Abstract
AIM
To assess the association between endogenous cortisol level and the risk of central serous chorioretinopathy (CSC).
METHODS
Case-control studies were systematically searched on PubMed, Embase, Cochrane, China National Knowledge Infrastructure (CNKI) for publishes between January 1990 and July 2017 to assess the association between endogenous cortisol level and CSC. The main endpoints were serum cortisol level at 8 a.m. and 24-hour urine 17-hydroxysteroids level. We assessed pooled data using a random-effects model.
RESULTS
Of 86 identified studies, 5 were eligible included in our analysis. The 5 studies included a total of 315 participants, of whom 187 had CSC. Statistically significant association was observed between serum cortisol level (summary SMD=0.77, 95%CI=0.55-0.99), 24-hour urine 17-hydroxysteroids level (summary SMD=0.95, 95%CI=0.61-1.30), and the risk of CSC.
CONCLUSION
Endogenous cortisol level is associated with an increased risk of CSC. Combined treatment targeting the serum cortisol level at 8 a.m. and 24-hour urine 17-hydroxysteroids level can be a potential preventive strategy for individuals who are at risk of CSC and therapeutic strategy for patients with CSC.
Keywords: endogenous cortisol, central serous chorio-retinopathy, serum cortisol, 24-hour urine 17-hydroxysteroids, Meta-analysis
INTRODUCTION
Central serous chorioretinopathy (CSC) was first described by Von Graefe as “relapsing idiopathic detachment of the macula” as early as 1866. And nearly 100y later, Klien[1] showed the leakage at the level of retinal pigmentation epithelium using fluorescence angiography. Nowadays, CSC gradually becomes the fourth most common retinopathy following age-related macular degeneration, diabetic retinopathy, and retinal vein occlusions[2]. The most common symptom in CSC is central scotoma. Other symptoms include dyschromatopsia, micropsia, reduced contrast sensitivity, metamorphopsia, and hypermetropization. Risk factors of this disease consist of genetic predisposition[3]–[4], cardiovascular diseases and hypertension[5], corticosteroids[6]–[7], A-type personality[8], gastroesophageal reflux[9], pregnancy[10] and obstructive sleep apnea[5]. Although these risk factors have been identified, their pathogenesis mechanisms of the disease are barely understood. Among these risk factors, the association between CSC and corticoids is probably one of the most intractable one. In the view of inflammation, glucocorticoids not only efficiently reduce macular edema but also help subretinal fluid to be absorbed[11]. But glucocorticoids can aggravate subretinal fluid accumulation in CSC patients.
Exogenous administration of glucocorticoids can cause CSC through different route, such as systemic intake (intravenous or oral)[12]–[14], triamcinolone intravitreal[15], periocular[16], and epidural[17], even localized topical using[18]. Given these controversies, there is much interest in the association between endogenous glucocorticoids and CSC. Endogenous glucocorticoids (cortisol in humans and corticosterone in rodents) are steroid hormones synthesized and released by the adrenal glands, which is regulated by the hypothalamic-pituitary-adrenal (HPA) axis[19]. Once glucocorticoids are released by adrenal glands, they play key roles in maintaining homeostasis by regulating physiologic processes[20], including metabolism, immune function, skeletal growth, cardiovascular function, reproduction, and cognition[21].
In 1993, Bouzas et al[22] reported a Cushing syndrome patient who had developed CSC. Besides Cushing syndrome, it is worth noted that A-type personality, pregnancy, and stress can also lead to the disease due to endogenous hypercortisolism[23]. Taken these together, it is of great interest whether the endogenous cortisol level in CSC patients is higher than normal. If the association between endogenous cortisol level and the risk of CSC is established, there may be more interventions to prevent and to treat this vision-threatening disease among working age people.
