Abstract
AIM
To clarify this controversy and to provide evidence for application of lipid lowering agents in treatment of diabetic retinopathy (DR).
METHODS
We searched the databases of PubMed, Embase and Cochrane Library Central Register of Controlled Trials (CENTRAL) and abstracts from main annual meetings up to January 1, 2017. Google scholar and ClinicalTrials.gov were also searched for unpublished relevant studies. We included randomized controlled trials (RCTs) that studied lipid-lowering agents in type 1 or type 2 diabetes in this Meta-analysis. The primary endpoint was the progression of DR, and the secondary endpoints included vision loss, development of diabetic macular edema (DME) and aggravation of hard exudates. The pooled odds ratios (OR) with corresponding 95% confidence intervals (95%CIs) were calculated.
RESULTS
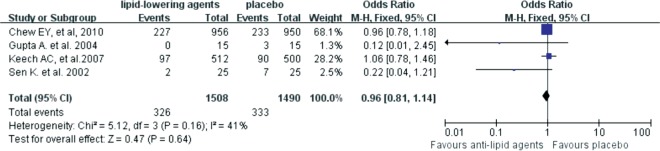
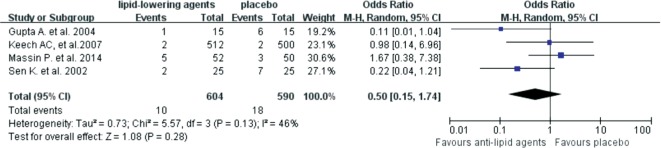
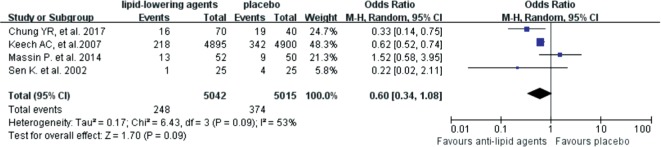
After systemic and manual literature search by two independent investigators, we included 8 RCTs from 7 published articles with 13 454 participants in this Meta-analysis. The results revealed that lipid-lowering drugs were associated with reduced risk in DR progression [OR=0.77 (95%CI: 0.62, 0.96), P=0.02]. Lipid-lowering agents might have protective effect on DME compared to placebo, although the difference was not statistically significant [OR=0.60 (95%CI: 0.34, 1.08), P=0.09]. However, no significant differences in the worsening of vision acuity [OR=0.96 (95%CI: 0.81,1.14), P=0.64] and hard exudates [OR=0.50 (95%CI:0.15, 1.74), P=0.28] were found between the lipid-lowering drugs and the placebo groups.
CONCLUSION
In DR patients, lipid-lowering agents show a protective effect on DR progression and might be associated with reduced risk in the development of DME. However, lipid-lowering agents have no effects on vision loss and hard exudates aggravation. Further clinical trials in larger scale are required to confirm the conclusion of this study and thus justify the use of intensive control lipids with anti-lipid agents at the early stages of DR.
Keywords: diabetic retinopathy, lipid-lowering agents, fibrates, statins, vision acuity, hard exudates, diabetic macular edema
INTRODUCTION
Diabetic retinopathy (DR) is the most frequently occurring chronic microvascular complication in patients with diabetes, which remains a leading cause of irreversible vision loss globally and has become an important public health problem[1]–[2]. The features of DR in the non-proliferative stage (NPDR) include the development of microaneurysms, petechial hemorrhage and hard and soft exudation. The diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR) occur in the late stage of the disease, which become refractory to treatment. Eventually, vitreous hemorrhage or tractional retinal detachment caused by retinal neovascularization will lead to partial or complete vision loss[3]. Therefore, developing effective therapies to prevent the onset and to delay the progression of DR at early stage has been considered as the most effective strategy for DR treatment[4].
Elevated blood lipid levels, in particular cholesterol and low-density lipoprotein (LDL), has been identified as a risk factor of DR and considered correlated to the development of retinal hard exudates and DME that lead to moderate vision loss, defined as three or more lines of vision loss compared with baseline[3],[5]–[9]. In recent years, some studies have revealed that intensive control of lipids in patients with diabetes could substantially delay the progression of DR and suggested that fibrates and statins, two types of widely prescribed lipid-lowering drugs, should be applied in the treatment of DR in diabetes[7],[10]–[14]. However, the results of some other studies did not demonstrate the beneficial effects of lipid-lowering drugs on the progression of DR[15]–[17]. In addition, there is no explicit statement in the application of lipid-lowering agents in the current guidelines for DR treatment[18]. Therefore, the relationship between antilipemic agents and the progression of DR deserves further investigation. In this study, we conducted a systematic review and Meta-analysis to assess the effects of two types of most widely used lipid-lowering drugs, fibrates and statins, on the development of DR, vision loss, hard exudates and DME.
