ABSTRACT
Mycetoma, a chronic and mutilating subcutaneous infection recognized by the WHO as a neglected tropical disease, has been reported in >25 countries in Africa, Asia, and South America. In Latin America, Trematosphaeria grisea is assumed to be the prevalent fungal agent. Recent molecular studies have shown that this is an environmental saprobe in Europe, where it is rarely implicated in human diseases. The aim of the present paper is to establish the identity of Latin American cases ascribed to Trematosphaeria grisea. Three cases analyzed were caused by Nigrograna mackinnonii. Data on an additional 21 strains in the literature revealed that N. mackinnonii rather than T. grisea is responsible for most cases of black grain eumycetoma in Latin America.
KEYWORDS: black grain, eumycetoma, Madurella grisea, Mexico, mycetoma, Nigrograna mackinnonii, Pyrenochaeta mackinnonii, Trematosphaeria grisea
INTRODUCTION
More than 50 fungal species have been recognized as causative agents of human eumycetoma, a chronic implantation disease endemic to the Tropic of Cancer (1). The infection may lead to extensive destruction of skin and subcutaneous tissues, and occasionally it extends to deeper anatomical structures, including muscles and bones, leading to massive deformity and disability (1, 2). The disease is acquired after a piercing injury, implanting the causative organism into the skin or subcutaneous tissue; therefore, rural workers, such as farmers and shepherds, are particularly at risk (3). Clinical presentations usually take months or years to develop and are characterized by subcutaneous necrosis with fungal granules that ultimately drain through sinuses (3, 4). The compact granules, or “grains,” are the core feature of mycetoma in both the fungal type (eumycetoma) and the bacterial type (actinomycetoma). However, the exact role and mechanism of the grain formation remain enigmatic (5); it is assumed to be a stress response to microaerobic conditions in host tissue and to serve as a resistant structure to enable survival of the host's immune system (5). The host is generally an immunocompetent individual with no apparent underlying disease, except that most mycetoma patients are in populations with low socioeconomic status in remote rural areas (3). Some authors have suggested that patients might have hidden deficiencies in their cell-mediated immunity (6), but as yet no clear predisposing factor has been uncovered.
Unlike other implantation mycoses, such as chromoblastomycosis, eumycetoma can be caused by a large diversity of species, and this list is steadily growing (1). Most cases occur in hot, arid climatic regions in Africa, Latin America, and Asia, with the highest endemicity in Sudan, Mexico, and India (7, 8). Inoculation of saprobic species that are resistant to environmental stress is the most likely cause. Since numerous extremotolerant desert fungi are known, a large diversity of species causing eumycetoma can be expected (1, 9).
The best-known and most important agents of eumycetoma are species of Madurella, a genus of sterile fungi lacking phenotypic characteristics that belong, with the genus Chaetomium, to the order Sordariales (10). Prior to molecular identification, all black grain eumycetomata were artificially classified in the genus Madurella. While Madurella mycetomatis was the main causative species in East Africa and India, Madurella grisea (currently known as Trematosphaeria grisea, order Pleosporales) was considered the most frequent agent in South America (11, 12, 16). The latter species was described by Mackinnon as phenotypically characterized by olive-gray colonies lacking a dark, diffusible pigment, with optimal growth at 30°C (12, 13). In 1976, Borelli described a coelomycetous agent of black grain eumycetoma from Venezuela and named it Pyrenochaeta mackinnonii after Juan Mackinnon (14, 16). This species also belonged to the order Pleosporales (14), and it is considered a rare cause of the disease, as reflected by the fact that in 22 years, a single case has been reported (15).
A significant change took place with the recent application of molecular and phylogenetic tools for the identification of pathogenic fungi. Using a multigene phylogenetic approach, Ahmed et al. (16) revised the taxonomic placement of eumycetoma agents from the order Pleosporales and concluded that T. grisea (syn., M. grisea) is actually not a common cause of eumycetoma and is rarely implicated in human infection. This conclusion is supported by the isolation of T. grisea strains from drinking water in temperate climate zones where mycetoma has never been reported; also, reidentification of a set of clinical T. grisea strains from reference collections has revealed a considerable number of misidentifications (16, 17).
Trematosphaeria grisea is phylogenetically affiliated with the family Trematosphaeriaceae, suborder Massarineae, in the order Pleosporales (16). The molecular characterization of type and authentic materials and the comparison of barcode sequences in public databases of this species have led to the discovery of novel taxa of eumycetoma agents in the family Trematosphaeriaceae that were previously misidentified as T. grisea (17). Moreover, a large number of provisionally identified T. grisea isolates were reidentified as Pyrenochaeta mackinnonii, belonging to a genus phylogenetically remote from Trematosphaeria. Since P. mackinnonii has been considered a rare cause of eumycetoma, in contrast to T. grisea, one might consider all previous reports of both Trematosphaeria grisea and Pyrenochaeta mackinnonii to be doubtful (16, 17). In a review of 3,933 mycetoma cases from Mexico, T. grisea was considered to be responsible for 28% of eumycetomata (13). López Martínez et al. (18) studied 2,105 cases of mycetoma from Mexico and phenotypically identified the agent in most of the eumycetoma cases as T. grisea, an identification that is also considered doubtful. Therefore, the aim of the present study is to revise the identification of T. grisea cases of black grain eumycetoma in Mexico and to clarify the etiology of the disease in Latin America.
MATERIALS AND METHODS
Case descriptions. (i) Case 1.
