Abstract
AIM
To examine the thickness of the ganglion cell-inner plexiform layer (GCIPL) in eyes with resolved macular edema (ME) in non-ischemic central retinal vein occlusion (CRVO), applying spectral-domain optical coherence tomography (SD-OCT), and its relationship with visual acuity.
METHODS
The retrospective observational case-control study included 30 eyes of non-ischemic CRVO patients with resolved ME (ME eyes) after treatment, and 30 eyes of non-ischemic CRVO patients without ME (non-ME eyes). The macular GCIPL thickness, peripapillary retinal nerve fiber layer (pRNFL) thickness and central macular thickness (CMT) were measured on a SD-OCT scan. Linear regression analyses were performed to determine the correlation between the thickness of each and the visual acuity (VA).
RESULTS
No significant difference in average GCIPL thickness, mean pRNFL thickness and CMT were observed between ME group and non-ME group (P=0.296, 0.183, 0.846). But, minimum GCIPL thickness was reduced in ME eyes compared with non-ME eyes (P=0.022). Final VA significantly correlated with the minimum GCIPL thickness in ME eyes (r=-0.482, P=0.007), whereas no correlation was found with average GCIPL thickness, average pRNFL thickness and mean CMT.
CONCLUSION
Minimum GCIPL thickness is reduced in ME eyes compared with non-ME eyes, and correlated with the VA in non-ischemic CRVO. These results propose that inner retinal damage occurring in patients with ME secondary to non-ischemic CRVO may lead to permanent visual defect after treatment.
Keywords: central retinal vein occlusion, ganglion cell-inner plexiform layer, optical coherence tomography
INTRODUCTION
Central retinal vein occlusion (CRVO) is a retinal vascular disease that has a significant effect on vision. In previous studies, about 2.5 million people worldwide are being influenced by CRVO, and it is estimated that approximately 13.9 million people are affected by branch retinal vein occlusion (BRVO)[1]. Two major complications of retinal vein occlusion (RVO) include macular edema (ME) and retinal non-perfusion. The usual cause of visual impairment in CRVO is mostly ME. This occurs primarily due to breaking of the blood-retinal barrier, which increases accumulation of the fluid in the intra-retinal layer in the macula[2]. The concentration of vascular endothelial growth factor (VEGF) in the vitreous cavity increases in CRVO patients with ME, and it is known that this is correlated with the extent of ME[3]. Several studies have reported that anti-VEGF intra-vitreal injections are an useful treatment for ME due to CRVO[4]–[7].
CRVOs have traditionally been classified as ischemic and non-ischemic according to the degree of capillary non-perfusion in fluorescein angiography. It is important to distinguish between the two types, because we can predict how this patient responds to natural history and its treatment. The ischemic subtype of CRVO shows that the visual acuity (VA) may not improve despite of proper treatment, due to irreversible damages that has already occurred due to severe ischemia in the inner retinal layer[8]. There was no significant relationship between resolution of ME and improvement of VA in ischemic CRVO patients[9]–[11]. But, in most non-ischemic CRVO cases, VA after treatment improves. However, therapeutic effects in all patients with non-ischemic CRVO even after ME resolution are not consistently observed. In the previous study there was a visual improvement at 58%-67% of the RVO eye after ME resolution, but in the case of 9%-42% it got even worse[12]. After non-ischemic CRVO the long-period causes of permanent visual loss are photoreceptor damage and permanent neuronal degeneration secondary to hypoxic damage on the retina. The inner retinal layers, and particularly the retinal ganglion cells (RGCs), are especially sensitive to hypoxic stress.
