Abstract
Cancer preventive activities of green tea and its main constituent, (−)-epigallocatechin gallate (EGCG) have been extensively studied by scientists all over the world. Since 1983, we have studied the cancer chemopreventive effects of EGCG as well as green tea extract and underlying molecular mechanisms. The first part of this review summarizes ground-breaking topics with EGCG and green tea extract: 1) Delayed cancer onset as revealed by a 10-year prospective cohort study, 2) Prevention of colorectal adenoma recurrence by a double-blind randomized clinical phase II trial, 3) Inhibition of metastasis of B16 melanoma cells to the lungs of mice, 4) Increase in the average value of Young’s moduli, i.e., cell stiffness, for human lung cancer cell lines and inhibition of cell motility and 5) Synergistic enhancement of anticancer activity against human cancer cell lines with the combination of EGCG and anticancer compounds. In the second part, we became interested in cancer stem cells (CSCs). 1) Cancer stem cells in mouse skin carcinogenesis by way of introduction, after which we discuss two subjects from our review on human CSCs reported by other investigators gathered from a search of PubMed, 2) Expression of stemness markers of human CSCs compared with their parental cells, and 3) EGCG decreases or increases the expression of mRNA and protein in human CSCs. On this point, EGCG inhibited self-renewal and expression of pluripotency-maintaining transcription factors in human CSCs. Human CSCs are thus a target for cancer prevention and treatment with EGCG and green tea catechins.
Keywords: AFM, Nanog, Oct4, Sox2, stemness
INTRODUCTION
The term “cancer chemoprevention” was introduced by Michael B. Sporn, at the US National Institutes of Health (NIH) in Bethesda, Maryland (Sporn et al., 1976), and Japanese cancer researchers became interested in screening for possible cancer preventive agents in the 1980s. We assumed that inhibitors of tumor promotion would be cancer preventive compounds, since they had been known to suppress experimentally induced tumor development in rodents (Boutwell, 1977). Since green tea is a daily beverage in Japan, we paid special attention to green tea catechins, especially its main constituent (−)-epigallocatechin gallate (EGCG), for our main experiments (Fujiki and Okuda 1992). In 1987, we reported for the first time that topical applications of EGCG significantly prevented tumor promotion in mouse skin induced by teleocidin, one of the 12-O-tetradecanoylphorbol-13-acetate (TPA)-type tumor promoters (Yoshizawa et al., 1987). Soon after publication, the British journal “New Scientist” introduced our research on cancer prevention with EGCG under the title of “Green tea cuts cancerous growths” (Editor, 1987). In quick response to this article, staff members of the Australian TV scientific series “Beyond 2000” visited us in Tokyo: They made a film about our experiments in cancer prevention with green tea catechins, and the film was released in Australia, U.S.A., England and Europe in 1988. It was followed by “Beyond Tomorrow” on American TV in 1988. Many overseas scientists then became greatly interested in cancer prevention with green tea, some of them even more interested than Japanese scientists.
Since then, numerous scientists have shown that EGCG and green tea extract in drinking water prevent carcinogenesis in various organs of rodents (Conney et al., 1992; Fujita et al., 1989; Fujiki and Suganuma, 2002; Gupta et al., 2001; Surh 2003; Yamane et al., 1995; Yang et al., 2009; Yoshizawa et al., 1992). The results are supported by our experiments showing that intubation of 3H-EGCG into mouse stomach distributed radioactivity in a wide range of target organs, and subsequent treatment of cells with 3H-EGCG showed radioactivity inside the cells (Okabe et al., 1997; Suganuma et al., 1998). Based on results showing that the inhibitory effects of EGCG and green tea extract on the growth of human lung cancer cell lines PC-9 and PC-14 were approximately 250-fold less effective than adriamycin (Komori et al., 1993), we conceived the development of EGCG and green tea extract as cancer preventives, rather than cancer therapeutic drugs.
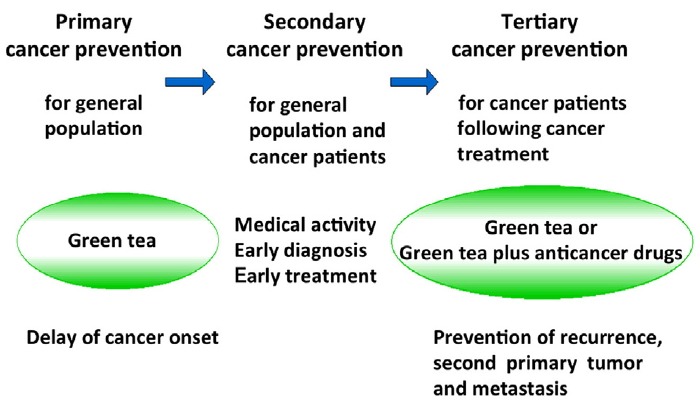
We have studied cancer prevention with green tea for over 30 years, and our collaborations have produced numerous significant results, both from basic studies and with cancer patients and the general human population (Fujiki et al., 2002; 2012). This review consists of two parts, and the first part summarizes epochal results by way of introduction: 1) A prospective cohort study revealed that drinking 10 Japanese-size cups (120 ml/cup) of green tea per day delayed cancer onset 7.3 years for female patients (Imai et al., 1997; Nakachi et al., 2000); 2) A randomized phase II clinical prevention trial showed that drinking 10 Japanese-size cups of green tea, supplemented with green tea tablets, significantly reduced tumor recurrence in patients with colorectal adenomas (Shimizu et al., 2008); 3) Peroral administration of EGCG in drinking water prevented both hematogenous and lymphogenous (spontaneous) lung metastases of B16 melanoma cells in male C57BL/6 mice (Taniguchi et al., 1992); 4) Treatment of B16-F10 mouse melanoma cells with EGCG increased the average value of Young’s moduli as assessed by the atomic force microscope (AFM), i.e., cell stiffness, and inhibited cell motility (Watanabe et al., 2012); and 5) The combination of EGCG and anticancer compounds induced apoptosis and increased efficacy of anticancer activity in rodents, and also showed synergistic enhancement of anti-cancer activity against human cancer cell lines (Suganuma et al., 1999; 2006; 2011). Green tea is a cancer preventive for primary cancer prevention, and green tea catechins act as synergist with anticancer drugs in tertiary cancer prevention (Fig. 1) (Fujiki, 2017; Fujiki et al., 2012; 2015a; 2015b).