MATERIALS AND METHODS
Search Strategy
We performed literature search using the online databases of PubMed, Embase, and the Cochrane Library Central Register of Controlled Trials (CENTRAL) for publications and abstracts from main annual meetings about the effects of two main types of lipid-lowering drugs, fibrates and statins, on the development of DR for the period of Jan. 1, 1990 through Jan. 1, 2017. Google scholar and ClinicalTrials.gov were also searched for unpublished relevant studies. We conducted the literature search using the following keywords and MeSH terms: “antilipemic agents”, “therapy for dyslipidemia”, “lipid lowering drugs”, “hypolipidemic”, “statins”, “statin”, “simvastatin”, “mevastatin”, “rosuvastatin”, “atorvastatin”, “cerivastatin”, “pravastatin”, “lovastatin”, “fibrate”, “fibrates”, “bezafibrate”, “fenofibrate”, “etofibrate” and “diabetic retinopathy”, “proliferative diabetic retinopathy”, “diabetic macular edema”, “diabetic macular oedema”, “diabetic maculopathy”, “retinal disorders”, “diabetic eye disease”, or “vision loss”. In addition, bibliographies of retrieved studies and recent review articles were checked for additional relevant studies. We limited the language of articles to English. The literature search process was conducted by two investigators independently.
Selection Criteria
Studies that meet the following criteria were included: 1) the study included type 1 or type 2 diabetes patients with or without DR; 2) the article compared the effect of lipid-lowering drugs with other reagents or placebo on DR or reported the relationship of lipid-lowering drugs and DR; 3) the study must include at least one of the following outcomes: progression of DR, incidence of DR, DME development or severity, aggravation of vision acuity loss or hard exudates and report the number of patients and events in each treatment group; 4) the study was a randomized controlled trial (RCT); 5) the period of follow-up was more than 3mo. The exclusion criteria were as following: 1) animal- and cell-based experimental studies; 2) crossover trials; 3) the study combined lipid-lowering reagents with other drugs, such as antihypertensive and hypoglycemic drugs.
Endpoints of Included Trials
The primary endpoint was the progression of DR. The secondary endpoints included 2-lines worsening in vision acuity, aggravation in DME and worsening of hard exudates. For studies employing the Early Treatment Diabetic Retinopathy Study (ETDRS) grading system, progression of at least two step changes was selected as the primary endpoint because most studies with ETDRS grading system used at least two step changes as criteria[19]. In addition, we performed subgroup analyses according to follow-up time and the number of participants to improve the specificity of the assessment.
Data Extraction and Quality Assessment
Two investigators (Shi R and Zhao L) independently reviewed the titles and abstracts of all retrieved articles and then selected the included studies. Any disagreement was solved by consensus or consultation. They then reviewed the full-text of all included articles and extracted the following data using a standardized excel-based data extraction form: first author's name, year of publication, types of diabetes, types and doses of drugs, number of participants and baseline information including mean age, duration of DM, HbA1c, total serum cholesterol (TC), LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and serum triglycerides (TGs), dropout rates and the number of events in the intervention groups. The quality of included articles was evaluated using the Review Manager 5.3 software (UK). Potential risks of bias in each included study were assessed using the Cochrane Collaboration's method that was composed of six domains: sequence generation, allocation concealment, masking of participants or outcome assessors, incomplete outcome, selective outcome reporting and other bias[20].
Statistical Analysis
The STATA statistical software (version 12.0, Stata Corp, USA) and the Review Manager software (v.5.3.0, Cochrane Community, UK) were employed to analyze the data and perform the Meta-analysis. The pooled odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated by using the bivariate binomial mixed model and used to compare the effects of treatments. The forest plot was used to illustrate the extent of effects and P<0.05 was considered as significant. The χ2-based Cochran's Q test and the Higgins' I2 statistics were used to assess the heterogeneity among included studies. P<0.1 and I2>50% were considered as indicators of significant heterogeneity. When heterogeneity was present, the random-effects model (DerSimonian-Laird method) was applied; otherwise the fixed-effects model (Mantel-Haenszel method) was carried out. Potential publication bias was assessed with the Begg's funnel plot and the Egger's linear regression test was applied to evaluate the asymmetry of the funnel plot. A P<0.05 indicated an asymmetric plot, which suggested the existence of possible risk of publication bias. All P values were two sided, and statistical significance was defined as P<0.05 unless indicated otherwise.
