Abstract
The biological significance and deregulation of the Hippo pathway during organ growth and tumorigenesis have received a surge of interest in the past decade. The Hippo pathway core kinases, MST1/2 and LATS1/2, are tumor suppressors that inhibit the oncogenic nuclear function of YAP/TAZ and TEAD. In addition to earlier studies that highlight the role of Hippo pathway in organ size control, cell proliferation, and tumor development, recent evidence demonstrates its critical role in cancer stem cell biology, including EMT, drug resistance, and self-renewal. Here we provide a brief overview of the regulatory mechanisms of the Hippo pathway, its role in cancer stem cell biology, and promising therapeutic interventions.
Keywords: cancer stem cell, Hippo pathway, metastasis, YAP/TAZ
INTRODUCTION
The Hippo pathway was originally identified by genetic screens to identify tumor suppressors involved in Drosophila melanogaster tissue growth control. Deletion of the Hippo pathway components in Drosophila resulted in dramatic tissue overgrowth phenotype that was later recapitulated in transgenic mouse models (Dong et al., 2007; Harvey et al., 2003; Huang et al., 2005; Wu et al., 2003; Xu et al., 1995). Recent advances in identification of the mammalian Hippo pathway components and functional implications highlight the role of Hippo pathway in organ development, tumorigenesis, tissue regeneration and stem cell self-renewal (Park and Guan 2013; Yu et al., 2015; Zanconato et al., 2016).
The core Hippo pathway components include a cytoplasmic kinase module and a nuclear transcriptional module. The kinase module is composed of mammalian STE20-like protein kinase 1 (MST1) and MST2, which phosphorylate and activate downstream kinases, large tumor suppressor 1 (LATS1) and LATS2. Recent studies indicate the MAP4K family as part of the kinase module parallel to MST1/2 by directly phosphorylating LATS (Meng et al., 2015). The major function of the Hippo kinase cascade is to inhibit the oncogenic transcriptional module composed of yes-associated protein (YAP), transcriptional co-activator with PDZ-binding motif (TAZ), and TEA domain family members (TEAD). YAP/TAZ function as transcriptional co-activators, which translocate between the cytoplasm and the nucleus, and induce target gene expression involved in cell proliferation and anti-apoptosis via interaction with the TEAD family of transcription factors. Genetic evidence in mice shows that YAP and TAZ (two homologs of Drosophila Yorkie) are functionally redundant during development and regeneration (Nishioka et al., 2009; Xin et al., 2013). When the cytoplasmic Hippo kinase module is ‘on’, MST1/2 activates LATS1/2, which in turn phosphorylates and inactivates YAP/TAZ. Phosphorylated YAP/TAZ are either retained in the cytoplasm via 14-3-3 interaction or subjected to proteasomal or autophagy-induced degradation, and consequently, TEAD-mediated gene transcription is suppressed. By contrast, when the Hippo kinases are inactive, dephosphorylated YAP/TAZ maneuver into the nucleus and induce TEAD target gene expression (Meng et al., 2016). In this view, it is well accepted that the core Hippo kinases are tumor suppressors and members of the transcriptional module are oncogenes (Fig. 1). Moreover, although the function of TEAD is largely regulated by YAP/TAZ (Zhao et al., 2008a), recent studies uncover the Hippo-YAP-independent regulatory mechanisms of TEAD that include post-translational modifications and changes in subcellular localization (Chan et al., 2016; Lin et al., 2017; Noland et al., 2016).
Fig. 1. Regulation of YAP and TAZ by the Hippo pathway.
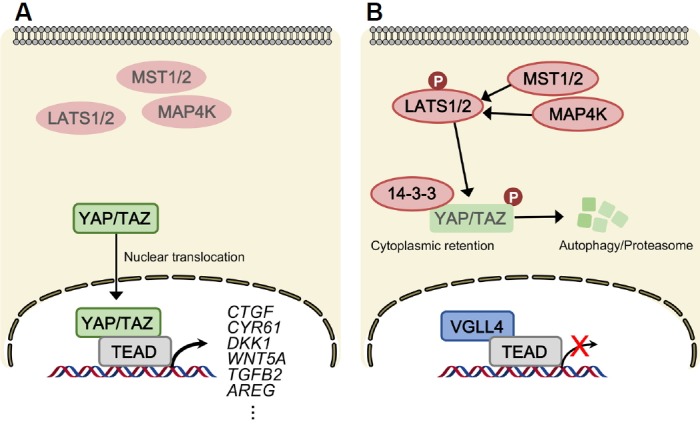
The core inhibitory kinase module of the Hippo pathway is composed of MST1/2, MAP4K, and LATS1/2. The transcriptional module is composed of YAP/TAZ and TEAD. (A) When the Hippo pathway is ‘off’, YAP/TAZ are dephosphorylated, accumulated, and they translocate into the nucleus to bind the transcription factors, TEAD1–TEAD4, which enable target gene transcription involved in cell proliferation. (B) When the Hippo pathway is turned ‘on’, LATS1/2 directly phosphorylate YAP/TAZ, which inhibit nuclear import of YAP/TAZ via 14-3-3-mediated cytoplasmic retention, and ubiquitination-mediated proteasomal and autolysosomal degradation. TEAD transcriptional activity is suppressed by VGLL4. LATS indicates large tumor suppressor; MST, mammalian STE20-like protein kinase; YAP, Yes-associated protein; TAZ, transcriptional co-activator with PDZ-binding motif; TEAD, TEA domain family members; VGLL4, transcription cofactor vestigial-like protein 4.
UPSTREAM SIGNALS OF THE HIPPO PATHWAY
Hippo pathway and soluble factors
G protein-coupled receptor (GPCR) Ligands
The G protein-coupled receptors (GPCRs) and its ligands, such as mitogenic hormones and growth factors, were discovered as the first soluble signals that regulate the Hippo pathway. YAP/TAZ are activated through Gα12/13-coupled GPCR ligands, including lysophosphatidic acid (LPA), sphingosine-1-phosphate (S1P), Wnt3a, Wnt5a/b, thrombin, thromboxane A2, as well as Kaposi sarcoma-associated herpesvirus (Feng et al., 2016; Liu et al., 2015; Miller et al., 2012; Mo et al., 2012; Park et al., 2015; Yu et al., 2012). Gα12/13 inhibits LATS1/2 and activates YAP/TAZ via Rho GTPase-dependent actin polymerization. Gαq/11-coupled GPCR ligands, such as endothelin-1 and estrogen also activate YAP/TAZ (Wang et al., 2017; Zhou et al., 2015). Moreover, constitutively activated YAP/TAZ are oncogenic drivers and therapeutic targets in patients with uveal melanoma harboring hyperactive Gαq/11 mutations (Feng et al., 2014; Yu et al., 2014). Different isoforms in the protein kinase C (PKC) family, key downstream effectors of Gαq/11, can both activate or inhibit YAP/TAZ (Gong et al., 2015).
