Abstract
AIM
To conduct a systematic review and quantitative Meta-analysis of the efficacy and safety of combined surgery for the eyes with coexisting cataract and open angle glaucoma.
METHODS
We performed a systematic search of the related literature in the Cochrane Library, PubMed, EMBASE, Web of Science databases, CNKI, CBM and Wan Fang databases, with no limitations on language or publication date. The primary efficacy estimate was identified by weighted mean difference of the percentage of intraocular pressure reduction (IOPR%) from baseline to end-point, the percentage of number of glaucoma medications reduction from pre- to post-operation, and the secondary efficacy evaluations were performed by odds ratio (OR) and 95% confidence interval (CI) for complete and qualified success rate. Besides, ORs were applied to assess the tolerability of adverse incidents. Meta-analyses of fixed or random effect models were performed using RevMan software 5.2 to gather the consequences. Heterogeneity was evaluated by Chi2 test and the I2 measure.
RESULTS
Ten studies enrolling 3108 patients were included. The combined consequences indicated that both glaucoma and combined cataract and glaucoma surgery significantly decreased IOP. For deep sclerectomy vs deep sclerectomy plus phacoemulsification and canaloplasty vs phaco-canaloplasty, the differences in IOPR% were not all statistically significant while trabeculotomy was detected to gain a quantitatively greater IOPR% compared with trabeculotomy plus phacoemulsification. Furthermore, there was no statistical significance in the complete and qualified success rate, and the rates of adverse incidents for trabeculotomy vs trabeculotomy plus phacoemulsification.
CONCLUSION
Compared with trabeculotomy plus phacoemulsification, trabeculectomy alone is more effective in lowering IOP and the number of glaucoma medications, while the two surgeries can not demonstrate statistical differences in the complete success rate, qualified success rate, or incidence of adverse incidents.
Keywords: open angle glaucoma, cataract, glaucoma surgery, phacoemulsification, combined surgery, Meta-analysis
INTRODUCTION
Glaucoma is the most important cause of irreversible blindness worldwide. At least 70 million people are suffering from glaucoma of which 10% are bilaterally blind[1]. Elevated intraocular pressure (IOP) is the most important risk factor in the development of the disease, although up to 30% of the patients never exceed an IOP within the normal range. Lowering the IOP is most important for glaucoma nowadays. Cataract is an age-related disease. According to the WHO, cataract is the main cause of reversible blindness worldwidely.
Within the aging population, it is increasingly frequent for cataract and glaucoma to coexist in the same patient. The treatment of coexistent cataract and glaucoma is a prevalently clinical challenge. The treatment of either condition can influence the course of the other.
In recent years, changes in surgical technique have greatly impacted the surgical method to patients with coexisting cataract and glaucoma. Especially, there has been an extensive tendency toward the application of combined phacoemulsification with intraocular lens (IOL) implantation and trabeculectomy (phacotrabeculectomy) as one choice of the surgical management for this situation[2].
This review article will evaluate the different aspects that affect the choice and result of surgical treatment in patients coexisting open angle glaucoma and cataract. The influence of trabeculectomy on the IOP will be discussed, and the most recent surgical pressure lowering techniques in combination with phacoemulsification will be reviewed.
MATERIALS AND METHODS
Search Strategy
Our Meta-analysis and systematic review were performed in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines[3]. To evaluate relevant studies, we searched the Cochrane Library, PubMed, EMBASE, Web of Science databases, CNKI, CBM and Wan Fang databases for observational studies of the comparision of effect on IOP between combined surgery and anti-glaucoma surgery in the patients with coexisting cataract and open angle glaucoma, with no limitations on language or publication date. The search period was through December 2016. Keywords included open angle glaucoma, glaucoma, combined glaucoma/cataract surgery, canaloplasty, non-penetrating glaucoma surgery, penetrating glaucoma surgery, punch trabeculectomy, deep sclerectomy, viscocanalostomy, glaucoma surgery, filtering surgery, trabeculectomy, bleb, filtering surgery, nonfiltering surgery, eye pressure, IOP, intraocular tension, ocular pressure, ocular tension, intraocular tension, eyeball pressure, eye internal pressure, intraocular hypertension, phacoemulsification, cataract extraction, IOP reduction mode. In addition, we also hand searched the reference of identified case reports and trial reports to seek out relevant articles. There were no language limitations in the search for articles.
Literature Selection
Two reviewers (Jiang N and Lin J) independently browsed the titles and abstracts to eliminate the articles which were not consistent with the inclusion criteria. Full text reports of the trials that may accord with the inclusion criteria were browsed for further evaluation. They reviewed the results, and decided whether the trial should be included or excluded by the third reviewer or discussion. We also got in touch with the authors to perfect the data.
Inclusion and Exclusion Criteria
The inclusion criteria involved:
Type of studies: All randomized and clinic controlled trials were appropriate for inclusion.
Type of participants: Participants with a diagnosis of open angle glaucoma, pseudoexfoliation glaucoma, or ocular hypertension (OH) which had the clinical features of open angle glaucoma and underwent glaucoma surgery or combined glaucoma/cataract surgery were involved in the trials. The trials with patients who were cyclopia, got uveitis, ocular operation and combined other ocular and systemic disease were excluded. There were no limitations concerning age, gender, ethnicity, the number of participants, use of adjunctive medications or co-morbidities.