RESULTS
Characteristics and Quality Assessment of Included Studies
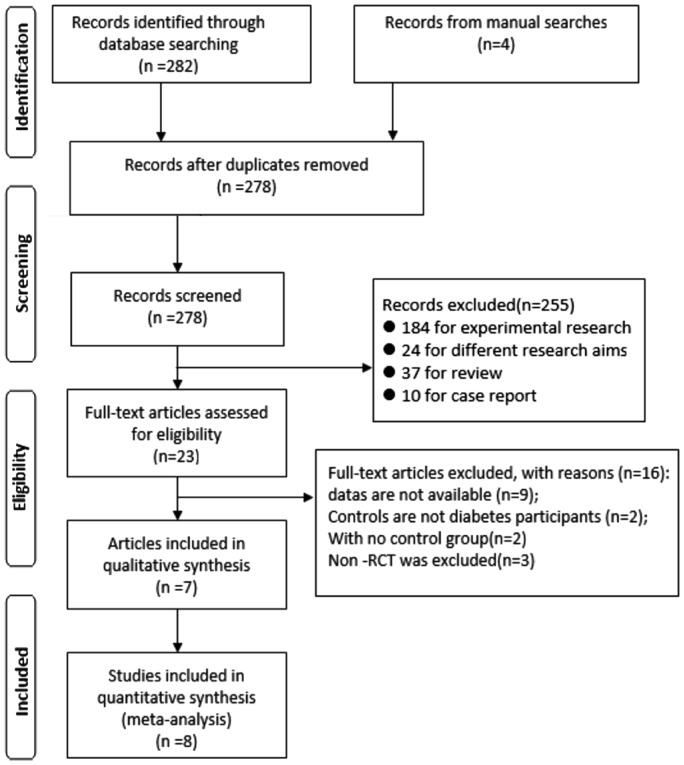
The initial literature search identified 282 articles through systematic search for online databases and 4 studies through manual review of the bibliographies of included studies and recently published reviews. Eight duplicate records were removed and 231 abstracts were further excluded because they were basic researches, for different aims reviews or case report. The remaining 23 studies were subjected to complete full-text review by 2 independent investigators, of which 16 studies were subsequently excluded based on the exclusion criteria. Finally, a total of 7 articles met the inclusion criteria and therefore were included in the present Meta-analysis[11],[15],[21]–[25]. One of the included articles reported 2 individual trials[21]; therefore, 8 clinical trials with 13 454 participants were included in this Meta-analysis. The process of literature search and selection was summarized in the PRISMA chart (Figure 1). The main characteristics of included studies were summarized in Table 1.
Figure 1. PRISMA 2009 flow diagram in our study.
Table 1. Main characteristics of 7 included articles in this Meta-analysis.
| Included study (year) | Study design | Sample size (lipid-lowering drug/control) | Intervention | Follow-up | Outcomes |
| Chung et al 2017[11] | RCT | 70/40 | Statin/no-statin | 5y | DME, hard exudates, progression steps |
| ACCORDION et al 2016[15] | RCT | 399/363 | Fibrate/placebo plus simvastatin | 8y | Progression steps |
| Keech et al 2007[21] | RCT | 4895/4900 | Fenofibrate/placebo | 5y | DME |
| 512/500 | Fenofibrate/placebo | VA, progression steps | |||
| Gupta et al 2004[22] | RCT | 15/15 | Atorvastatin/no-lipid lowering agents | 18wk | VA, progression steps |
| Sen et al 2002[23] | RCT | 25/25 | Simvastatin/placebo | 6mo | VA, hard exudates, progression steps, DME |
| Chew et al 2010[24] | RCT | 806/787 | Fibrate/placebo plus simvastatin | 4y | VA and progression steps |
| Massin et al 2014[25] | RCT | 52/50 | Fenofibrate/placebo | 1y | DME, exudates, progression steps |
RCT: Randomized controlled trial; DME: Diabetic macular edema; VA: Vision acuity; NA: Not available. Outcomes: VA=2-line worsen in visual acuity, progression steps=at least 2 steps progression. DR status was defined according to the eye with the highest level on the ETDRS Final Severity Scale, as follows: no DR, a level of less than 20; mild DR, a level of 20; moderate NPDR, a level above 20 but less than 53; severe DR, a level of 53; and PDR, a level of 60 or higher[24].
Among the included trials, 6 studies involved type 2 diabetes patients, 2 studies recruited both type 1 and 2 diabetes patients. Regarding the types of lipid-lowering reagents, 5 included trials investigated the effects of fibrates on DR and the other 3 trials studied statins. All studies reported the baseline levels of all or some of the following blood lipid parameters: HbA1c (%), serum TC, TG, LDL and HDL. The data for DR progression was reported in 6 trials with 3629 participants; 4 included studies described DME according to the definitions by the ETDRS study[19]; 4 trials provided the data for the alterations in vision acuity during the lipid-lowering drugs therapy; and the worsening of hard exudates was examined in 4 articles. Baseline characteristics of the included trials in the present Meta-analysis were summarized in Table 2.