In the previous studies have interested on the survey of the retinal nerve fiber layer (RNFL), getting thinner following BRVO[13]. RNFL contains RGCs axons, and the ganglion cell layer and inner plexiform layer consist of nuclei and dendrites of the RGCs, respectively. RGCs play the role of transferring the received visual information to the brain via through axonal transport. Using the ganglion cell analysis (GCA) algorithm on Cirrus spectral-domain optical coherence tomography (SD-OCT) (Carl Zeiss Meditec, Dublin, California, USA), it is possible to measure only the thicknesses of ganglion cell-inner plexiform layer (GCIPL), which is the complex of ganglion cell layer and inner plexiform layer[14]. The quantitative measurement of the GCIPL thickness by SD-OCT allows one to dependably and objectively analyzing GCIPL changes in the macula, since more than 50% of the ganglion cell bodies are located in the macula. Even the dependable quantitative assessment of retinal structures on OCT images in the presence of ME is difficult, assessing retinal layers on a dry retina after resolution of ME seems a valuable alternative.
The authors aimed to analyze the quantitative changes of the inner retina in eyes with ME secondary to non-ischemic CRVO, applying the automatic OCT-based GCIPL measurement and to evaluate the relation with the VA, and to investigate whether this value may be used as indicators for prognosis of non-ischemic CRVO.
SUBJECTS AND METHODS
Patient Criteria
We reviewed the charts of 60 patients, retrospectively diagnosed with non-ischemic CRVO at the Chosun University Hospital between September 2012 and February 2016. We included 60 eyes of 60 non-ischemic CRVO patients (30 patients with ME and 30 patients without ME) in this case-control study. The subject group (ME Group) included only the patients who were diagnosed with non-ischemic CRVO with ME that treated with intravitreal anti-VEGF injection, thereby enabling follow-up observation of at least 6mo. The control group (non-ME Group) consisted of age-matched non-ischemic CRVO eyes without ME. Controls with the history of intravitreal injection or intraocular surgery were excluded. The major exclusion criteria were as follows: 1) history of or clinical evidence of neurological diseases; 2) presence of another retinal disease except for non-ischemic CRVO; 3) presence of other diseases that can cause macular thickening such as age-related macular degeneration, or vitreomacular traction, or epiretinal membrane; 4) presence of pathologic myopia of greater than -6.0 diopter; 5) previous treatment for ME with focal/gridlaser; 6) history of pan-retinal photocoagulation; 7) within 6mo of any intraocular surgery or previous pars plana vitrectomy; and 8) severe media opacity or cataracts, which could have an influence on performing OCT. In this study, ischemic CRVO was excluded because ischemic CRVO has no correlation with VA in spite of anti-VEGF treatment. Non-ischemic CRVO was defined as a CRVO with an area of non-perfusion less than 10 disc diameters based on fluorescein angiography that was performed at 3mo.
The study was approved by the Institutional Review Board of Chosun University Hospital, and it was carried out according to the tenets of the Declaration of Helsinki. Every data was assessed by a chart review, including sex, age, best-corrected visual acuity (BCVA) after refraction, refractive power, duration of disease, previous ophthalmologic treatments (pan-retinal or macular grid photocoagulation, intravitreal injections). Fluorescein angiography was reviewed to exclude ischemic CRVO and ischemic maculo pathy (defined by an enlargement of the foveal avascular zone >1000 µm in at least one diameter).
Cirrus Spectral-domain Optical Coherence Tomography Measurement
After pupillary dilation, SD-OCT scans were carried by using the Cirrus HD-OCT in a dark room by a single skilled examiner. All subjects were analyzed by using the macular cube 512×128 scan protocol. The GCA algorithm was applied to the macular cube scans. The GCA algorithm confirms the outer boundary of the RNFL and the outer boundary of the inner plexiform layer (IPL) and provides measurements of GCIPL thickness. The GCA reports the average GCIPL thickness over six sectorial areas (superior, superotemporal, superonasal, inferior, inferonasal, and inferotemporal) that form an elliptical annulus around the fovea, also the total average for the annulus. The GCA also reports the minimum GCIPL thickness. This is the lowest GCIPL thickness on a single meridian crossing the annulus[15]. optic disc cube 200×200 scan provides the peripapillary RNFL thickness in the circular section with the diameter of 3.46 mm on the center of the optic disc, and the average thickness and thickness of each quadrant were used in analysis. As the existence of scan with signal strength less than or equal to 6 (maximum 10), scan not centered, no uniform brightness, RNFL discontinuity or drift, fire crackdown or blinking artifact or algorithm segmentation failure defined quality was excluded.
