ABSTRACT
The performance characteristics of the ceftolozane-tazobactam (C-T) Etest (bioMérieux, Marcy l'Etoile, France), MIC test strips (MTS; Liofilchem, Italy), and disk diffusion (Hardy, Santa Ana, CA) were evaluated for a collection of 308 beta-lactam-resistant isolates of Pseudomonas aeruginosa recovered from three institutions in Los Angeles, CA. Reference testing was performed by the reference broth microdilution (rBMD) method. MIC and disk results were interpreted using Clinical and Laboratory Standards Institute breakpoints. Overall, 72.5% of the isolates were susceptible to C-T by rBMD. Etest and disk diffusion demonstrated acceptable performance, whereas MTS yielded a greater than acceptable percentage of minor errors. Categorical agreement was 96.8% for Etest, 87.0% for MTS, and 92.9% for disk diffusion. No very major errors were observed by any test, and no major errors (ME) were observed by Etest or disk diffusion. Two ME (0.9% of susceptible isolates) were observed by MTS. The incidence of minor errors was 3.2%, 12.3%, and 7.1% for Etest, MTS, and disk diffusion, respectively. Essential agreement (EA) for Etest was excellent, at 97.7%, whereas the MICs obtained by MTS tended to be 1 to 2 dilutions higher than those obtained by rBMD, with an EA of 87.0%.
KEYWORDS: categorical agreement, ceftolozane-tazobactam, disk diffusion, Etest, MIC test strip, very major error, major error, susceptibility testing
INTRODUCTION
Ceftolozane-tazobactam (C-T) is a newer beta-lactam–beta-lactamase inhibitor combination agent with U.S. Food and Drug Administration (FDA) approval for the treatment of complicated urinary tract infections and complicated intra-abdominal infections, including those caused by Pseudomonas aeruginosa. C-T has excellent activity against resistant P. aeruginosa isolates, including those resistant to other beta-lactam–beta-lactamase inhibitor combinations, antipseudomonal cephalosporins, and carbapenems (1–4). However, susceptibility testing of C-T has been a challenge for the clinical laboratory, as only manual methods (disk, gradient strips, and Sensititre custom panels) have been cleared by the FDA for the testing of clinical isolates. These include Etest (bioMérieux, Marcy l'Etoile, France), MIC test strips (MTS; Liofilchem, Italy), Sensititre panels (Thermo Fisher, Lenexa, KS), and disks (Hardy Diagnostics, Santa Ana, CA). Clinical microbiology laboratories anecdotally have documented the disappointing performance of both the gradient strips and disks compared to the reference broth microdilution (rBMD) method, and one study demonstrated high rates of very major errors (VME; false susceptibility) for the research-use-only (RUO)-labeled Etest (5). In this study, we evaluated the performance characteristics of C-T Etest, MTS, and disk compared to the Clinical and Laboratory Standards (CLSI) rBMD with a collection of highly resistant P. aeruginosa isolates.
MATERIALS AND METHODS
Bacterial isolates.
P. aeruginosa isolates were collected from 2015 to 2017 from three clinical laboratories in Los Angeles, CA: the University of California, Los Angeles (UCLA), Ronald Reagan Medical Center, Kindred Hospital Los Angeles, and Huntington Memorial Hospital. Isolates were selected on the basis of resistance to one or more antipseudomonal beta-lactam agents, including ceftazidime, cefepime, meropenem, imipenem, or piperacillin-tazobactam. Additionally, 22 isolates previously evaluated by Flynt and colleagues (5) were obtained from the Henry Ford Health System and included in this study. Eleven of these isolates were documented to have VME or major errors (ME) by Etest in the prior study of Flynt et al. (5), and an equal number of isolates that demonstrated no errors were randomly selected from the study of Flynt et al. (5) for evaluation. Isolates were stored at −70°C in brucella broth with 10% glycerol (Hardy Diagnostics, Santa Ana, CA) and subcultured to sheep's blood agar plates twice prior to testing.
Susceptibility testing.