Fig. 1. Primary, secondary, and tertiary cancer prevention in humans.
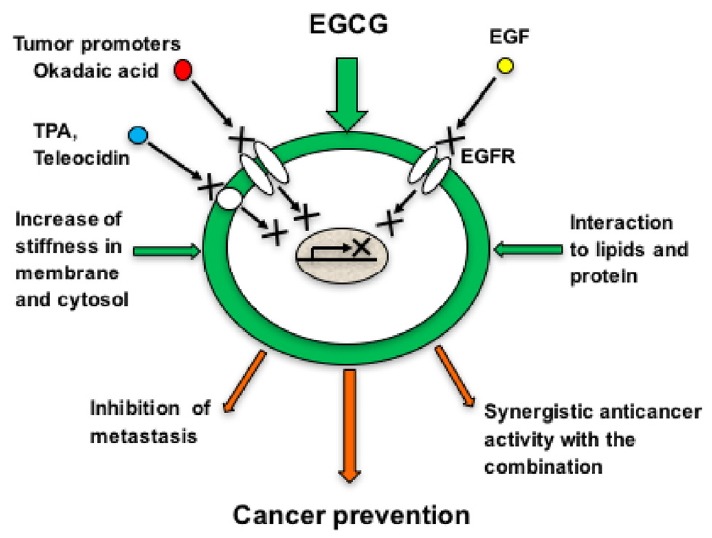
The second part of this review shows the molecular mechanisms of anticancer activity of EGCG against human cancer stem cells (CSCs). We first found that repeated applications of 5 mg EGCG before each treatment with 1 μg okadaic acid - a potent tumor promoter and inhibitor of protein phosphatases 1 and 2A - completely prevented tumor promotion in mouse skin, in two-stage carcinogenesis experiments initiated with 7,12-dimethylbenz(a)anthracene (DMBA), and that treatment with DMBA plus okadaic acid produced tumors in 73.3% of mice at week 20 (Fujiki and Suganuma, 1993; Suganuma et al., 1988). We believe that EGCG treatment inhibited the interaction of tumor promoters, such as okadaic acid, TPA and teleocidin with their receptors, and theorized that EGCG can interrupt the interaction of ligand with its receptor on cell membrane. This is now called the “Sealing effects of EGCG” (Fig. 2) (Yoshizawa et al., 1992): We thus decided to study cancer stem cells in mouse skin as a target of cancer prevention. In 2012, Srivastava’s group reported that EGCG inhibited viability of human pancreatic CSCs in primary and secondary spheroids, as well as expression of pluripotency-maintaining factors in the CSCs (Tang et al., 2012). Recently we published a review article on the anticancer activity of EGCG against various human CSCs enriched from cancer cell lines (Fujiki et al., 2017). We then discuss: 1) Cancer stem cells in mouse skin carcinogenesis, 2) Expression of stemness markers of human CSCs enriched from colorectal and nasopharyngeal cancer cell lines, compared with their parental cells, and 3) Decrease or increase in the expression of mRNA and protein in human CSCs of breast, lung and colorectal cancers after treatment with EGCG. The inhibitory effects of EGCG on self-renewal and expression of transcription factors in human CSCs derived from various cancer tissues are emphasized. It has now become clear that human CSCs are effective targets for prevention and treatment using EGCG and green tea extract.
Fig. 2. Schematic illustration of mechanisms of EGCG in relation to “Sealing effects of EGCG”.
DELAYED CANCER ONSET REVEALED BY 10-YEAR PROSPECTIVE COHORT STUDY
Nakachi and Imai found a total of 419 cancer patients, 175 females and 244 males, during 10 years, from a survey living habits of 8,552 individuals aged over 40 living in Saitama Prefecture, including their daily consumption of green tea (Imai et al., 1997; Nakachi et al., 2000). They reported that cancer onset in female patients who had consumed over 10 Japanese-size cups (120 ml/cup) of green tea per day was 7.3 years later than that of patients who had consumed less than three cups per day (Table 1). The difference between females and males may be partly due to higher tobacco consumption by males. Consuming over 10 cups of green tea per day (corresponding to 2.5 g green tea extract) also significantly prevented lung cancer, with a relative risk of 0.33, followed by cancers of the colorectum, liver and stomach, in that order (Imai et al., 1997; Nakachi et al., 2000). Results of two research groups: Green tea catechins at 600 mg per day were effective for treating premalignant lesions before prostate cancer development, research conducted at University of Parma, Italy (Bettuzzi et al., 2006): and green tea extract showed preventive effects on oral premalignant leukoplakia in patients, at the University of Texas M.D. Anderson Cancer Center (Tsao et al., 2009).
Table 1.
Daily green tea consumption and average age at cancer onset*
| Gender | Daily consumption of green tea (cups) | ||
|---|---|---|---|
|
|
|||
| ≤3 | 4 – 9 | ≥10 | |
| Average age at cancer onset (% of patients) | |||
| Female (175) | 67.0 ± 1.7 (28.0%) | 66.4 ± 1.3 (58.3%) | 74.3 ± 2.2 (13.7%)** |
| Male (244) | 65.0 ± 1.5 (24.2%) | 67.2 ± 1.0 (46.7%) | 68.2 ± 1.1 (29.1%) |
All different types of cancer are included,
P < 0.01
Nakachi and Imai also studied recurrence of breast cancer among the 472 cancer patients: Stages I and II cancer patients consuming over five cups of green tea per day (average 8 cups) showed a lower recurrence rate, 16.7%, and a longer disease-free period, 3.6 years, than those consuming less than four cups per day (average 2 cups), 24.3% and 2.8 years (Table 2) (Nakachi et al., 1998). However, in Stage III breast cancer patients, green tea did not show any decreased recurrence because Stage III breast cancer includes more accumulated genetic changes in the cells than are found in Stages I and II. The results were later confirmed at Aichi Cancer Center (Inoue et al., 2001), and at Harvard T.H. Chan School of Public Health in the United States (Ogunleye et al., 2010).
Table 2.