Table 2. Baseline data of the included clinical trials in the Meta-analysis.
| Included study (year) | Sample size | Interventions | Baseline characteristics |
|||||||
| Age (y) | Diabetes duration (y) | VA | HbA1c (%) | TC | LDL-C | HDL-C | TG | |||
| Chung et al 2017[11] | 70 | Statin | 58.1 (11.6) | 12.4 (8.0) | NA | 8.1 (1.7) | 4.4 (2.6) | 2.1 (0.8) | 1.1 (0.3) | 1.9 (1.4) |
| 40 | No-statin | 52.3 (12.2) | 8.6 (8.2) | NA | 8.0 (1.5) | 4.4 (1.5) | 3.0 (1.3) | 1.1 (0.3) | 2.1 (1.5) | |
| ACCORDION et al 2016[15] | 399 | Fibrate plus simvastatin | 61.8 (5.8) | 9.8 (6.5) | NA | 8.2 (1.0) | NA | 2.79 (0.78) | 0.98 (0.19) | 2.1 (1.14) |
| 363 | Placebo plus simvastatin | 61.1 (5.6) | 9.4 (6.6) | NA | 8.1 (0.9) | NA | 2.84 (0.87) | 1.0 (0.19) | 2.06 (1.18) | |
| Keech et al 2007[21] | 4895 | Fenofibrate | NA | NA | NA | NA | 5.04 | 3.07 | 1.1 | NA |
| 4900 | Placebo | NA | NA | NA | NA | 5.04 | 3.07 | 1.1 | NA | |
| 512 | Fenofibrate | NA | NA | NA | NA | 5.04 | 3.07 | 1.1 | NA | |
| 500 | Placebo | NA | NA | NA | NA | 5.04 | 3.07 | 1.1 | NA | |
| Gupta et al 2004[22] | 15 | Atorvastatin | 55.53 (8.29) | 10.99 (5.42) | NA | 8.38 | 5.95 (0.96) | 3.29 (0.56) | NA | 2.13 (1.05) |
| 15 | No-lipid lowering agents | 52.73 (7.27) | 14.07 (4.36) | NA | 8.32 | 6.07 (1.78) | 3.16 (0.75) | NA | 2.41 (1.52) | |
| Sen et al 2002[23] | 25 | Simvastatin | 54.9 (7.8) | NA | NA | 7.4 (0.14) | 5.8 (0.69) | 4.1 (0.65) | 1 (0.1) | 1.4 (0.6) |
| 25 | Placebo | 53.0 (10.2) | NA | NA | 7.3 (0.26) | 5.5 (0.45) | 4 (0.53) | 1 (0.13) | 1.3 (0.31) | |
| Chew et al 2010[24] | 806 | Fibrate plus simvastatin | 61.9 (6.2) | 9.7 (6.8) | 76.2 (9.7) | 8.2 (1.0) | NA | 2.49 (0.76) | 0.99 (0.20) | 2.14 (1.25) |
| 787 | Placebo plus simvastatin | 61.5 (6.5) | 9.8 (7.2) | 76.2 (10.7) | 8.2 (1.0) | NA | 2.50 (0.77) | 0.99 (0.20) | 2.12 (1.27) | |
| Massin et al 2014[25] | 52 | Fenofibrate | 62.6 (6.3) | 14.3 | 0.28 (0.27) | 7.8 (1.1) | NA | 3.16 (1.07) | 1.23 (0.33) | 2.25 (1.29) |
| 50 | Placebo | 60.6 (8.8) | 13.3 | 0.23 (0.27) | 8.0 (1.0) | NA | 3.05 (1.16) | 1.21 (0.34) | 2.18 (0.92) | |
Data shown was the mean (SD) of each parameter except for the study by Keech et al[21] that only provided the mean. TC: Total cholesterol; TG: Triglyceride; LDL-C: Low-density lipoprotein cholesterol; HDL: High-density lipoprotein cholesterol. In three articles, mg/dL instead of mmol/L was used, therefore, interchange of units was performed according the following calculation: TC: 100 mg/dL=2.58 mmol/L, TG: 100 mg/dL=1.13 mmol/L, LDL: 100 mg/dL=2.59 mmol/L, HDL: 100 mg/dL= 2.58 mmol/L. Mixed=D1M and D2M. Baseline data of the first article[11] was exchanged from the table in the paper (Table 2: Baseline characteristics of participants requiring or not requiring laser treatment during the study).
mean±SD
The quality of included articles was assessed using the Cochrane Collaboration's method for the potential risks of bias, and the results showed that all the included RCTs had low risk of bias (Table 3).