MICs were determined by CLSI rBMD on panels prepared in-house using cation-adjusted Mueller-Hinton broth (Difco, BD, Sparks, MD). The following antimicrobials were included on the panels: ceftazidime, cefepime, imipenem, meropenem, piperacillin-tazobactam, and ceftolozane-tazobactam. Concentrations spanned the doubling dilution range from 32 μg/ml to 0.25 μg/ml for all antimicrobials with the exception of piperacillin, which was tested at concentrations ranging from 128 to 1 μg/ml. Tazobactam was held at 4 μg/ml when it was used in combination with both piperacillin and ceftolozane. All antimicrobial powders were obtained from Sigma-Aldrich, with the exception of ceftolozane, which was obtained from Merck. Quality control (QC) testing was performed using Escherichia coli ATCC 25922, P. aeruginosa ATCC 27853, and Klebsiella pneumoniae ATCC 700603. All values for QC strains were at the middle of the acceptable CLSI range for QC isolates. The breakpoints used to interpret MICs were those published by CLSI (6).
MTS (Liofilchem) and disk diffusion (disk potency, 30 μg ceftolozane and 10 μg tazobactam; Hardy) were performed according to the manufacturer's FDA-cleared instructions. Research-use-only (RUO)-labeled Etest strips were obtained from International Health Management Associates (IHMA). The RUO Etest used in this study is the same formulation as the recently 510(k)-cleared, in vitro diagnostic (IVD)-labeled product currently available in the United States (bioMérieux, personal communication to R.M.H.). The reading instructions recommended by bioMérieux during the clinical trial to support 510(k) submission of the C-T Etest were used in this study. Tests that resulted in colonies within the zone of inhibition were repeated in parallel by rBMD, disk diffusion, Etest, and MTS. If the result was reproducible, the inner, colony-free zone of growth inhibition was evaluated as the endpoint for all three tests. All three tests were performed on commercially prepared Mueller-Hinton agar (BBL, BD, Sparks, MD).
Study design.
The BMD, Etest, MTS, and disk diffusion tests were performed using bacterial suspensions at the same 0.5 McFarland standard. rBMD results were used as the “gold standard,” against which the performance characteristics of Etest, MTS, and disk diffusion were compared. MICs between the typical log2 dilution MICs obtained by Etest and MTS were rounded up to the nearest log2 dilution. Any isolates for which a VME (false susceptible) or ME (false resistant) was obtained were retested by all four methods.
VME rates were calculated using the number of isolates with resistant MICs by BMD as the denominator, ME rates were calculated using the number of susceptible isolates as the denominator, and minor error (mE) rates were calculated using the total number of isolates evaluated as the denominator. Essential agreement (EA) and categorical agreement (CA) were calculated using BMD as the reference. EA was defined as the number of isolates with MICs ±1 log2 dilution of the BMD MIC.
RESULTS
Three-hundred eight isolates of P. aeruginosa were evaluated in this study. Twelve percent (n = 37) were susceptible to imipenem, 16% (n = 49) were susceptible to meropenem, 21% (n = 64) were susceptible to piperacillin-tazobactam, 25% (n = 76) were susceptible to ceftazidime, and 26% (n = 80) were susceptible to cefepime (Table 1). In contrast, 73% (n = 224) were susceptible to C-T (Table 1) when tested by rBMD.
TABLE 1.
Activity of beta-lactams for 308 P. aeruginosa evaluated in this study, as determined by rBMD
| Beta-lactama | No. (%) of isolates |
|
|---|---|---|
| Susceptible | Resistant | |
| Ceftazidime | 76 (24.6) | 209 (67.6) |
| Cefepime | 80 (25.9) | 149 (48.2) |
| Imipenem | 37 (12.0) | 259 (83.8) |
| Meropenem | 49 (15.9) | 228 (73.8) |
| TZP | 64 (20.7) | 183 (59.2) |
| C-T | 224 (72.5) | 66 (21.4) |
C-T, ceftolozane-tazobactam; TZP, piperacillin-tazobactam.