Recurrence rate of breast cancer in relation to daily consumption of green tea
| Daily green tea consumption | ||
|---|---|---|
|
|
||
| Parameter | ≤4 cups | ≥5 cups |
| Stages I and II (390 patients) | ||
| Recurrence rate (%) | 24.3 | 16.7* |
| Disease-free period (years) | 2.8 | 3.6 |
| Stage III (82 patients) | ||
| Recurrence rate (%) | 48.8 | 58.5 |
| Disease-free period (years) | 1.9 | 1.9 |
P < 0.05 in terms of the Cox proportional hazards model.
Since humans are always at risk of tumor promotion induced by inflammation, we need to establish a cancer prevention strategy that can reduce TNF-α, IL-1 and other proinflammatory cytokines, and inactivate NF-κB (Fujiki et al., 2013). The key point is: Drinking green tea contributes to primary cancer prevention (Fig. 1).
PREVENTION OF COLORECTAL ADENOMA RECURRENCE WITH 10 JAPANESE-SIZE CUPS OF GREEN TEA PER DAY SUPPLEMENTED WITH GREEN TEA TABLETS
The Saitama Prefectural Tea Research Institute began to produce tablets of green tea extract (G.T.E), which is the dried form of green tea beverage. One tablet is equivalent to approximately 2 Japanese-size cups of green tea. One hundred two healthy citizens of Saitama Prefecture joined the preclinical safety trial of G.T.E, with informed consent. The blood examination did not show any serious effects, and 93% of the participants were able to continue drinking green tea beverage and also taking G.T.E (Fujiki et al., 2001). Because some of the subjects had very mild temporary disorders, the Tea Research Institute subsequently reduced the caffeine content of the tablets from 5% to less than 3% without using an organic solution.
Moriwaki’s group at Gifu University conducted a double-blind randomized clinical phase II prevention trial of colorectal adenoma recurrence with subjects drinking 10 cups of green tea supplemented with tablets of G.T.E, with informed consent. Patients without colon adenomas were then double-blind randomized into two groups: Control group maintained daily consumption of green tea beverage only, without a placebo, and G.T.E group took the daily beverage plus 3 tablets (equivalent to 6 cups) per day, corresponding to over 10 cups, about 2.5 g green tea extract, for 12 months. The incidence of recurrent adenomas was determined by end-point colonoscopy 12 months later: Control group showed 31.0% recurrence rate, while the G.T.E group rate was 15.0%, and the average size of relapsed adenomas was 3.0 ± 1.0 mm in the G.T.E. group and 4.0 ± 1.3 mm in control group (P < 0.001) (Table 3). Thus, drinking 10 Japanese-size cups of green tea, supplemented with G.T.E, significantly, 51.6%, reduced recurrence of colorectal adenomas (Shimizu et al., 2008).
Table 3.
Phase II prevention trial of colorectal adenoma recurrence of patients drinking a combination of daily green tea beverage and tablets of G.T.E
| Study in Japan | ||
|---|---|---|
|
| ||
| Groups (cases) | Recurrence rate % | Size of relapsed adenomas (mm) |
| Control (20/65) | 31.0 | 4.0 ± 1.3 |
| G.T.E (9/60) | 15.0* | 3.0 ± 1.0** |
|
| ||
| Study in Korea | ||
|
| ||
| Groups (cases) | Recurrence rate % | Size of the largest polyps (mm) |
|
| ||
| Control (43/71) | 60.6 | 4.8 ± 2.4 |
| G.T.E (20/72) | 27.8** | 4.8 ± 2.3 |
P < 0.05
P < 0.001
Similar results were confirmed at different institutions: drinking green tea extract prevented 44.2% of colorectal adenoma recurrence in Korean patients at Seoul National University (Table 3) (Shin et al., 2017), and the flavonoid mixture (daily standard dose, 20 mg apigenin and 20 mg EGCG) reduced the recurrence rate of colon neoplasia in patients with resected colon cancer at the Hospital of Gross-Gerau, Germany (Hoensch et al., 2008). And a plan for the first large-scale placebo-controlled prevention trial for metachronous adenoma recurrence in the colorectum of patients, using green tea extract for three years, is conducted at University Ulm, Germany (Stingl et al., 2011). All the results show that drinking green tea is effective for tertiary cancer prevention (Fig. 1).
INHIBITION OF METASTASES OF B16 MELANOMA CELLS TO THE LUNGS OF MICE BY DRINKING EGCG
Taniguchi’s group reported for the first time that oral administration of EGCG inhibited lung metastases of two different B16 melanoma variants in two experimental models (Taniguchi et al., 1992). Hematogenous metastasis was induced with intravenous injection of highly metastatic B16-F10 cells in male C57BL/6 mice given a solution of 0.05% and 0.1% EGCG. Lymphogenous (spontaneous) metastasis was induced by inoculation of highly metastatic and invasive B16-BL6 cells into the right foot pads of male C57BL/6 mice, given the same solutions of EGCG. EGCG reduced the average number of lung nodules (Table 4) (Taniguchi et al., 1992). To understand the inhibitory effects of EGCG on metastasis, Suganuma’s group further studied the biophysical effects of EGCG on cell stiffness and motility (Suganuma et al., 2016).
Table 4.
Inhibition of lung metastasis of B16 melanoma cells with peroral administration of EGCG
| Hematogenous metastasis with B16-F10 cells | Lymphogenous metastasis with B16-BL6 cells | |||
|---|---|---|---|---|
|
|
||||
| Groups | Average number of lung nodules | % of inhibition | Average number of lung nodules | % of inhibition |
| Control | >150* | 25* | ||
| 0.05% EGCG | 107* | >29% | 7* | 72% |
| 0.1% EGCG | 76* | >50% | 10* | 60% |
P < 0.01
GREEN TEA AND EGCG INCREASED THE AVERAGE VALUE OF YOUNG’S MODULUS OF CANCER CELLS (CELL STIFFNESS) AND INHIBITED CELL MOTILITY
Cell stiffness can be determined using atomic force microscope (AFM), which quantitatively provides the average value of Young’s modulus of cancer cells. In 2007, Gimzewski’s group at UCLA reported that metastatic cells in pleural fluids obtained from lung, breast, and pancreatic cancer patients have significantly lower average values of Young’s modulus with less stiffness (equivalent to smaller elasticity) - determined using AFM - than normal mesothelial cells in the body fluids (Cross et al., 2007). They also found that green tea extract dramatically increased cell stiffness of metastatic cancer cells, from 0.43 kPa to 2.53 kPa, about 6.2-fold, based on the average value of Young’s moduli in nine cancer cell lines (Cross et al., 2011). The results indicated that AFM can measure the changes in cell stiffness induced by EGCG.
