]. In Japan, there is a current trend of procreating later in life; thus, some women who develop EC/AEH wish to prolong their fertility. This poses an issue, as the standard treatment for EC/AEH is surgical therapy, including hysterectomy, resulting in total sterility.
Hormonal therapy using progesterone is considered effective in patients with early EC and AEH who wish to preserve their fertility. The commonly used drugs include medroxyprogesterone acetate (MPA) and megestrol acetate, as oral medicines, and the levonorgestrel-releasing intrauterine system, as an intrauterine treatment option [2]. In general, fertility-preserving therapeutic options are indicated in cases of AEH or grade 1 endometrioid carcinoma (G1) localized in the endometrium, without myometrial invasion or extrauterine lesions [3,4]. In a meta-analysis of 151 AEH and 408 early EC cases, the tumor disappearance rates associated with hormonal therapy were 85.6% and 76.2% for AEH and early EC, respectively [5]. For cases in which the tumor does not disappear and tumor progression is observed, total hysterectomy is required. Furthermore, many patients experience relapse following hormonal therapy, with recurrence rates of 26.0% and 40.6% for AEH and early EC, respectively [5]. Although some researchers and clinicians assert that hysterectomy should be performed in recurrent cases, others propose that repeated hormonal therapy can be performed for cases of recurrent AEH or G1 without myometrial invasion or extrauterine lesions [6]. However, few reports on the repeated administration of MPA against relapsed early EC and AEH have been published.
Therefore, the aim of this study was to clarify the outcomes of repeated high-dose MPA therapy in cases of intrauterine recurrence after fertility-preserving therapy with high-dose MPA against early EC/AEH.
MATERIALS AND METHODS
1. Study design and patients
A retrospective study was conducted with data collected from medical records. The study was approved by the Ethics Committee at Keio University School of Medicine (approval No. 20110237).
Patients with AEH or G1 without myometrial invasion or extrauterine lesions who underwent high-dose MPA therapy for primary lesions or intrauterine recurrence from 1998 to 2013 at Keio University Hospital were enrolled. The patients were divided into 2 groups: the initial treatment group, comprising 162 patients who underwent MPA therapy for primary lesions; and the repeated treatment group, comprising 82 patients who underwent the re-administration of high-dose MPA for intrauterine recurrence of AEH or G1, without myometrial invasion or extrauterine lesions after first-line high-dose MPA therapy. A total of 80.5% of the patients in the repeated treatment group underwent both the initial and repeated treatments at our institution. The diagnosis of the histological type was based on the total endometrial curettage performed under anesthesia before the initial treatment, and not on the histological type at the time of recurrence. Patients in both the initial and repeated treatment groups fulfilled the following 2 conditions: 1) diagnosed with AEH or G1 on total endometrial curettage, and 2) no myometrial invasion, cervical involvement, or extrauterine lesions observed on pelvic magnetic resonance imaging (MRI) and chest abdominal computed tomography (CT). Patients with myometrial invasion, cervical stromal invasion, or extrauterine lesions were treated with a standard treatment, including hysterectomy, and were excluded. Furthermore, patients who did not wish to preserve their fertility and those who had recurrent lesions that were not indicated for repeated MPA administration underwent hysterectomy and were excluded.
2. Treatment
Oral MPA administration (400–600 mg/day) was started after all intrauterine lesions had been sufficiently removed via total endometrial curettage. The patients underwent interviews, transvaginal ultrasonography, endometrial cytology, and endometrial biopsy every month during MPA administration, to confirm the absence of disease progression and the presence of histological dyshormonal changes. Adverse events (AEs) were assessed using the Common Terminology Criteria for Adverse Events (CTCAE; ver. 4.0; National Institutes of Health, Bethesda, MA, USA), and were based on the interviews and blood testing. After at least 4 months of MPA treatment, hysterofiberscopy (HFS) was performed to evaluate the localization of the intrauterine lesions, and total endometrium curettage was again performed.
If the endometrial lesions had pathologically disappeared on total endometrial curettage, the oral administration of MPA was terminated. Otherwise, MPA therapy was continued, and total endometrial curettage was performed every 2 months until lesion disappearance was confirmed. Tumor disappearance was defined as no apparent endometrial hyperplasia or adenocarcinoma, and was considered to be complete response (CR). If MPA therapy was discontinued and hysterectomy was performed, the histological type and surgical staging were compared to those before treatment; equivalency was considered to represent stable disease (SD), while worse post-treatment histological type or surgical staging compared to that before treatment was considered to represent progressive disease (PD). The MPA treatment period (MTP) was defined as the period from treatment initiation to termination, and the time-to-tumor disappearance (TTD) was defined as the period from treatment initiation to tumor disappearance. When tumor disappearance was pathologically confirmed, infertility treatment was started in the patients who hoped to conceive; otherwise, low-dose cyclic progestin therapy was started.