Case series have shown a controversial result of the level of endogenous glucocorticoids in CSC patients. In one hand, Haimovici et al[24] reported fifty percent of patients with acute CSC showed elevated 24-hour urine cortisol level. However, other studies have not shown this result. Tufan et al[25] showed negative association between serum cortisol and testosterone levels with CSC. A series of clinical studies have compared the serum cortisol levels at 8 a.m. and 24-hour urine 17-hydroxysteroids levels (the major metabolite of cortisol metabolism) between CSC patients and non-CSC age- and sex-matched controls. Considering that individual studies might not be able to provide sufficient data, we aimed to objectively assess the potential role of endogenous glucocorticoids in CSC patients by doing a Meta-analysis of randomized controlled studies.
SUBJECTS AND METHODS
Search Strategy and Selection Criteria
This Meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. We selected relevant studies by searching PubMed, Embase, Cochrane, and China National Knowledge Infrastructure (CNKI) using the MeSH terms including “central serous chorioretinopathy” and “glucocorticoids”. The complete search used for PubMed was (Central Serous Chorioretinopathy[MeSH] OR Central Serous Chorioretinopathies[Title/Abstract] OR Chorioretinopathies, Central Serous[Title/Abstract] OR Chorioretinopathy, Central Serous[Title/Abstract] OR Serous Chorioretinopathies, Central[Title/Abstract] OR Serous Chorioretinopathy, Central[Title/Abstract] OR Central Serous Retinopathy[Title/Abstract] OR Central Serous Retinopathies[Title/Abstract] OR Retinopathies, Central Serous[Title/Abstract] OR Retinopathy, Central Serous[Title/Abstract] OR Serous Retinopathies, Central[Title/Abstract] OR Serous Retinopathy, Central[Title/Abstract]) AND (Glucocorticoids[MeSH] OR Glucocorticoid Effect[Title/Abstract] OR Effect, Glucocorticoid[Title/Abstract] OR Glucocorticoid Effects[Title/Abstract] OR Effects, Glucocorticoid[Title/Abstract] OR Catatoxic Steroids[Title/Abstract] OR Steroids, Catatoxic[Title/Abstract]). All potentially eligible studies were considered irrespective of the primary outcome or language.
Study Selection and Data Extraction
Inclusion criteria were as follows: case-control studies; studies done in adults with CSC; studies done in adults without systemic intake and local usage; blood samples taken at 8 a.m.; serum cortisol levels or 24-hour urine 17-hydroxysteroids levels compared between CSC patients and non-CSC age- and sex-matched controls. Exclusion criteria were as follows: observational and retrospective studies; studies done in adults with systemic intake and local usage; blood samples not taken at 8 a.m.; no controls. We compared the endogenous glucocorticoids levels between CSC patients and non-CSC age- and sex-matched controls, with no limitation on disease course. The outcomes assessed were as follows: serum cortisol levels at 8 a.m. and 24-hour urine 17-hydroxysteroids levels.
Two independent investigators (Qu JF and Huang LZ) looked through the titles and abstracts of trails. If the trails met the inclusion criteria, full-text assessment would be retrieved for full-text assessment. Disagreement was resolved by the third investigator (Zhao MW). Data including total number of participants, age (mean±SD), sex, disease course (mean±SD), and number of first attack (%) were extracted from each selected trial.
Statistical Analysis
Serum cortisol levels at 8 a.m. and 24-hour urine 17-hydroxysteroids levels were compared between CSC patients and non-CSC age- and sex-matched controls, which were regarded as continuous variables. Pooled estimates of the mean differences in serum cortisol levels and 24-hour urine 17-hydroxysteroids levels between CSC and non-CSC groups was calculated using a random-effects model (I-V heterogeneity) to fully account for the additional uncertainty associated with inter-trial variability in testing the blood and urine sample.
Because the number of studies included in our Meta-analysis was less than 10, funnel plot to assess heterogeneity between studies and Begg and Egger tests to defined significant publication bias were not necessary. The Cochran Q test and Galbraith Plot were used to assess heterogeneity between studies. I2 testing with values greater than 50% were considered moderate-to-high heterogeneity[26]–[27]. Stata (version 12.0, USA) was used for all statistical analyses.