Apart from Gα12/13 and Gαq/11, the Gαs-coupled GPCR ligands such as glucagon and epinephrine, as well as the downstream effectors, cAMP and protein kinase A (PKA), inhibit YAP/TAZ activity (Kim et al., 2013; Yu et al., 2012; 2013). The omega-3 polyunsaturated fatty acids, docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) inhibit YAP/TAZ via GPR40/120-Gαs-PKA (Zhang et al., 2016). On the other hand, Gαs is an oncogene frequently mutated in human cancers (O’Hayre et al., 2013). The status and function of Hippo and YAP/TAZ activity are yet unknown in these hyperactive Gαs-driven cancers.
Wnt signaling
Among various morphogens important in development, the Wnt proteins are particularly noteworthy. Recent studies have revealed a complexed cross talk between the Wnt and Hippo-YAP/TAZ pathways. Both canonical Wnt/β-catenin and β-catenin-independent alternative Wnt signaling activate YAP/TAZ via distinct mechanisms. Upon Wnt3a stimulation, canonical Wnt signaling activates both YAP/TAZ and β-catenin similarly through dissociation from the destruction complex (Azzolin et al., 2012; 2014). In this context, YAP/TAZ activity is indispensable for Wnt-induced biological responses. However, Wnt can activate YAP/TAZ via β-catenin-independent mechanisms. APC, a critical component of the destruction complex, regulates Hippo-YAP activity independent of β-catenin (Cai et al., 2015). Alternative Wnt signaling triggered by both Wnt3a and Wnt5a/b ligands activates YAP/TAZ via the FZD/ROR-Gα12/13-Rho GTPases-LATS1/2 pathway which in turn inhibits β-catenin-induced transcription (Park et al., 2015). Mechanistically, YAP/TAZ have been shown to inhibit Wnt/β-catenin signaling by inhibition of DVL nuclear translocation or induction of secreted Wnt inhibitors such as DKK1 and Wnt5a/b (Barry et al., 2013; Park et al., 2015; Seo et al., 2013; Varelas et al., 2010a). Inhibition of Wnt/β-catenin signaling by YAP/TAZ is important during intestinal stem cell regeneration (Barry et al., 2013; Gregorieff et al., 2015), mesenchymal stem cell differentiation (Park et al., 2015; Seo et al., 2013), and induction of naïve pluripotent stem cells (Qin et al., 2016). Similarly, YAP/TAZ-dependent liver growth and HCC formation were suppressed by Wnt/β-catenin signaling (Kim et al., 2017). The context-dependent regulation between YAP/TAZ and Wnt/β-catenin signaling requires further investigation.
PHYSICAL CUES
Cell density and contact inhibition
Stringent regulation of cell growth upon cell-cell contact is critical for proper organ growth and function since loss of cell contact inhibition of proliferation (CIP) leads to tissue overgrowth, hyperplasia, and tumorigenesis. The Hippo pathway has been shown to mediate cell-cell contact-induced growth inhibitory signals (Ota and Sasaki 2008; Zhao et al., 2008b). During cell-cell contact, components of adherens junction and tight junctions, such as E-cadherin, α-catenin, and Crumbs, activate the Hippo pathway and inhibit YAP/TAZ-induced cell proliferation (Kim et al., 2011; Schlegelmilch et al., 2011; Varelas et al., 2010). In normal cells, YAP/TAZ activation by loss of cell-cell contact during cell migration and proliferation is important for wound healing. In addition, YAP-TEAD transcriptional regulation by contact inhibition is important for proper embryonic development (Nishioka et al., 2009). However, hyperactive YAP/TAZ during epithelial-to-mesenchymal transition (EMT) results in tumorigenesis due to loss of contact inhibition (Zhao et al., 2007).
Mechanotransduction
Mechanical cues including extracellular matrix (ECM) stiffness, cell attachment or detachment, cell geometry, and cytoskeletal tension are potent regulators of YAP/TAZ (Aragona et al., 2013; Dupont et al., 2011; Zhao et al., 2012). Physical attachment of cells to stiff matrix are widely spread out and cells harbor active nuclear YAP/TAZ, whereas, YAP/TAZ are sequestered in the cytoplasm when cells are grown on soft ECM and poorly spread. Similarly, cell attachment to ECM activates YAP/TAZ through Rho GTPases or FAK-Src-PI3K pathway, whereas cell detachment inhibits YAP/TAZ in a LATS-dependent manner, leading to anoikis (Kim and Gumbiner 2015; Zhao et al., 2012). Integrins are critical mediators that sense ECM stiffness and relay signals for cell survival and proliferation. Integrins and the focal adhesion complex have been shown to regulate YAP/TAZ (Elbediwy et al., 2016; Serrano et al., 2013; Wang et al., 2016c). Mechanical strain has been shown to induce E-cadherin-dependent, β-catenin-independent YAP activation, which is required for cell cycle entry (Benham-Pyle et al., 2015).
YAP/TAZ in vascular endothelial cells are activated by disturbed blood flow-induced shear stress, whereas laminar flow-induced unidirectional shear stress inhibits YAP/TAZ (Wang et al., 2016b; 2016c). YAP is also required for shear stress-induced migration and invasion of cancer cells (Lee et al., 2017). These implicate YAP/TAZ as mediators of mechanotransduction that are involved in a wide range of pathological conditions.
STRESS SIGNALS
Nutrient stress
Extracellular nutrients such as glucose and amino acids regulate cell metabolism and proliferation. Cancer cells rely on glycolysis, the Warburg effect, as their main energy source to generate ATP and this regulates YAP/TAZ activity. Glucose starvation-induced energy stress activates LATS1/2 and AMP-activated protein kinase (AMPK), which in turn phosphorylate and inhibit YAP/TAZ activity (Mo et al., 2015; Wang et al., 2015). AMPK can also directly phosphorylate AMOTL1, which regulates LATS and YAP simultaneously (DeRan et al., 2014). Interestingly, recent findings indicate a critical role of YAP in glucose metabolism in cancer cells. YAP promotes glucose transporter 3-induced glucose uptake, which can further activate YAP by promoting YAP-TEAD interaction via direct binding of phosphofructokinase (PFK-1) to TEAD (Enzo et al., 2015; Wang et al., 2015).