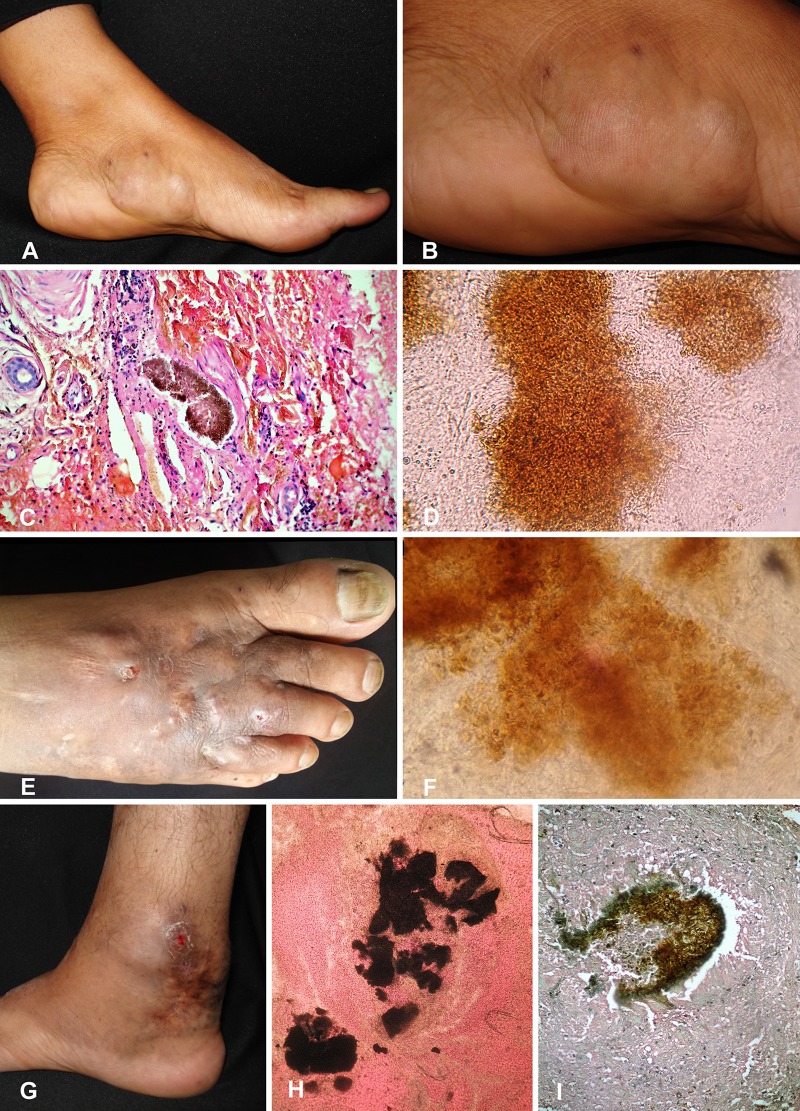
A 43-year-old woman from Tapachula, Chiapas, Mexico, presented with two 3- and 5-cm draining nodules and dark-brown patches in the left plantar aspect of the foot for 4 years. The preceding trauma was recalled by the patient. She went to a regional hospital in Chiapas, where mycetoma was clinically diagnosed and treatment with trimethoprim-sulfamethoxazole (co-trimoxazole) at 160 and 800 mg/day, respectively, for 30 days was started, with no clinical improvement. Laboratory findings were within normal ranges. Direct examination revealed black grains with thick branching hyphae. Histopathologically, black grains were observed. The fungus was initially identified as T. grisea on the basis of colony morphology and microscopic appearance. Treatment with itraconazole at 400 mg/day was started for a 1-year course and showed significant clinical improvement, but as yet no complete mycological cure has been achieved (Fig. 1).
FIG 1.
Clinical picture, histology, and direct microscopy for case 1 (A to D), case 2 (E and F), and case 3 (G to I).
(ii) Case 2.
A 44-year-old male farmer from Zirandaro, Guerrero, Mexico, presented to our consultation with a 30-year swelling and deformity on the anterior aspect of the left leg region as well as hyperpigmentation patches. No nodules were observed. The lesions were mainly distributed on the lower third of the left leg. Surgical excision had been performed 19 years ago with mild improvement. In 2006, the infection relapsed after treatment with trimethoprim-sulfamethoxazole for 1 year. Laboratory findings were within normal ranges. Direct examination in 10% KOH revealed a large grain of approximately 120 by 150 μm, in addition to thick hyphae. On histopathological examination, a cicatricial process was observed. A diagnosis of eumycetoma caused by T. grisea was proposed, and treatment with itraconazole at 400 mg/day was initiated. After 18 months of follow-up, the therapeutic outcome was satisfactory, with clinical and microbiological improvement (Fig. 1).
(iii) Case 3.
A 49-year-old male farmer from Veracruz, Mexico, presented with an 11-year swelling, atrophic scars, and hyperchromic brown patches on the dorsum of the right foot. The lesion was progressively increasing in size and was often accompanied by a serohematic discharge. The patient was previously diagnosed with mycetoma caused by Madurella mycetomatis at another hospital and was treated with itraconazole, fluconazole, and amoxicillin-clavulanic acid, with no clinical improvement. Laboratory findings were within normal ranges. In our department, direct examination showed vesiculous black grains. On histopathological examination, black grains were reported, and the isolated fungus was phenotypically identified as T. grisea. Treatment with itraconazole at 400 mg/day was given for 10 months, with clinical improvement (Fig. 1).
Ethics statement.