RESULTS
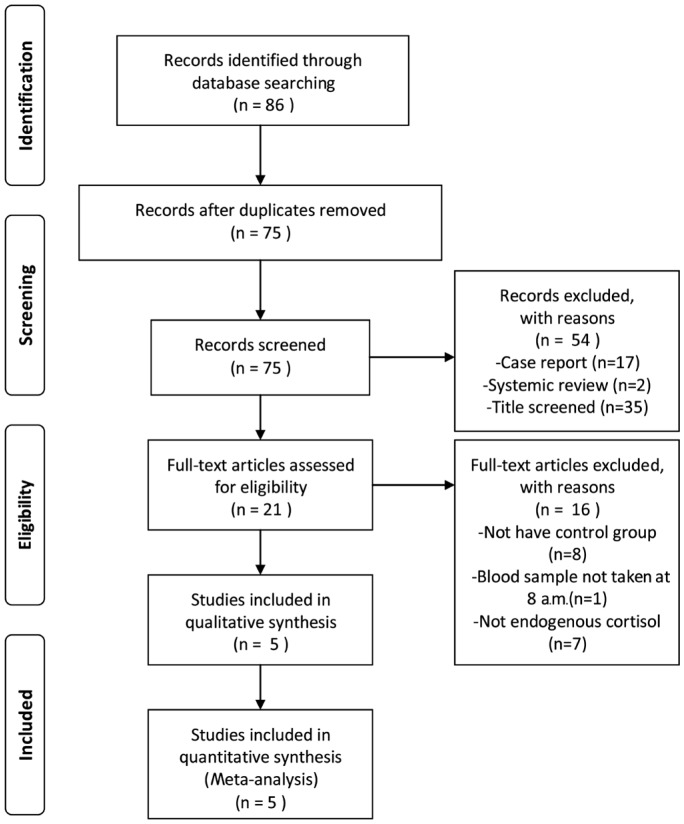
We identified 86 potentially relevant articles from our search of the published literature. Five studies[28]–[32] with 340 participants were included in this study (Figure 1). The 5 studies were published between March 1994 and December 2009 (Table 1). We found no related or relevant Meta-analyses in the Cochrane library. Mean participants age was 37.9±7.6y. All blood samples were taken at 8 a.m. In three studies, age and sexed matched healthy adult without any systemic or ocular diseases were included as controls[28]–[30]. One trial used patients with acute unilateral retinal detachment[31], while the other trial controls used 10 retinal detachment patients and one central retinal artery occlusion and one vitreous hemorrhage patient as controls[32]. In one trial cortisol analysis was done on the spectrophotometer based on the Porter-Silber reaction while other studies tested cortisol by radioimmunoassay (RIA).
Figure 1. Flow chart of study selection.
Table 1. Characteristics of included studies.
| Parameters |
Study groups |
Control groups |
||||||||||
| Study | Year | No. | M:F | Mean age (y) | Mean weight (kg) | Disease course (mo) | Number of patients of first attack | No. | M:F | Mean age (y) | Mean weight (kg) | Health condition |
| Garg | 1997 | 30 | 30:0 | 28.6±5.59 | Not available | 8.56±4.19 | 30 (100%) | 30 | 30:0 | 29.27±5.07 | Not available | Unhealthya |
| Zarkir | 2009 | 23 | 22:1 | 37.1±9.7 | 53.80±5.44 | 25.13±12.11 | 22 (100%) | 12 | 11:1 | 35.7±9.2 | 51.87±6.95 | Unhealthyb |
| Shang | 1999 | 44 | 40:4 | 38.79±8.27 | Not available | 2-8 | 44 (100%) | 41 | 37:4 | 38.63±8.16 | Not available | Healthy |
| Wang | 2007 | 50 | 41:9 | 38.5±14.2 | Not available | <10 | Not available | 40 | 32:8 | 38.8±13.9 | Not available | Healthy |
| Xu | 1994 | 40 | 28:12 | 38.3 | Not available | 3-120 | 33 (82.5%) | 30 | 21:9 | 36.4 | Not available | Healthy |
aAcute unilateral retinal detachment; bTen had retinal detachment (cases), one had central retinal artery occlusion and one had vitreous hemorrhage.