Amino acids can regulate YAP/TAZ through the TSC-mTORC1 pathway. Loss-of-function TSC mutations activate YAP by mTORC1-mediated inhibition of autophagy (Liang et al., 2014). In addition, YAP-induced miR-29 expression suppresses PTEN translation, which in turn activates the PI3K-mTOR pathway (Tumaneng et al., 2012). Also, under nutrient-limiting conditions, YAP/TAZ activates mTORC1 via inducing the TEAD target gene, LAT1 amino acid transporter (Hansen et al., 2015). Inhibition of cholesterol synthesis can also suppress YAP/TAZ activity by inducing defect in isoprenylation and membrane localization of Rho GTPases (Sorrentino et al., 2014).
Cellular and environmental stresses
Cells and tissues are constantly challenged by various cellular and environmental stresses, and the Hippo pathway has been suggested to play critical roles against stress signals. Cytokinesis failure and extra centrosomes activate the Hippo pathway, which induces YAP/TAZ inhibition and p53 stabilization, thereby preventing tumorigenesis by G1 phase cell cycle arrest (Ganem et al., 2014). In addition, mitotic arrest by anti-tubulin drugs regulates YAP via cyclin-dependent kinase 1 (CDK1)-induced phosphorylation (Yang et al., 2013; Zhao et al., 2014). ER stress dynamically regulates the Hippo pathway. During the adaptive stage of UPR, PERK-eIF2α-ATF4 axis activates YAP to prevent cell death, however, activation of Hippo signaling by prolonged ER stress inhibits YAP and promotes apoptosis (Wu et al., 2015).
In addition to intracellular stresses, Hippo-YAP/TAZ pathway responds to various external environmental stresses. Oxidative stress activates the Hippo pathway, which in turn, antagonizes YAP-FOXO1 complex that leads to cell death in the mouse heart (Shao et al., 2014a). By contrast, hypoxia inhibits the Hippo pathway by LATS2 degradation through SIAH2-induced ubiquitination, which in turn activates YAP/TAZ (Ma et al., 2015). Hyperosmotic stress elicits TAZ-NFAT5 interaction by ABL kinase-induced tyrosine phosphorylation of TAZ (Jang et al., 2012). Osmotic pressure and gravity also regulate YAP (Hong et al., 2017; Porazinski et al., 2015). In addition to YAP/TAZ, a recent study highlights the effect of cellular and environmental stresses on TEAD localization. High cell density and osmotic stress promote TEAD cytoplasmic translocation via p38 MAPK-independent and -dependent mechanisms, respectively (Lin et al., 2017).
HIPPO PATHWAY IN TUMORIGENESIS
Cancer stem cell
A specialized subset of tumor cells that had undergone epithelial-to-mesenchymal transition (EMT) has been proposed to harbor unique properties, such as self-renewal and tumor-initiating potential. These cancer stem cells (CSCs) are responsible for drug resistance, metastasis, and recurrence, which are the major causes of cancer mortality (Shibue and Weinberg 2017). Therefore, exploring CSC-specific signaling mechanisms and characteristics is clinically important for better-targeted anticancer treatment.
The Hippo-YAP pathway regulates cell fate and differentiation of progenitor cells during normal organ development and in the context of cancer. YAP can also dedifferentiate and expand undifferentiated stem/progenitor cells such as mature hepatocytes into progenitor cells (Yimlamai et al., 2014). Similarly, YAP/TAZ activation leads to induction of CSC properties in a wide range of human cancers (Fig. 2). In breast cancer cells, YAP has been shown to occupy mammary stem cell signature gene promoters to induce breast CSCs (Kim et al., 2015). In addition, gene expression data from breast cancer tissues with high CSC content overlap with YAP/TAZ-induced gene expression, and TAZ expression is enriched in breast CSCs with CD44high/CD24low phenotype, which is required to sustain their self-renewal and tumor-initiating capacities (Cordenonsi et al., 2011). YAP is also a major inducer of CSC properties by direct upregulation of SOX9 (Song et al., 2014). Similarly, YAP activation by SOX2 is important in maintaining CSCs in osteosarcoma and glioblastoma (Basu-Roy et al., 2015). Glucocorticoid hormone-induced YAP activation expands chemoresistant breast CSCs (Sorrentino et al., 2017). Together, ample amount of evidence indicates that YAP/TAZ play critical roles in CSCs maintenance and cancer progression. However, CSC-specific regulatory mechanisms of YAP/TAZ and Hippo pathway remain unclear.
Fig. 2. Overview of YAP/TAZ regulation and function in cancer stem cells.
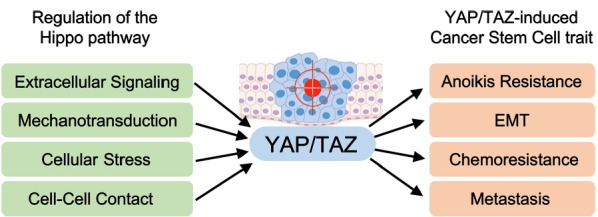
The Hippo pathway regulates YAP/TAZ activation in cancer cells through a wide range of upstream stimuli, such as extracellular ligands, mechanotransduction, environmental stress, energy stress, and cell-cell contact. Aberrant activation of YAP/TAZ via dysregulation of the Hippo pathway results in tumorigenesis and confers cancer stem cell traits that lead to anoikis resistance, EMT, drug resistance, and metastasis.
EMT
The epithelial-to-mesenchymal transition (EMT) is a normal developmental program that has been hijacked by cancer cells to trigger abnormal cell migration and invasion. Therefore, EMT has emerged as a critical regulator of CSC phenotype and prerequisite for metastasis (Overholtzer et al., 2006; Shibue and Weinberg 2017). Importantly, induction of EMT in epithelial cells results in CSC characteristics, such as increased mesenchymal traits, increased stem-cell marker expression, enhanced ability to form mammosphere, and more efficient tumor initiation in vivo.