Statistical Analyses
SPSS 19.0 (SPSS Inc., Chicago, IL, USA) were used for statistical analyzing. Results are expressed as the mean±SD. Data were analyzed using the Mann-Whitney test to compare. The Spearman test and simple linear regression analysis were used to test correlations between variables. It was considered to be statistically significant if the value of P is less than 0.05.
RESULTS
In this study, 30 eyes of 30 non-ischemic CRVO patients with resolved ME after treatment (ME eyes) and 30 eyes of 30 non-ischemic CRVO patients without ME (non-ME eyes) were included. The baseline characteristics of 60 patients with non-ischemic CRVO are summarized in Table 1. There was no statistically significant difference between the ME and no-ME eyes for age, sex, refractive errors and period for treatment. Final BCVA was statistically significantly better in the non-ME group in comparison to that of ME group. The average number of anti-VEGF injections in ME group was 3.70±2.05. There was no significant difference in mean central macular thickness (CMT) between the two groups (P=0.846).
Table 1. Clinical characteristics of ME group and non-ME group.
| Characteristics | ME | Non-ME | aP |
| No. of patients (eyes) | 30 | 30 | |
| Sex (M/F) | 16/14 | 17/13 | |
| Age (y) | 60.23±11.47 | 61.83±10.84 | 0.581 |
| BCVA (logMAR) | 0.51±0.23 | 0.31±0.18 | <0.001 |
| SE (diopters) | -0.05±1.66 | 0.31±1.49 | 0.377 |
| Follow up period (mo) | 15.63±6.30 | 13.2±4.12 | 0.082 |
| Intravitreal anti-VEGF injection (n) | 3.70±2.05 | N/A | |
| CMT (µm) | 252.63±18.41 | 251.57±23.73 | 0.846 |
BCVA: Best-corrected visual acuity; SE: Spherical equivalent; CMT: Central macular thickness. aP: Mann-Whitney test.
Table 2 shows the average GCIPL thickness in ME and non-ME eyes. There was no significant difference between the two groups, but the average GCIPL thickness of the retina decreased with the ME eye (77.63±12.64 µm) versus non-ME eyes (81.39±14.95 µm). Furthermore, a significant difference was observed in the minimum thickness of GCIPL in ME eyes and non-ME eyes (54.07±22.17 µm vs 66.83±19.88 µm; P=0.022). Of the six macular sectors, ME group was measured thinner than non-ME group in all regions, however, these differences were not statistically significant (P>0.05). Evaluation of the outer thickness of the retina (full retinal thickness-GCIPL thickness) did not show a significant difference between the ME and non ME eyes.
Table 2. Comparison of macular GCIPL parameter between ME group and non-ME group measured by Cirrus OCT.
| Thickness (µm) | ME (n=30) | Non-ME (n=30) | aP |
| Average GCIPL | 77.63±12.64 | 81.39±14.95 | 0.296 |
| Minimum GCIPL | 54.07±22.17 | 66.83±19.88 | 0.022 |
| Superotemporal | 76.07±17.23 | 81.03±15.95 | 0.251 |
| Superior | 78.70±16.66 | 82.73±24.10 | 0.454 |
| Superonasal | 79.03±14.28 | 82.43±17.05 | 0.406 |
| Inferonasal | 79.67±11.80 | 81.67±18.67 | 0.622 |
| Inferior | 78.90±19.01 | 81.67±18.67 | 0.926 |
| Inferotemporal | 75.80±19.94 | 83.03±15.32 | 0.121 |
GCIPL: Ganglion cell-inner plexiform layer. aP: Mann-Whitney test.
Table 3 shows the average peripapillary RNFL thickness in ME and non-ME eyes. Although no significant difference was observed between both groups, the average peripapillary RNFL thickness of the retina was reduced in ME eyes versus non-ME eyes (97.38±7.56 µm vs 100.16±8.41 µm; P=0.183). Of the four quadrant sectors, ME group was measured thinner than non-ME group in all regions, however, these differences were not statistically significant (P>0.05).