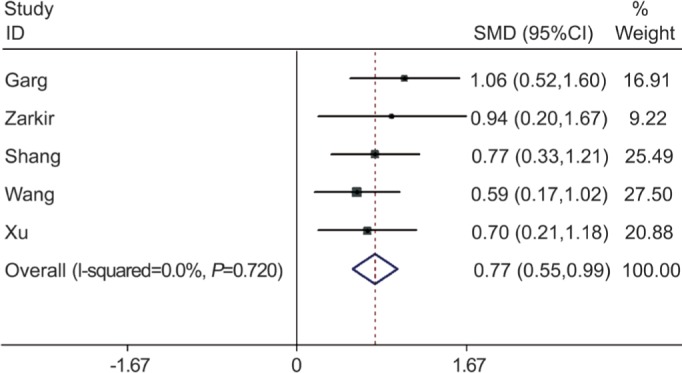
In a pooled analysis of all 5 studies, the serum cortisol levels at 8 a.m. in CSC group were much higher than that in non-CSC group. There was significant association between serum cortisol level (summary SMD=0.77, 95%CI=0.55-0.99) and the risk of CSC in case-control studies, without statistically significant between-study heterogeneity (I2=0, P=0.720) (Figure 2).
Figure 2. Meta-analysis of the serum cortisol levels at 8 a.m. in CSC group and in non-CSC group.
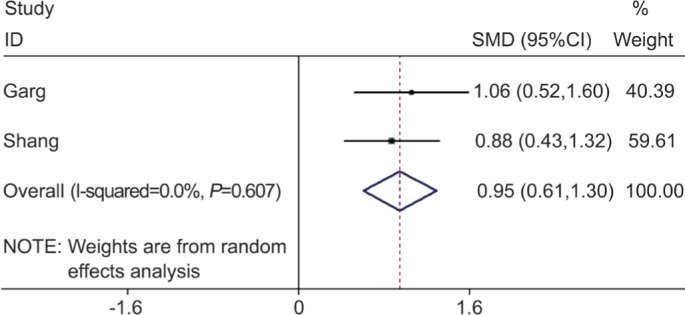
In a pooled analysis of the 2 studies that assessed the 24-hour urine cortisol values, there was a significant association between 24-hour urine cortisol values (summary SMD=0.95, 95%CI=0.61-1.30) and the risk of CSC in case-control studies, without statistically significant between-study heterogeneity (I2=0, P=0.607) (Figure 3).
Figure 3. Meta-analysis of the 24-hour urine cortisol values in CSC group and in non-CSC group.
DISCUSSION
This Meta-analysis shows that compared with non-CSC controls, the endogenous cortisol level is higher in patients with CSC. According to the pooled crude standardized mean difference from the included studies, both serum cortisol levels at 8 a.m. and 24-hour urine 17-hydroxysteroids levels are significantly higher in patients with CSC. Accumulation of subretinal fluid in CSC patients is associated with high levels of endogenous glucocorticoids. However, the mechanisms that glucocorticoids cause CSC still remain unclear.