Numerous studies indicate that YAP/TAZ activation drives cell transformation by inducing EMT. YAP/TAZ are well known to promote EMT and metastasis via activation of TEAD transcription factors (Lamar et al., 2012; Lei et al., 2008; Overholtzer et al., 2006; Zhang et al., 2009a; Zhao et al., 2008a). Loss of Hippo kinases or induction of YAP/TAZ activates TEAD-mediated target gene transcription, which results in increased expression of mesenchymal markers, such as vimentin and N-cadherin, and inhibition of epithelial markers, including E-cadherin, simultaneously. Nuclear accumulation of YAP/TAZ is required for epithelial cells to undergo TGFβ-induced EMT, and YAP-TEAD interaction promotes tumor growth and metastasis of breast cancer and melanoma cells (Lamar et al., 2012; Varelas et al., 2010). In addition, YAP interacts with ZEB1, an EMT-inducing transcriptional repressor, and together, ZEB1/YAP target genes promote CSC traits and predict poor survival in breast cancer (Lehmann et al., 2016). Similarly, EMT-inducing transcription factors, Snail/Slug, bind YAP/TAZ-TEAD to control mesenchymal stem cell self-renewal (Tang et al., 2016). Interestingly, YAP expression was critical for the progression of various KRAS-driven cancers, and YAP/KRAS converged on FOS to promote EMT that contributes to KRAS oncogenic addiction (Shao et al., 2014b).
Drug resistance
Chemotherapy and targeted therapy are currently the major treatments for patients with cancer. Unfortunately, these conventional therapies often fail to eradicate carcinoma cells that enter the CSC state, thereby permitting CSC-mediated clinical relapse.
Tumor cells with high YAP/TAZ activity display resistance to chemotherapeutics, which is in part due to the CSC characteristics acquired by YAP/TAZ activation. YAP activation transforms prostate epithelial cells and confers an androgen-insensitive state and castration resistance in vivo (Zhang et al., 2015). Recently, it was shown that inhibition of LATS2 by miR-302/367 induces YAP activation and confers CSC state in prostate cancer cells (Guo et al., 2017). YAP/TAZ activation was shown to induce metastatic activity and drug resistance to chemotherapeutic drugs such as paclitaxel and doxorubicin in breast CSCs (Bartucci et al., 2015; Cordenonsi et al., 2011). Numerous studies suggest that Hippo pathway inhibition or YAP/TAZ activation sustains cancer cell survival against DNA-damaging agents such as UV, radiation, cisplatin, taxol, and fluorouracil (5-FU) in a wide number of tumor types (Cheng et al., 2016; Ciamporcero et al., 2016; Fernandez et al., 2012; Mao et al., 2014; Zhao et al., 2014). YAP/TAZ also promote resistance to targeted therapies. YAP promotes resistance to drugs targeting RAF and MEK in tumor cells harboring BRAF, KRAS, and NRAS mutations, which is a major clinical challenge (Lin et al., 2015). YAP activation by actin remodeling is one of the mechanisms by which YAP induces resistance to BRAF inhibitors in melanoma cells (Kim et al., 2016).
Importantly, well-established YAP/TAZ-TEAD target genes of secreted ligands mediate drug resistance. Connective tissue growth factor (CTGF) confers resistance to chemotherapeutic drugs including doxorubicin and paclitaxel in breast cancer, osteosarcoma, and glioblastoma multiforme (GBM) (Tsai et al., 2014; Wang et al., 2009; Yin et al., 2010). In addition, the prototype alternative Wnt ligand WNT5A, as well as its receptors Ror1/2, has been shown to induce resistance to targeted BRAF inhibitors in melanoma cells (Anastas et al., 2014; O’Connell et al., 2013; Park et al., 2015). Moreover, in PIK3CA mutant breast tumors, WNT5A is one of the most upregulated genes, which correlates with activation of YAP/TAZ-WNT5A axis by PIK3CA H1047R mutation (Cizkova et al., 2010; Park et al., 2015). The ligand for epidermal growth factor receptor (EGFR), amphiregulin (AREG), is also a transcriptional target of YAP, which is known to cause resistance to chemotherapy and receptor tyrosine inhibitors such as gefitinib in non-small-cell lung cancers (Busser et al., 2010; Tung et al., 2017; Zhang et al., 2009b). These indicate that targeting YAP/TAZ-induced secreted ligands could improve the efficacy in patients with cancer via non-cell-autonomous mechanisms.
PHARMACOLOGICAL INTERVENTIONS
Cancer remains to be one of the leading causes of mortality worldwide. CSCs that have undergone EMT, are capable of metastasizing, and confer drug resistance. As discussed above, the Hippo-YAP/TAZ pathway has emerged as an oncogenic driver that confers CSC traits in cancer cells. An ideal target for small-molecule therapeutics is protein kinases, which function as oncogenes. Unfortunately, since the Hippo core kinases, MST1/2 and LATS1/2, are tumor suppressors, researchers have been searching for therapeutic options beyond the Hippo kinases (Fig. 3). However, recent progress suggests that inhibition of Hippo kinases may also be beneficial in cancer treatment.
Fig. 3. Therapeutic targeting of the Hippo pathway.
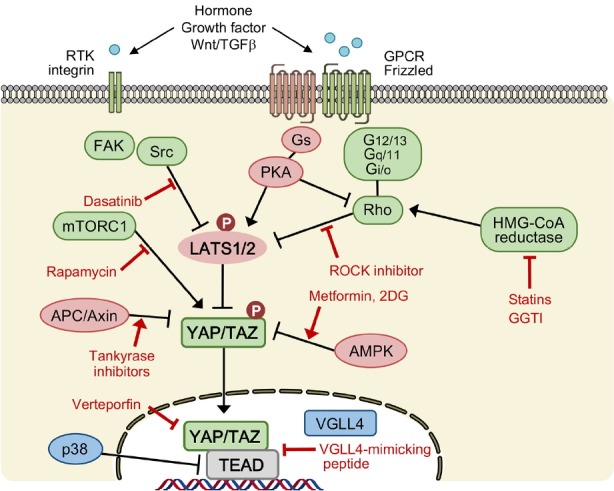
Strategies for targeting Hippo-YAP/TAZ activity are indicated. Dasatinib, ROCK inhibitors, statins, GGTI (inhibitors of geranylgeranyl transferase-1) represent compounds that inhibit YAP/TAZ activity via activating LATS. Rapamycin, tankyrase inhibitors, metformin, 2-DG represent compounds that inhibit YAP/TAZ activity independent of LATS. Verteporfin and VGLL4-mimicking peptide represent compounds that inhibit the interaction between YAP/TAZ and TEAD. ROCK indicates Rho-associated protein kinase; FAK, focal adhesion kinase; PKA, protein kinase A; APC, adenomatous polyposis coli; AMPK, AMP-activated protein kinase; mTORC1, mechanistic target of rapamycin complex 1; RTK, receptor tyrosine kinase; GPCR, G-protein coupled receptor.