Table 3. Comparison of peripapillary RNFL parameter between ME group and non-ME group using Cirrus OCT.
| Thickness (µm) | ME (n=30) | Non-ME (n=30) | aP |
| Average RNFL | 97.38±7.56 | 100.16±8.41 | 0.183 |
| Superior quadrant | 122.60±10.91 | 124.17±10.37 | 0.571 |
| Inferior quadrant | 122.93±14.94 | 129.20±16.25 | 0.125 |
| Temporal quadrant | 78.53±13.10 | 78.83±13.24 | 0.930 |
| Nasal quadrant | 65.43±10.73 | 68.20±11.35 | 0.336 |
RNFL: Retinal nerve fiber layer. aP: Mann-Whitney test.
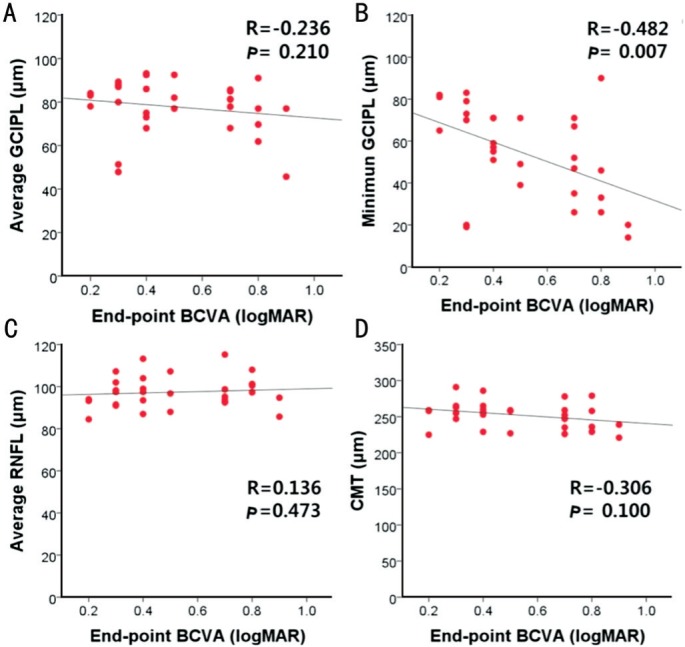
The Figure 1 illustrates the simple linear regression between the average GCIPL thickness and the BCVA (Figure 1A), the minimum GCIPL thickness and the BCVA (Figure 1B), the average RNFL thickness and the BCVA (Figure 1C), and the mean CMT and BCVA (Figure 1D) in ME eyes. The final BCVA significantly correlated with the minimum GCIPL thickness in ME eyes (r=-0.482, P=0.007), whereas no correlation was found with average GCIPL thickness (r= -0.236, P=0.210), average RNFL thickness (r=0.136, P=0.473) and mean CMT (r=-0.306, P=0.100).
Figure 1. The simple linear regression between the average GCIPL thickness (A), the minimum GCIPL thickness (B), the average RNFL thickness (C), and the mean CMT (D) and BCVA in ME eyes.