Endogenous glucocorticoids may impair the functions of retinal pigment epithelium (RPE), Bruch's membrane, and choroidal circulation[20]. Glucocorticoids can directly damage the RPE cells or their tight junctions and may decelerate or destroy some process in damaged RPE cells[33], which will cause fluid to accumulate under the retina and the leakage from choroidal vessels cross the damaged junctions[6]. In fact, Arndt et al[34] found that the baseline transepithelial potential and resistance were remarkably decreased when hydrocortisone was added on the apical side of the RPE in porcine and bovine tissue. Glucocorticoids can also inhibit formation of collagen, which is the main component of Bruch's membrane[35]–[36]. Lack of collagen will predispose a patient to serous retinal detachment. Increased choroidal thickness has been reported in both affected and fellow eyes of CSC patients. As a result, choroidal vessels are considered as the site of primary damage of CSC. Glucocorticoids excess may cause increased capillary fragility and hyper-permeability, which will damage the function of choroidal vessels and cause accumulation of leaked fluid in the subretinal space[6]. In addition, glucocorticoids affect the production of nitric oxide[37], prostaglandins, and free radicals, which may change the status of circulation of choroid[6]. Schubert et al[38] proposed that as the major cell-cell adhesion molecule in vascular endothelium, cadherin 5 (CDH5) was downregulated by corticosteroids, which probably increased permeability of choroidal vasculature. Some reports also suggest that activated mineralocorticoid receptor (MR) may play an important role in the course of CSC. Activation of the MR pathway by glucocorticoids results upregulation of the endothelial vasodilatory potassium channel KCa2.3, which leads to the hyperpolarization of endothelial cells and underlying smooth muscle cells[39]–[41]. Subsequently, choroid vasodilation will occur and fluid will be accumulated at the subretinal space of posterior pole of the fundus, creating a circumscribed area of serous retinal detachment. However, an exact pathogenesis mechanism of the disease remains unclear due to lack of relevant experimental data.
The current study has some limitations which may affect the final conclusions. First, the number of included studies is small, which causes the conclusion of this Meta-analysis to be limited to certain extent. Second, the selection bias is inevitable given the search strategy was limited to the articles published in English or Chinese missing potentially high-quality data published in other languages. Third, all studies are conducted among Asian countries, where CSC is endemic. To some extent, therefore, this Meta-analysis cannot implicate the associations between endogenous cortisol level and the risk of CSC around the world. Fourth, the possibility of selection biases and unidentified confounding biases cannot be excluded because all the included studies were case-controlled studies. Finally, only serum cortisol levels at 8 a.m. and 24-hour urine 17-hydroxysteroids levels were included in this analysis. Morning cortisol levels at 8 a.m. were analyzed because cortisol levels follow a relatively predictable circadian rhythm with a morning peak after awakening almost around 8 a.m. But it may not represent the serum cortisol level of the whole day. A study designed to analyze the serum cortisol of various time points rather than 8 a.m. only is needed to confirm this association. There are other biomarkers to assess endogenous cortisol level including 24-hour urine unary free cortisol, 24-hour urine 17-ketosteriods, serum adreno-cortico-tropic-hormone (ACTH), and serum corticotropin releasing hormone (CRH). They were not included because there were less than 2 studies for each biomarker.
In conclusion, we found that endogenous cortisol level at 8 a.m. was associated with an increased risk of CSC in parts of Asia. Frequent examination of patients with high risk of CSC, such as cardiovascular diseases, hypertension, A-type personality, gastroesophageal reflux, obstructive sleep apnea and pregnancy, may be useful for timely prognosis of the disease and implementing preventive treatment. Future studies should focus on the mechanisms of corticosteroid-caused CSC and whether the MR inhibitor can serve at treatment for CSC patients.
Acknowledgments
Conflicts of Interest: Liang ZQ, None; Huang LZ, None; Qu JF, None; Zhao MW, None.