3. Follow-up
In order to confirm a lack of recurrence, follow-up examinations, including interviews, transvaginal ultrasonography, endometrial cytology, endometrial biopsy, and tumor marker analysis, were performed every 3–4 months for 2 years after the last hormonal treatment, and every 6 months thereafter. AEH or EC diagnosed on endometrial biopsy or total endometrial curettage during the follow-up period was defined as intrauterine recurrence. MRI and CT were performed for cases of suspected extrauterine recurrence on transvaginal ultrasound. The period from the end of the previous treatment to recurrence was defined as the treatment-free interval (TFI). The recurrence-free interval was defined as the period from termination of prior treatment to the time at which recurrence was pathologically confirmed, and was used to calculate recurrence-free survival (RFS). Upon satisfying the eligibility criteria of high-dose MPA treatment at the time of recurrence, the treatment was repeated; however, when the eligibility criteria were not met or the need for fertility preservation was absent, standard treatment, including hysterectomy, was performed.
4. Statistical analysis
The clinicopathological factors and prognostic information extracted from the medical records at our institution were analyzed. Statistical analyses were performed using SPSS Statistics ver. 24 (IBM Corp., Armonk, NY, USA). Fisher's exact test and Pearson's χ2 test were used to compare frequencies between treatment groups, while Student's t-test and non-parametric test were used to compare outcomes. RFS was analyzed using the Kaplan-Meier method, with group differences evaluated using standard log-rank tests. In addition, a multivariate analysis was performed using a Cox proportional hazard model. The p-values <0.05 were considered statistically significant.
RESULTS
1. Patient characteristics
Patient characteristics are shown in Table 1. There were 65 and 97 cases of AEH and G1, respectively, in the initial treatment group. In the repeated treatment group, there were 28 and 54 patients diagnosed with AEH and G1, respectively, at the time of the initial treatment; at the time of relapse, 51 and 31 patients were diagnosed with AEH and G1, respectively. The initial and repeated treatment groups did not significantly differ in histological types at the time of the initial treatment; however, significantly more patients had AEH at the time of the repeated treatment than at the time of the initial treatment within the repeated treatment group. Body mass index (BMI) and the frequencies of pregnancy, polycystic ovary (PCO), diabetes mellitus, and family history of cancer were not significantly different between the groups. The median follow-up duration was 71.3 (4.5–208.7) months.
Table 1. Patient's characteristics.
| Characteristics | Initial treatment group | Repeated treatment group | p-value | ||
|---|---|---|---|---|---|
| Histological type | |||||
| At initial treatment | AEH | 65 | 28 | n.s. | |
| G1 | 97 | 54 | |||
| At repeated treatment | AEH | - | 51 | 0.002 | |
| G1 | - | 31 | |||
| Age (year) | |||||
| At initial treatment | AEH | 36 (20–45) | 35 (26–45) | n.s. | |
| G1 | 35 (19–44) | 34 (19–44) | n.s. | ||
| At repeated treatment | AEH | - | 36 (21–49) | n.s. | |
| G1 | - | 35 (24–44) | n.s. | ||
| BMI (kg/m2) | |||||
| At initial treatment | AEH | 21.3 (16.4–40.2) | 20.2 (16.4–31.6) | n.s. | |
| G1 | 21.9 (15.8–41.4) | 22.1 (17.6–41.4) | n.s. | ||
| At repeated treatment | AEH | - | 20.4 (16.5–36.0) | n.s. | |
| G1 | - | 23.8 (17.6–40.3) | n.s. | ||
| No gravida (%) | AEH | 86.1 | 89.2 | n.s. | |
| G1 | 85.6 | 88.9 | n.s. | ||
| PCO (%) | AEH | 20.0 | 21.4 | n.s. | |
| G1 | 29.9 | 38.9 | n.s. | ||
| Diabetes mellitus (%) | AEH | 1.5 | 0.0 | n.s. | |
| G1 | 8.2 | 13.0 | n.s. | ||
| Family history of cancer (%) | AEH | 30.8 | 39.3 | n.s. | |
| G1 | 37.1 | 44.4 | n.s. | ||
| Median follow up period (mo) | 71.3 (4.5–208.7) | ||||
AEH, atypical endometrial hyperplasia; BMI, body mass index; G1, grade 1 endometrioid carcinoma; n.s., not significant; PCO, polycystic ovary.