Type of interventions: All pre- and post-operative trials included glaucoma/cataract surgery (Table 1).
Table 1. Characteristics of included studies.
| First author (year) | Country | Design | No. of paients | No. of eyes | Mean age±SD (y) |
M/F (n) |
Intervention regimen | Follow up (mo) | ||
| Glaucoma surgery | Combined surgery | Glaucoma surgery | Combined surgery | |||||||
| Bilgin (2014) | Turkey | CCT | 49 | 52 | 69.6±7.1 | 66.6±9.5 | 15/11 | 12/11 | NPDS vs phaco-NPDS | 30.9/28.7 |
| Bull (2011) | Germany | RCT | 101 | 101 | 67.3±9.9 | 67.3±9.9 | - | - | Canaloplasty vs phacocanaloplasty | 36/36 |
| Cillino (2004) | Italy | RCT | 65 | 65 | 68.6±1.7 | 71.7±2.0 | 9/8 | 8/7 | NPDS vs phaco-NPDS | 24/24 |
| 71.3±1.2 | 74.6±1.1 | 8/10 | 9/6 | PT vs phaco-PT | 24/24 | |||||
| D'Eliseo (2003) | Italy | RCT | 42 | 42 | 71.5 | 79 | 15/6 | 8/13 | DS vs Phaco-DS | 12/12 |
| Ting (2012) | USA | Pro | 713 | 713 | 68±15 | 74±9 | 181/256 | 104/155 | Trabeculectomy vs cataract extraction and trabeculectomy | 12/12 |
| Wishart (2003) | England | Pro | 151 | 192 | 66.9±10.1 | 78.9±12.3 | 13/7 | 23/33 | VC vs phaco-VC | 36.4/32.2 |
| 67±11.7 | 77±8.9 | 21/22 | 17/18 | DS vs phaco-DS | 36.3/35.1 | |||||
| Wishart (2002) | UK | Pro | 73 | 101 | 75.2 | 75.2 | - | - | VC vs phaco-VC | 36/36 |
| Parikh (2016) | USA | Retro | 753 | 753 | 69±11 | 72±9 | - | - | Trabeculectomy vs phaco-trabeculectomy | 12/12 |
| Tetz (2015) | Germany | Retro | 112 | 112 | 63.5±9.9 | 74.8±9.0 | 44/38 | 12/18 | Canaloplasty vs phacocanaloplasty | 36/36 |
| Sałaga-Pylak (2013) | Poland | Retro | 122 | 122 | 70.8±6.3 | 70.7±7.0 | 32/40 | 12/38 | TrabMMC vs phaco-trabMMC | 18/18 |
| Chihara (2011) | Japan | Retro | 789 | 789 | 60.2±17.6 | 68.7±13.9 | - | - | TrabMMC vs phaco-trabMMC | 6/6 |
| 60.2±17.6 | 71.4±9.6 | VC vs phaco-VC | ||||||||
| Rotchford (2007) | UK | Retro | 63 | 63 | 72.8±7.6 | 79.2±7.5 | 17/13 | 16/16 | MT vs phaco-MT | 43.5/41.8 |
| Marek (2006) | Poland | Retro | 35 | 67 | - | - | - | - | DS vs phaco-DS | 12/12 |
| Uretmen (2003) | Turkey | Retro | 40 | 40 | 71.8±7.7 | 71.1±6.4 | 10/10 | 12/8 | VC vs phaco-VC | 12/12 |
CCT: Clinical controlled trial; RCT: Prospective randomized controlled trial; Pro: Prospective non-randomized; Retro: Retrospective; NPDS: Non-penetrating deep sclerectomy; Phaco: Phacoemulsification; DS: Deep sclerectomy; VC: Viscocanalostomy; MT: Microtrabeculectomy; PMT: Phaco-microtrabeculectomy; TrabMMC: Trabeculectomy with mitomycin C; PT: Punch trabeculectomy.
Type of outcome measures: At least one of the following: pre- and post-operative IOP, percentage of intraocular pressure reduction (IOPR%), visual acuity, complete success rate, qualified success rate and adverse events.
Data Extraction and Analysis
Two reviewers (Jiang N and Lin J) separately extracted the data that conformed to the inclusion criteria. The full papers of selected trials were read to estimate whether they contain useful information. The investigators resolved any disagreement by discussion to reach a consensus. The following data were collected from each trial: 1) publication data: the first author's last name, year of publication, country of origin; 2) features of the participants: gender, age, the setting, sample size; 3) interventions: all surgical methods of lens extraction; 4) follow-up time; 5) outcome calculation: the number of IOP reduction, C-values, visual acuity, complete success rate, qualified success rate and complications.