REFERENCES
- 1.Klien BA. Macular diseases: clinical manifestations. Trans Am Acad Ophthalmol Otolaryngol. 1965;69:614–622. [PubMed] [Google Scholar]
- 2.Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 3.Amalric P, Gourinat P, Rebière P. Is central serous choroiditis sometimes hereditary? Bull Soc Ophtalmol Fr. 1971;71(2):163–168. [PubMed] [Google Scholar]
- 4.Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F. Pachychoroid: an inherited condition? Retina. 2015;35(1):10–16. doi: 10.1097/IAE.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 5.Eom Y, Oh J, Kim SW, Huh K. Systemic factors associated with central serous chorioretinopathy in Koreans. Korean J Ophthalmol. 2012;26(4):260–264. doi: 10.3341/kjo.2012.26.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cusani M. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2004;49(1):128–129. doi: 10.1016/j.survophthal.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Han JM, Hwang JM, Kim JS, Park KH, Woo SJ. Changes in choroidal thickness after systemic administration of high-dose corticosteroids: a pilot study. Invest Ophthalmol Vis Sci. 2014;55(1):440–445. doi: 10.1167/iovs.13-12854. [DOI] [PubMed] [Google Scholar]
- 8.Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986;84:799–845. [PMC free article] [PubMed] [Google Scholar]
- 9.Mansuetta CC, Mason JO, 3rd, Swanner J, Feist RM, White MF, Jr, Thomley ML, McGwin G, Jr, Emond TL. An association between central serous chorioretinopathy and gastroesophageal reflux disease. Am J Ophthalmol. 2004;137(6):1096–1100. doi: 10.1016/j.ajo.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Errera MH, Kohly RP, da Cruz L. Pregnancy-associated retinal diseases and their management. Surv Ophthalmol. 2013;58(2):127–142. doi: 10.1016/j.survophthal.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Noma H, Funatsu H, Mimura T, Shimada K. Comparison of the efficacy of intravitreal triamcinolone acetonide for cystoid macular edema with versus without serous retinal detachment in branch retinal vein occlusion: influence on macular sensitivity and morphology. BMC Ophthalmol. 2012;12:39. doi: 10.1186/1471-2415-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai DC, Chen SJ, Huang CC, Chou P, Chung CM, Chan WL, Huang PH, Lin SJ, Chen JW, Chen TJ, Leu HB. Risk of central serous chorioretinopathy in adults prescribed oral corticosteroids: a population-based study in Taiwan. Retina. 2014;34(9):1867–1874. doi: 10.1097/IAE.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 13.Valls Pascual E, Martínez-Costa L, Santander F. Central serous chorioretinopathy and systemic corticosteroids in rheumatic diseases: report of three cases. BMC Musculoskelet Disord. 2015;16:378. doi: 10.1186/s12891-015-0843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geoffroy M, Afriat M, Fauconier M, Eschard JP, Salmon JH. Adverse effect of corticosteroid therapy: central serous chorioretinopathy. Joint Bone Spine. 2018;85(1):127–128. doi: 10.1016/j.jbspin.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Imasawa M, Ohshiro T, Gotoh T, Imai M, Iijima H. Central serous chorioretinopathy following vitrectomy with intravitreal triamcinolone acetonide for diabetic macular oedema. Acta Ophthalmol Scand. 2005;83(1):132–133. doi: 10.1111/j.1600-0420.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 16.Baumal CR, Martidis A, Truong SN. Central serous chorioretinopathy associated with periocular corticosteroid injection treatment for HLA-B27-associated iritis. Arch Ophthalmol. 2004;122(6):926–928. doi: 10.1001/archopht.122.6.926. [DOI] [PubMed] [Google Scholar]
- 17.Iida T, Spaide RF, Negrao SG, Carvalho CA, Yannuzzi LA. Central serous chorioretinopathy after epidural corticosteroid injection. Am J Ophthalmol. 2001;132(3):423–425. doi: 10.1016/s0002-9394(01)00970-9. [DOI] [PubMed] [Google Scholar]
- 18.Chan LY, Adam RS, Adam DN. Localized topical steroid use and central serous retinopathy. J Dermatolog Treat. 2016;27(5):425–426. doi: 10.3109/09546634.2015.1136049. [DOI] [PubMed] [Google Scholar]