2. Differences in outcomes between the initial and repeated treatment groups
Tumor and pregnancy outcomes are presented in Table 2. Among the patients with AEH, the CR rates were 98.5% and 96.4% for the initial and repeated treatment groups, respectively, with no significant group difference. Among the patients with G1, the CR rates were 90.7% and 98.1% in the initial and repeated treatment groups, respectively, with a tendency toward a higher CR in the repeated treatment than in the initial treatment group (p=0.097). In the initial treatment group, the SD and PD rates were 3.1% and 0%, respectively, among patients with AEH, and were 6.2% and 3.1%, respectively, among patients with G1. In the repeated treatment group, the SD and PD rates were 3.6% and 0%, respectively, among patients with AEH, and were 0% and 1.9%, respectively, among patients with G1. No significant group differences in the SD and PD rates were observed.
Table 2. Outcome of initial and repeated hormonal treatment.
| Characteristics | Initial treatment group | Repeated treatment group | p-value | |
|---|---|---|---|---|
| CR rate | AEH | 98.5% (64/65) | 96.4% (27/28) | n.s. |
| G1 | 90.7% (88/97) | 98.1% (53/54) | 0.097 | |
| Median MTP (mo) | AEH | 4.9 (1.9–15.0) | 5.1 (2.7–16.8) | n.s. |
| G1 | 6.1 (2.5–28.8) | 6.6 (3.7–17.3) | n.s. | |
| Median TTD (mo) | AEH | 2.7 (0.1–18.8) | 3.8 (0.9–15.9) | n.s. |
| G1 | 4.5 (0.6–24.9) | 4.4 (0.7–15.1) | n.s. | |
| Recurrence rate | AEH | 42.1% (27/64) | 66.6% (18/27) | 0.024 |
| G1 | 63.2% (55/87) | 81.1% (43/53) | 0.036 | |
| Pregnancy rate | AEH | 29.2% (19/65) | 11.1% (3/27) | 0.107 |
| G1 | 22.7% (20/88) | 20.8% (11/53) | n.s. |
AEH, atypical endometrial hyperplasia; CR, complete response; G1, grade 1 endometrioid carcinoma; MTP, medroxyprogesterone acetate treatment period; n.s., not significant; TTD, time-to-tumor disappearance.
The initial and repeated treatment groups did not significantly differ in the MTP (AEH: 4.9 vs. 5.1 and G1: 6.1 vs. 6.6 months, respectively) and TTD (AEH: 2.7 vs. 3.8 and G1: 4.5 vs. 4.4 months, respectively). However, the recurrence rate in the repeated treatment group was significantly higher than that in the initial treatment group for both AEH and G1 cases (AEH: 42.1% vs. 66.6%; p=0.024 and G1: 63.2% vs. 81.1%; p=0.036, respectively). Among patients with AEH, the pregnancy rate in the repeated treatment group tended to be lower than that in the initial treatment group (p=0.107), while no significant group difference was found among patients with G1 (AEH: 11.1% vs. 29.2% and G1: 20.8% vs. 22.7%, respectively).
3. Differences in adverse effects between the initial and repeated treatment groups
Among the AEs obtained from the medical records, only those graded 2 and above are shown in Supplementary Table 1. Weight gain occurred in 5 cases, liver dysfunction in 1 case, deep venous thrombosis in 1 case, allergic reactions in 2 cases, hypertension in 1 case, and other AEs in 2 cases. MPA administration was discontinued due to AEs in 4 cases. In the remaining cases, the AEs were relatively mild; although the dosage was reduced to 200–400 mg/day in some cases, the AEs did not require treatment interruption.
4. Differences in overall survival (OS) and RFS between the initial and repeated treatment groups
In the initial treatment group, 2-, 5-, and 10-year OS rates were 100.0%, 100.0%, and 95.0%, respectively, among patients with AEH, and were 99.0%, 99.0%, and 99.0%, respectively, among patients with G1. In the repeated treatment group, 2-, 5-, and 10-year OS rates were 100.0%, 100.0%, and 92.3%, respectively, among patients with AEH, and were 100.0%, 100.0%, and 100.0%, respectively, among patients with G1. There were no significant differences in OS between AEH and G1 cases in the initial and repeated treatment groups.