Verteporfin
Verteporfin, a FDA-approved photosensitizer to treat macular degeneration, is currently the most widely used YAP inhibitor in research laboratories (Liu-Chittenden et al., 2012). Verteporfin inhibits YAP-TEAD-induced target gene transcription via disrupting the binding between YAP and TEAD, or upregulating 14-3-3 expression (Liu-Chittenden et al., 2012; Wang et al., 2016a). Verteporfin blocks YAP-induced liver tumorigenesis and uveal melanoma in vivo, thereby demonstrating the therapeutic significance of disrupting YAP/TAZ-TEAD interactions in cancer cells (Feng et al., 2014; Liu-Chittenden et al., 2012; Yu et al., 2014). Verteporfin exerts its anti-tumor effect via directly binding to YAP. In addition to TEADs, other transcription factors, such as SMAD, p73, and AP1 serves as transcriptional partners of YAP/TAZ (Strano et al., 2001; Varelas et al., 2008; Zanconato et al., 2015), however, the effect of verteporfin on their transcriptional activity is unknown. Also, YAP-independent cytotoxic effect of verteporfin has been reported, which suggests caution when interpreting the effect of verteporfin on YAP activity (Dasari et al., 2017).
VGLL4-mimicking peptide
VGLL4, which is the only member of VGLL family that carries two TDU domains, was initially reported to bind TEAD1 in cardiac myocytes and suppress TEAD1-dependent α1-adrenergic activation (Chen et al., 2004). Recently, VGLL4 was identified as an endogenous inhibitor of YAP-TEAD interaction by competing with YAP, thereby preventing TEAD-induced cell growth and tumorigenesis in D. melanogaster as well as mouse models (Koontz et al., 2013; Zhang et al., 2014). Unlike other negative regulators of YAP/TAZ, such as Hippo kinases, angiomotin, 14-3-3, and α-catenin that are located in the cytoplasm, VGLL4 is one of the few endogenous YAP inhibitors that resides in the nucleus. Based on the structure of VGLL4-TEAD4 complex, a peptide that mimics VGLL4 inhibitory function against YAP has recently been developed (Jiao et al., 2014). Treatment with VGLL4-mimicking peptide suppresses human primary gastric cancer cell growth, providing a rationale for peptide-based therapeutic strategy to treat YAP-driven human cancers.
Statins
Statins were identified as potent YAP inhibitors from FDA-approved drug library screening to search for drugs that induce cytoplasmic translocation of YAP/TAZ in breast cancer cells (Sorrentino et al., 2014). Statins are drugs that are used to lower cholesterol levels in patients with hypercholesterolemia by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. Inhibiting the conversion of HMG-CoA to mevalonate reduces geranylgeranyl pyrophosphate synthesis required for proper Rho GTPase activity. Consequently, inactivation of Rho GTPases by treatment with statins inhibits YAP/TAZ (Sorrentino et al., 2014; Wang et al., 2014). Statin treatment of breast cancer cells decreased their CSC properties, such as self-renewal, and inhibited in vitro and in vivo tumor initiation. In addition to clinical studies showing the anti-inflammatory effects of statins, the inhibitory mechanisms of YAP may provide a rationale to reposition statin as an anticancer drug (Gronich and Rennert 2013). Moreover, recent studies indicate the involvement of YAP/TAZ in atherosclerotic lesion development due to aberrant blood flow, which suggests that statins may act as anti-atherosclerotic drugs in part via YAP/TAZ inhibition (Wang et al., 2016b; 2016c).
LATS kinase inhibitors
Hippo core kinases are well established as tumor suppressors since loss of NF2, Sav1, MST1/2, and Mob1 in mouse models as well as in humans leads to cancer development (Yu et al., 2015). Since small-molecule kinase activators are hardly available, Hippo kinases remain to be poorly druggable targets. However, a recent study identified an unexpected role of LATS1/2 kinase in suppressing anti-tumor immunity (Moroishi et al., 2016). LATS1/2 suppressed Toll-like receptors-MYD88/TRIF pathway-mediated type I interferon response, thereby enhancing anti-tumor immune response. Therefore, inhibition of upstream Hippo kinases as a monotherapy or in combination with immune checkpoint inhibitors can be an attractive strategy to suppress tumor progression. In addition to anticancer effects, drugs targeting the Hippo pathway may be valuable for wound healing and tissue regeneration.
FUTURE PERSPECTIVE
The Hippo pathway plays an important role in tumorigenesis by promoting CSC characteristics, such as EMT, chemoresistance, metastatic potential, and self-renewal. Despite its relevance in tumorigenesis, oncogenic driver mutations in the core components of the Hippo pathway are relatively rare. Therefore, in addition to targeted therapy, pharmacological modulation of signal transduction pathways that crosstalk with the Hippo pathway or inhibition of YAP/TAZ target genes by combination therapy may be promising approaches to target YAP/TAZ activity in CSCs. More importantly, limitations of current CSC markers hinder precise isolation of CSCs from bulk heterogeneous cancer cell populations. Therefore, identifying the CSC-specific oncogenic signaling network of YAP/TAZ will provide attractive avenues to eradicate CSC and treat cancer progression.
ACKNOWLEDGMENTS
We apologize to those colleagues whose work was not cited because of space limitation. This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C1560), and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MOE) (2017R1D1A1B03034797) and (MSIP) (2017R1A4A1015328) to H.W.P.