- 19.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson BP, Atchison E, Idris AA, Bakri SJ. Central serous chorioretinopathy and glucocorticoids: an update on evidence for association. Surv Ophthalmol. 2018;63(1):1–8. doi: 10.1016/j.survophthal.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am. 2016;42(1) doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouzas EA, Scott MH, Mastorakos G, Chrousos GP, Kaiser-Kupfer MI. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol. 1993;111(9):1229–1233. doi: 10.1001/archopht.1993.01090090081024. [DOI] [PubMed] [Google Scholar]
- 23.Giovansili I, Belange G, Affortit A. Cushing disease revealed by bilateral atypical central serous chorioretinopathy: case report. Endocr Pract. 2013;19(5):e129–e133. doi: 10.4158/EP12389.CR. [DOI] [PubMed] [Google Scholar]
- 24.Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110(4):698–703. doi: 10.1016/S0161-6420(02)01975-9. [DOI] [PubMed] [Google Scholar]
- 25.Tufan HA, Gencer B, Comez AT. Serum cortisol and testosterone levels in chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):677–680. doi: 10.1007/s00417-012-2075-8. [DOI] [PubMed] [Google Scholar]
- 26.Crippa A, Khudyakov P, Wang M, Orsini N, Spiegelman D. A new measure of between-studies heterogeneity in Meta-analysis. Stat Med. 2016;35(21):3661–3675. doi: 10.1002/sim.6980. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang WY. The variation of serum glucocorticoids level and catecholamine of central serous chorioretinopathy patients. J Med Theor & Prac. 2007;20(10):1185–1186. [Google Scholar]
- 29.Xu QW JK, Zhang GJ, Tian FL, Wang XY. Serum cortisol level by radioimmunoassay of central serous chorioretinopathy patients. Journal of Jining Medical College. 1994;17(1):7–8. [Google Scholar]
- 30.Shang Q, Liu C, Wei S, Shi F, Li Y, Qiao L. Determination of cortisol in plasma and 24-hour urine of patients with central serous chorioretinopathy. Zhonghua Yan Ke Za Zhi. 1999;35(4):297–299. [PubMed] [Google Scholar]
- 31.Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81(11):962–964. doi: 10.1136/bjo.81.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zakir SM, Shukla M, Simi ZU, Ahmad J, Sajid M. Serum cortisol and testosterone levels in idiopathic central serous chorioretinopathy. Indian J Ophthalmol. 2009;57(6):419–422. doi: 10.4103/0301-4738.57143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polak BC, Baarsma GS, Snyers B. Diffuse retinal pigment epitheliopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1995;79(10):922–925. doi: 10.1136/bjo.79.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arndt C, Sari A, Ferre M, Parrat E, Courtas D, De Seze J, Hache J, Matran R. Electrophysiological effects of corticosteroids on the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42(2):472–475. [PubMed] [Google Scholar]
- 35.Oikarinen AI, Uitto J, Oikarinen J. Glucocorticoid action on connective tissue: from molecular mechanisms to clinical practice. Med Biol. 1986;64(5):221–230. [PubMed] [Google Scholar]
- 36.Norouzpour A, Abrishami M. Central serous chorioretinopathy: from glucocorticoids to light intensity. Int J Ophthalmol. 2016;9(2):312–314. doi: 10.18240/ijo.2016.02.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worrall NK, Misko TP, Sullivan PM, Hui JJ, Rodi CP, Ferguson TB., Jr Corticosteroids inhibit expression of inducible nitric oxide synthase during acute cardiac allograft rejection. Transplantation. 1996;61(2):324–328. doi: 10.1097/00007890-199601270-00028. [DOI] [PubMed] [Google Scholar]
- 38.Schubert C, Pryds A, Zeng S, Xie Y, Freund KB, Spaide RF, Merriam JC, Barbazetto I, Slakter JS, Chang S, Munch IC, Drack AV, Hernandez J, Yzer S, Merriam JE, Linneberg A, Larsen M, Yannuzzi LA, Mullins RF, Allikmets R. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. 2014;35(7):859–867. doi: 10.1002/humu.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao M, Valamanesh F, Celerier I, Savoldelli M, Jonet L, Jeanny JC, Jaisser F, Farman N, Behar-Cohen F. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Müller glial cells. FASEB J. 2010;24(9):3405–3415. doi: 10.1096/fj.09-154344. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672–2679. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, Jaisser F, Behar-Cohen F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]