Table 3. Risk of bias summary: each risk of bias item for each included study.
| Included study (year) | Random sequence generation | Allocation concealment | Masking of participants or outcome assessors | Incomplete outcome | Selective outcome reporting | Other bias |
| Chung et al 2017[11] | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk |
| ACCORDION et al 2016[15] | Low risk | High Risk | Unclear | Low risk | Low risk | Low risk |
| Keech et al 2007[21] | Low risk | Low risk | Unclear | High Risk | Low risk | Low risk |
| Gupta et al 2004[22] | High Risk | High Risk | Low risk | Low risk | unclear | Low risk |
| Sen et al 2002[23] | Low risk | Low risk | Low risk | High Risk | Low risk | Low risk |
| Chew et al 2010[24] | Low risk | Low risk | Low risk | High Risk | Low risk | Low risk |
| Massin et al 2014[25] | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk |
The Effects of Lipid-lowering Reagents on the Development and Progression of Diabetic Retinopathy
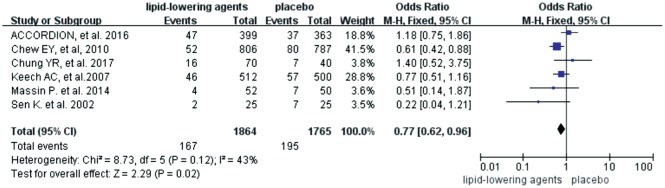
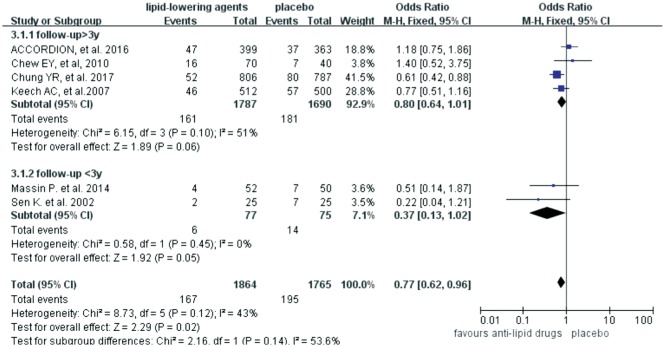
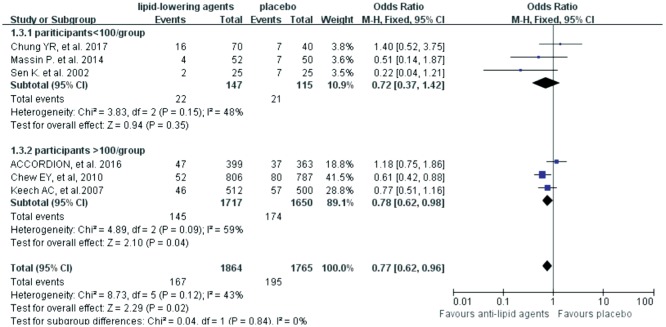
Six trials investigated the roles of lipid-lowering drugs (4 trials for fibrates, 2 trials for statins) in the progression of DR. The Meta-analysis revealed that lipid-lowering drugs were associated with reduced risk of DR progression [OR=0.77 (95%CI: 0.62, 0.96), P=0.02] (Figure 2). Subgroup analyses were then carried out based on the follow-up time (< 3y or ≥3y) and the number of participants (n<100 or n≥100). However, no difference was found between the trials with different follow-up time (test for subgroup differences: P=0.14, I2=53.6%) (Figure 3) and different scales (test for subgroup differences: P=0.84, I2=0) (Figure 4).
Figure 2. Effects of lipid-lowering agents on DR progression.
Figure 3. Subgroup analyses effects of lipid-lowering agents on diabetic retinopathy progression according to the follow-up period.
Three years was used as the grouping factor as previously described[26].
Figure 4. Subgroup analyses effects of lipid-lowering agents on DR progression according to number of participants.
We then explored the effects of lipid-lowering reagents on secondary endpoints, including vision acuity, hard exudes and DME. The results suggested that lipid-lowering drugs did not reduce the risk of vision acuity worsening [OR=0.96 (95%CI: 0.81,1.14), P=0.64] (Figure 5). Patients on lipid-lowering drugs had no significant difference in developing severe hard exudates compared with patients who didn't use these reagents [OR=0.50 (95%CI: 0.15, 1.74), P=0.28] (Figure 6). Although no statistically significant difference was found in the severity of DME between the lipid-lowering drugs and the placebo groups [OR=0.60 (95%CI: 0.34, 1.08), P=0.09], anti-lipid drugs might be able to reduce DME aggravation according to its OR value (Figure 7).
Figure 5. Forest plots for the effects of lipid-lowering reagents on the worsening of vision acuity, which was defined as 2-line worsening in visual acuity.
Figure 6. Forest plots for the effects of lipid-lowering reagents on hard executes.
Figure 7. Forest plots for the effects of lipid-lowering reagents on the severity of DME.
All included studies described DME according to the definitions by the Early Treatment Diabetic Retinopathy Study (ETDRS) study[19].