Outcome Measures
The primary result for efficacy was IOPR% and the percentage of the number of glaucoma medications reduction from pre- to post-operation. We used mean and standard deviation (SD) of IOP and IOPR reported directly. When these were not found, we would measure these in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions: IOPR=IOPbaseline - IOPend point, SDIOPR=(SDbaseline2 + SDend point2 -SDbaseline × SDend point)1/2. IOPR% and SD of IOPR% (SDIOPR%) were assessed by IOPR%=IOPR/IOPbaseline and SDIOPR%=SDIOPR/IOPbaseline, apartly. For the percentage of the number of glaucoma medications reduction from pre- to post-operation, we used the same data processing. We used the proportions of qualified and complete success to estimate the efficacy. The target end point IOP without medications was regarded as complete success, and the target end point IOP with or without medications was regarded as qualified success. Besideds, we estimated the result of the incidence of adverse incidents, including flat anterior chamber, bleb leakage, hypotony and choroidal effusion.
Statistical Analysis
Statistical analyses were executed using RevMan 5.2 software. The pooled odds ratio (OR) was measured for dichotomous outcomes, and weighted mean difference (WMD) was for continuous outcomes. We estimated the heterogeneity among trials by examination of graphical presentations and using the Chi2 and I2 measure[4]. We defined P<0.05 for Chi-square or the I2 measure >50% as significant heterogeneity. We applied a fixed effects model to collect results when there was no significant heterogeneity; otherwise, a random effects model was applied (inverse of variance method and DerSimonian and Laird method). P<0.05 demonstrated statistical significance on the test for whole effect. We conducted subgroup analysis to estimate the effect of methodological features in study designs, which were distinguished as prospective (Pro), retrospective (Retro), randomized and nonrandomized.
RESULTS
Study Selection
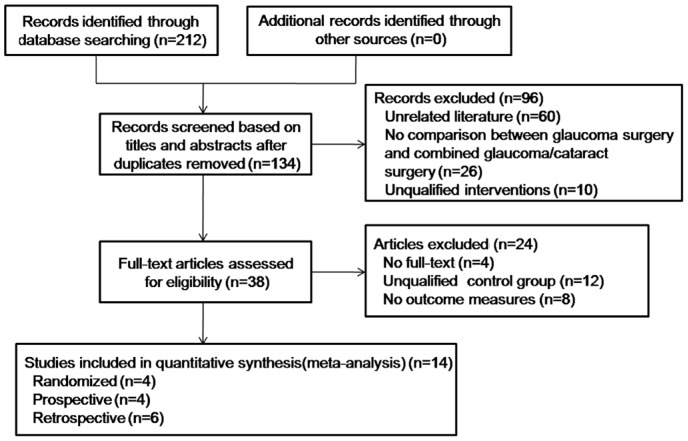
In total, 212 papers were determined by the literature search. Of 212, 78 papers were repetitions; thus, these were eliminated. We read the titles and abstracts of the remaining 134 papers and eliminated 96 papers for the reasons outlined in Figure 1. Then, 24 papers were eliminated owing to unqualified control groups and short of required outcomes. At last, 14 appropriate controlled clinical trials that accorded with our inclusion criteria were involved in this systematic review[5]–[18].
Figure 1. Flow chart of literature search and study selection.
Quality and Features of the Included Studies
Of the 14 included studies, 5 reported the application of deep sclerectomy vs combined deep sclerectomy with phacoemulsification, 5 reported the use of canaloplasty vs phaco-canaloplasty, nevertheless the other 4 reported the trabeculotomy vs combined trabeculotomy with phacoemulsification. The characteristics of the included studies were pooled in Table 1. Four studies were randomized controlled trials (RCTs)[5]–[8], which were taken on in Turkey, Germany, Italy, while the other 3 were Pro nonrandomized[9]–[10],[15] and 7 were Retro[11]–[14],[16]–[18]. A sum of 3212 eyes of 3108 patients were included. The follow-up period was from 6 to 43.5mo. The average ages of patients were from 60.2 to 79.2y. As for quality estimation, Downs and Blacks scores of all the included studies were >16 (50%). Quality assessment was generalized in Table 2.
Table 2. Quality scoring components for 14 clinical trials included.
| First author (year) | Quality score component |
Score |
|||||
| I | II | III | IV | V | Over all | Percentage (%) | |
| Bilgin (2014) | 10 | 3 | 5 | 6 | 1 | 25 | 78.13 |
| Bull (2011) | 11 | 2 | 5 | 4 | 1 | 23 | 71.88 |
| Cillino (2004) | 11 | 1 | 7 | 5 | 1 | 25 | 78.13 |
| D'Eliseo (2003) | 9 | 1 | 5 | 4 | 1 | 20 | 62.5 |
| Ting (2012) | 9 | 1 | 5 | 3 | 1 | 19 | 59.36 |
| Wishart (2003) | 9 | 1 | 5 | 4 | 4 | 23 | 71.88 |
| Wishart (2002) | 9 | 1 | 5 | 3 | 4 | 22 | 68.75 |
| Parikh (2016) | 9 | 1 | 5 | 4 | 4 | 23 | 71.88 |
| Tetz (2015) | 9 | 1 | 5 | 4 | 3 | 22 | 68.75 |
| Sałaga-Pylak (2013) | 9 | 1 | 5 | 4 | 2 | 21 | 65.63 |
| Chihara (2011) | 10 | 1 | 5 | 3 | 5 | 24 | 75 |
| Rotchford (2007) | 10 | 1 | 5 | 3 | 1 | 20 | 62.5 |
| Marek (2006) | 9 | 1 | 5 | 3 | 1 | 19 | 59.38 |
| Uretmen (2003) | 9 | 1 | 4 | 3 | 1 | 18 | 56.25 |
Results of Meta-analysis
IOPR% (deep sclerectomy vs combined deep sclerectomy with phacoemulsification)
Five studies including 254 eyes contrasted deep sclerectomy with deep sclerectomy plus phacoemulsification regarding IOPR%. Moderate heterogeneity was examined between these studies (P=0.12, I2=46%). The combined results indicated the two agents greatly decreased IOP. The differences in IOPR% were not all statistically great (WMD=2.85, 95%CI: -0.69, 6.39; Table 3). We then separated the studies into 3 subgroups in accordance with study design (Pro and Retro nonrandomized, randomized). A statistically significant result was obtained in Retro but not in the Pro randomized and nonrandomized trial (Table 4).