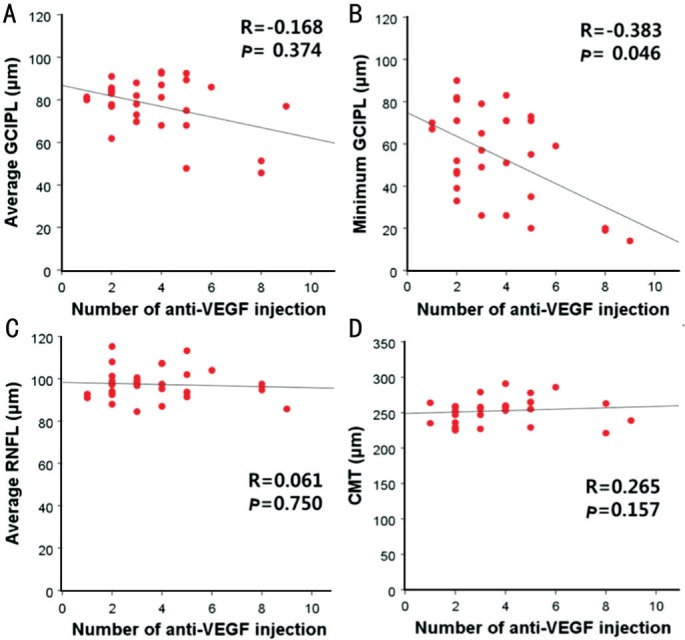
The Figure 2 illustrates the simple linear regression between the number of anti-VEGF intravitreal injection and each parameter (average GCIPL thickness, minimum GCIPL thickness, average RNFL thickness, mean CMT) in ME eyes. The number of intravitreal injection significantly correlated with the minimum GCIPL thickness in ME eyes (r=-0.383, P=0.046), whereas no correlation was found with average GCIPL thickness (r=-0.168, P=0.374), average peripapillary RNFL (pRNFL) thickness (r=0.061, P=0.750) and mean CMT (r=0.265, P=0.157).
Figure 2. The number of anti-VEGF intravitreal injection and average GCIPL thickness (A), minimum GCIPL thickness (B), average RNFL thickness (C) and mean CMT (D) in ME eyes.
DISCUSSION
The most frequent cause of visual loss after CRVO is ME, which benefits from treatment such as intravitreal injection of steroid or anti-VEGF agents[16]. However, therapeutic effects are not consistently reached in all patients with CRVO even after resolved ME. After CRVO, the long-term causes of permanent visual loss are damage of photoreceptors and permanent neuronal degeneration secondary to hypoxic of retina. Intraretinal fluid accumulation by itself makes Müller cell ballooning and retinal degeneration. If edema continues, irreversible neuronal degeneration may be induced due to necrosis of Müller cells[17]. This is also known that the status of photoreceptors and external limiting membrane are useful for predicting the final prognosis of visual outcome after intravitreal anti-VEGF treatment in patients with CRVO. Although an association between outer retinal damages induced by ME and the poor visual prognosis has known in the past, to our knowledge, in CRVO eyes with ME, the relation between inner retinal layers and the VA has not been investigated[18]. In this study, we compared the thicknesses of peripapillary RNFL and macular GCIPL between the ME group and non-ME group in non-ischemic CRVO patients. In the visual pathway, we trust not only the integrity of the photoreceptors and external limiting membrane, but also, and more importantly, the status of GCIPL and RNFL, should be carefully studied in determining treatment strategies in patients with CRVO.
RGCs are located in the inner retinal layer, and their axons from each of the cells pass throughtheoptic nerve and connect to the lateral geniculate body and cerebral cortex. Since more than 50% of the ganglion cell bodies are located in the macula, it is possible to measure macular GCIPL thickness which is the complex of ganglion cell layer and inner plexiform layer by SD-OCT. Describing quantitative changes of the thicknesses of pRNFL and macular GCIPL measured in eyes with resolved ME is the purpose of this study. The authors analyzed in eyes with ME secondary to non-ischemic CRVO, applying the automatic OCT-based thickness profiles measurement and to be evaluated the its relation with the VA. Our study found an interesting relation between the GCIPL thickness and the VA, although this finding was not significant but do not imply a causative effect. In fact, in this study damage to the outer retinal layer was not assessed, but it can contribute to the reduction of VA after fruiting of ME. As mentioned in the Results section, the thickeness of outer retinal layer were not significantly different between both groups. We aimed to evaluate inner retinal damages for cause of permanent visual loss. The thicknesses of the macular GCIPL and peripapillary RNFL were thinner in ME eyes, although the difference was not statistically significant. As the result of the correlation analysis between these thicknesses and the final VA, the final VA significantly correlated with the minimum GCIPL thickness in ME eyes. Previously, the GCIPL algorithm has been applyed and approved to analyze the loss of local ganglion cell in early stage of glaucoma. Interestingly, in this study it is shown that the minimum GCIPL thickness can distinguish healthy eyes from mean glaucomatous eyes than average GCIPL thickness[19]. Our results are similar to these results. In this study, a high correlation coefficient (r=-0.482) between minimum GCIPL thickness and BCVA could be used as an important indicator of visual function. Since the GCIPL thickness is averaged along each radius coming out of the center, the minimum GCIPL thickness reflects extreme values of very thin sectors, especially sensitive to cell loss. This value could serve as a compass indicating the location of local RGC loss in eyes with ME. This high correlation supports the hypothesis that the minimum GCIPL thickness can more accurately detect the loss of RGC with the eyes with ME.