Test of Heterogeneity and Publication Bias
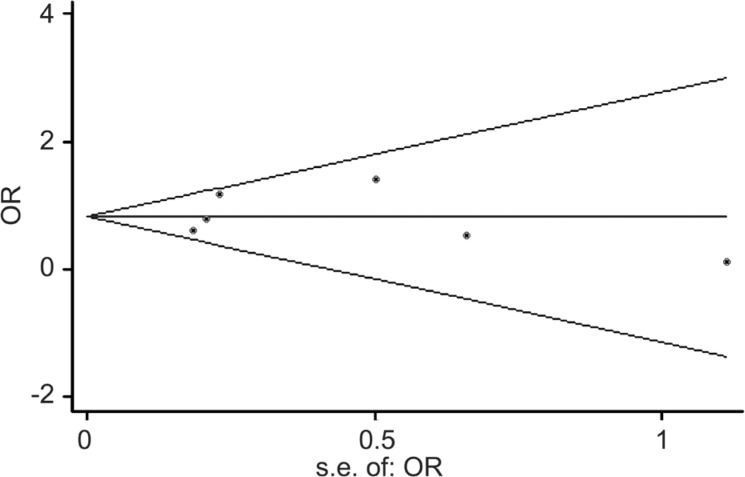
The spearman correlation coefficient was 0.371 (P=0.468), suggesting that no obvious threshold effect was detected. The Cochran Q value of DOR was 12.30 (P=0.031) and the inconsistency index (I2) was <50%, suggesting the absence of obvious non-threshold effects. In together, these results indicated that no evidence of significant heterogeneity existed among the included studies when the progression of DR was set as the primary endpoint. The Begg's funnel plot and the Egger's regression test were performed to explore any potential publication bias in this Meta-analysis (Figure 8). The slope coefficient reflected a P value of 0.935 in the overall studies, suggesting the symmetry of data and the absence of significant publication bias.
Figure 8. The Begg's funnel plot for potential publication bias.
DISCUSSION
A total of 8 RCTs from 7 articles[11],[15],[21]–[25] with 13 454 participants were included in the present Meta-analysis. The large number of participants made our research more reliable in estimating the effect of lipid-lowering reagents on the development and progression of DR, compared with single-centered studies and previously published systemic reviews[27]. Our Meta-analysis revealed that lipid-lowering agents were associated with significantly reduced risks in DR progression (Figure 2, Figure 8), and might delay DME aggravation in diabetic patients (Figure 7). However, no beneficial effects of lipid-lowering agents were discovered in terms of vision acuity and hard exudates in DR patients (Figures 5, 6).
In this Meta-analysis lipid-lowering drugs including fibrates and statins were shown to significantly reduce the risk in DR progression in 6 trials with 3629 participants, for which estimates were adjusted for baseline or follow-up characteristics (Table 2). This finding was in consistent with the reports by Chew et al[24] and Gupta et al[22]. However, due to the limited number of included studies, subgroup analysis based on the types of lipid-lowering agents, fibrates and statins, was not carried out in this Meta-analysis, which represented the main methodological limitation in our study. However, there was no significant heterogeneity among included studies, suggesting that the type of lipid-lowering drugs unlikely affected the results across the included trials. We also performed a subgroup analysis according to the number of participants of the included trials, and the results revealed that the size of trials did not influence the final results.
The underlying mechanism(s) of the protective effects of lipid-lowering drugs was unlikely solely due to lowering the plasma lipids concentrations and several potential mechanisms have been proposed. Some studies suggested that these drugs might exert protective effect via anti-inflammation property, because they could reduce the expression of inflammatory factors, such as IL-6, prostacyclin, cyclooxygenase (COX)-2 and VEGF in vascular smooth muscles[28] and therefore relieve the leakage of endothelium and regulate the function of blood-retina barrier[29]–[31]. In addition, reducing endothelial cell apoptosis and affecting antioxidant pathway were also involved in the protective effect of fibrates[32]. However, the exact molecular mechanism(s) for this effect was still unclear. Further studies are needed to explicit it.
Regarding the duration of the protective effects offered by lipid-lowering drugs, there were variations among reported studies. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study demonstrated that treatment with 200 mg fenofibrate per day (n=4895), compared with placebo (n=4900), resulted in a 31% reduction in the need for the first course of laser treatment for DR over 5y[21]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study reported the most updated follow-up data that fenofibrate plus simvastatin slowed the progression of DR in patients with type 2 diabetes in 4y, but fenofibrate provided no benefit at 8y[15]. To further identify the protective effect of lipid-lowering agents on DR progression, we did a subgroup analysis for different follow-up time and the period of 3y was chosen as the criterion as described in a previous study[26]. Our Meta-analysis revealed that lipid-lowering therapy had significant protection against the progression of DR in both subgroups (I2=53.6%, P=0.14), particularly within 3y follow-up (OR=0.37, I2=0, P=0.05), similar to what the ACCORD study has reported[15]. We proposed that at late stages of DR, ischemia led to irreversible vascular dysfunction and proliferation, thus the resultant retina neovascularization and DME needed more potent treatments beyond anti-lipidemic therapy. In addition, the effectiveness of anti-lipid drugs might also be related to the baseline status of DR before the participants were recruited into the trials. Further experimental studies or clinical trials with long-term follow-ups are guaranteed to uncover such mechanism(s).