The Institutional Review Board and Ethics Committee of the Hospital General of México waived the need for ethical approval because this was only a report of three cases and no experimental intervention was performed. Written informed consent was obtained from the patients for the publication of this study and any accompanying images. Patient data were kept anonymous to ensure confidentiality and privacy.
Molecular identification.
Strains from patient materials were inoculated on Sabouraud dextrose agar (Oxoid, UK) and were maintained at 30°C. For DNA extraction, strains were transferred to 2% malt extract agar and were incubated for 1 week at 25°C. About 1 cm2 of the colony was transferred to 2-ml sterile tubes containing 490 μl CTAB buffer (2% cetyltrimethylammonium bromide, 100 mM Tris-HCl, 20 mM EDTA, 1.4 M NaCl) and acid-washed glass beads. Proteinase K was then added (10 μl) to the tubes, and the mixture was vortexed for 10 min, followed by incubation at 60°C for 60 min. After incubation, 500 μl of Sevag (chloroform–isoamyl alcohol [24:1]) was added, and the mixture was shaken for 2 min and then spun at 14,000 rpm for 10 min. Supernatants were collected in new Eppendorf tubes containing ∼270 μl ice-cold isopropanol and were spun again. Pellets were washed in 70% ethanol, vacuum dried, and resuspended in 50 μl Tris-EDTA (TE) buffer. The quality and concentration of the extracted DNA were verified using a NanoDrop spectrophotometer (ND-1000; Thermo Scientific, Wilmington, DE).
The ribosomal DNA (rDNA) internal transcribed spacer region (ITS) was amplified using primers ITS1 and ITS4 (19), and the large ribosomal subunit (LSU) was amplified using primers LRoR and LR5 (20). PCR conditions were those described by Ahmed et al. (16). The amplified products were sequenced using a BigDye Terminator cycle sequencing kit, v3.1 (Thermo Fisher Scientific, Waltham, MA, USA) and were analyzed with a 3730xl DNA analyzer (Thermo Fisher Scientific). Consensus sequences of the forward and reverse primers were generated using the Lasergene software package and the SeqMan assembly program (DNAStar, Madison, WI, USA). The sequences obtained were compared to each other and to the sequences present in GenBank using the BLAST tool. Since the three clinical isolates showed high similarity (99%) to each other and yielded similar BLAST results, the ITS and LSU sequences of case 1 were chosen as representative. The first hits resulting from the BLAST searches of the case 1 ITS and LSU sequences were collected for further phylogenetic analysis. In addition, available Biatriospora (Nigrograna) sequences were retrieved from GenBank and from research databases at the Westerdijk Fungal Biodiversity Institute and were included in the analysis. The sequence alignments were generated using the MUSCLE tool, available at the EMBL-EBI Web server (https://www.ebi.ac.uk/Tools/msa/muscle/), and were edited manually using BioEdit software, v7.1.3 (21). Phylogenetic trees were constructed using the maximum-likelihood approach, with an approximate likelihood ratio test (aLRT) performed in PhyML (22). Sporormia lignicola CBS 363.69 and Sporormiella minima CBS 524.50 were used as outgroups for the ITS analysis.
Accession number(s).
The sequences generated in this study were deposited in GenBank, and the accession numbers are provided in Table 1.
TABLE 1.
Nigrograna mackinnonii clinical strains
| Strain | Phenotypic identification | Clinical presentation | Origin | Yr of isolation | GenBank accession no. |
Source or reference | |
|---|---|---|---|---|---|---|---|
| ITS | LSU | ||||||
| Case 1 strain | T. grisea | Eumycetoma | Tapachula, Chiapas, Mexico | 2011 | MG063814 | MG064456 | This study |
| Case 2 strain | T. grisea | Eumycetoma | Zirandaro, Guerrero, Mexico | 2014 | MG063816 | MG064454 | This study |