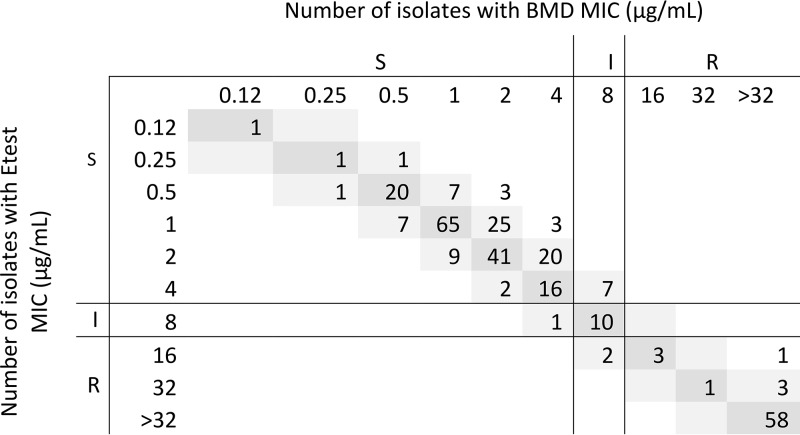
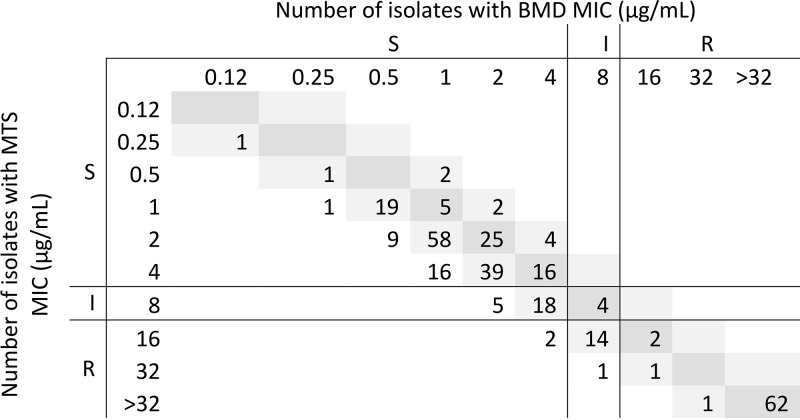
The performance of all Etest and disk diffusion assays was excellent, whereas the performance of MTS fell short of acceptable, according to U.S. Food and Drug Administration criteria (Table 2). Essential agreement (EA) was 96.8% for Etest and 89.0% for MTS. Etest MICs were 1 log2 dilution lower than rBMD MICs for 20% of isolates and 1 log2 dilution higher for 10% of isolates. Seven isolates had an Etest MIC 2 log2 dilutions lower than the rBMD MIC (i.e., out of EA) (Fig. 1). The MICs observed by MTS were generally higher than those observed by rBMD (Fig. 2). The absolute agreement of the MTS and rBMD MICs was 37.0%, with 49.0% of MTS MICs being 1 log2 dilution higher and 11.0% being 1 log2 dilution lower than the rBMD MICs. The MICs for all 34 (11.0%) isolates with MTS MICs out of EA were 2 log2 dilutions higher than the rBMD MICs (Fig. 2).
TABLE 2.
Performance of disk diffusion, Etest, and MTS compared to rBMD for 308 P. aeruginosa isolatesa
| Assay | EA (%) | CA (%) | No. (%) of isolates with: |
||
|---|---|---|---|---|---|
| VME | ME | mE | |||
| Hardy disk | NA | 92.9 | 0 (0) | 0 (0) | 22 (7.1) |
| Etest | 96.8 | 96.8 | 0 (0) | 0 (0) | 10 (3.2) |
| MTS | 89.0 | 87.0 | 0 (0) | 2 (0.9) | 38 (12.3) |
NA, not applicable; EA, essential agreement; CA, categorical agreement; VME, very major errors; ME, major errors; mE, minor errors.
FIG 1.
rBMD and Etest MIC distributions for 308 P. aeruginosa isolates. S, susceptible; R, resistant; I, intermediate.
FIG 2.
rBMD and MTS MIC distributions for 308 P. aeruginosa isolates. S, susceptible; R, resistant; I, intermediate.
No VMEs were observed by any of the test methods. Major errors (ME) were observed only with MTS (n = 2, 0.9%). These MEs included one for an isolate with a MIC of 4 μg/ml (susceptible) by rBMD and Etest and a disk zone of 19 mm (intermediate) but an MTS MIC of 16 μg/ml (resistant). The second ME was for an isolate with MICs of 4 μg/ml (susceptible) by rBMD and 6 μg/ml (intermediate) by Etest and a disk zone of 16 mm (intermediate) but an MTS MIC of 12 μg/ml (resistant). These two ME were confirmed by repeat testing.
Several minor errors (mE) were observed in the study. Ten (3.2%) were observed by Etest; for 7 of these, the Etest MIC was 1 log2 dilution lower than the rBMD MIC (Fig. 1). Similarly, for all 38 mE observed by MTS, MTS MICs were 1 to 2 log2 dilutions higher than rBMD MICs (Fig. 2).