Table 3. Percentage IOP decline from baseline comparing glaucoma surgery with combined glaucoma/cataract surgery.
| Trails | Glaucoma surgery |
Combined glaucoma/cataract surgery |
WMD (fixed) (95%CI) | ||
| No. of eyes | IOPR% [Mean (SD)] | No. of eyes | IOPR% [Mean (SD)] | ||
| Deep sclerectomy vs combined deep sclerectomy with phacoemulsificationa | |||||
| Bilgin (2014) | 26 | 37.87 (18.85) | 23 | 38.7 (29.76) | 0.83 (-13.33, 14.99) |
| Cillino (2004) | 17 | 41.06 (8.36) | 15 | 41.04 (7.20) | -0.02 (-5.41, 5.37) |
| D'Eliseo (2003) | 21 | 35.34 (12.4) | 21 | 42.44 (12.58) | 7.10 (-0.45, 14.65) |
| Wishart (2003) | 52 | 40.99 (19.32) | 35 | 38.02 (23.26) | -2.97 (-12.29, 6.35) |
| Marek (2006) | 21 | 29.59 (17.73) | 23 | 41.39 (13.63) | 11.80 (2.39, 21.21) |
| Total | 137 | 117 | 2.85 (-0.69, 6.39) | ||
| Canaloplasty vs phaco-canaloplastyb | |||||
| Tetz (2015) | 82 | 33.76 (16.93) | 30 | 42.13 (19.63) | -8.37 (-16.29, -0.45) |
| Bull (2011) | 82 | 32.61 (17.22) | 16 | 42.39 (21.58) | -9.78 (-20.99, 1.43) |
| Wishart (2003) | 27 | 35.63 (28.61) | 78 | 30.83 (19.56) | -4.80 (-16.43, 6.83) |
| Wishart (2002) | 26 | 35.38 (22.52) | 75 | 33.89 (17.57) | 1.49 (-8.04, 11.02) |
| Uretmen (2003) | 20 | 34.45 (20.49) | 20 | 38.4 (24.95) | -3.95 (-18.10, 10.20) |
| Total | 237 | 219 | -3.78 (-8.38, 0.81) | ||
| Trabeculotomy vs combined trabeculotomy with phacoemulsificationc | |||||
| Parikh (2016) | 255 | 26.73 (27.94) | 498 | 21.32 (25.74) | 5.41 (1.30, 9.52) |
| Sałaga-Pylak (2013) | 72 | -4.55 (31.37) | 50 | -13.79 (29.65) | 9.24 (-1.72, 20.20) |
| Ting (2012) | 450 | 34.12 (26.83) | 263 | 21.61 (23.63) | 12.51 (8.73, 16.29) |
| Chihara (2011) | 145 | 52.04 (24.19) | 116 | 24.07 (16.22) | 27.97 (23.05, 32.89) |
| Rotchford (2007) | 37 | 45.12 (28.88) | 37 | 42.98 (18.97) | 2.14 (-8.99, 13.27) |
| Cillino (2004) | 18 | 47.04 (7.29) | 15 | 36.30 (6.94) | 10.74 (5.87, 15.61) |
| Total | 977 | 979 | 12.65 (10.56, 14.74) | ||
aTest for heterogeneity Chi2=7.35, df=4 (P=0.12); I2=46%, Test for overall effect: Z=1.58, P=0.12; bTest for heterogeneity Chi2=5.66, df=4 (P=0.23); I2=29%, Test for overall effect: Z=1.61, P=0.11; cTest for heterogeneity Chi2=53.56, df=5 (P<0.00001); I2=91%, Test for overall effect: Z=11.87, P<0.00001.