REFERENCES
- Anastas J.N., Kulikauskas R.M., Tamir T., Rizos H., Long G.V., von Euw E.M., Yang P.T., Chen H.W., Haydu L., Toroni R.A., et al. WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J Clin Invest. 2014;124:2877–2890. doi: 10.1172/JCI70156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., Cordenonsi M., Piccolo S. Role of TAZ as Mediator of Wnt Signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Barry E.R., Morikawa T., Butler B.L., Shrestha K., de la Rosa R., Yan K.S., Fuchs C.S., Magness S.T., Smits R., Ogino S., et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartucci M., Dattilo R., Moriconi C., Pagliuca A., Mottolese M., Federici G., Di Benedetto A., Todaro M., Stassi G., Sperati F., et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- Basu-Roy U., Bayin N.S., Rattanakorn K., Han E., Placantonakis D.G., Mansukhani A., Basilico C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle B.W., Pruitt B.L., Nelson W.J. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busser B., Sancey L., Josserand V., Niang C., Favrot M.C., Coll J.L., Hurbin A. Amphiregulin Promotes BAX Inhibition and Resistance to Gefitinib in Non-small-cell Lung Cancers. Mol Ther. 2010;18:528–535. doi: 10.1038/mt.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Maitra A., Anders R.A., Taketo M.M., Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P., Han X., Zheng B.H., Deran M., Yu J.Z., Jarugumilli G.K., Deng H., Pan D.J., Luo X.L., Wu X. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat Chem Biol. 2016;12:282. doi: 10.1038/nchembio.2036. + [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.H., Mullett S.J., Stewart A.F. Vgl-4, a novel member of the vestigial-like family of transcription cofactors, regulates alpha1-adrenergic activation of gene expression in cardiac myocytes. J Biol Chem. 2004;279:30800–30806. doi: 10.1074/jbc.M400154200. [DOI] [PubMed] [Google Scholar]
- Cheng H.Y., Zhang Z.F., Rodriguez-Barrueco R., Borczuk A., Liu H.J., Yu J.Y., Silva J.M., Cheng S.K., Perez-Soler R., Halmos B. Functional genomics screen identifies YAP1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget. 2016;7:28976–28988. doi: 10.18632/oncotarget.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciamporcero E., Shen H., Ramakrishnan S., Ku S.Y., Chintala S., Shen L., Adelaiye R., Miles K.M., Ullio C., Pizzimenti S., et al. YAP activation protects urothelial cell carcinoma from treatment-induced DNA damage. Oncogene. 2016;35:1541–1553. doi: 10.1038/onc.2015.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizkova M., Cizeron-Clairac G., Vacher S., Susini A., Andrieu C., Lidereau R., Bieche I. Gene expression profiling reveals new aspects of PIK3CA mutation in ERalpha-positive breast cancer: major implication of the Wnt signaling pathway. Plos One. 2010;5 doi: 10.1371/journal.pone.0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C., Inui M., Montagner M., Parenti A.R., Poletti A., et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Dasari V.R., Mazack V., Feng W., Nash J., Carey D.J., Gogoi R. Verteporfin exhibits YAP-independent anti-proliferative and cytotoxic effects in endometrial cancer cells. Oncotarget. 2017;8:28628–28640. doi: 10.18632/oncotarget.15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRan M., Yang J., Shen C.H., Peters E.C., Fitamant J., Chan P., Hsieh M., Zhu S., Asara J.M., Zheng B., et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S.A., Gayyed M.F., Anders R.A., Maitra A., Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–U212. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Elbediwy A., Vincent-Mistiaen Z.I., Spencer-Dene B., Stone R.K., Boeing S., Wculek S.K., Cordero J., Tan E.H., Ridgway R., Brunton V.G., et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzo E., Santinon G., Pocaterra A., Aragona M., Bresolin S., Forcato M., Grifoni D., Pession A., Zanconato F., Guzzo G., et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–1370. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X.D., Degese M.S., Iglesias-Bartolome R., Vaque J.P., Molinolo A.A., Rodrigues M., Zaidi M.R., Ksander B.R., Merlino G., Sodhi A., et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Liu P., Zhou X., Li M.T., Li F.L., Wang Z., Meng Z.P., Sun Y.P., Yu Y., Xiong Y., et al. Thromboxane A2 activates YAP/TAZ protein to induce vascular smooth muscle cell proliferation and migration. J Biol Chem. 2016;291:18947–18958. doi: 10.1074/jbc.M116.739722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L.A., Squatrito M., Northcott P., Awan A., Holland E.C., Taylor M.D., Nahle Z., Kenney A.M. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N.J., Cornils H., Chiu S.Y., O’Rourke K.P., Arnaud J., Yimlamai D., Thery M., Camargo F.D., Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R., Hong A.W., Plouffe S.W., Zhao B., Liu G.B., Yu F.X., Xu Y.H., Guan K.L. Opposing roles of conventional and novel PKC isoforms in Hippo-YAP pathway regulation. Cell Res. 2015;25:985–988. doi: 10.1038/cr.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A., Liu Y., Inanlou M.R., Khomchuk Y., Wrana J.L. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- Gronich N., Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Na Re Clin Oncol. 2013;10:625–642. doi: 10.1038/nrclinonc.2013.169. [DOI] [PubMed] [Google Scholar]
- Guo Y., Cui J., Ji Z., Cheng C., Zhang K., Zhang C., Chu M., Zhao Q., Yu Z., Zhang Y., et al. miR-302/367/LATS2/YAP pathway is essential for prostate tumor-propagating cells and promotes the development of castration resistance. Oncogene. 2017;36:6336–6347. doi: 10.1038/onc.2017.240. [DOI] [PubMed] [Google Scholar]
- Hansen C.G., Ng Y.L.D., Lam W.L.M., Plouffe S.W., Guan K.L. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299–1313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K.F., Pfleger C.M., Hariharan I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Hong A.W., Meng Z.P., Yuan H.X., Plouffe S.W., Moon S., Kim W., Jho E.H., Guan K.L. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep. 2017;18:72–86. doi: 10.15252/embr.201642681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.B., Wu S., Barrera J., Matthews K., Pan D.J. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Jang E.J., Jeong H., Han K.H., Kwon H.M., Hong J.H., Hwang E.S. TAZ suppresses NFAT5 activity through tyrosine phosphorylation. Mol Cell Biol. 2012;32:4925–4932. doi: 10.1128/MCB.00392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S., Wang H.Z., Shi Z.B., Dong A.M., Zhang W.J., Song X.M., He F., Wang Y.C., Zhang Z.Z., Wang W.J., et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Kim N.G., Gumbiner B.M. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210:503–515. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.G., Koh E., Chen X., Gumbiner B.M. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Kim M., Lee S., Kuninaka S., Saya H., Lee H., Lee S., Lim D.S. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Yang S.J., Hwang D., Song J., Kim M., Kim S.K., Kang K., Ahn J., Lee D., Kim M.Y., et al. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nat Commun. 