Abstract
Objectives
Vestibular loss is a debilitating condition, and despite its high prevalence in older adults, the quality of life (QoL) burden of vestibular loss in older individuals has not been well‐studied. This report quantifies the impact on overall QoL and identifies domains of health most affected. We hypothesize vestibular loss will be associated with impairment in diverse domains of health‐related QoL.
Study Design
Prospective, case‐control study.
Methods
A convenience sample of 27 patients age ≥60 years with vestibular physiologic loss was recruited from an academic neurotology clinic. The patients did not have any identifiable cause of their vestibular loss other than aging. The convenience sample was compared to an age‐matched cross‐sectional sample of the general US population (n = 1266). The main outcome was QoL measured by the Ontario Health Utilities Index Mark III (HUI3).
Results
Compared to the general population, patients with vestibular loss had significantly lower overall unadjusted HUI3 scores (−0.32, p < 0.001). Multivariate regression analysis showed vestibular loss was significantly associated with poorer performance in vision (−0.11 p < 0.0001), speech (−0.15, p < 0.0001), dexterity (−0.13, p < 0.0001), and emotion (−0.07, p = 0.0065). Adjusted aggregate HUI3 was also significantly lower for vestibular loss (−0.15, p = 0.0105). These QoL decrements resulted in an average loss of 1.30 Quality‐Adjusted Life Years (QALYs). When using a $50,000/QALY willingness‐to‐pay threshold, vestibular loss was associated with a $64,929 lifetime economic burden per affected older adult, resulting in a total lifetime societal burden of $227 billion for the US population ≥60 years of age.
Conclusions
Loss of vestibular function with aging significantly decreases quality of life across multiple domains of well‐being. These QoL reductions are responsible for heavy societal economic burdens of vestibular loss, which reveal potential benefits of prompt diagnosis and treatment of this condition.
Level of Evidence
3
Keywords: Vestibular loss, aging, health‐related quality of life
INTRODUCTION
The vestibular system is integral to balance control, locomotion, and spatial navigation. Loss of vestibular function can be a debilitating condition that causes imbalance, unsteady vision, and a 12‐fold increased risk of falls and fall‐associated morbidity.1 Individuals with vestibular loss have difficulty carrying out activities of daily living such as walking, climbing stairs, and driving, and these individuals report increased dependence on others, reduced productivity, and decreased life satisfaction.2, 3, 4, 5 As with other sensory systems, vestibular function declines with age, and older individuals are disproportionately affected by vestibular loss. Some degree of physiologic vestibular impairment occurs in 50% of older adults age ≥ 60 years,1 and symptoms of vestibular loss such as imbalance with ambulation or unsteady vision (oscillopsia) are commonly reported by community‐dwelling older adults.6, 7, 8, 9, 10 The increased prevalence of vestibular loss in the older population has potentially substantial economic and societal consequences.
Despite the greater prevalence of vestibular loss in older adults and its associated functional limitations, the quality of life (QoL) burden of vestibular loss in this vulnerable population has not been well‐studied. The few studies that have reported QoL outcomes considered the broader symptom of dizziness rather than specifically vestibular physiologic impairment,7, 11, 12, 13, 14, 15 did not have a normative‐age‐matched comparison group available,11 or were conducted across a broad age range including younger age groups.7, 13, 16
In this report, we quantify the independent impact of vestibular physiologic loss on overall QoL and identify domains of health including vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain affected by this condition in a sample of older adults with vestibular loss seen in a Neurotology clinic. We compare results in this sample to QoL attainment among age‐matched peers from the general US population. Lastly, we report the individual and societal economic burden of vestibular loss in aging adults using the difference in QoL attainment between the study sample and normative control groups.
MATERIALS AND METHODS
Study Design and Study Population
Approval for this study was obtained from the Hospital Institutional Review Board. Patients were recruited from the Otology–Neurotology practice within the Department of Otolaryngology–Head and Neck Surgery. Eligible participants were age ≥60 years who presented with dizziness and imbalance, and had evidence of vestibular impairment confirmed by vestibular physiologic testing. A cutoff of ≥60 years of age was used as a defined criteria for an older population.17 Vestibular testing procedures consisted of standard clinical assessments including head impulse testing (HIT; either qualitative or quantitative using video‐oculography), measurement of cervical vestibular‐evoked myogenic potentials (cVEMP), caloric testing, and/or rotatory chair testing. The patients did not have a specific vestibular diagnosis, such as Menière's disease or benign paroxysmal positional vertigo, as this was a study of vestibular loss primarily due to advanced age. Demographic, socioeconomic, and medical history factors were collected for each patient through a medical chart review.