| Case 3 strain | T. grisea | Eumycetoma | Veracruz, Mexico | 2016 | MG063815 | MG064455 | This study |
| Strain reported by Hughart et al. | Phaeohyphomycosis | From USA, but patient traveled to Colima, Mexico | 2016 | 23 | |||
| CBS 674.75T | N. mackinnonii | Eumycetoma | Venezuela | 1975 | NR_132037 | GQ387613 | 16 |
| CBS 110022 | N. mackinnonii | Eumycetoma | Mexico | 1999 | KF015653 | GQ387614 | 16 |
| IP1034.71 | Medicopsis romeroi | Eumycetoma | Costa Rica | 1971 | LT160874 | LT160875 | 17 |
| IP72.65 | T. grisea | Eumycetoma | Venezuela | 1965 | LT160876 | LT160877 | 17 |
| IP67.52 | T. grisea | Eumycetoma | Venezuela | 1952 | LT160878 | LT160879 | 17 |
| NCPF 2290 | T. grisea | Eumycetoma | Venezuela | 1981 | LT160880 | LT160881 | 17 |
| NCPF 2292 | T. grisea | Eumycetoma | Venezuela | 1981 | LT160882 | LT160883 | 17 |
| NCPF 2294 | T. grisea | Eumycetoma | Venezuela | 1981 | LT160884 | LT160885 | 17 |
| NCPF 2297 | T. grisea | Eumycetoma | Venezuela | 1981 | LT160886 | LT160887 | 17 |
| NCPF 2323 | T. grisea | Eumycetoma | Venezuela | 1982 | LT160888 | LT160889 | 17 |
| NCPF 2628 | Eumycetoma | Venezuela | 1986 | LT726688 | 24 | ||
| NCPF 2629 | Eumycetoma | Venezuela | 1986 | LT726689 | 24 | ||
| NCPF 2631 | Eumycetoma | Venezuela | 1986 | LT726690 | 24 | ||
| NCPF 2633 | Eumycetoma | Venezuela | 1986 | LT726685 | 24 | ||
| NCPF 2637 | Eumycetoma | Venezuela | 1986 | 24 | |||
| NCPF 2638 | Eumycetoma | Venezuela | 1986 | LT726691 | 24 | ||
| NCPF 2734 | Eumycetoma | Unknown | 1989 | LT726692 | 24 | ||
| NCPF 2737 | T. grisea | Eumycetoma | Venezuela | 1989 | LT726686 | 24 | |
| L3396 | Mycelia sterilia | Cyst, nodule | Brazil | 1996–2009 | KC288117 | KC288125 | 25 |
| UTHSC DI16-241 | Superficial tissue | USA | LN907384 | 26 | |||
RESULTS
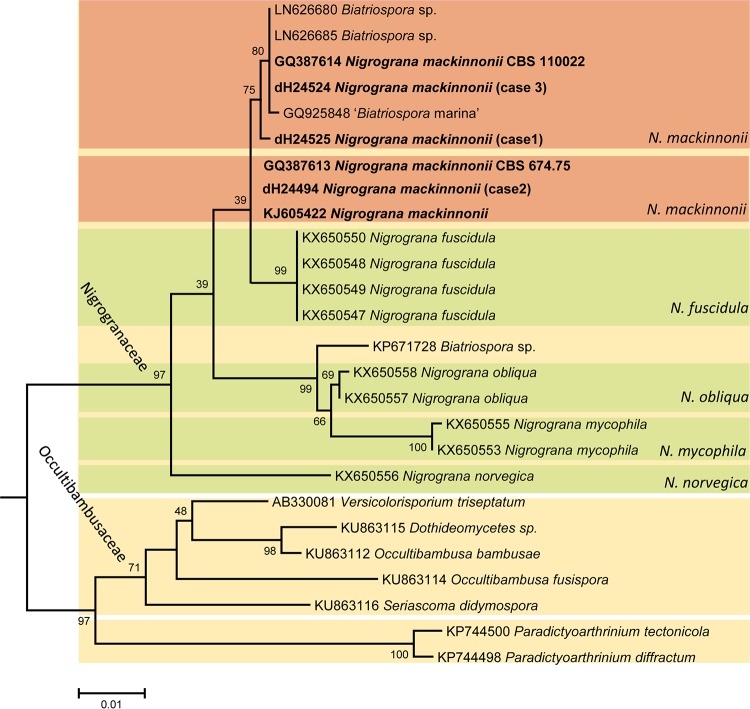
The three clinical isolates from eumycetoma patients showed dark olive-gray colonies, with no diffusible pigments being exuded into the agar. Microscopically, only brown, thick-walled hyphae were observed, without conidia or conidiomata. Based on these features, the strains had been provisionally identified as Trematosphaeria grisea. With ITS sequencing and BLAST searches against the NCBI database, none of the isolates yielded 100% homology to deposited sequences, but high similarities were found to several unidentified fungal species, with best hits (99% identity) to “Pleosporales sp.,” “fungal sp.,” “uncultured fungi,” “Dothideomycetes sp.,” and Biatriospora mackinnonii. Biatriospora (Nigrograna) mackinnonii was the only identified species among the first 10 best matches of our ITS sequence, and the similarity with the T. grisea ITS (reference strain CBS 332.50) was <70%. The LSU BLAST search of the case 1 strain resulted in 100% identity with one strain deposited as N. mackinnonii (CBS 110022) and isolated from a eumycetoma patient from Mexico. The LSU alignment was constructed for 26 sequences representing three phylogenetically close families in the order Pleosporales: Nigrogranaceae, Occultibambusaceae, and Paradictyoarthriniaceae. The alignment length was 790 bp, including gaps. In Nigrogranaceae, the newly described species of the genus Nigrograna formed well-supported lineages in the tree. Strains identified as Biatriospora (Nigrograna) mackinnonii and our clinical isolates were polyphyletic, forming two clades, one with 75% support and the other one unsupported. These strains clustered with Nigrograna fuscidula (only 39% support) paraphyletic to other Nigrograna species (Fig. 2).
FIG 2.
Phylogram generated from analyses of LSU sequences of Nigrogranaceae and their closest neighbor. The tree is constructed using an approximate likelihood ratio test in PhyML. The strains of the three cases reported in the present paper and clinical Nigrograna (Biatriospora) mackinnonii strains are shown in boldface.