DME is the most common cause of vision loss in diabetes patients and may occur at any stage of DR[33], and it is defined as retinal thickening in the macular area of either eye, according to the ETDRS scale. Four trials with 10 057 participants were included in this Meta-analysis to investigate the effect of lipid-lowering agents on DME aggravation, which was defined as occurrence of DME or the increased thickness of macular during the follow-up time[11]. Lipid-lowering agents seemed to improve the outcome of DME compared with placebo (Figure 7), although this result was not statistically significant (P=0.09). This finding was in accordance with the results of previous studies[21], which showed that fenofibrate had no significant effect on DME. However, Das et al[27] has revealed a strong relationship between lipid levels and the severity of DME, and dyslipidemia is known to be an important risk factor for DME[34]. Therefore, evidence for the direct comparisons between anti-lipid drugs for DR is guaranteed in future studies.
The ophthalmic outcome of most interest to patients is how well they can see. However, our results suggested that lipid-lowering agents had no significant effect on improving vision acuity in diabetes patients with DR. That was in line with the two biggest clinical trials, the FIELD substudy over 5y and the ACCORD-Eye substudy over 4y, which also reported that lipid-lowering drugs was not expected to significantly reduce PDR-related vision loss. Here we proposed three possible explanations. Firstly, hard exudates in macular might be an important reason for worsening in vision acuity at the early stage of DR, which was related to high serum lipid level[6]. However, we found no significant effect of lipid-lowering agents on hard exudates. Therefore, there might be other mechanism(s). Another possible explanation for the absence of a significant effect of lipid-lowering agents on DR in diabetes patients was the lens status of all the participants. The average age of participants in all the included trials was about 60y, and poor lens status or pre-existing cataract might be another risk factor which will influence the results of the clinical trials. Thirdly, moderate heterogeneity and the limited number of included trials might be other factors influencing the results.
The main limitation of this Meta-analysis was the small number of included studies. For example, some subgroup analyses had small numbers of participants, which may lead to inaccuracy of estimates. Additionally, because of the small number of relevant studies, funnel plot and Egger's test had little power to correctly detect the risk of publication bias. Finally, most included trials didn't analyze the difference in response to lipid-lowing agents between types 1 and 2 diabetes. Moreover, the trials that involved hard exudates and DME only calculated the rate of developing hard exudates and DME, but the baseline data and the diagnostic criteria were not provided.
In conclusion, despite the above-mentioned limitations, this Meta-analysis revealed that in patients with DR, lipid-lowering agents could reduce the risk in progression of DR and might delay DME development, but they did not have protective effects on vision acuity and hard exudates. These findings provided important evidence that intensive control of blood lipid levels at early stage of DR potentially represented a novel therapeutic strategy for delaying DR development. Further large-scale prospective studies are warranted to confirm our results. In addition, previous studies have revealed that the effects of lipid-lowering agents on DR progression were not only related to their lipid-lowering function, therefore, whether DR patients without dyslipidemia should be administrated with oral lipid-lowering drugs still remain controversial.
Acknowledgments
Authors' contributions: Shi R, Wang F and Li R designed the study; Chen Z, Li R and Liu Y collected data and performed the analyses; Shi R and Zhao L drafted the manuscript; Liu F as an professional statistician controlled the quality of this study; Zhao L, Shi R and Liu F contributed to the discussion and revised the manuscript.
Foundations: Supported by the National Natural Science Foundation of China (No.81500726); Science & Technology project for Social development of Shaanxi Province in China (No.2017SF-249).
Conflicts of Interest: Shi R, None; Zhao L, None; Wang F, None; Liu F, None; Chen Z, None; Li R, None; Liu Y, None; Lin R, None.