Table 4. Subgroup analysis estimating the effect of trial design on percentage IOP decline.
| Subgroup | No. of studies | WMD (fixed) (95%CI) | Heterogeneity |
Overall effect |
||||||
| Chi2 | P | I2 (%) | Z | P | ||||||
| Deep sclerectomy vs combined deep sclerectomy with phacoemulsification | ||||||||||
| All trials | 5 | 2.85 (-0.69, 6.39) | 7.35 | 0.12 | 46 | 1.58 | 0.12 | |||
| RCTs | 3 | -2.25 (-6.44, 1.95) | 2.30 | 0.32 | 13 | 1.05 | 0.29 | |||
| Pro | 1 | 2.97 (-6.35, 12.29) | - | - | - | 0.62 | 0.53 | |||
| Retro | 1 | -11.80 (-21.21, -2.39) | - | - | - | 2.46 | 0.01 | |||
| Canaloplasty vs phaco-canaloplasty | ||||||||||
| All trials | 5 | -3.78 (-8.38,0.81) | 5.66 | 0.23 | 29 | 1.61 | 0.11 | |||
| RCTs | 1 | -9.78 (-20.99,1.43) | - | - | - | 1.71 | 0.09 | |||
| Pro | 2 | 2.82 (-4.55, 10.19) | 0.19 | 0.67 | 0 | 0.75 | 0.45 | |||
| Retro | 2 | -7.31 (-14.23, -0.40) | 0.29 | 0.59 | 0 | 2.07 | 0.04 | |||
| Trabeculotomy vs combined trabeculotomy with phacoemulsification | ||||||||||
| All trials | 6 | 12.65 (10.56, 14.74) | 53.56 | <0.00001 | 91 | 11.87 | <0.00001 | |||
| RCTs | 1 | 10.74 (5.87, 15.61) | - | - | - | 4.33 | <0.00001 | |||
| Pro | 1 | 12.51 (8.73, 16.29) | - | - | - | 6.48 | <0.00001 | |||
| Retro | 4 | 13.42 (10.50, 16.35) | 52.70 | <0.00001 | 94 | 9 | <0.00001 | |||
Percentage of intraocular pressure reduction (canaloplasty vs phaco-canaloplasty)
Five studies including 456 eyes compared canaloplasty with canaloplasty plus phacoemulsification regarding IOPR%. Mild heterogeneity was indicated between studies (P=0.23, I2=29%). The combined results indicated that the two factors greatly declined IOP. The differences in IOPR% were not all statistically obvious (WMD=-3.78, 95%CI: -8.38, 0.81; Table 3). We then separated the studies into 3 subgroups in terms of the design (Pro and Retro nonrandomized, and randomized). There was statistically significant result in the Retro subgroup (Table 4).
Percentage of intraocular pressure reduction (trabeculotomy vs combined trabeculotomy with phacoemulsification)
Six studies involving 1956 eyes compared trabeculotomy with trabeculotomy plus phacoemulsification regarding IOPR%. High statistical heterogeneity was indicated between studies (P<0.00001, I2=91%). The combined results indicated that the two factors significantly declined IOP. Trabeculotomy obtained a numerically greater IOPR% from baseline, and the differences in IOPR% were statistically significant (WMD=12.65, 95%CI: 10.56, 14.74; Table 3). We then separated the studies into 3 subgroups in line with the design (Retro and randomized). The three subgroups indicated distinct results (Table 4).
Percentage of the number of glaucoma medications reduction in all subgroups
Three studies involving 1088 eyes compared trabeculotomy with trabeculotomy plus phacoemulsification regarding percentage of the number of glaucoma medications reduction. Statistical heterogeneity was not applicable between studies. The combined results indicated that both the factors didn't reduce the number of glaucoma medications after surgery. The differences in two retrospective subgroups were not statistically obvious (WMD=1.55, 95%CI: -5.06, 8.16). Two studies including 152 eyes were used to compare canaloplasty with phaco-canaloplasty. There was no difference between two subgroups (WMD=-12.87, 95%CI: -29.65, 3.91; Table 5).
Table 5. Subgroup analysis estimating the effect of trial design on percentage of the number of glaucoma medications reduction.
| Subgroup | No. of studies | WMD (fixed) (95%CI) | Heterogeneity |
Overall effect |
|||
| Chi2 | P | I2 (%) | Z | P | |||
| Canaloplasty vs phaco-canaloplasty | |||||||
| All trials (Retro) | 2 | -12.87 (-29.65, 3.91) | 3.17 | 0.07 | 68 | 1.5 | 0.13 |
| Trabeculotomy vs combined trabeculotomy with phacoemulsification | |||||||
| All trials (Retro) | 3 | 1.55 (-5.06, 8.16) | 9.46 | 0.002 | 89 | 0.46 | 0.65 |
Complete and Qualified Success (trabeculotomy vs combined trabeculotomy with phacoemulsification)
In two researches studied the possibility of complete success, there was no significant difference between the two groups [pooled OR=1.13 (95%CI: 0.51, 2.53); Table 6]. Furthermore, no significant difference was found between trabeculotomy and trabeculotomy plus phacoemulsification in the subgroup analyses in terms of study design [pooled OR=1.43 (95%CI: 0.36, 5.66) for randomized and [pooled OR=1.00 (95%CI: 0.37, 2.71)] for Retro nonrandomized]. Two researches also studied the proportion of patients reaching target IOP with or without medications at follow-up endpoint; the difference in qualified success rate between the two groups was not statistically obvious [pooled OR=0.48 (95%CI: 0.14, 1.72)]. For the subgroup analysis in terms of study design, the difference between groups was not statistically obvious [pooled OR=0.57 (95%CI: 0.05, 7.00)] for randomized and [pooled OR=0.46 (95%CI: 0.10, 1.98)] for Retro nonrandomized] (Table 6).