Reasons for GCIPL thinning can be several things, including vascular changes and primary neuronal degeneration. Permanent damages to the retinal neuronal cells due to ischemia and damages to RGCs due to repetitive anti-VEGF injection could be considered as the cause of the damages to the inner retinal layers in CRVO. Firstly, ischemic changes induce degeneration of RGCs. Increased levels of VEGF, nitric oxide, free oxygen radicals, glutamate, and inflammatory cytokines result in hypoxia-ischemic damage to the retina. Damage to RGC occurring in the eyes of CRVO may be due to cell death of neurons secondary to hypoxic damage over time. Or RGC can disappear to progressive hypoxia-reperfusion venous closure. Two-thirds of the inner retina receives its blood supply from the retinal blood vessels and one-third of the outer retina from the retina and choroid. Since the inner retinal layer is oxygenated by the retinal vasculature, there may be a risk of particularly low oxygen damage. The retinal vascular system is relatively rare compared to the choroidal circulation that supplies most external retina. Diabetic retinopathy, RVO, ischemia-related retinal diseases such as retinal artery occlusion and severe hypertension retinopathy is responsible for thinning of the retinal layer[20]. Ebneter et al[21] reported that the layers of the outer retina in the ischemic retinal thickeness area is well preserved, on the other hand that of the inner retina layer is reduced, in animal models of RVO. Secondly, potential toxic effects of treatment of anti-VEGF on ganglion cells could occur. In this study, most eyes of the subject group were treated with drugs containing anti-VEGF. VEGF is a famous angiogenic and neurotrophic factor. In theory, long-term suppression of neurotrophic cytokines in chronic anti-VEGF treated eyes can bring harmful downstream effects to RNFL, can cause RNFL thin baggage[22]–[25]. In theoretical perspective, anti-VEGF may inhibit the autocrine VEGF-induced survival of RGCs. Tatlipinar et al[26] reported that repetition of intravitreal anti-VEGF injections induced the reduction in the diameter of retinal blood vessels. Martinez-de-la-Casa et al[27] reported that repeated injection of intravitreal anti-VEGF exacerbated RNFL due to direct drug toxicity and changes in intraocular pressure (IOP) from patients with wet age related macular degeneration. On the contrary, several studies on the long-term stability of anti-VEGF injection were reported. Shin et al[28] reported that repeated intravitreal anti-VEGF injections did not bring about significant change in RNFL thickness with various retinal disease. Many experimental studies that repeated anti-VEGF injections had no toxic effect on the retina[29]. In this study, the number of intravitreal injection significantly correlated with the minimum GCIPL thickness in ME eyes, whereas no correlation was found with average GCIPL thickness, the average RNFL thickness and the mean CMT.
We acknowledge that there are some limitations in our research. First of all, this was a retrospective study to register patients visiting a single hospital, there may be a selection bias that this is inherent in retrospective studies. Second, due to the absence of a long-term follow-up observation, changes in GCIPL and RAFL thickeness could not be analyzed over time. However, this has no purpose of this case-control study. Third, we could not directly compare the thickness of RNFL and GCIPL at same retinal areas, because currently the software of the Cirrus device does not have this capability. Thickness of the RNFL was measured in the frame of the optic nerve, while the thickness of the macula and GCIPL was measured in the macula. Fourth, this study showed a small sample size of 60 eyes, so diversity of non-ischemic CRVO eyes was not satisfiable in the study. Analysis of our inadequate data should be revealed by larger scale studies. The automated segmentation of macular structures on OCT images may be questionable in the presence of ME, and the originality of this study relied on the investigation of inner retina changes on a dried retina after resolution of ME. This method prevents fragmentation errors and can reliably and automatically objectively evaluate GCIPL changes in the macula.