2015;6:10186. doi: 10.1038/ncomms10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.H., Kim J., Hong H., Lee S.H., Lee J.K., Jung E., Kim J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016;35:462–478. doi: 10.15252/embj.201592081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Khan S.K., Gvozdenovic-Jeremic J., Kim Y., Dahlman J., Kim H., Park O., Ishitani T., Jho E.H., Gao B., et al. Hippo signaling interactions with Wnt/beta-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127:137–152. doi: 10.1172/JCI88486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz L.M., Liu-Chittenden Y., Yin F., Zheng Y., Yu J., Huang B., Chen Q., Wu S., Pan D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar J.M., Stern P., Liu H., Schindler J.W., Jiang Z.G., Hynes R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Diaz M.F., Price K.M., Ozuna J.A., Zhang S., Sevick-Muraca E.M., Hagan J.P., Wenzel P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat Commun. 2017;8:14122. doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann W., Mossmann D., Kleemann J., Mock K., Meisinger C., Brummer T., Herr R., Brabletz S., Stemmler M.P., Brabletz T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7:10498. doi: 10.1038/ncomms10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q.Y., Zhang H., Zhao B., Zha Z.Y., Bai F., Pei X.H., Zhao S., Xiong Y., Guan K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N., Zhang C., Dill P., Panasyuk G., Pion D., Koka V., Gallazzini M., Olson E.N., Lam H., Henske E.P., et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249–2263. doi: 10.1084/jem.20140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.P., Sabnis A.J., Chan E., Olivas V., Cade L., Pazarentzos E., Asthana S., Neel D., Yan J.J., Lu X.Y., et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.C., Moroishi T., Meng Z.P., Jeong H.S., Plouffe S.W., Sekido Y., Han J.H., Park H.W., Guan K.L. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol. 2017;19:996–1002. doi: 10.1038/ncb3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Yu F.X., Kim Y.C., Meng Z., Naipauer J., Looney D.J., Liu X., Gutkind J.S., Mesri E.A., Guan K.L. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2015;34:3536–3546. doi: 10.1038/onc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y., Huang B., Shim J.S., Chen Q., Lee S.J., Anders R.A., Liu J.O., Pan D.J. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Gene Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Chen Y., Chen L., Cheng H.C., Mu C.L., Li J., Gao R.Z., Zhou C.Q., Cao L., Liu J.H., et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- Mao B., Hu F., Cheng J., Wang P., Xu M., Yuan F., Meng S., Wang Y., Yuan Z., Bi W. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468–1474. doi: 10.1038/onc.2013.88. [DOI] [PubMed] [Google Scholar]
- Meng Z., Moroishi T., Mottier-Pavie V., Plouffe S.W., Hansen C.G., Hong A.W., Park H.W., Mo J.S., Lu W., Lu S., et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z.P., Moroishi T., Guan K.L. Mechanisms of Hippo pathway regulation. Gene Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E., Yang J.Y., DeRan M., Wu C.L., Su A.I., Bonamy G.M.C., Liu J., Peters E.C., Wu X. Identification of Serum-Derived Sphingosine-1-Phosphate as a Small Molecule Regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mo J.S., Yu F.X., Gong R., Brown J.H., Guan K.L. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Gene Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J.S., Meng Z., Kim Y.C., Park H.W., Hansen C.G., Kim S., Lim D.S., Guan K.L. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T., Hayashi T., Pan W.W., Fujita Y., Holt M.V., Qin J., Carson D.A., Guan K.L. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. 2016;167:1525–1539. doi: 10.1016/j.cell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N., Inoue K., Adachi K., Kiyonari H., Ota M., Ralston A., Yabuta N., Hirahara S., Stephenson R.O., Ogonuki N., et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Noland C.L., Gierke S., Schnier P.D., Murray J., Sandoval W.N., Sagolla M., Dey A., Hannoush R.N., Fairbrother W.J., Cunningham C.N. Palmitoylation of TEAD transcription factors is required for their stability and function in Hippo pathway signaling. Structure. 2016;24:179–186. doi: 10.1016/j.str.2015.11.005. [DOI] [PubMed] [Google Scholar]
- O’Connell M.P., Marchbank K., Webster M.R., Valiga A.A., Kaur A., Vultur A., Li L., Herlyn M., Villanueva J., Liu Q., et al. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Discov. 2013;3:1378–1393. doi: 10.1158/2159-8290.CD-13-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hayre M., Vazquez-Prado J., Kufareva I., Stawiski E.W., Handel T.M., Seshagiri S., Gutkind J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M., Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- Overholtzer M., Zhang J., Smolen G.A., Muir B., Li W., Sgroi D.C., Deng C.X., Brugge J.S., Haber D.A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.W., Guan K.L. Regulation of the Hippo pathway and implications for anticancer drug development. Trends Pharmacol Sci. 2013;34:581–589. doi: 10.1016/j.tips.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.W., Kim Y.C., Yu B., Moroishi T., Mo J.S., Plouffe S.W., Meng Z.P., Lin K.C., Yu F.X., Alexander C.M., et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porazinski S., Wang H.J., Asaoka Y., Behrndt M., Miyamoto T., Morita H., Hata S., Sasaki T., Krens S.F.G., Osada Y., et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521:217–221. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Hejna M., Liu Y., Percharde M., Wossidlo M., Blouin L., Durruthy-Durruthy J., Wong P., Qi Z., Yu J., et al. YAP induces human naive pluripotency. Cell Rep. 2016;14:2301–2312. doi: 10.1016/j.celrep.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K., Mohseni M., Kirak O., Pruszak J., Rodriguez J.R., Zhou D., Kreger B.T., Vasioukhin V., Avruch J., Brummelkamp T.R., et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E., Basu-Roy U., Gunaratne P.H., Coarfa C., Lim D.S., Basilico C., Mansukhani A. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3:2075–2087. doi: 10.1016/j.celrep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano I., McDonald P.C., Lock F., Muller W.J., Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D., Zhai P.Y., Del Re D.P., Sciarretta S., Yabuta N., Nojima H., Lim D.S., Pan D.J., Sadoshima J. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014a;5:3315. doi: 10.1038/ncomms4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D.D., Xue W., Krall E.B., Bhutkar A., Piccioni F., Wang X., Schinzel A.C., Sood S., Rosenbluh J., Kim J.W., et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014b;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T., Weinberg R.A. EMT, CSCs, and drug resistance, the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.M., Ajani J.A., Honjo S., Maru D.M., Chen Q.R., Scott A.W., Heallen T.R., Xiao L.C., Hofstetter W.L., Weston B., et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014;74:4170–4182. doi: 10.1158/0008-5472.CAN-13-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino G., Ruggeri N., Specchia V., Cordenonsi M., Mano M., Dupont S., Manfrin A., Ingallina E., Sommaggio R., Piazza S., et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- Sorrentino G., Ruggeri N., Zannini A., Ingallina E., Bertolio R., Marotta C., Neri C., Cappuzzello E., Forcato M., Rosato A., et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. 