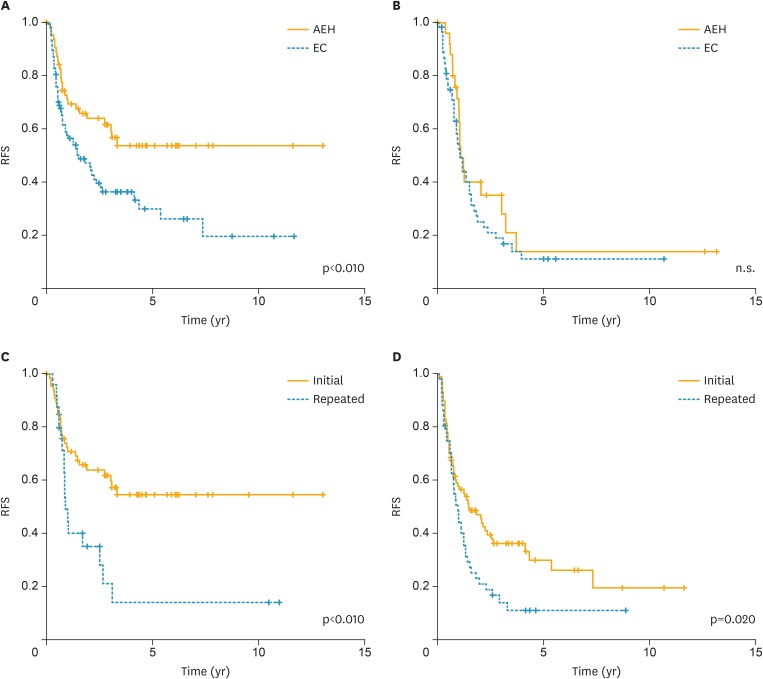
In the initial treatment group, the 2-, 5-, and 10-year RFS rates were 65.8%, 53.7%, and 53.7%, respectively, among patients with AEH, and were 48.6%, 33.2%, and 19.6%, respectively, among patients with G1. Furthermore, the RFS was significantly worse in G1 cases compared to that in AEH cases (p=0.003, Fig. 1A). In the repeated treatment group, the 2-, 5-, and 10-year RFS rates were 40.1%, 14.0%, and 14.0%, respectively, among patients with AEH, and were 25.1%, 11.2%, and 11.2%, respectively, among patients with G1, with no significant differences between AEH and G1 cases (Fig. 1B). However, the RFS was significantly worse in the repeated treatment group than in the initial treatment group for both AEH and G1 cases (p<0.01 and 0.02, respectively; Fig. 1C and D).
Fig. 1.
RFS following the initial and repeated treatments with high-dose MPA. (A) Initial treatment group, (B) repeated treatment group, (C) AEH, (D) early G1 endometrial carcinoma.
AEH, atypical endometrial hyperplasia; EC, endometrial cancer; G1, grade 1 endometrioid carcinoma; MPA, medroxyprogesterone acetate; n.s., not significant; RFS, recurrence-free survival.
5. Relationships between clinicopathological factors and initial and repeated treatment outcomes
Univariate analysis evaluating associations between clinicopathological factors (age, BMI, PCO, hyperprolactinemia, family history of cancer, histological type, and TTD) and RFS following the initial treatment revealed significant associations for PCO, histological type, family history of cancer, and TTD ≥6 months. In addition, a multivariate Cox regression analysis revealed that histological type and cancer family history were prognostic factors for RFS following the initial treatment (Table 3).
Table 3. Univariate and multivariate analysis between clinicopathological factors and RFS.