Flynt and colleagues previously demonstrated 50% VME and 2.6% ME by the C-T Etest (5). In an effort to understand the significantly different outcomes from their study and ours, the investigators kindly sent us 22 isolates evaluated in their study, including all isolates that yielded VME or ME (n = 11) and an equal number that yielded categorically concordant results (n = 11). We retested these isolates by Etest, MTS, and rBMD and did not observe any VME or ME (Table 3). Subcultures of the 22 isolates tested in our laboratory were resent to Flynt and colleagues for repeat testing by Etest and rBMD in their laboratory, and they were unable to reproduce any of the previously reported errors (not shown). For all 6 isolates where initial testing revealed a VME, i.e., resistant by rBMD but susceptible by Etest, our repeat testing revealed a susceptible or intermediate MIC by our rBMD method (Table 3), as well as by the Etest and MTS. In contrast, for the two isolates that initially showed ME with an Etest MIC of >256 μg/ml and an rBMD MIC of 1 μg/ml, we observed an Etest MIC of 0.5 or 0.75 μg/ml (Table 3), which correlated with the rBMD MICs obtained concurrently.
TABLE 3.
MIC data for P. aeruginosa isolates that demonstrated errors by Flynt et al.a
| Isolate no. | Data from Flynt et al. (5) |
UCLA data from repeat testing |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original Etest |
Original rBMD |
Original error | rBMD |
Etest |
Etest Error vs. BMD | MTS |
MTS error vs rBMD result | ||||||
| MIC (μg/ml) | Interpretation | MIC (μg/ml) | Interpretation | MIC (μg/ml) | Interpretation | MIC (μg/ml) | Interpretation | MIC (μg/ml) | Interpretation | ||||
| 16-40-303 | 3 | S | >128 | R | VME | 1 | S | 0.75 | S | None | 2 | S | None |
| 16-40-305 | 1.5 | S | 32 | R | VME | 4 | S | 3 | S | None | 8 | I | mE |
| 16-40-309 | 3 | S | 16 | R | VME | 4 | S | 3 | S | None | 6 | I | mE |
| 16-40-310 | 3 | S | 16 | R | VME | 8 | I | 4 | S | mE | 8 | I | None |
| 16-40-312 | 4 | S | 32 | R | VME | 8 | I | 8 | I | None | 16 | R | mE |
| 16-40-314 | 2 | S | 16 | R | VME | 8 | I | 8 | I | None | 16 | R | mE |
| 16-40-299 | >256 | R | 1 | S | ME | 1 | S | 0.75 | S | None | 1.5 | S | None |
| 16-40-311 | >256 | R | 1 | S | ME | 0.5 | S | 0.5 | S | None | 1 | S | None |
| 16-40-313 | 1.5 | S | 8 | I | mE | 4 | S | 3 | S | None | 6 | I | mE |
| 16-40-307 | 1 | S | 8 | I | mE | >32 | R | 24 | R | None | 48 | R | None |
| 16-40-308 | 8 | I | 64 | R | mE | >32 | R | 48 | R | None | 96 | R | None |
| 16-40-300 | 32 | R | >128 | R | None | >32 | R | 128 | R | None | 128 | R | None |
| 16-40-301 | 1 | S | 1 | S | None | 1 | S | 1 | S | None | 2 | S | None |
| 16-40-302 | 0.75 | S | 1 | S | None | 2 | S | 1 | S | None | 2 | S | None |
| 16-40-304 | 0.5 | S | 0.5 | S | None | 1 | S | 0.75 | S | None | 1.5 | S | None |
| 16-40-306 | 0.5 | S | 1 | S | None | 1 | S | 0.75 | S | None | 1.5 | S | None |
| 16-40-315 | >256 | R | >64 | R | None | >32 | R | >256 | R | None | >256 | R | None |
| 16-40-316 | 0.75 | S | 1 | S | None | 2 | S | 1.5 | S | None | 3 | S | None |
| 16-40-317 | 0.5 | S | 1 | S | None | 2 | S | 1 | S | None | 3 | S | None |
| 16-40-318 | 1 | S | 1 | S | None | 2 | S | 1 | S | None | 2 | S | None |
| 16-40-319 | 1 | S | 0.5 | S | None | 2 | S | 1 | S | None | 2 | S | None |
| 16-40-320 | 2 | S | 1 | S | None | 2 | S | 1 | S | None | 2 | S | None |
S, susceptible; R, resistant; I, intermediate; VME, very major errors; ME, major errors; mE, minor errors.