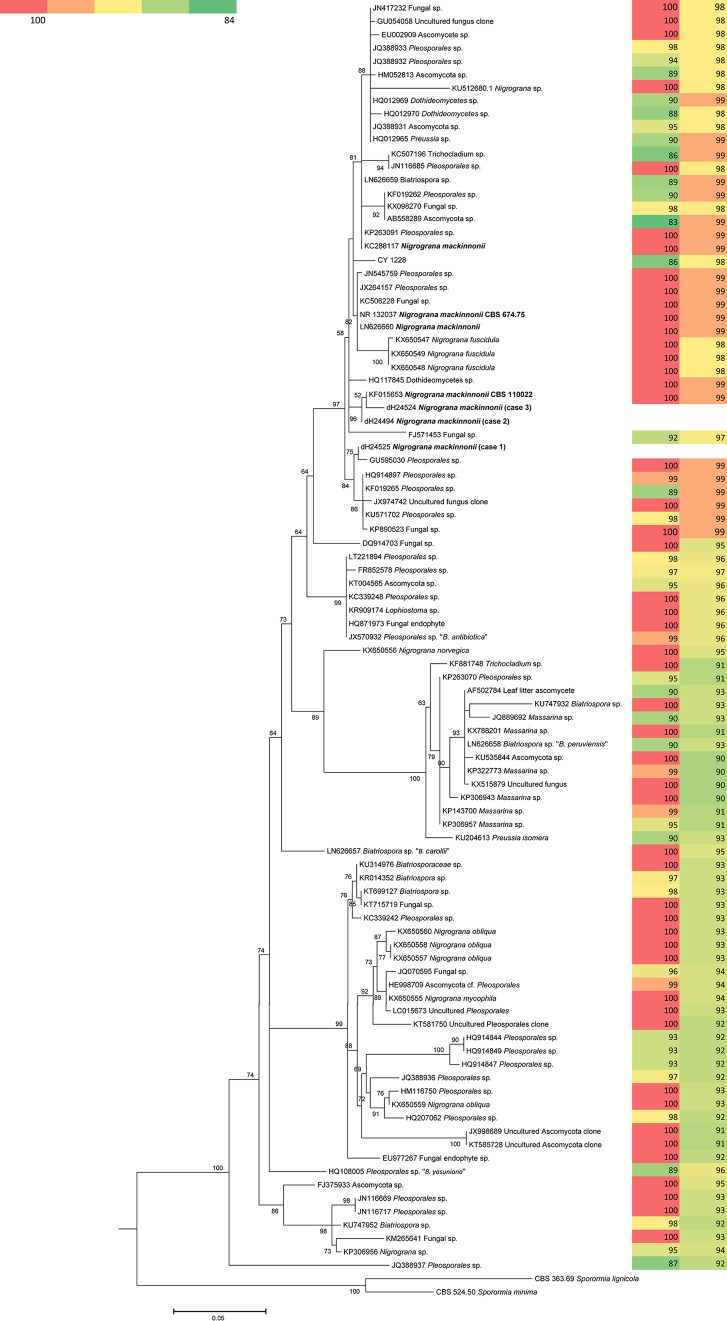
The ITS analyses were performed for a larger number of taxa, and the alignment included 97 sequences with a length of 490 bp, including gaps. The ITS tree (Fig. 3) showed a topology similar to the LSU tree, and Biatriospora (Nigrograna) mackinnonii was also found to be polyphyletic. Our clinical isolates clustered in a single clade (97% support) containing the known species Biatriospora (Nigrograna) mackinnonii and the plant-inhabiting species Nigrograna fuscidula. The BLAST results agreed with phylogeny in that the sequences derived from clinical strains in this clade showed 98 to 99% identity to each other; the maximum variability within the clade was 2% (Fig. 3). Two of our clinical isolates clustered in a subclade (96% support) with an authentic strain of N. mackinnonii (CBS 110022) from Mexico. In addition, another two strains, one from Costa Rica and one from Venezuela, were found in this clade, but we excluded them from the analysis because of the short coverage (data not shown). The third clinical isolate clustered (75% support) with a Pleosporales sp. isolated from a mangrove on the south coast of China.
FIG 3.
Phylogram resulting from analyses of ITS sequences of our clinical strains and their best matches in the public DNA repositories. The tree was generated using an approximate likelihood ratio test in PhyML. The strains of the three cases reported in the present paper and the clinical Nigrograna mackinnoni strains retrieved from GenBank are shown in boldface. Color coding represents the degree of similarity (left) and the query coverage of the BLAST search of ITS sequences (right).
Since both phylogeny and the best match of our isolates showed that these isolates are close to N. mackinnonii, we report them as N. mackinnonii. Nevertheless, in both the ITS and LSU trees, Biatriospora (Nigrograna) mackinnonii appears to be polyphyletic; the type strain, CBS 674.75, from a eumycetoma patient in Venezuela, clustered with three unidentified environmental strains. Furthermore, a large number of unidentified operational taxonomic units (OTUs) were found in the Biatriospora (Nigrograna) complex and were deposited in GenBank (Fig. 3). In total, 80 unnamed sequences were found in the best-hit match with the case 1 strain ITS sequence; this hinders the identification of our clinical strain by use of the BLAST tool, and a proper description of these taxa is paramount. While the clinical strains are distributed in several lineages among these environmental saprophytes, we still do not know if the environmental strains are actually introduced into humans and cause eumycetoma or whether a specific pathogenicity factor is needed to render the strain an infectious agent.