| Characteristics | Initial treatment | Repeated treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Univariate analysis | Multivariate analysis | No. | Univariate analysis | Multivariate analysis | ||||||
| Median RFS (95% CI) | p-value | HR (95% CI) | p-value | Median RFS (95% CI) | p-value | HR (95% CI) | p-value | ||||
| Age (yr) | ≥35 | 82 | 60.3 (25.1–95.5) | n.s. | - | 25 | 10.9 (9.8–14.9) | n.s. | - | ||
| <35 | 69 | 26.7 (14.8–38.7) | 37 | 16.3 (10.0–22.6) | |||||||
| BMI (kg/m2) | ≥25 | 36 | 26.8 (11.8–41.8) | n.s. | - | 17 | 16.3 (6.1–26.6) | n.s. | - | ||
| <25 | 112 | 45.1 (14.8–75.2) | 45 | 12.8 (9.7–15.9) | |||||||
| PCO | + | 38 | 26.7 (1.9–51.6) | 0.040 | - | 20 | 16.6 (6.1–27.2) | n.s. | - | ||
| − | 113 | 48.8 (6.7–90.8) | 42 | 12.8 (10.8–14.8) | |||||||
| Hyperprolactinemia | + | 17 | 40.3 (0–82.6) | n.s. | - | 9 | 11.6 (6.2–19.4) | n.s. | - | ||
| − | 61 | 12.4 (3.3–21.5) | 37 | 16.3 (8.5–24.1) | |||||||
| Family history of cancer | + | 51 | 13.8 (6.0–21.7) | 0.010 | 1.9 (1.1–3.2) | 0.040 | 28 | 11.0 (7.3–14.6) | n.s. | - | |
| − | 98 | 48.8 (24.0–73.6) | 34 | 16.6 (8.1–25.1) | |||||||
| Histological type | G1 | 87 | 21.4 (9.4–33.5) | <0.010 | 2.1 (1.1–3.7) | 0.020 | 43 | 11.6 (7.9–15.4) | n.s. | - | |
| AEH | 64 | - | 19 | 25.0 (3.1–46.9) | |||||||
| Initial TTD (mo) | ≥6 | 51 | 20.3 (12.3–28.3) | 0.020 | - | 27 | 10.9 (8.6–13.1) | n.s. | - | ||
| <6 | 100 | 60.4 (13.3–107.4) | 35 | 16.3 (7.8–24.8) | |||||||
| 2nd TTD (mo) | ≥6 | - | 18 | 8.8 (6.8–10.8) | n.s. | - | |||||
| <6 | - | 44 | 16.3 (10.3–22.4) | ||||||||
AEH, atypical endometrial hyperplasia; BMI, body mass index; CI, confidence interval; G1, grade 1 endometrioid carcinoma; HR, hazard ratio; n.s., not significant; PCO, polycystic ovary; RFS, recurrence-free survival; TTD, time-to-tumor disappearance.
In contrast, there were no significant associations between the abovementioned clinicopathological factors and RFS following repeated therapy in the univariate and multivariate analyses.
6. Relationships between the TTD and RFS following the initial and repeated treatment
The relationship between the TTD of the initial treatment and the recurrence rate was evaluated. The recurrence rates among patients with AEH were 42.5%, 42.9%, 45.5%, and 47.8% for a TTD<4, 4≤TTD<6, 6≤TTD<9, and TTD≥9 months, respectively. Furthermore, the recurrence rates among patients with G1 were 71.1%, 74.3%, 79.1%, and 79.8% for a TTD <4, 4–6, 7–9, and >9 months, respectively.
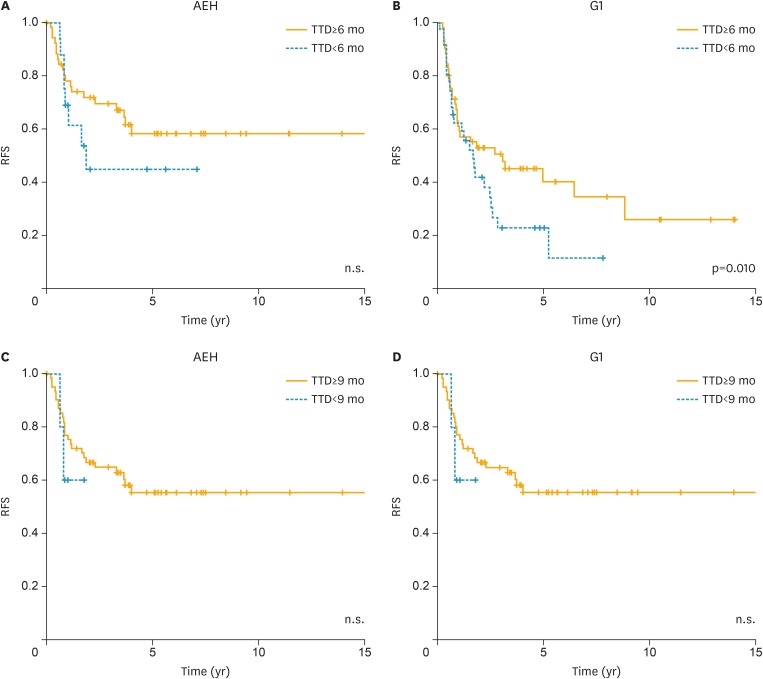
The relationship between the TTD of the initial treatment and RFS following the initial treatment was also evaluated. Among patients with AEH, there was no significant association (Fig. 2A and C); however, among patients with G1, RFS tended to be worse in cases with a TTD ≥6 months than in cases with a TTD <6 months (p=0.100; Fig. 2B). Furthermore, RFS was significantly worse in cases with a TTD ≥9 months than in cases with a TTD <9 months (p=0.020; Fig. 2D).