The MIC trends observed for the clinical isolates by the test methods were reflected by the QC strains. The mode MICs (n = 10 readings) for E. coli ATCC 25922 were 0.125 μg/ml, 0.5 μg/ml, and 0.25 μg/ml by Etest, MTS, and rBMD, respectively. The acceptable MIC range is 0.12 to 0.5 μg/ml. The modal zone size was 25 mm (acceptance range, 24 to 32 mm) for E. coli ATCC 25922. Similarly, the mode MICs for P. aeruginosa ATCC 27853 were 0.38 μg/ml, 1.0 μg/ml, and 0.5 μg/ml by Etest, MTS, and rBMD, respectively. The acceptable MIC range for this QC strain is 0.25 to 1 μg/ml. The modal zone size was 25 mm (acceptance range, 24 to 32 mm).
DISCUSSION
Treatment of P. aeruginosa infections can be challenging due to this organism's low permeability to many antimicrobial agents and multiple resistance mechanisms. As such, C-T is an important addition to our arsenal against P. aeruginosa. Large surveillance studies have demonstrated 86 to 95% rates of susceptibility of P. aeruginosa to C-T (7, 8). However, real-world outcomes of treating multidrug-resistant (MDR) P. aeruginosa infections with C-T are limited. A recent case series reported the outcomes of C-T treatment of 35 patients with MDR P. aeruginosa infections (9). C-T susceptibility testing was not performed on 5 of the cases, due to a lack of available FDA-cleared susceptibility tests for C-T at the time. Two of the patients without susceptibility data were among 6 clinical failures, and the remaining 4 patients were infected with isolates that were not susceptible to C-T (9). This study highlights the critical importance of antimicrobial susceptibility testing (AST) of C-T and other new agents, particularly where it is known that not all isolates are susceptible. In a recent study of 309 P. aeruginosa isolates resistant to one or more antipseudomonal beta-lactams, the clinical scenario in which C-T is most likely to be considered for therapy, 73.4% were susceptible, 6.1% were intermediate, and 20.5% were resistant (10). Local testing of C-T is desirable, as the turnaround time to results when reference laboratories are used may be prolonged.
Clinical microbiology laboratories are reluctant to test C-T for multiple reasons (personal communications to R.M.H.). These primarily include the absence of C-T on the test panels used with automated AST methods and concerns regarding the complexity of performing the verification studies required by Clinical Laboratory Improvement Amendments prior to implementing C-T ASTs. The latter point cannot be overemphasized, as the investment required to perform a verification study is substantial, particularly in smaller laboratories with limited resources. As the analytical performance of ASTs is crucial for their use in patient care, laboratories are more likely to evaluate and implement in their laboratories tests that have a good probability of satisfactory performance in verification studies. As such, reports of poor performance of a test from colleagues may dissuade a laboratory from investing the time to evaluate that test. Flynt and colleagues previously documented the poor performance of the C-T Etest (5). We tested their isolates and were unable to reproduce the errors. Upon repeat testing by the investigators at Henry Ford Hospital, the errors likewise did not reproduce. The reason for this variability in performance is not clear. In a discussion of the methods used by Flynt and colleagues (5) in the original study, it was revealed that the source of the C-T powder used for preparation of stock solutions for rBMD was pharmacy-grade C-T powder obtained from the investigator's pharmacy. This stock was then serially diluted for preparation of rBMD panels. This is problematic, as the tazobactam component of the test must be held constant at 4 μg/ml and not serially diluted along with the ceftolozane component, as was done in their study. However, upon repeat testing of the isolates using the same method, the investigators could not reproduce their original data. Since all of the isolates tested were not resistant due to the limited concentration of tazobactam, it suggests that there may be additional resistance mechanisms in those 11 isolates. It is also possible that the isolate's susceptibility or resistance characteristics may have changed during storage, although this theory cannot be evaluated.