To study the relationship between increased average value of Young’s moduli and reduction of cell motility by Transwell assay, we used three metastatic B16 mouse melanoma variants for experiments: B16-F10 cells are most motile, B16-BL6 cells, medium motile and B16-F1 cells, least motile. Young’s modulus of the most motile B16-F10 cells showed significantly lower cell stiffness, i.e., more soft elasticity, than those of B16-BL6 cells and B16-F1 cells. Furthermore, treatment of B16-F10 cells with 100 μM EGCG increased the average value of Young’s modulus to 0.68 ± 0.03 from 0.44 ± 0.01 kPa of non-treated B16-F10 cells (Table 5). The results indicated that EGCG increased the average value of Young’s modulus for B16-F10 cells (0.68 kPa), which was comparable to that of the least motile B16-F1 cells (0.72 kPa) without EGCG, showing that treatment with EGCG increased stiffness. Moreover, treatment of B16-F10 cells with EGCG (50 – 200 μM) dose-dependently reduced the motility of the cells to 57.1, 30.3 and 12.6%, respectively (Table 5), without affecting viability of the cells (Watanabe et al., 2012). Thus, EGCG simultaneously increased cell stiffness and enhanced inhibition of cell motility (Fig. 2).
Table 5.
Average values of Young’s moduli and inhibition of cell motility for B16-F10 mouse melanoma cells
| EGCG (μM) | ||||
|---|---|---|---|---|
|
|
||||
| 0 | 50 | 100 | 200 | |
| Young’s moduli (kPa) | 0.44 ± 0.01 | 0.58 ± 0.03* | 0.68 ± 0.03* | 0.80 ± 0.02* |
| Cell motility (%) | 100 | 57.1 | 30.3* | 12.6** |
P < 0.001
P < 0.0001
Suganuma’s group found that treatment of human non-small cell lung cancer cell lines H1299 and Lu99 with EGCG (5 – 50 μM) for 4 h significantly increased the average value of Young’s moduli from 1.24 ± 0.05 to 2.25 ± 0.11 kPa in H1299 cells, and from 1.29 ± 0.11 to 2.28 ± 0.09 kPa in Lu99 cells, showing a 2-fold increase of cell stiffness (Table 6) (Suganuma et al., 2016; Takahashi et al., 2014). The results showed that EGCG reduces membrane fluidity - increases rigidification of cell membrane - the cell stiffness of H1299 and Lu99, indicating that EGCG can reduce highly metastatic potential of both cell lines (Fig. 2).
Table 6.
Increase of average values of Young’s moduli for human lung cancer cell lines by treatment with EGCG
| EGCG (μM) | |||
|---|---|---|---|
|
|
|||
| Cell lines | 0 | 5 | 50 |
| H1299 (kPa) | 1.24 ± 0.05 | 2.30 ± 0.07* | 2.25 ± 0.11* |
| Lu99 (kPa) | 1.29 ± 0.11 | 1.63 ± 0.08* | 2.28 ± 0.09* |
P < 0.0001
SYNERGISTIC ENHANCEMENT OF ANTICANCER ACTIVITY AGAINST HUMAN CANCER CELL LINES WITH THE COMBINATION OF EGCG AND ANTICANCER COMPOUNDS
In 2011, Suganuma’s group published a review article entitled “New cancer treatment strategy using combination of green tea catechins and anticancer drugs” in Cancer Sci. (Suganuma et al., 2011). The Publisher, Wiley-Blackwell at the Annual Meeting of Japanese Cancer Association, announced that our review article was No. 1 among most read articles and No. 2 among most cited articles in 2012. Since then, numerous scientists around the world have become greatly interested in the combination. We briefly showed that the combinations of EGCG or other green tea catechins and 46 anticancer drugs all synergistically induced in vitro anticancer effects in 58 human cancer cell lines (Fujiki et al., 2015a; 2015b).
It is important to note that the enhanced anticancer activity of the combination was demonstrated by reduction of tumor volume in xenograft mouse models in 13 in vivo experiments: human cancer cell lines from head and neck, lung, breast, prostate, liver, and stomach were implanted in experiments conducted by numerous investigators (Fujiki et al., 2015a; 2015b). It is striking that the combinations of EGCG and paclitaxel, and EGCG and docetaxel, completely eliminated tumors of human prostate cancer cell line PC-3ML in vivo (Table 7) (Stearns and Wang, 2011). In addition, average reduction of tumor volume (% of control) for the groups treated with vehicle (control), EGCG alone, anti-cancer drugs alone, and combinations were 100%, 73.5%, 66.3%, and 29.7%, respectively (Table 7). Thus, the combinations of EGCG and anticancer drugs significantly and synergistically reduced tumor volume by 70.3%, while treatment with EGCG or green tea extract alone was slightly less effective than that with anticancer drugs alone. When the amount of EGCG necessary for the complete elimination of tumors in mice is converted to that for humans, it would be 6 – 9 Japanese-size-cups of green tea, i.e., 1.37 – 2.05 g EGCG/day/person (Fujiki, 2017; Fujiki et al., 2015a; 2015b).
Table 7.