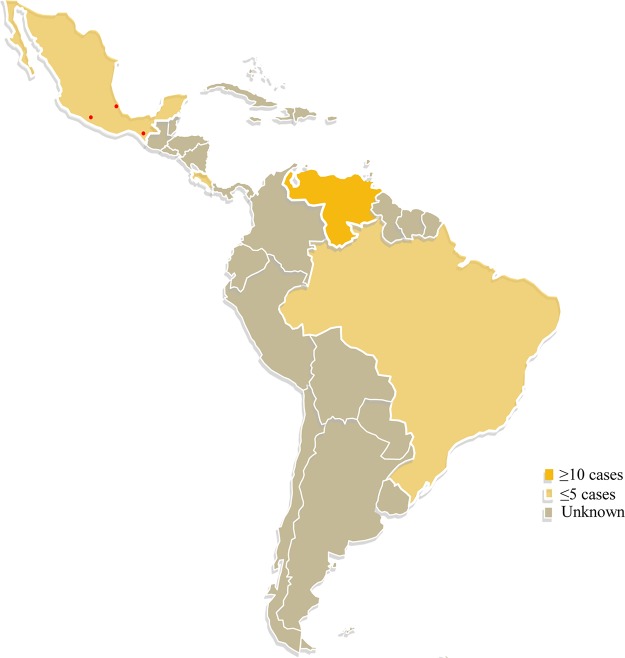
In total, 24 sequences of clinical isolates identified as Biatriospora (Nigrograna) mackinnonii were found in GenBank: 21 originated from human patients with eumycetoma, while 2 were isolated in cases of phaeohyphomycosis and 1 in a case of superficial infection (Table 1) (23–26). Most of these strains, including the isolates of our cases, were from Venezuela and Mexico (Fig. 4). Of the 21 strains from human eumycetoma, 11 were morphologically identified in the past as T. grisea, 8 of which were reported by Borman et al. (17) and 3 of which are reported in the current study.
FIG 4.
Distribution of cases of Nigrograna mackinnonii eumycetoma in Latin America. Red dots indicate the origins of the three cases reported here. (The template for the map was obtained from YourFreeTemplates.com and used with permission.)
DISCUSSION
Mycetoma is a chronic localized infection that is characterized by slow progression but in advanced stages is associated with high morbidity and severe disability. The disease was originally referred to as “Madura foot,” because feet are the most commonly affected body parts. The three patients reported in the present study also had pedal infections (3). Patients probably develop the disease after transcutaneous trauma, which may, however, occur unnoticed; in our case series, only the case 1 patient reported a preceding injury at the site of infection. One of the major problems of mycetoma is the late presentation of the patient and poor treatment compliance, sometimes leading to amputation. This is enhanced by the slow progression and painless nature of the disease. Our case series presented with 4-, 11-, and 30-year durations of subcutaneous masses. Despite this chronicity, the infections remain localized, with no systemic spread. This is typical for eumycetoma and contrasts with actinomycetoma, where the disease progresses faster and tends to be more aggressive (8).
In Mexico, actinomycetoma is more common than eumycetoma; the former was reported for 92% of the patients reviewed by Bonifaz et al. (3). In other regions of endemicity, such as Sudan and India, eumycetoma is prevalent (7). Clinicians in areas of endemicity easily recognize black grain mycetoma, because with black grains, the causative agent is invariably of a fungal nature, whereas pale grains are found in both eumycetomata and actinomycetomata. The hyphal substructure of the grains in histology is a further indicator (3) but provides no clue as to species identification. The grains for case 1 revealed a typical hyphal substructure and a tissue reaction characteristic of eumycetoma. Exuded grains allow culture of the causal agent, molecular analysis of which is necessary for reliable species identification (27).
In the present study, we applied the fungal barcode markers rDNA ITS and LSU for identification. Originally, case 3 was diagnosed as caused by Madurella mycetomatis, but later, this patient and the other two patients were all diagnosed with T. grisea eumycetoma. This has been due to the lack of characteristic sporulation, especially in the freshly isolated clinical strains, since the majority of these strains remain sterile, without sporulation (28). Our sequencing results showed that N. mackinnonii was concerned in all three cases. In the literature, this is considered a rare entity of the disease. This species was first described in the genus Pyrenochaeta as P. mackinnonii, characterized by coelomycetous (conidial) fruit bodies with an unknown sexual stage (14). In the era of molecular taxonomy, the generic affiliation was changed to Nigrograna mackinnonii, because the species was found to be phylogenetically remote from the type species of Pyrenochaeta, Pyrenochaeta nobilis (29), a member of Cucurbitariaceae; thus, the name P. mackinnonii appeared inappropriate. Nigrograna was proposed, a genus with unestablished phylogenetic affinities (30). However, the multilocus studies of Ahmed et al. (16) demonstrated that this species is closely similar to the Biatriospora species Biatriospora marina; the clade containing B. marina and N. mackinnonii was later accommodated in a new family, Biatriosporaceae (30, 31), a family of the order Pleosporales. The fungi in this clade have been of significance to biotechnology since the discovery of volatile organic compounds produced by an endophytic strain of B. mackinnonii that appeared to be a useful source of biofuels (32, 33). Four additional endophytic species have been described in Biatriospora, i.e., B. antibiotica, B. carollii, B. peruviensis, and B. yasuniana, which may be potential sources of a wide range of compounds, including antibiotics (34). Recently, Jaklitsch and Voglmayr (35) showed that the strain that was identified as B. marina and formed the basis of the phylogenetic description of Biatriospora and Biatriosporaceae was not the type strain of B. marina; not only that, but they also doubted the identity of this strain. Thus, the name of the clade containing these species was changed back to the oldest existing generic name, Nigrograna, and a novel family, Nigrogranaceae, was described (35). However, according to Tibpromma et al. (36) Biatriosporaceae is a morphologically distinct family; therefore, these authors recommended further sampling and evaluation of the family. Until further updates, the nomenclaturally correct name of Pyrenochaeta mackinnonii is Nigrograna mackinnonii. Other Biatriospora species in the ITS tree were labeled “Biatriospora sp.” (Fig. 3).