Fig. 2.
The relationship between the TTD following the initial treatment and RFS following the initial treatment. (A, C) AEH and (B, D) early G1.
AEH, atypical endometrial hyperplasia; G1, grade 1 endometrioid carcinoma; n.s., not significant; RFS, recurrence-free survival; TTD, time-to-tumor disappearance.
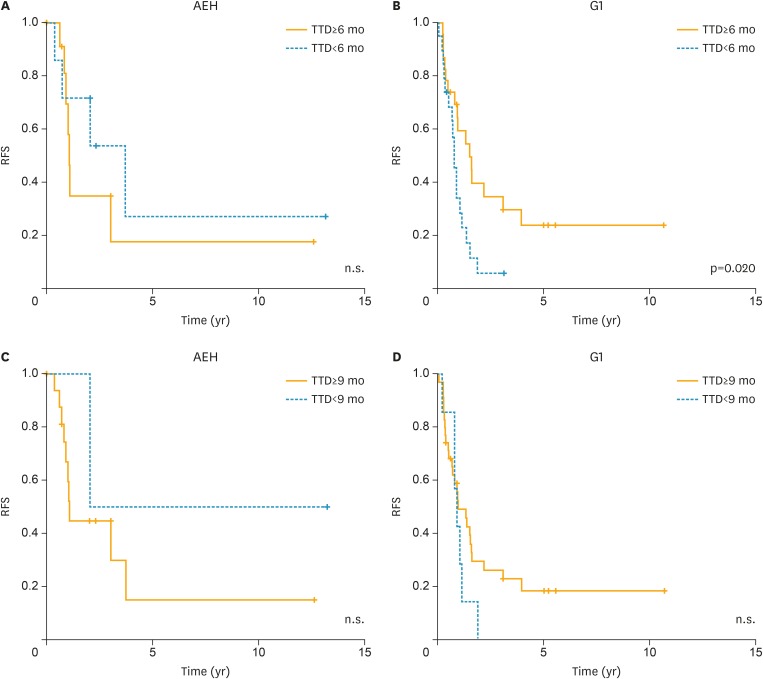
In addition, the relationship between the TTD of the initial treatment and RFS following the repeated treatment was evaluated. Among patients with AEH, there was no significant association (Fig. 3A and C); however, among patients with G1, RFS was significantly worse in cases with a TTD ≥6 months than in cases with a TTD <6 months (p=0.020; Fig. 3B).
Fig. 3.
The relationship between the TTD of the initial treatment and RFS following the repeated treatment. (A, C) AEH and (B, D) early G1.
AEH, atypical endometrial hyperplasia; G1, grade 1 endometrioid carcinoma; n.s., not significant; RFS, recurrence-free survival; TTD, time-to-tumor disappearance.
7. Relationship between the TFI after the initial treatment and RFS following the repeated treatment
Three cut points (i.e., 6, 12, and 18 months) were used to classify TFI into 2 groups: short TFI (<6, <12, and <18 months) and long TFI (≥6, ≥12, and ≥18 months). Then, the 2 groups were compared by using each of the 3 cut points, and the relationship between the TFI after the initial treatment and RFS following the repeated treatment was evaluated, according to histological types. However, no significant association between the TFI and RFS following the repeated treatment was observed (Supplementary Table 2).