REFERENCES
- 1.Hammes HP, Simó R, Lois N. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156–186. doi: 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Simó R, Hernández C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res. 2015;48:160–180. doi: 10.1016/j.preteyeres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Silva PS, Cavallerano JD, Sun JK, Aiello LM, Aiello LP. Effect of systemic medications on onset and progression of diabetic retinopathy. Nat Rev Endocrinol. 2010;6(9):494–508. doi: 10.1038/nrendo.2010.122. [DOI] [PubMed] [Google Scholar]
- 4.Pusparajah P, Lee LH, Abdul KK. Molecular markers of diabetic retinopathy: potential screening tool of the future? Front Physiol. 2016;7:200. doi: 10.3389/fphys.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks FM, Hermans MP, Fioretto P, et al. Association between plasma triglycerides and high-density lipoproteincholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-controlstudy in 13 countries. Circulation. 2013;129(9):999–1008. doi: 10.1161/CIRCULATIONAHA.113.002529. [DOI] [PubMed] [Google Scholar]
- 6.Golubovic-Arsovska M. Association of dyslipidaemia with macular oedema and hard exudates in diabetic maculopathy. Prilozi. 2007;28(2):149–160. [PubMed] [Google Scholar]
- 7.Valensi P, Picard S. Lipids, lipid-lowering therapy and diabetes complications. Diabetes Metab. 2011;37(1):15–24. doi: 10.1016/j.diabet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Papavasileiou E, Davoudi S, Roohipoor R, Cho H, Kudrimoti S, Hancock H, Wilson JG, Andreoli C, Husain D, James M, Penman A, Chen CJ, Sobrin L. Association of serum lipid levels with retinal hard exudate area in African Americans with type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):509–517. doi: 10.1007/s00417-016-3493-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang NK, Lai CC, Wang JP, Wu WC, Liu L, Yeh LK, Tseng HJ, Chang CJ, Lo FS, Chang Gung Juvenile Diabetes Eye Study Group Risk factors associated with the development of retinopathy 10 yr after the diagnosis of juvenile-onset type 1 diabetes in Taiwan: a cohort study from the CGJDES. Pediatr Diabetes. 2016;17(6):407–416. doi: 10.1111/pedi.12312. [DOI] [PubMed] [Google Scholar]
- 10.Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, Ambrosius WT, Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121(12):2443–2451. doi: 10.1016/j.ophtha.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung YR, Park SW, Choi SY, Kim SW, Moon KY, Kim JH, Lee K. Association of statin use and hypertriglyceridemia with diabetic macular edema in patients with type 2 diabetes and diabetic retinopathy. Cardiovasc Diabetol. 2017;16(1):4. doi: 10.1186/s12933-016-0486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modjtahedi BS, Bose N, Papakostas TD, Morse L, Vavvas DG, Kishan AU. Lipids and diabetic retinopathy. Semin Ophthalmol. 2016;31(1-2):10–18. doi: 10.3109/08820538.2015.1114869. [DOI] [PubMed] [Google Scholar]
- 13.Ioannidou E, Tseriotis VS, Tziomalos K. Role of lipid-lowering agents in the management of diabetic retinopathy. World J Diabetes. 2017;8(1):1–6. doi: 10.4239/wjd.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks FM. After the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: implications for fenofibrate. Am J Cardiol. 2008;102(12A):34L–40L. doi: 10.1016/j.amjcard.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 15.Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and the Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) follow-on study. Diabetes Care. 2016;39(7):1089–1100. doi: 10.2337/dc16-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2(11):894–900. doi: 10.1016/S2213-8587(14)70173-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, McGwin G., Jr Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol. 2007;125(8):1096–1099. doi: 10.1001/archopht.125.8.1096. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Erratum. Diabetic retinopathy: a position statement by the American diabetes association. Diabetes Care 2017;40: 412-418. Diabetes Care. 2017;40(9):1285. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group; Cochrane Statistical Methods Group The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG, FIELD study investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137(4):675–682. doi: 10.1016/j.ajo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56(1):1–11. doi: 10.1016/s0168-8227(01)00341-2. [DOI] [PubMed] [Google Scholar]
- 24.ACCORD Study Group; ACCORD Eye Study Group. Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC, Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massin P, Peto T, Ansquer JC, Aubonnet P, MacuFEN Study Investigators FT Effects of fenofibric acid on diabetic macular edema: the MacuFen study. Ophthalmic Epidemiol. 2014;21(5):307–317. doi: 10.3109/09286586.2014.949783. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL, Wang YG. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and Meta-analysis. Lancet Diabetes Endocrinol. 2015;3(4):263–274. doi: 10.1016/S2213-8587(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 27.Das R, Kerr R, Chakravarthy U, Hogg RE. Dyslipidemia and diabetic macular edema: a systematic review and Meta-analysis. Ophthalmology. 2015;122(9):1820–1827. doi: 10.1016/j.ophtha.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393(6687):790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009;89(1):71–78. doi: 10.1016/j.exer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshaq RS, Wright WS, Harris NR. Oxygen delivery, consumption, and conversion to reactive oxygen species in experimental models of diabetic retinopathy. Redox Biol. 2014;2:661–666. doi: 10.1016/j.redox.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnes CM, Fannon M, Laforme AM, Chaponis DM, Folkman J, Kieran MW. PPAR agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Nat Acad Sci U S A. 2008;105(3):985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammes HP, Welp R, Kempe HP, Wagner C, Siegel E, Holl RW, DPV Initiative-German BMBF Competence Network Diabetes Mellitus Risk factors for retinopathy and DME in type 2 diabetes-results from the German/Austrian DPV Database. PLoS One. 2015;10(7):e0132492. doi: 10.1371/journal.pone.0132492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatziralli IP. The role of dyslipidemia control in the progression of diabetic retinopathy in patients with type 2 diabetes mellitus. Diabetes Ther. 2017;8(2):209–212. doi: 10.1007/s13300-017-0240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]