Reduction of tumor volume in xenograft mouse models implanted using human cancer cell lines after treatment with the combination of EGCG and anticancer drugs
| Tumor volume (% of control) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cancer cell line | Name of drugs | Vehicle (control) | EGCG alone | Anticancer drugs alone | Combinations | References |
| Prostate cancer cell lines | ||||||
| PC-3ML | Paclitaxel | 100 | 40.9a | 44.3 | 0 | Stearns and Wang, 2011 |
| Docetaxel | 100 | 54.1a | 42.4 | 0 | Stearns and Wang, 2011 | |
|
| ||||||
| Average reduction of tumor volume (% of control) | 100 | 73.5a | 66.3 | 29.7 | Fujiki et al., 2015a | |
228 mg/kg EGCG
CANCER STEM CELLS IN MOUSE SKIN CARCINOGENESIS
Two-stage chemical carcinogenesis in mouse skin, initiation and tumor promotion, is a useful model for studying tumor promotion and cancer chemoprevention (Boutwell, 1977). We found that repeated applications of 5 mg EGCG before each treatment with 1 μg okadaic acid completely prevented tumor promotion in mouse skin initiated with DMBA (Yoshizawa et al., 1992). At that time, the dark basal keratinocytes in normal epidermal cells were assumed to be stem cells in two-stage carcinogenesis experiments in mouse skin (Slaga and Klein-Szanto, 1983). The epidermis is believed to contain two types of proliferating cells: stem cells and cells with a lower capacity for self-renewal and higher probability of undergoing terminal differentiation (transit amplifying cells) (Jones and Watt, 1993). The removal of the interfollicular epidermis from carcinogen-exposed mice, using an abrasion technique, reduced by half the number of papillomas, while the number of carcinomas remained the same in both abraded and unabraded mice (Morris et al., 2000). Stem cells are generally characterized by slow cycling, unlimited self-renewal, and multipotentiality, and they can commit to a variety of cell lineages that comprise the tissue of origin. Initiated label-retaining cells are clonogenic and differentiation-resistant, and hence a likely target for the mutagenic activity of chemical initiators and ultraviolet light (Gerdes and Yuspa, 2005). Mouse keratinocyte stem cells with CD34+ and cytokeratin 15+ (K15) are located only in the outer root sheath of a specific niche within the hair follicle defined as “the bulge,” which is thought to contain stem cells (Affara et al., 2006). Trempus’s group reported that the back skin of CD34 knockout mice (CD34KO) initiated with DMBA and promoted with TPA failed to develop papillomas by week 20, compared with the wild-type mice. This suggests that CD34 is required for TPA-induced hair follicle stem cell activation and tumor formation in mice (Trempus et al., 2007). Blanpain’s group showed that cancer stem cells (CSCs) of skin papillomas are localized in a perivascular niche, and that vascular endothelial growth factor (VEGF) affects skin tumor growth by promoting cancer stemness (the ability to self-renew and differentiate) and symmetric CSC division, leading to CSC expansion (Beck et al., 2011). Furthermore, they reported that SRY (sex determining region Y)-box 2 (Sox2) is the most upregulated transcription factor in the CSCs of squamous skin tumors in mice, and that Sox2 is absent in normal epidermis but begins to be expressed in the vast majority of mouse and human pre-neoplastic skin tumors (Boumahdi et al., 2014). The results indicated a strong relationship between cancer stem cells and expression of pluripotency-maintaining transcription factors.
EGCG and green tea extract inhibit the growth of human cancer cell lines in culture and in rodents (Fujiki et al., 2012; Okabe et al., 1999). Since inhibition of tumor promotion by EGCG is assumed to be strongly related to the non-toxic downregulation of CSCs, we gathered numerous reports from a search of PubMed and published our review article of the literature to provide a broad selection for the effects of EGCG on about 20 human CSCs enriched from cancer cell lines (Fujiki et al., 2017). In the next section, the expression of stemness markers in colorectal and nasopharyngeal CSCs will be introduced as examples, followed by the decrease or increase in stemness markers of human breast, lung and colorectal CSCs by EGCG.
HUMAN CSCS EXPRESS STEMNESS MARKERS DIFFERENTLY FROM THEIR PARENTAL CELLS
Human CSCs enriched from primary and secondary spheroids are capable of undergoing self-renewal. The quantitative differences in the levels of stemness markers between CSCs and parental cells were studied. The spheroid-derived CSCs, designated HCT116-SDCSCs, exhibit approximately 4.5-fold and 3.2-fold higher expression of stem cell markers, octamer-binding transcription factor 4 (Oct4) and Nanog homeobox protein (Nanog), respectively, than the parental cells (Toden et al., 2016). Oct4, Nanog, and Sox2 are transcription factors required for the maintenance of pluripotency by coordinated networks of transcription factors (Boumahdi et al., 2014; Kashyap et al., 2009; Sarkar and Hochedlinger, 2013). Moreover, the expression levels of the surface marker CD44 and self-renewal markers Notch homolog (Notch), B-lymphoma Moloney murine leukemia virus integration site 1 homologue (Bmi-1), CD133, and aldehyde dehydrogenase 1 (ALDH1) are higher in HCT116-SDCSCs than in parental cells (Table 8).
Table 8.
Human CSCs express stemness markers differentially
| Cancers types and names of CSCs | Markers of increased expression | Markers of decreased expression | References | |
|---|---|---|---|---|
| Colorectal cancer | ||||
| HCT116-SDCSCs | mRNAs: | Oct4, Nanog, | Toden et al., 2016 | |
| Proteins: | CD44, Notch, Bmi-1, CD133, ALDH1 | |||
|
| ||||
| Nasopharyngeal cancer | ||||
| TW01 sphere | mRNAs: | Sox2, Oct4, KLf-4, Twist, Snail, Vimentin, N-cadherin | E-cadherin | Lin et al., 2012 |
Compared with parental cells, human nasopharyngeal sphere-derived cells CSCs, designated TW01, express relatively high levels of the stem cell markers Sox2, Oct4, and Krüppel-like factor (Klf4), plus epithelial-mesenchymal transition (EMT) markers including Twist family BHLH transcription factor (Twist), Snail family transcriptional repressor (Snail), and vimentin, along with N-cadherin. However, decreased expression of E-cadherin in TW01 sphere-derived cells was detected (Table 8) (Lin et al., 2012).
EGCG DECREASES THE EXPRESSION OF MRNAS AND PROTEINS THAT SERVE AS STEMNESS MARKERS OF HUMAN CSCS
Breast CSCs
EGCG (40 μg/ml, 87.3 μM) inhibits the expression of genes that promote growth and contribute to the transformed phenotype and survival of SUM-190 spheres: In SUM-149 and SUM-190 cells, EGCG decreases the levels of mRNAs of the proliferation markers cyclin D1 (CCND1); ras homolog family member C (RHOC); and B-cell lymphoma-extra large (BCL-XL), which is a major antiapoptotic protein of the B-cell lymphoma 2 (Bcl2) family. EGCG also decreases ATP levels. In contrast, the levels of Fibronectin 1 (FN1), E-cadherin (CDHI), and Vimentin decreased only in SUM-149 cells (Table 9). These results indicate that tumorsphere formation was inhibited by EGCG (Mineva et al., 2013).
Table 9.