In conclusion, despite favorable anatomic response and restoration of CMT after resolution of ME, the minimum GCIPL thickness was lower than the control group and correlated with the VA in non-ischemic CRVO eyes. These results suggest that inner retinal changes could be associated with ME and there is sustained visual impairment after treatment. Analyzing the GCIPL thickness by SD-OCT and analyzing it as a prognostic factor for visual recovery could be an interesting next step to investigate.
Acknowledgments
Foundation: Supported by Research Fund from Chosun University, 2016
Conflicts of Interest: Kim HJ, None; Yoon HG, None; Kim ST, None.
REFERENCES
- 1.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. [PMC free article] [PubMed] [Google Scholar]
- 2.Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T, Jian K, Sakamoto I, Nakano K, Yamashita H, Minamoto A, Mishima HK. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005;140(2):256–261. doi: 10.1016/j.ajo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Noma H, Funatsu H, Mimura T, Harino S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116(1):87–93. doi: 10.1016/j.ophtha.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, Rundle AC, Rubio RG, Murahashi WY, CRUISE Investigators Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124–1133.e1. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Liu ZL, Sun P, Gu F. Intravitreal bevacizumab for treatment of macular edema secondary to central retinal vein occlusion: eighteen-month results of a prospective trial. J Ocul Pharmacol Ther. 2011;27(6):615–621. doi: 10.1089/jop.2011.0050. [DOI] [PubMed] [Google Scholar]
- 6.Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ, Kazmi H, Ma Y, Stemper B, Zeitz O, Sandbrink R, Haller JA. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014;121(7):1414–1420.e1. doi: 10.1016/j.ophtha.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U, Lorenz K, Honda M, Vitti R, Berliner AJ, Hiemeyer F, Stemper B, Zeitz O, Sandbrink R, GALILEO Study Group Intravitreal Aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the Phase 3 GALILEO study. Ophthalmology. 2014;121(1):202–208. doi: 10.1016/j.ophtha.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Bashshur ZF, Ma'luf RN, Allam S, Jurdi FA, Haddad RS, Noureddin BN. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Arch Ophthalmol. 2004;122(8):1137–1140. doi: 10.1001/archopht.122.8.1137. [DOI] [PubMed] [Google Scholar]
- 9.Gregori NZ, Rosenfeld PJ, Puliafito CA, Flynn HW, Jr, Lee JE, Mavrofrides EC, Smiddy WE, Murray TG, Berrocal AM, Scott IU, Gregori G. One-year safety and efficacy of intravitreal triamcinolone acetonide for the management of macular edema secondary to central retinal vein occlusion. Retina. 2006;26(8):889–895. doi: 10.1097/01.iae.0000237111.82357.30. [DOI] [PubMed] [Google Scholar]
- 10.Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Arch Ophthalmol. 2002;120(9):1217–1219. [PubMed] [Google Scholar]
- 11.Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, Sorenson J, Slakter JS, Freund KB, Cooney M, Fine HF. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26(3):279–284. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, Nguyen HP, Wang JJ, Wong TY. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094–1101.e5. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 13.Kim CS, Shin KS, Lee HJ, Jo YJ, Kim JY. Sectoral retinal nerve fiber layer thinning in branch retinal vein occlusion. Retina. 2014;34(3):525–530. doi: 10.1097/IAE.0b013e3182a2e746. [DOI] [PubMed] [Google Scholar]
- 14.Pierro L, Gagliardi M, Iuliano L, Ambrosi A, Bandello F. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012;53(9):5912–5920. doi: 10.1167/iovs.11-8644. [DOI] [PubMed] [Google Scholar]