Nigrograna mackinnonii is the only species in the genus Nigrograna that is known exclusively from cases of eumycetoma in Latin America, including Costa Rica (n = 1), Venezuela (n = 15), and Mexico (n = 4) (14–17). It has also been implicated in phaeohyphomycosis in a renal transplant recipient in Brazil and in a U.S. citizen who traveled to Mexico (23, 25). Molecular identification of a large set of strains from the Fungus Testing Laboratory in San Antonio, TX, revealed a strain of N. mackinnonii from a superficial infection (26). Given that in cases where molecular verification of the identity of the etiologic agent was possible, N. mackinnonii was identified so many times, it may be concluded that this species is not a rare cause of human infection. The species seems prevalent in Latin America, and more cases may be expected in the future. In addition, the large number of unidentified OTUs that are close to N. mackinnonii and are all from the environment, sharing the same origin with this species, is remarkable. The unstable taxonomic status of the family Nigroganaceae and the fact that only recently have sequences of the type and authentic strains of Nigrogana become available in the public databases might have contributed to the delayed description of these unnamed taxa. Despite the high similarity of some of these unidentified sequences and strains to N. mackinnonii, we still do not know if they represent an environmental form of the species, since for most eumycetoma agents, the exact environmental origin is still unknown (9).
In the three cases presented in this study, treatment with 400 mg itraconazole/day was applied, and clinical improvement was achieved in two cases. In addition, a patient with a published case of N. mackinnonii phaeohyphomycosis was treated with itraconazole and excision, which resulted in a complete cure (23). Borman et al. (17) tested the in vitro susceptibilities of 10 strains of N. mackinnonii to amphotericin B, itraconazole, voriconazole, and caspofungin and reported MIC ranges of 0.25 to 2, 0.25 to 2, 0.13 to 1, and 2 to >16, respectively. Similar MICs were obtained by Ahmed et al. (37) when testing the type strain of N. mackinnonii and an additional isolate from Mexico. Since amphotericin B is not recommended for chronic, localized infections, azoles seem to be appropriate for therapy.
REFERENCES
- 1.Ahmed SA. 2016. New insights into a disfiguring fungal disease, eumycetoma. Ph.D. thesis. University of Amsterdam, Amsterdam, The Netherlands. [Google Scholar]
- 2.Zijlstra EE, van de Sande WW, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH. 2016. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis 16:100–112. doi: 10.1016/S1473-3099(15)00359-X. [DOI] [PubMed] [Google Scholar]
- 3.Bonifaz A, Tirado-Sánchez A, Calderón L, Saúl A, Araiza J, Hernández M, González GM, Ponce RM. 2014. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis 8:e3102. doi: 10.1371/journal.pntd.0003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahal AH, Suliman SH. 1994. Clinical presentation of mycetoma. Sudan Med J 32:46–66. [Google Scholar]
- 5.Kloezen W, van Helvert-van Poppel M, Fahal AH, van de Sande WWJ. 2015. A Madurella mycetomatis grain model in Galleria mellonella larvae. PLoS Negl Trop Dis 9:e0003926. doi: 10.1371/journal.pntd.0003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahgoub ES, Gumaa SA, El Hassan AM. 1977. Immunological status of mycetoma patients. Bull Soc Pathol Exot 70:48–54. [PubMed] [Google Scholar]
- 7.van de Sande WWJ. 2013. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis 7:e2550. doi: 10.1371/journal.pntd.0002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed AO, van Leeuwen W, Fahal A, van de Sande WWJ, Verbrugh H, van Belkum A. 2004. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect Dis 4:566–574. doi: 10.1016/S1473-3099(04)01131-4. [DOI] [PubMed] [Google Scholar]
- 9.de Hoog GS, Ahmed SA, Najafzadeh MJ, Sutton DA, Keisari MS, Fahal AH, Eberhardt U, Verkleij GJ, Xin L, Stielow B, van de Sande WW. 2013. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis 7:e2229. doi: 10.1371/journal.pntd.0002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Hoog GS, Adelmann D, Ahmed AO, van Belkum A. 2004. Phylogeny and typification of Madurella mycetomatis, with a comparison of other agents of eumycetoma. Mycoses 47:121–330. doi: 10.1111/j.1439-0507.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 11.Hospenthal DR. 2010. Agents of mycetoma, p 3281–3285. In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases, 7th ed Elsevier Churchill Livingstone, Philadelphia, PA. [Google Scholar]
- 12.Mackinnon JE, Ferrada-Urzúa LV, Montemayor L. 1949. Madurella grisea n. sp. Mycopathologia 4:384–393. doi: 10.1007/BF01237166. [DOI] [Google Scholar]
- 13.López-Martínez R, Méndez-Tovar LJ, Bonifaz A, Arenas R, Mayorga J, Welsh O, Vera-Cabrera L, Padilla-Desgarennes MC, Contreras Pérez C, Chávez G, Estrada R, Hernández-Hernández F, Manzano-Gayosso P. 2013. Update on the epidemiology of mycetoma in Mexico. A review of 3,933 cases. Gac Med Mex 149:586–592. (In Spanish.) [PubMed] [Google Scholar]
- 14.Borelli D. 1976. Pyrenochaeta mackinnonii nova species agente de micetoma. Castellania 4:227–234. [Google Scholar]
- 15.Serrano JA, Pisani ID, Lopez FA. 1998. Black grain minimycetoma caused by Pyrenochaeta mackinnonii. The first clinical case of eumycetoma reported in Barinas State, Venezuela. Clinical-histological features and case treatment. J Mycol Med 8:34–39. [Google Scholar]