EGCG decreases or increases the expression of stemness marker mRNAs and proteins in human CSCs.
| Cancer types and names of CSCs | Inhibited expression of stemness markers (mRNAs and proteins) | References | |
|---|---|---|---|
| Breast CSCs | |||
| SUM-149 & SUM-190 | mRNAs: | CCND1, RHOC, BCL-XL | Mineva et al., 2013 |
| SUM-149 | mRNAs: | FN1, CDHI, Vimentin | |
|
| |||
| Lung CSCs | |||
| A549 & H1299 | mRNAs: | CD133, CD44, ALDH1A1, Nanog, Oct4 | Zhu et al., 2017 |
| Proteins: | CD133, CD44, ALDH1A1, Nanog, Oct4, PCNA, CyclinD1, Bcl2, β-Catenin, c-Myc | ||
| Increased | Bax, Caspase8, Cleaved Caspase-3 and -9 | ||
|
| |||
| Colorectal CSCs | |||
| HCT116-5FUR & SW480-5FUR | mRNAs: | Oct4, Nanog | Toden et al., 2016 |
| Proteins: | Notch 1, cleaved-Notch 1, c-Myc, Bmi-1, Suz12, Ezh2 | ||
The phenotypes of human estrogen receptor (ER)-negative MDA-MB-231 and MDA-MB-436 cells reflect tumors with a poor prognosis. In ER-negative breast cancer cell lines, ER-α36 is overexpressed and is associated with malignant growth (Zhang et al., 2011). EGCG (10 – 40 μM) inhibits tumorsphere formation and down-regulates ER-α36 expression at 24 h, which is consistent with down-regulation of the epidermal growth factor receptor (EGFR). EGCG inhibits the growth of ER-negative human breast CSCs through down-regulation of ER-α36 expression, indicating that EGCG treatment will result in longer survival of patients with mammary cancers (Pan et al., 2016). The longer survival of patients who drink green tea was reported by Nakachi’s group, as noted in the Introduction (Nakachi et al., 1998).
Lung CSCs
EGCG (0 – 100 μM) reduces the mRNA and protein levels of the lung CSC markers CD133, CD44, ALDH1A1, Nanog, and Oct4 in CSC-A549 and CSC-H1299 cells, and also the protein levels of markers proliferating cell nuclear antigen (PCNA) and Cyclin D1 as well as that of Bcl2. In addition, EGCG reduces the protein levels of β-Catenin and v-Myc avian myelocytomatosis viral oncogene homolog (c-Myc). However, EGCG increases the levels of Bcl-2-associated X protein (Bax), Caspase 8, and cleaved Caspases-3 and -9 (Table 9). These results show that EGCG inhibits proliferation and induces apoptosis of lung CSCs (Zhu et al., 2017).
Colorectal CSCs
Compared with parental cells, 5-fluorouracil (5FU)-resistant (5FUR) CRC cells exhibit an increased ability to form spheroids, indicating the presence of a larger CSC population. EGCG (50EμM) inhibits tumorspheroid formation and the expression of the mRNAs of the stem cell markers Oct4 and Nanog. Treatment of 5FUR CRCs with EGCG inhibits expression of all of the following: self-renewal markers that are components of the Notch homolog 1 (Notch) signaling pathway - Notch1, cleaved Notch1, and c-Myc - as well as that of the polycomb repressive complex subunits, Bmi-1, polycomb protein SUZ (Suz12), and enhancer of zeste homologue (Ezh2) (Table 9) (Toden et al., 2016).
The 30-year history of our studies is summarized in Fig. 1: Primary cancer prevention with green tea is for the general population, and it results in delayed cancer onset and reduced cancer incidence; secondary cancer prevention means early cancer diagnosis and treatment for the general population as well as cancer patients at clinics; and tertiary cancer prevention with the combination of green tea catechin and anticancer compounds is for cancer patients following cancer treatment (Fujiki et al., 2012; 2015a; 2015b; 2017).
ACKNOWLEDGMENTS
This work was supported by the Smoking Research Foundation, Urakami Foundation, the Princess Takamatsu Cancer Research Fund, Takeda Science Foundation, and the Japan Society for the Promotion of Science (JSPS) under the Japan-Korea Basic Scientific Cooperation Program between Prof. In Kyoung Lim at Ajou University and Prof. Masami Suganuma at Saitama University. The authors thank late Prof. Takuo Okuda and Dr. Takashi Yoshida at Okayama University for providing green tea catechins, Drs. Kei Nakachi and Kazue Imai at Saitama Cancer Center Research Institute, Dr. Shun’ichiro Taniguchi at Shinshu University, Prof. Hisataka Moriwaki, Drs. Mitsuo Ninomiya and Masahito Shimizu at Gifu University, Mr. Mituhiro Furuhasi at Saitama Prefectural Office, Messrs. Yoshiaki Kitaoka and Kenta Nakajima, and Dr. Atsushi Takahashi at the Saitama Prefectural Tea Research Institute for their stimulating collaborations, Prof. Koichi Shudo at University of Tokyo and Director of National Institute of Health Sciences for his professional support, Prof. James K Gimzewski at University of California, Los Angeles and Prof. Otmar D. Wiestler, former Chair of the German Cancer Research Center, Heidelberg for their fruitful discussions. We also thank Dr. Takashi Sugimura at the National Cancer Center, Dr. Kunio Aoki at Aichi Cancer Center and the late Dr. Haruo Sugano at The Cancer Institute of the Japanese Foundation for Cancer Research for their warm encouragement. We express our thanks to the editorial board members of this Journal Molecules and Cells for their refined English editing of this manuscript.