- 15.Mwanza JC, Durbin MK, Budenz DL, Girkin CA, Leung CK, Liebmann JM, Peace JH, Werner JS, Wollstein G, Cirrus OCT Normative Database Study Group Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(11):7872–7879. doi: 10.1167/iovs.11-7896. [DOI] [PubMed] [Google Scholar]
- 16.Beutel J, Ziemssen F, Luke M, Partsch M, Bartz-Schmidt KU, Bevacizumab Study Group. Gelisken F. Intravitreal bevacizumab treatment of macular edema in central retinal vein occlusion: one-year results. Int Ophthalmol. 2010;30(1):15–22. doi: 10.1007/s10792-008-9282-7. [DOI] [PubMed] [Google Scholar]
- 17.Yanoff M, Fine BS, Brucker AJ, Eagle RC., Jr Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28 Suppl:505–511. doi: 10.1016/0039-6257(84)90233-9. [DOI] [PubMed] [Google Scholar]
- 18.Kang HM, Chung EJ, Kim YM, Koh HJ. Spectral-domain optical coherence tomography (SD-OCT) patterns and response to intravitreal bevacizumab therapy in macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):501–508. doi: 10.1007/s00417-012-2067-8. [DOI] [PubMed] [Google Scholar]
- 19.Takayama K, Hangai M, Durbin M, Nakano N, Morooka S, Akagi T, Ikeda HO, Yoshimura N. A novel method to detect local ganglion cell loss in early glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(11):6904–6913. doi: 10.1167/iovs.12-10210. [DOI] [PubMed] [Google Scholar]
- 20.Ota M, Tsujikawa A, Ojima Y, Miyamoto K, Murakami T, Ogino K, Akagi-Kurashige Y, Muraoka Y, Yoshimura N. Retinal sensitivity after resolution of the macular edema associated with retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2012;250(5):635–644. doi: 10.1007/s00417-011-1860-0. [DOI] [PubMed] [Google Scholar]
- 21.Ebneter A, Agca C, Dysli C, Zinkernagel MS. Investigation of retinal morphology alterations using spectral domain optical coherence tomography in a mouse model of retinal branch and central retinal vein occlusion. PLoS One. 2015;10(3):e0119046. doi: 10.1371/journal.pone.0119046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilton RG, Chang KC, LeJeune WS, Stephan CC, Brock TA, Williamson JR. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Invest Ophthalmol Vis Sci. 1999;40(3):689–696. [PubMed] [Google Scholar]
- 23.Carmeliet P, Ruiz de Almodovar C. VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci. 2013;70(10):1763–1778. doi: 10.1007/s00018-013-1283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foxton RH, Finkelstein A, Vijay S, Dahlmann-Noor A, Khaw PT, Morgan JE, Shima DT, Ng YS. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013;182(4):1379–1390. doi: 10.1016/j.ajpath.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel N, Kumar V, Ghosh B. Ischemic maculopathy following intravitreal bevacizumab for refractory diabetic macular edema. Int Ophthalmol. 2011;31(1):39–42. doi: 10.1007/s10792-010-9390-z. [DOI] [PubMed] [Google Scholar]
- 26.Tatlipinar S, Dinc UA, Yenerel NM, Gorgun E. Short-term effects of a single intravitreal bevacizumab injection on retinal vessel calibre. Clin Exp Optom. 2012;95(1):94–98. doi: 10.1111/j.1444-0938.2011.00662.x. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F, Reche-Frutos J, Calvo-Gonzalez C, Donate-Lopez J, Garcia-Feijoo J. Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012;53(10):6214–6218. doi: 10.1167/iovs.12-9875. [DOI] [PubMed] [Google Scholar]
- 28.Shin HJ, Shin KC, Chung H, Kim HC. Change of retinal nerve fiber layer thickness in various retinal diseases treated with multiple intravitreal antivascular endothelial growth factor. Invest Ophthalmol Vis Sci. 2014;55(4):2403–2411. doi: 10.1167/iovs.13-13769. [DOI] [PubMed] [Google Scholar]
- 29.Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26(3):270–274. doi: 10.1097/00006982-200603000-00003. [DOI] [PubMed] [Google Scholar]