Table 6. Complete success and qualified success comparing trabeculotomy with combined trabeculotomy with phacoemulsification.
| Trial | Studies (n) | Success rate, n/N |
OR (95%CI) | Heterogeneity |
Overall effect |
|||||
| Trabeculotomy | Phaco-trabe | Chi2 | P | I2 (%) | Z | P | ||||
| Complete success (≤21 mm Hg) | ||||||||||
| All trials | 2 | 36/55 | 33/52 | 1.13 (0.51, 2.53) | 0.17 | 0.68 | 0 | 0.30 | 0.77 | |
| RCT | 1 | 10/18 | 7/15 | 1.43 (0.36, 5.66) | - | - | - | 0.51 | 0.61 | |
| Retro | 1 | 26/37 | 26/37 | 1.00 (0.37, 2.71) | - | - | - | 0 | 1.00 | |
| Qualified success (≤21 mm Hg) | ||||||||||
| All trials | 2 | 47/55 | 48/52 | 0.48 (0.14, 1.72) | 0.02 | 0.88 | 0 | 1.13 | 0.26 | |
| RCT | 1 | 16/18 | 14/15 | 0.57 (0.05, 7.00) | - | - | - | 0.44 | 0.66 | |
| Retro | 1 | 31/37 | 34/37 | 0.46 (0.10, 1.98) | - | - | - | 1.05 | 0.29 | |
Adverse Events
No significant differences in the incidence of shallow/flat anterior chamber (AC), hyphema and choroid detachment were found between deep sclerectomy and deep sclerectomy plus phacoemulsification, with the pooled ORs being 2.21 (95%CI: 0.09, 55.42), 1.22 (95%CI: 0.28, 5.38), and 2.23 (95%CI: 0.22, 22.06) respectively (Table 7). Furthermore, the rates of adverse events did not greatly differ between trabeculotomy and trabeculotomy plus phacoemulsification, with pooled ORs of 1.28 (95%CI: 0.68, 2.42), 1.19 (95%CI: 0.70, 2.05) and 1.17 (95%CI: 0.33, 4.17) for hyphema, hypotony and choroid detachment, respectively.
Table 7. Subgroup analysis evaluating frequency of postoperative complications in study group.
| Adverse events | Studies (n) | Crude event rate, n |
OR (95%CI) | Heterogeneity |
Overall effect |
||||||
| Glaucoma surgery | Combined cataract/glaucoma surgery | Chi2 | P | I2 (%) | Z | P | |||||
| Deep sclerectomy vs combined deep sclerectomy with phacoemulsification | |||||||||||
| Shallow/flat anterior chamber | 2 | 1/69 | 0/50 | 2.21 (0.09, 55.42) | - | - | - | 0.48 | 0.63 | ||
| Hyphema | 2 | 5/69 | 3/50 | 1.22 (0.28, 5.38) | - | - | - | 0.27 | 0.79 | ||
| Choroid detachment | 2 | 3/69 | 1/50 | 2.23 (0.22, 22.06) | - | - | - | 0.68 | 0.49 | ||
| Trabeculotomy vs combined trabeculotomy with phacoemulsification | |||||||||||
| Hyphema | 2 | 25/103 | 24/120 | 1.28 (0.68, 2.42) | - | - | - | 0.77 | 0.44 | ||
| Hypotony | 2 | 43/103 | 45/120 | 1.19 (0.70, 2.05) | - | - | - | 0.65 | 0.52 | ||
| Choroid detachment | 2 | 5/103 | 5/120 | 1.17 (0.33, 4.17) | - | - | - | 0.25 | 0.80 | ||
DISCUSSION
With the emergence of the aging of the population, at least 4% to 10% of the elderly patients with significant cataracts have glaucoma or OH[19]–[21]. For glaucoma and cataracts, there are many similar risk elements, including gender, age, smoking, prior trauma, use of topical medication, eye surgery and diabetes. So the question emerges: “How to size up the options for these combined problems of cataract and glaucoma?”
Many surgeries are optional for patients with cataract accompanied with glaucoma. Cataract surgery is a vision-restoring and sometimes life-transforming operation, nevertheless glaucoma filtering procedures were actualized to lessen the IOP in order to lower the risk of visual loss. The surgery of combined phacoemulsification with IOL implantation and trabeculectomy (phacotrabeculectomy) is extensively used as one surgical treatment for this condition. The advantages of combined surgery are an immediate increase of visual acuity and economical aspects. It may also be important in patients whose medical conditions are too poor to endure multiple surgical procedures.
Systematic reviews of the medical literature can help practitioners determine how to provide the best care[22]. Nevertheless, there is short of reliable evidence-based conclusions in clinical practice. Therefore, we resorted to Meta-analysis to estimate the clinical safety and efficacy of the combined surgery in patients with coexisting cataract and open angle glaucoma.