REFERENCES
- Affara N.I., Trempus C.S., Schanbacher B.L., Pei P., Mallery S.R., Bauer J.A., Robertson F.M. Activation of Akt and mTOR in CD34+/K15+ keratinocyte stem cells and skin tumors during multistage mouse skin carcinogenesis. Anticancer Res. 2006;26:2805–2820. [PubMed] [Google Scholar]
- Beck B., Driessens G., Goossens S., Youssef K.K., Kuchnio A., Caauwe A., Sotiropoulou P.A., Loges S., Lapouge G., Candi A., et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Boumahdi S., Driessens G., Lapouge G., Rorive S., Nassar D., Le Mercier M., Delatte B., Caauwe A., Lenglez S., Nkusi E., et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- Boutwell R.K. The role of the induction of ornithine decarboxylase in tumor promotion. In: Hiatt H.H., Watson J.D., Winsten J.A., editors. Origins of Human Cancer. New York: Cold Spring Harbor Laboratory; 1977. pp. 773–783. [Google Scholar]
- Conney A.H., Wang Z.Y., Huang M.T., Ho C.T., Yang C.S. Inhibitory effect of oral administration of green tea on tumorigenesis by ultraviolet light, 12-O-tetradecanoylphorobol-13-acetate and N-nitrosodiethylamine in mice. In: Wattenberg L.W., Lipkin M., Boone C.W., Kelloff G.J., editors. Cancer Chemoprevention. Florida: CRC Press; 1992. pp. 361–373. [Google Scholar]
- Cross S.E., Jin Y.S., Rao J.K., Gimzewski J.K. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- Cross S.E., Jin Y.S., Lu Q.Y., Rao J.Y., Gimzewski J.K. Green tea extract selectively targets nanomechanics of liver metastatic cancer cells. Nanotechnology. 2011;22:215101. doi: 10.1088/0957-4484/22/21/215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green tea cuts cancerous growths. New Scientist. 1987;116:1586, 32. Editor. [Google Scholar]
- Fujiki H. Green tea cancer prevention. In: Schwab M., editor. Encylopedia of Cancer. Berlin Heidelberg: Springer-Verlag; 2017. pp. 1960–1965. [Google Scholar]
- Fujiki H., Okuda T. (−)-Epigallocatechin gallate. Drugs Future. 1992;17:462–464. [Google Scholar]
- Fujiki H., Suganuma M. Tumor promotion by inhibitors of protein phosphatases 1 and 2A: the okadaic acid class of compounds. Adv Cancer Res. 1993;61:143–194. doi: 10.1016/s0065-230x(08)60958-6. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M. Green tea and cancer prevention. Proc Jpn Acad. 2002;78 (B):263–270. [Google Scholar]
- Fujiki H., Suganuma M., Okabe S., Sueoka E., Sueoka N., Fujimoto N., Goto Y., Matsuyama S., Imai K., Nakachi K. Cancer prevention with green tea and monitoring by a new biomarker, hnRNP B1. Mutat Res. 2001;480–481:299–304. doi: 10.1016/s0027-5107(01)00189-0. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M., Imai K., Nakachi K. Green tea: cancer preventive beverage and/or drug. Cancer Lett. 2002;188:9–13. doi: 10.1016/s0304-3835(02)00379-8. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Imai K., Nakachi K., Shimizu M., Moriwaki H., Suganuma M. Challenging the effectiveness of green tea in primary and tertiary cancer prevention. J Cancer Res Clin Oncol. 2012;138:1259–1270. doi: 10.1007/s00432-012-1250-y. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Sueoka E., Suganuma M. Tumor promoters: from chemicals to inflammatory proteins. J Cancer Res Clin Oncol. 2013;139:1603–1614. doi: 10.1007/s00432-013-1455-8. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Sueoka E., Watanabe T., Suganuma M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J Cancer Res Clin Oncol. 2015a;141:1511–1522. doi: 10.1007/s00432-014-1899-5. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Sueoka E., Watanabe T., Suganuma M. Primary cancer prevention by green tea, and tertiary cancer prevention by the combination of green tea catechins and anticancer compounds. J Cancer Prev. 2015b;20:1–4. doi: 10.15430/JCP.2015.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Sueoka E., Rawangkan A., Suganuma M. Human cancer stem cells are a target for cancer prevention using (−)-epigallocatechin gallate. J Cancer Res Clin Oncol. 2017 doi: 10.1007/s00432-017-2515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Yamane T., Tanaka M., Kuwata K., Okuzumi J., Takahashi T., Fujiki H., Okuda Y. Inhibitory effect of (−)-epigallocatechin gallate on carcinogenesis with N-ethyl-N′-nitro-N-nitrosoguanidine in mouse duodenum. Jpn J Cancer Res. 1989;80:503–505. doi: 10.1111/j.1349-7006.1989.tb01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes M.J., Yuspa S.H. The contribution of epidermal stem cells to skin cancer. Stem Cell Rev. 2005;1:225–231. doi: 10.1385/SCR:1:3:225. [DOI] [PubMed] [Google Scholar]
- Gupta S., Hastak K., Ahmad N., Lewin J.S., Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoensch H., Groh B., Edler L., Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J Gastroenterol. 2008;14:2187–2193. doi: 10.3748/wjg.14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Suga K., Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- Inoue M., Tajima K., Mizutani M., Iwata H., Iwase T., Miura S., Hirose K., Hamajima N., Tominaga S. Regular consumption of green tea and the risk of breast cancer recurrence: follow-up study from the Hospital-based Epidemiological Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001;167:175–182. doi: 10.1016/s0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- Jones P.H., Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kashyap V., Rezende N.C., Scotland K.B., Shaffer S.M., Persson J.L., Gudas L.J., Mongan N.P. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093–1108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori A., Yatsunami J., Okabe S., Abe S., Hara K., Suganuma M., Kim S.J., Fujiki H. Anticarcinogenic activity of green tea polyphenols. Jpn J Clin Oncol. 1993;23:186–190. [PubMed] [Google Scholar]
- Lin C.H., Shen Y.A., Hung P.H., Yu Y.B., Chen Y.J. Epigallocatechin gallate, polyphenol present in green tea, inhibits stem-like characteristics and epithelial-mesenchymal transition in nasopharyngeal cancer cell lines. BMC Complement Alern Med. 2012;12:201. doi: 10.1186/1472-6882-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineva N.D., Paulson K.E., Naber S.P., Yee A.S., Sonenshein G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS One. 2013;8:e73464. doi: 10.1371/journal.pone.0073464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.J., Tryson K.A., Wu K.Q. Evidence that the epidermal targets of carcinogen action are found in the interfollicular epidermis or infundibulum as well as in the hair follicles. Cancer Res. 2000;60:226–229. [PubMed] [Google Scholar]