- 16.Ahmed SA, van de Sande WWJ, Stevens DA, Fahal A, van Diepeningen AD, Menken SB, de Hoog GS. 2014. Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia 33:141–154. doi: 10.3767/003158514X684744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borman AM, Desnos-Ollivier M, Campbell CK, Bridge PD, Dannaoui E, Johnson EM. 2016. Novel taxa associated with human fungal black-grain mycetomas: Emarellia grisea gen. nov., sp. nov., and Emarellia paragrisea sp. nov. J Clin Microbiol 54:1738–1745. doi: 10.1128/JCM.00477-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López Martínez R, Méndez Tovar LJ, Lavalle P, Welsh O, Saúl A, Macotela Ruíz E. 1992. Epidemiology of mycetoma in Mexico: study of 2105 cases. Gac Med Mex 128:477–481. (In Spanish.) [PubMed] [Google Scholar]
- 19.White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc, New York, NY. [Google Scholar]
- 20.Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser (Oxford) 41:95–98. [Google Scholar]
- 22.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughart R, Merrick M, Adelaja OT, Bleasdale SC, Harrington A, Tsoukas M. 2016. Cutaneous phaeohyphomycosis caused by Biatriospora mackinnonii in a renal transplant recipient. JAAD Case Rep 2:230–232. doi: 10.1016/j.jdcr.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser M, Borman AM, Johnson EM. 2017. Rapid and robust identification of the agents of black-grain mycetoma by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 55:2521–2528. doi: 10.1128/JCM.00417-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos DW, Padovan AC, Melo AS, Gonçalves SS, Azevedo VR, Ogawa MM, Freitas TV, Colombo AL. 2013. Molecular identification of melanised non-sporulating moulds: a useful tool for studying the epidemiology of phaeohyphomycosis. Mycopathologia 175:445–454. doi: 10.1007/s11046-012-9608-x. [DOI] [PubMed] [Google Scholar]
- 26.Valenzuela-Lopez N, Sutton DA, Cano-Lira JF, Paredes K, Wiederhold N, Guarro J, Stchigel AM. 2017. Coelomycetous fungi in the clinical setting: morphological convergence and cryptic diversity. J Clin Microbiol 55:552–567. doi: 10.1128/JCM.02221-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed SA, van den Ende BHGG, Fahal AH, van de Sande WWJ, de Hoog GS. 2014. Rapid identification of black grain eumycetoma causative agents using rolling circle amplification. PLoS Negl Trop Dis 8:e3368. doi: 10.1371/journal.pntd.0003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed SA, Stevens DA, van de Sande WW, Meis JF, de Hoog GS. 2014. Roussoella percutanea, a novel opportunistic pathogen causing subcutaneous mycoses. Med Mycol 52:689–698. doi: 10.1093/mmy/myu035. [DOI] [PubMed] [Google Scholar]
- 29.de Gruyter J, Woudenberg JH, Aveskamp MM, Verkley GJ, Groenewald JZ, Crous PW. 2013. Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, et al. . 2013. Families of Dothideomycetes. Fungal Divers 63:1–313. doi: 10.1007/s13225-013-0263-4. [DOI] [Google Scholar]
- 31.Liu JK, Phookamsak P, Dai DQ, Tanaka K, Jones EBG, Xu JC, Chukeatirote E, Hyde KD. 2014. Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gennov., Roussoella and Roussoellopsis. Phytotaxa 181:1–33. doi: 10.11646/phytotaxa.181.1.1. [DOI] [Google Scholar]
- 32.Shaw JJ, Spakowicz DJ, Dalal RS, Davis JH, Lehr NA, Dunican BF, Orellana EA, Narváez-Trujillo A, Strobel SA. 2015. Biosynthesis and genomic analysis of medium-chain hydrocarbon production by the endophytic fungal isolate Nigrograna mackinnonii E5202H. Appl Microbiol Biotechnol 99:3715–3728. doi: 10.1007/s00253-014-6206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stodůlková E, Man P, Kuzma M, Černý J, Císařová I, Kubátová A, Chudíčková M, Kolařík M, Flieger M. 2015. A highly diverse spectrum of naphthoquinone derivatives produced by the endophytic fungus Biatriospora sp. CCF. 4378. Folia Microbiol (Praha) 60:259–267. [DOI] [PubMed] [Google Scholar]
- 34.Kolařík M, Spakowicz DJ, Gazis R, Shaw J, Kubátová A, Nováková A, Chudíčková M, Forcina GC, Kang KW, Kelnarová I, Skaltsas D, Portero CE, Strobel SA, Narváez-Trujillo A. 2017. Biatriospora (Ascomycota: Pleosporales) is an ecologically diverse genus including facultative marine fungi and endophytes with biotechnological potential. Plant Syst Evol 303:35. doi: 10.1007/s00606-016-1350-2. [DOI] [Google Scholar]
- 35.Jaklitsch WM, Voglmayr H. 2016. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Stud Mycol 85:35–64. doi: 10.1016/j.simyco.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SS, Liu J-K, Bhat DJ, Jones EB, McKenzie EH, Camporesi E, Bulgakov TS, Doilom M, Santiago AL, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang J-B, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KN, Latha KP, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li J, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KW, Luo Z-L, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, et al. . 2017. Fungal diversity notes 491-602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261. doi: 10.1007/s13225-017-0378-0. [DOI] [Google Scholar]
- 37.Ahmed SA, de Hoog GS, Stevens D, Fahal AH, van de Sande WWJ. 2015. In vitro antifungal susceptibility of coelomycete agents of black grain mycetoma to eight antifungals. Med Mycol 53:295–301. doi: 10.1093/mmy/myu098. [DOI] [PubMed] [Google Scholar]