Evaluation of ASTs is complex, as the method relies on the behavior of live bacteria in vitro and visual interpretation of results for all methods described here, including the rBMD method. Some variables, such as slight changes in the incubation temperature or the length of incubation, small differences in drug powder or stock solution potency, or the manufacturer source of Mueller-Hinton medium, generally result in the rBMD method having an accepted reproducibility of ±1 log2 dilution (i.e., 3 possible MICs for a given isolate). However, recent data compiled by the Clinical and Laboratory Standards Institute (CLSI) demonstrated that MIC reproducibility can be strain dependent, with some isolates yielding a range of 4 or more different MICs by rBMD when tested on the same day, using the same panels and bacterial source plate (CLSI, January 2016 Agenda Book). As such, it is critical when evaluating different AST systems that testing be performed in parallel rather than sequentially and preferably with a single inoculum suspension. At the very least, confirmation of discrepancies by parallel testing should be done. Furthermore, when publishing studies on the analytical performance of ASTs, it is crucial that detailed methods be included in the published paper so that the results can be interpreted. Unfortunately, in this era of minimizing manuscript lengths, details on the methodology for the studies are often omitted from published papers, as was the case for the paper of Flynt et al. (5). Additionally, differences in evaluating MIC endpoints and zones of growth inhibition for disk diffusion can result in different interpretations of AST performance. We did not encounter any such difficulties with the present collection of isolates, although others have commented that certain strains of P. aeruginosa yield haze growth in rBMD panels, which may be difficult to detect and interpret (Laura Koeth, personal communication with R.M.H.).
In the present study, we demonstrate the excellent activity of Etest and disk diffusion for evaluation of the activity of C-T against a population of beta-lactam-resistant P. aeruginosa isolates. The C-T MIC distribution for the isolates evaluated in this study reflects that documented in larger databases, including the European Committee on Antimicrobial Susceptibility Testing (EUCAST) data (https://mic.eucast.org/Eucast2/regShow.jsp?Id=36537). The highest probability of testing errors would be for those isolates with MICs near the breakpoint, i.e., 8 to 32 μg/ml, as slight downward differences in MICs may result in VMEs. However, such isolates are rare (6.6% of all P. aeruginosa included in the EUCAST database and 7.5% included in the present study). In contrast, the MTS had a tendency to yield MICs that were 1 to 2 log2 dilutions higher than rBMD MICs, and as such, laboratories may consider carefully evaluating isolates with MICs in the intermediate range if using MTS, as these may be susceptible by rBMD.
ACKNOWLEDGMENTS
Funding for this study was provided by Merck, in the form of an investigator-initiated research grant to R.M.H.
We thank the Kindred Hospital laboratories for isolates included in this study.
REFERENCES
- 1.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation beta-lactam/beta-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livermore DM, Mushtaq S, Meunier D, Hopkins KL, Hill R, Chaundhry A, Pike R, Staves P, Woodford N. 2017. Activity of ceftolozane/tazobactam against surveillance and ‘problem’ Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 72:2278–2289. doi: 10.1093/jac/dkx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Bassetti M, Duncan LR, Castanheira M. 2017. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing urinary tract and intraabdominal infections in Europe: report from an antimicrobial surveillance programme (2012–15). J Antimicrob Chemother 72:1386–1395. doi: 10.1093/jac/dkx009. [DOI] [PubMed] [Google Scholar]
- 4.Shortridge D, Castanheira M, Pfaller MA, Flamm RK. 2017. Ceftolozane-tazobactam activity against Pseudomonas aeruginosa clinical isolates from U.S. hospitals: report from the PACTS antimicrobial surveillance program (2012–2015). Antimicrob Agents Chemother 61:e00465-17. doi: 10.1128/AAC.00465-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynt LK, Veve MP, Samuel LP, Tibbetts RJ. 2017. Comparison of Etest to broth microdilution for testing of susceptibility of Pseudomonas aeruginosa to ceftolozane-tazobactam. J Clin Microbiol 55:334–335. doi: 10.1128/JCM.01920-16. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing, 27th ed M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.Farrell DJ, Sader HS, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 43:533–539. doi: 10.1016/j.ijantimicag.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. 2014. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12). J Antimicrob Chemother 69:2713–2722. doi: 10.1093/jac/dku184. [DOI] [PubMed] [Google Scholar]
- 9.Munita JM, Aitken SL, Miller WR, Perez F, Rosa R, Shimose LA, Lichtenberger PN, Abbo LM, Jain R, Nigo M, Wanger A, Araos R, Tran TT, Adachi J, Rakita R, Shelburne S, Bonomo RA, Arias CA. 2017. Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis 65:158–161. doi: 10.1093/cid/cix014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphries RM, Hindler JA, Wong-Beringer A, Miller SA. 2017. Activity of ceftolozane-tazobactam and ceftazidime-avibactam against beta-lactam-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 61:e01858-17. doi: 10.1128/AAC.01858-17. [DOI] [PMC free article] [PubMed] [Google Scholar]