In the Meta-analysis, we reviewed the clinically relevant outcome measures of 14 controlled clinical trials. Concerning IOP, this study discovered that both trabeculotomy and trabeculotomy plus phacoemulsification greatly reduced IOP, but in comparison with trabeculotomy plus phacoemulsification, trabeculotomy alone reduced IOP more efficiently, resulting in a greatly higher percentage of IOP and glaucoma medications reduction. One possible reason for the finding may be that combined surgery prolongs the operation time and increases the risk of postoperative complication[7]. There was no significant difference in complete and qualified success rates between the two groups. It is difficult to draw credible conclusions due to the reason for the small sample size and short follow-up duration.
In all glaucoma surgical techniques, trabeculectomy has been the most popular surgery to be combined with phacoemulsification. The main risk of trabeculectomy and phacotrabeculectomy is ocular hypotony and its consequences[5]. Adverse events were similar concerning the two types. It may also be related with a small sample size and short follow-up duration.
Trabeculectomy is the preferred filtration procedure in combined surgery. However, new filtering surgery techniques known as “non-perforating” have been introduced, such as ab externo trabeculectomy, deep sclerectomy and Stegmann's viscocanalostomy[23]. They all make a scleral ablation towards Schlemm's canal to create a space for the aqueous humour defluxion[24]. So, in our Meta-analysis, we observe the IOP lowering efficacy in canaloplasty vs phaco-canaloplasty and deep sclerectomy vs combined deep sclerectomy with phacoemulsification subgroups.
As for IOP assessment, we also found both deep sclerectomy and deep sclerectomy plus phacoemulsification significantly decreased IOP, but for the outcomes of deep sclerectomy alone and deep sclerectomy plus phacoemulsification, there were no statistically significant differences in IOPR%. The result was similar concerning IOP lowering efficacy in canaloplasty and canaloplasty plus phacoemulsification groups. Moreover, no statistical difference was found in evaluating frequency of postoperative complications between deep sclerectomy and combined deep sclerectomy with phacoemulsification group. This may be related with a small sample size and short follow-up duration.
Our study had some advantages. Firstly, we strictly conformed to the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, involving the literature search, data extraction, quality assessment and statistical analysis. This procedure makes our conclusions more reliable and scientific. Secondly, we actualized the subgroup analysis in accordance with study design in order to make the conclusions credible.
Besides, there were several limitations: 1) Randomization: There were 4 RCTs, 3 Pro nonrandomized, and 7 Retro in the 14 included studies. Only 3 RCT studies included sufficient information on how to actualize RCTs and describe the performance of allocation concealment. The potential selection bias may occur if inappropriate methods of RCTs were performed. 2) Masking: Only 1 study used masking, while others did not find masking, which may lead to measurement and performance bias. 3) Heterogeneity: Significant heterogeneity in the studies may indicate differences in age, gender, sample size, differences in definition of complete and qualified success, and outcome of measurements. A random effects model was used when statistically significant heterogeneity was fit. 4) Publication bias: We used manual and electronic searches to gather all potential relevant trials to prevent publication bias. Nevertheless, we may not have consisited some papers, especially those published in languages other than Chinese or English. Besides, the test for publication bias was not done due to a limited number of trials involved in our Meta-analysis. 5) Follow-up: The short follow-up time may influence the long-term results.
As a result, this Meta-analysis firstly answers the question of whether combined cataract/glaucoma surgery is more effective and safer than glaucoma surgery. The results suggest that trabeculectomy alone is more effective in lowering IOP than trabeculotomy plus phacoemulsification; however, trabeculotomy is comparable with trabeculotomy plus phacoemulsification concerning qualified success rate, complete success rate and incidence of adverse events. Besides, there was no statistically significant concerning IOP lowering efficacy in deep sclerectomy and deep sclerectomy plus phacoemulsification, and there was a similar result in terms of IOP lowering efficacy in canaloplasty and canaloplasty plus phacoemulsification groups. Although there are some limitations, we believe that the results of this Meta-analysis are sufficient credibility and can give enlightenment to future clinical practice. Besides, we require the more RCTs with larger sample sizes and systematic studies to further confirm the presented results.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.8170080; No.81470609); the Natural Science Foundation of Shandong Province (No.ZR2017MH008)
Conflicts of Interest: Jiang N, None; Zhao GQ, None; Lin J, None; Hu LT, None; Che CY, None; Wang Q, None; Xu Q, None; Li C, None; Zhang J, None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casson RJ, Salmon JF. Combined surgery in the treatment of patients with cataract and primary open-angle glaucoma. J Cataract Refract Surg. 2001;27(11):1854–1863. doi: 10.1016/s0886-3350(01)01127-0. [DOI] [PubMed] [Google Scholar]