DISCUSSION
The present study investigated the efficacy and safety of repeated MPA therapy for the treatment of EC/AEH in women who wish to preserve their fertility. It is difficult to produce evidence related to this issue, as assessing the outcomes of fertility-preserving therapy is complex, and phase III trials cannot be planned using standard treatment as a control arm. The prognosis following hormonal therapy for early EC/AEH is obviously worse in terms of tumor prognosis than that following standard treatment. Sufficient evidence for the efficacy of initial hormonal treatment has not yet been established, and the evidence for the efficacy of repeated hormonal treatment is poorer still. The present study obtained 82 cases with repeated hormonal therapy (28 and 54 cases for AEH and EC, respectively), and represents the largest study to date, despite being a retrospective study conducted at a single institution.
In the present study, the CR rate in patients with AEH did not significantly differ between the 2 treatment groups; however, the CR rate in patients with early EC tended to be higher in the repeated treatment group than in the initial treatment group. This result was probably due to the fact that patients who were not responsive to hormonal therapy often decided to undergo surgery after the initial treatment, and were thus not included in the repeated treatment group. However, there was no correlation between the MTP/TTD values following the initial treatment and those following the repeated treatment (data not shown). Thus, the MTP/TTD following the repeated treatment could not be predicted from the MTP/TTD values following the initial treatment.
Among patients with AEH, the pregnancy rate tended to be lower in the repeated treatment group than in the initial treatment group, while there was no significant group difference among patients with EC. This result was probably related to the much worse RFS in patients with EC than that in patients with AEH. AEs during the repeated treatment were not increased compared to that for the initial treatment.
Based on the abovementioned data, repeated hormonal treatment for intrauterine recurrence after hormonal therapy appears to be an acceptable treatment option. In patients with early EC, the CR rate for repeated therapy tends to be equal to or better than that of the initial treatment, and the pregnancy rate associated with repeated treatment is at least equivalent to that associated with the initial treatment. However, in patients with AEH or EC, the recurrence rate following repeated treatment was significantly higher than that following the initial treatment, and RFS following the repeated treatment was significantly worse than that following the initial treatment. Thus, it appears necessary to follow a stricter follow-up regime after repeated treatment than after initial treatment to ensure that patients remain eligible for the treatment.
Age, BMI, and TTD have been reported as factors predictive of recurrence following initial and repeated treatments in previous reports [7,8]. In the present study, the histological type and family history of cancer were recognized as predictive factors for recurrence following the initial treatment in both the univariate and multivariate analyses; however, no factor was found to be predictive of recurrence following the repeated treatment in either the univariate or multivariate analysis. Although the TTD affected RFS following the initial treatment, regardless of histological type (as shown in the univariate analysis), there was a significant association for EC cases only (in both initial and repeated treatments). In EC cases, cases with a longer TTD following initial treatment appear likely to recur after the initial and repeated treatments. In contrast, TFI did not affect the recurrence rate or RFS, unlike that in cytotoxic chemotherapy [9].
Previous reports on repeated treatment for intrauterine recurrence after hormonal therapy are summarized in Supplementary Table 3 [10,11,12,13,14,15]. Combining the results of previous studies (in which treatment outcomes for both the initial and repeated treatments were reported) yielded a CR rate of 79.8% (99/124) for the initial treatment and 85.2% (52/61) for the repeated treatment, with no significant difference between the treatment groups. In addition, the recurrence rates were 36.3% (82/240) and 28.8% (15/42) for the initial treatment and repeated treatment groups, respectively, with no significant difference between the treatment groups, which is contrary to the findings of the present study. In these previous reports, it is possible that no significant differences were found due to the small numbers of cases and shortened follow-up period in the repeated treatment.
The present study has a few limitations that should be discussed. First, this was a single-center retrospective study. It is possible that the treatment outcomes in the present study differ from those in previous studies. However, the current study has an advantage in that the treatment protocols were relatively similar, as 2 gynecological oncologists at a single institution planned all treatments. Secondly, in some cases, it was not possible to collect data on all AEs and clinical factors, as we only used the medical records.
In conclusion, repeated treatment is sufficiently effective for intrauterine recurrence after hormonal therapy, as previously reported in smaller studies. However, some previous reports have suggested that in 2%–4% of patients, there is a risk of duplicated ovarian cancer or an increase in cancer stage (≥stage II). Therefore, when intrauterine recurrence is identified after hormone therapy, it is necessary to confirm whether the tumor is limited to the endometrium and to clarify whether the histological type is AEH or G1. Pelvic MRI, chest-pelvic CT (or positron emission tomography-CT), and total endometrial curettage are needed to ensure that the patient meets the eligibility criteria for the initial and repeated hormonal treatments.