- Nakachi K., Suemasu K., Suga K., Takeo T., Imai K., Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89:254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakachi K., Matsuyama S., Miyake S., Suganuma M., Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. BioFactor. 2000;13:49–54. doi: 10.1002/biof.5520130109. [DOI] [PubMed] [Google Scholar]
- Ogunleye A.A., Xue F., Michels K.B. Green tea consumption and breast cancer risk or recurrence: a meta-analysis. Breast Cancer Res Treat. 2010;119:477–484. doi: 10.1007/s10549-009-0415-0. [DOI] [PubMed] [Google Scholar]
- Okabe S., Suganuma M., Hayashi M., Sueoka E., Komori A., Fujiki H. Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols. Jpn J Cancer Res. 1997;88:639–643. doi: 10.1111/j.1349-7006.1997.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S., Ochiai Y., Aida M., Park K., Kim S.J., Nomura T., Suganuma M., Fujiki H. Mechanistic aspects of green tea as a cancer preventive: effect of components on human stomach cancer cell lines. Jpn J Cancer Res. 1999;90:733–739. doi: 10.1111/j.1349-7006.1999.tb00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhao B., Song Z., Han S., Wang M. Estrogen receptor-α36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J Pharmacol Sci. 2016;130:85–93. doi: 10.1016/j.jphs.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Sarkar A., Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Fukutomi Y., Ninomiya M., Nagura K., Kato T., Araki H., Suganuma M., Fujiki H., Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–3025. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- Shin C.M., Lee D.H., Seo A.Y., Lee H.J., Kim S.B., Son W.C., Kim Y.K., Lee S.j., Park S.H., Kim N., et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: A randomized clinical trial. Clin Nutr. 2017 doi: 10.1016/j.clnu.2017.01.014. Pii:S0261-5614(17)30038-9. [DOI] [PubMed] [Google Scholar]
- Slaga T.J., Klein-Szanto A.J. Initiation-promotion versus complete skin carcinogenesis in mice: importance of dark basal keratinocytes (stem cells) Cancer Invest. 1983;1:425–436. doi: 10.3109/07357908309048511. [DOI] [PubMed] [Google Scholar]
- Sporn M.B., Dunlop N.M., Newton D.L., Smith J.M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- Stearns M.E., Wang M. Synergistic effects of the green tea extract epigallocatechin-3-gallate and taxane in eradication of malignant human prostate tumors. Transl Oncol. 2011;4:147–156. doi: 10.1593/tlo.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J.C., Ettrich T., Muche R., Wiedom M., Brockmöller J., Seeginger A., Seufferlein T. Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (MIRACLE): a randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population. BMC Cancer. 2011;11:360. doi: 10.1186/1471-2407-11-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci USA. 1988;85:1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma M., Okabe S., Oniyama M., Tada Y., Ito H., Fujiki H. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Okabe S., Kai Y., Sueoka N., Sueoka E., Fujiki H. Synergistic effects of (−)-epigallocatechin gallate with (−)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–47. [PubMed] [Google Scholar]
- Suganuma M., Kurusu M., Suzuki K., Tasaki E., Fujiki H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int J Cancer. 2006;119:33–40. doi: 10.1002/ijc.21809. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Saha A., Fujiki H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011;102:317–323. doi: 10.1111/j.1349-7006.2010.01805.x. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Takahashi A., Watanabe T., Iida K., Matsuzaki T., Yoshikawa H.Y., Fujiki H. Biophysical approach to mechanisms of cancer prevention and treatment with green tea catechins. Molecules. 2016;21 doi: 10.3390/molecules21111566. pii: E1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Watanabe T., Mondal A., Suzuki K., Kurusu-Kanno M., Li Z., Yamazaki T., Fujiki H., Suganuma M. Mechanism-based inhibition of cancer metastasis with (−)-epigallocatechin gallate. Biochem Biophys Res Commun. 2014;443:1–6. doi: 10.1016/j.bbrc.2013.10.094. [DOI] [PubMed] [Google Scholar]
- Tang S.N., Fu J., Nall D., Rodova M., Shankar S., Srivastava R.K. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131:30–40. doi: 10.1002/ijc.26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S., Fujiki H., Kobayashi H., Go H., Miyado K., Sadano H., Shimokawa R. Effect of (−)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992;65:51–54. doi: 10.1016/0304-3835(92)90212-e. [DOI] [PubMed] [Google Scholar]
- Toden S., Tran H.M., Tovar-Camargo O.A., Okugawa Y., Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7:16158–16170. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempus C.S., Morris R.J., Ehinger M., Elmore A., Bortner C.D., Ito M., Cotsarelis G., Nijhof J.G.W., Peckham J., Flagler N., et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A.S., Liu D., Martin J., Tang X.M., Lee J.J., El-Naggar A.K., Wistuba I., Culotta K.S., Mao L., Gillenwater A., et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res. 2009;2:931–941. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Kuramochi H., Takahashi A., Imai K., Katsuta N., Nakayama T., Fujiki H., Suganuma M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (−)-epigallocatechin gallate-treated cells. J Cancer Res Clin Oncol. 2012;138:859–866. doi: 10.1007/s00432-012-1159-5. [DOI] [PubMed] [Google Scholar]
- Yamane T., Takahashi T., Kuwata K., Oya K., Inagake M., Kitao Y., Suganuma M., Fujiki H. Inhibition of N-methyl-N′-nitro-N-nitrosoguanidine-induced carcinogenesis by (−)-epigallocatechin gallate in the rat glandular stomach. Cancer Res. 1995;55:2081–2084. [PubMed] [Google Scholar]
- Yang C.S., Wang X., Lu G., Picinich S.C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa S., Horiuchi T., Fujiki H., Yoshida T., Okuda T., Sugimura T. Antitumor promoting activity of (−)-epigallocatechin gallate, the main constituent of “tannin” in green tea. Phytother Res. 1987;1:44–47. [Google Scholar]
- Yoshizawa S., Horiuchi T., Suganuma M., Nishiwaki S., Yatsunami J., Okabe S., Okuda T., Muto Y., Frenkel K., Troll W., et al. Penta-O-galloyl-β-D-glucose and (−)-epigallocatechin gallate cancer preventive agents. ACS Symposium Series. 1992;501:316–325. [Google Scholar]
- Zhang X.T., Kang L.G., Ding L., Vranic S., Gatalica Z., Wang Z.T. A positive feedback loop of ER-α36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30:770–780. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Jiang Y., Yang X., Wang S., Xie C., Li X., Li Y., Chen Y., Wang X., Meng Y., et al. Wnt/β-catenin pathway mediates (−)-epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochemical Biophys Res Commun. 2017;482:15–21. doi: 10.1016/j.bbrc.2016.11.038. [DOI] [PubMed] [Google Scholar]