- 3.Topouzis F, Wilson MR, Harris A, Founti P, Yu F, Anastasopoulos E, Pappas T, Koskosas A, Salonikiou A, Coleman AL. Risk factors for primary open-angle glaucoma and pseudoexfoliative glaucoma in the Thessaloniki eye study. Am J Ophthalmol. 2011;152(2):219–228.e1. doi: 10.1016/j.ajo.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 4.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C, BO17704 Study Group Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21(9):1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilgin G, Karakurt A, Saricaoglu MS. Combined non-penetrating deep sclerectomy with phacoemulsification versus non-penetrating deep sclerectomy alone. Semin Ophthalmol. 2014;29(3):146–150. doi: 10.3109/08820538.2013.874466. [DOI] [PubMed] [Google Scholar]
- 6.Bull H, von Wolff K, Körber N, Tetz M. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study results. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1537–1545. doi: 10.1007/s00417-011-1728-3. [DOI] [PubMed] [Google Scholar]
- 7.Cillino S, Di Pace F, Casuccio A, Calvaruso L, Morreale D, Vadalà M, Lodato G. Deep sclerectomy versus punch trabeculectomy with or without phacoemulsification: a randomized clinical trial. J Glaucoma. 2004;13(6):500–506. doi: 10.1097/01.ijg.0000137869.18156.81. [DOI] [PubMed] [Google Scholar]
- 8.D'Eliseo D, Pastena B, Longanesi L, Grisanti F, Negrini V. Comparison of deep sclerectomy with implant and combined glaucoma surgery. Ophthalmologica. 2003;217(3):208–211. doi: 10.1159/000068974. [DOI] [PubMed] [Google Scholar]
- 9.Wishart PK, Wishart MS, Porooshani H. Viscocanalostomy and deep sclerectomy for the surgical treatment of glaucoma: a longterm follow-up. Acta Ophthalmol Scand. 2003;81(4):343–348. doi: 10.1034/j.1600-0420.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 10.Wishart MS, Shergill T, Porooshani H. Viscocanalostomy and phacoviscocanalostomy: long-term results. J Cataract Refract Surg. 2002;28(5):745–751. doi: 10.1016/s0886-3350(01)01285-8. [DOI] [PubMed] [Google Scholar]
- 11.Chihara E, Hayashi K. Different modes of intraocular pressure reduction after three different nonfiltering surgeries and trabeculectomy. Jpn J Ophthalmol. 2011;55(2):107–114. doi: 10.1007/s10384-010-0923-9. [DOI] [PubMed] [Google Scholar]
- 12.Rotchford AP, Vernon SA. Phaco-microtrabeculectomy: technique and intraocular pressure control in comparison with microtrabeculectomy. Clin Exp Ophthalmol. 2007;35(9):812–817. doi: 10.1111/j.1442-9071.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 13.Marek R, Joanna W, Lewczuk K, Siemiatkowska A, Stankiewicz A. Efficacy and safety of deep sclerectomy and phacoemulsification and deep sclerectomy in clinical material of Military Health Service Institute-yearly observations. Klin Oczna. 2006;108(10-12):385–391. [PubMed] [Google Scholar]
- 14.Uretmen O, Ateş H, Güven S, Andaç K. Comparison of outcomes of viscocanalostomy and phacoviscocanalostomy. Can J Ophthalmol. 2003;38(7):580–586. doi: 10.1016/s0008-4182(03)80112-6. [DOI] [PubMed] [Google Scholar]
- 15.Ting JL, Damji KF, Stiles MC, Trabectome Study Group Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg. 2012;38(2):315–323. doi: 10.1016/j.jcrs.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Sałaga-Pylak M, Kowal M, Zarnowski T. Deterioration of filtering bleb morphology and function after phacoemulsification. BMC Ophthalmol. 2013;13:17. doi: 10.1186/1471-2415-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tetz M, Koerber N, Shingleton BJ, von Wolff K, Bull H, Samuelson TW, Lewis RA. Phacoemulsification and intraocular lens implantation before, during,or after canaloplasty in eyes with open-angle glaucoma: 3-year results. J Glaucoma. 2015;24(3):187–194. doi: 10.1097/IJG.0b013e318285ff13. [DOI] [PubMed] [Google Scholar]
- 18.Parikh HA, Bussel II, Schuman JS, Brown EN, Loewen NA. Coarsened exact matching of phaco- trabectome to trabectome in phakic patients: lack of additional pressure reduction from phacoemulsification. PLoS One. 2016;11(2):e0149384. doi: 10.1371/journal.pone.0149384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman DS, Wolfs RC, O'Colmain BJ, Klein BE, Taylor HR, West S, Leske MC, Mitchell P, Congdon N, Kempen J, Eye Diseases Prevalence Research Group Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, O'Colmain B, Wu SY, Taylor HR, Eye Diseases Prevalence Research Group Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487–494. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 21.Leske MC, Wu SY, Nemesure B, Hennis A, Barbados Eye Studies Group Causes of visual loss and their risk factors: an incidence summary from the Barbados Eye Studies. Rev Panam Salud Publica. 2010;27(4):259–267. doi: 10.1590/s1020-49892010000400004. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA. 1994;271(1):59–63. doi: 10.1001/jama.271.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg. 1999;25(3):316–322. doi: 10.1016/s0886-3350(99)80078-9. [DOI] [PubMed] [Google Scholar]
- 24.Schwenn O, Dick B, Pfeiffer N. Trabeculotomy, deep sclerectomy and viscocanalostomy. Non-fistulating microsurgical glaucoma operation ab externo. Ophthalmologe. 1998;95(12):835–843. doi: 10.1007/s003470050363. [DOI] [PubMed] [Google Scholar]