ACKNOWLEDGMENTS
The authors are grateful to Ms. Tomomi Noda and Ms. Keiko Abe of Keio University School of Medicine for their secretarial help.
Footnotes
Funding: This work was supported, in part, by Grants-in-Aid from the Japan Society for the Promotion of Science (Kiban-C-16K11156, N.S.).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Y.W., S.N., A.D.
- Data curation: N.H., K.F., H.A.
- Formal analysis: M.T., S.K.
- Funding acquisition: S.N.
- Investigation: Y.W., S.N.
- Methodology: Y.W., S.N.
- Project administration: A.D.
- Resources: M.T., S.K.
- Software: Y.W.
- Supervision: B.K., A.D.
- Validation: Y.W.
- Visualization: A.D.
- Writing - original draft: Y.W.
- Writing - review & editing: Y.W., S.N., H.A., B.K., A.D.
SUPPLEMENTARY MATERIALS
AE during and after MPA therapy
RFS was not associated with TFI at initial treatment
Outcomes after progestin repeated treatment in patients with recurrent disease
References
- 1.Yamagami W, Aoki D. Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2015;41:1861–1869. doi: 10.1111/jog.12833. [DOI] [PubMed] [Google Scholar]
- 2.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int J Clin Oncol. 2016;21:419–434. doi: 10.1007/s10147-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (US) NCCN Clinical Practice Guidelines in Oncology. Uterine neoplasms, version 2 [Internet] Fort Washington, PA: National Comprehensive Cancer Network; 2017. [cited 2017 Sep 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. [Google Scholar]
- 5.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266.e1–266.12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Rodolakis A, Biliatis I, Morice P, Reed N, Mangler M, Kesic V, et al. European Society of Gynecological Oncology Task Force for Fertility Preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer. 2015;25:1258–1265. doi: 10.1097/IGC.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Jin Y, Li Y, Bi Y, Shan Y, Pan L. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynaecol Obstet. 2016;132:34–38. doi: 10.1016/j.ijgo.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 8.Wang CJ, Chao A, Yang LY, Hsueh S, Huang YT, Chou HH, et al. Fertility-preserving treatment in young women with endometrial adenocarcinoma: a long-term cohort study. Int J Gynecol Cancer. 2014;24:718–728. doi: 10.1097/IGC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 9.Nagao S, Nishio S, Okada S, Otsuki T, Fujiwara K, Tanabe H, et al. What is an appropriate second-line regimen for recurrent endometrial cancer? Ancillary analysis of the SGSG012/GOTIC004/Intergroup study. Cancer Chemother Pharmacol. 2015;76:335–342. doi: 10.1007/s00280-015-2793-9. [DOI] [PubMed] [Google Scholar]
- 10.Gotlieb WH, Beiner ME, Shalmon B, Korach Y, Segal Y, Zmira N, et al. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet Gynecol. 2003;102:718–725. doi: 10.1016/s0029-7844(03)00667-7. [DOI] [PubMed] [Google Scholar]
- 11.Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 12.Yu M, Yang JX, Wu M, Lang JH, Huo Z, Shen K. Fertility-preserving treatment in young women with well-differentiated endometrial carcinoma and severe atypical hyperplasia of endometrium. Fertil Steril. 2009;92:2122–2124. doi: 10.1016/j.fertnstert.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Eftekhar Z, Izadi-Mood N, Yarandi F, Shojaei H, Rezaei Z, Mohagheghi S. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: our experiences with 21 patients. Int J Gynecol Cancer. 2009;19:249–252. doi: 10.1111/IGC.0b013e31819c5372. [DOI] [PubMed] [Google Scholar]
- 14.Perri T, Korach J, Gotlieb WH, Beiner M, Meirow D, Friedman E, et al. Prolonged conservative treatment of endometrial cancer patients: more than 1 pregnancy can be achieved. Int J Gynecol Cancer. 2011;21:72–78. doi: 10.1097/IGC.0b013e31820003de. [DOI] [PubMed] [Google Scholar]
- 15.Park JY, Lee SH, Seong SJ, Kim DY, Kim TJ, Kim JW, et al. Progestin re-treatment in patients with recurrent endometrial adenocarcinoma after successful fertility-sparing management using progestin. Gynecol Oncol. 2013;129:7–11. doi: 10.1016/j.ygyno.2012.12.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AE during and after MPA therapy
RFS was not associated with TFI at initial treatment
Outcomes after progestin repeated treatment in patients with recurrent disease