2017;8:14073. doi: 10.1038/ncomms14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S., Munarriz E., Rossi M., Castagnoli L., Shaul Y., Sacchi A., Oren M., Sudol M., Cesareni G., Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- Tang Y., Feinberg T., Keller E.T., Li X.Y., Weiss S.J. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat Cell Biol. 2016;18:917–929. doi: 10.1038/ncb3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.C., Huang C.Y., Su H.L., Tang C.H. CTGF increases drug resistance to paclitaxel by upregulating survivin expression in human osteosarcoma cells. Biochim Biophys Acta. 2014;1843:846–854. doi: 10.1016/j.bbamcr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Tumaneng K., Schlegelmilch K., Russell R.C., Yimlamai D., Basnet H., Mahadevan N., Fitamant J., Bardeesy N., Camargo F.D., Guan K.L. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung S.L., Huang W.C., Hsu F.C., Yang Z.P., Jang T.H., Chang J.W., Chuang C.M., Lai C.R., Wang L.H. miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis. 2017;6:e3226. doi: 10.1038/oncsis.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X., Sakuma R., Samavarchi-Tehrani P., Peerani R., Rao B.M., Dembowy J., Yaffe M.B., Zandstra P.W., Wrana J.L. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B.G., Rossant J., Wrana J.L. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Wang M.Y., Chen P.S., Prakash E., Hsu H.C., Huang H.Y., Lin M.T., Chang K.J., Kuo M.L. Connective tissue growth factor confers drug resistance in breast cancer through concomitant up-regulation of Bcl-xL and cIAP1. Cancer Res. 2009;69:3482–3491. doi: 10.1158/0008-5472.CAN-08-2524. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wu Y., Wang H., Zhang Y., Mei L., Fang X., Zhang X., Zhang F., Chen H., Liu Y., et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA. 2014;111:E89–98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xiao Z.D., Li X., Aziz K.E., Gan B., Johnson R.L., Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhu X.Y., Feng W.W., Yu Y.H., Jeong K.J., Guo W., Lu Y.L., Mills G.B. Verteporfin inhibits YAP function through up-regulating 14-3-3 sigma sequestering YAP in the cytoplasm. Am J Cancer Res. 2016a;6:27–37. [PMC free article] [PubMed] [Google Scholar]
- Wang L., Luo J.Y., Li B.C., Tian X.Y., Chen L.J., Huang Y.H., Liu J., Deng D., Lau C.W., Wan S., et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016b;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- Wang L., Luo J.Y., Li B.C., Tian X.Y., Chen L.J., Huang Y.H., Liu J., Deng D., Law C.W., Wan S., et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016c;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- Wang Z., Liu P., Zhou X., Wang T.X., Feng X., Sun Y.P., Xiong Y., Yuan H.X., Guan K.L. Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ. Cancer Res. 2017;77:2413–2423. doi: 10.1158/0008-5472.CAN-16-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Huang J.B., Dong J.X., Pan D.J. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Wu H.T., Wei L.Y., Fan F.Q., Ji S.Y., Zhang S.H., Geng J., Hong L.X., Fan X., Chen Q.H., Tian J., et al. Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat Commun. 2015;6:6239. doi: 10.1038/ncomms7239. [DOI] [PubMed] [Google Scholar]
- Xin M., Kim Y., Sutherland L.B., Murakami M., Qi X.X., McAnally J., Porrello E.R., Mahmoud A.I., Tan W., Shelton J.M., et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T.A., Wang W.Y., Zhang S., Stewart R.A., Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yang S.P., Zhang L., Liu M., Chong R., Ding S.J., Chen Y.H., Dong J.X. CDK1 phosphorylation of YAP promotes mitotic defects and cell motility and is essential for neoplastic transformation. Cancer Res. 2013;73:6722–6733. doi: 10.1158/0008-5472.CAN-13-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D., Christodoulou C., Galli G.G., Yanger K., Pepe-Mooney B., Gurung B., Shrestha K., Cahan P., Stanger B.Z., Camargo F.D. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Chen W., O’Kelly J., Lu D., Ham M., Doan N.B., Xie D., Wang C., Vadgama J., Said J.W., et al. Connective tissue growth factor associated with oncogenic activities and drug resistance in glioblastoma multiforme. Int J Cancer. 2010;127:2257–2267. doi: 10.1002/ijc.25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H., Zhao J.G., Yuan H.X., Tumaneng K., Li H.R., et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Zhang Y.F., Park H.W., Jewell J.L., Chen Q., Deng Y.T., Pan D.J., Taylor S.S., Lai Z.C., Guan K.L. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Gene Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Luo J., Mo J.S., Liu G.B., Kim Y.C., Meng Z.P., Zhao L., Peyman G., Ouyang H., Jiang W., et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Zhao B., Guan K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F., Forcato M., Battilana G., Azzolin L., Quaranta E., Bodega B., Rosato A., Bicciato S., Cordenonsi M., Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu C.Y., Zha Z.Y., Zhao B., Yao J., Zhao S., Xiong Y., Lei Q.Y., Guan K.L. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009a;284:13355–13362. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.M., Ji J.Y., Yu M., Overholtzer M., Smolen G.A., Wang R., Brugge J.S., Dyson N.J., Haber D.A. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009b;11:1444–U1134. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.J., Gao Y.J., Li P.X., Shi Z.B., Guo T., Li F., Han X.K., Feng Y., Zheng C., Wang Z.Y., et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yang S.P., Chen X.C., Stauffer S., Yu F., Lele S.M., Fu K., Datta K., Palermo N., Chen Y.H., et al. The Hippo Pathway Effector YAP Regulates Motility, Invasion, and Castration-Resistant Growth of Prostate Cancer Cells. Mol Cell Biol. 2015;35:1350–1362. doi: 10.1128/MCB.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Hu Z., Qi H., Shi Z., Chang Y., Yao Q., Cui H., Zheng L., Han Y., Han X., et al. G-protein-coupled receptors mediate omega-3 PUFAs-inhibited colorectal cancer by activating the Hippo pathway. Oncotarget. 2016;7:58315–58330. doi: 10.18632/oncotarget.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Gene Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J.D., Wang C.Y., Chinnaiyan A.M., et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008a;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Ye X., Yu J.D., Li L., Li W.Q., Li S.M., Yu J.J., Lin J.D., Wang C.Y., Chinnaiyan A.M., et al. TEAD mediates YAP-dependent gene induction and growth control. Gene Dev. 2008b;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Wang L., Wang C.Y., Yu J.D., Guan K.L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Gene Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.L., Khanal P., Savage P., She Y.M., Cyr T.D., Yang X.L. YAP-Induced Resistance of Cancer Cells to Antitubulin Drugs Is Modulated by a Hippo-Independent Pathway. Cancer Res. 2014;74:4493–4503. doi: 10.1158/0008-5472.CAN-13-2712. [DOI] [PubMed] [Google Scholar]
- Zhou X., Wang S.Y., Wang Z., Feng X., Liu P., Lv X.B., Li F.L., Yu F.X., Sun Y.P., Yuan H.X., et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125:2123–2135. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]