Patients in this study completed a paper‐based QoL survey. The survey included the Health Utilities Index Mark 3 (HUI3) questionnaire, which is a 15‐item, population‐based, validated health utility instrument that measures the respondent's general health status and health‐related quality of life along 8 specific domains of function: vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain.18 It has been used extensively in health economic analyses, including studies of cochlear implantation19, 20 and bilateral vestibular deficiency in younger adults.21 In the present study, each respondent's individual domain and overall health utility were calculated using methods prescribed for analysis of HUI3 data,18 yielding scores ranging from 1 (“perfect health”) to 0 (“death”) on the individual domains and 1 (“perfect health”) to −0.371 (a state “worse than death”) on the overall index. The unit for health utility from the HUI3 is the quality‐adjusted life year (QALY).
Normative age‐matched data from the general US population was collected from the 2002–2003 Joint Canada/United States Survey of Health (JCUSH), which is a cross‐sectional random‐digit‐dialed telephone survey conducted in Canada and the United States, administered via a computer‐assisted telephone interview (CATI).22 The HUI3 is also administered as part of JCUSH. A total of 8,145 people of all ages took part in JCUSH, of which 5,859 participants were US residents. Of these, 1,369 were at least 60 years of age at time of study. Of the eligible adults, 104 (7.6%) participants did not complete the HUI3 questionnaire, resulting in a sample size of 1,265 participants. There were no significant differences between included and excluded participants in the HUI3 survey with respect to gender or race. Included participants, however, were more likely to be younger and single.
Assessment of Comorbidities
A history of smoking was defined in JCUSH based on smoking greater than 100 cigarettes in one's lifetime, and in patients based on a positive smoking history ascertained from the medical record. Hypertension was defined in JCUSH based on responding yes to the question “Have you ever been told by a doctor or other health professional that you have high blood pressure, also called hypertension?” and in patients based on a diagnosis of hypertension in the medical record. Diabetes mellitus was defined in JCUSH based on responding yes to the question “Have you ever been told by a doctor or other health professional that you have diabetes?” and in patients based on a diagnosis of diabetes mellitus in the medical record. Vision loss was defined in JCUSH as a positive response to the question “Do you have problems with vision (whether corrected or uncorrected with glasses or lenses)?” and in patients based on a diagnosis of vision loss from the medical record. Hearing loss was defined in JCUSH based on a positive response to the question “Do you have difficulty hearing (whether corrected or uncorrected with hearing aids) and in patients based on a diagnosis of hearing loss (either self‐reported, or PTA > 25 dB in the better‐hearing ear) ascertained from the medical record. A history of stroke was defined in JCUSH based on a positive response to the question “What condition or health problem causes you to have difficulty?” with the patient's answer as “Stroke problem.” JCUSH did not probe directly about a stroke history, but rather a history of stroke was ascertained based on a participant having difficulty in their daily life that they attributed to a history of stroke. As such, 481 individuals in JCUSH were missing data for the stroke variable. We carried out sensitivity analyses including and excluding the stroke variable from analyses to evaluate the impact of these missing data. In general the findings changed very little; therefore we included history of stroke in all analyses.
Discounting and Time Horizon
Total individual QALYs lost due to vestibular loss were calculated and averaged across all study patients by compounding the yearly adjusted health utility loss associated with vestibular loss across an individual's remaining exact age life expectancy stratified by gender.23 The discount rate is the factor by which individuals preferentially value costs and benefits incurred in the present to those incurred in the future (e.g., one would rather receive $100 today than $100 dollars a year from now). A discount rate of 3% was utilized in all base QALY estimates, as it represents the average US government's borrowing rate, which has been argued as the most appropriate intra‐generational discounting metric for use in cost‐benefit analyses.24, 25 In this case, a 3% discount rate implies that the magnitude of the annual QALY losses associated with vestibular loss (and conversely potential benefits from restoring vestibular function) will decrease with every subsequent year by a factor of 1/1.03 of the prior year's value.
Perspective
Following collection of individual health‐utility data, a societal perspective analysis was performed to calculate the overall economic burden of vestibular loss using a commonly accepted $50,000 Willingness‐to‐Pay (WTP) threshold to gain one QALY.26
Economic Burden and Sensitivity Analysis
The total economic burden of vestibular loss resulting from the above QALY loss was calculated for the total patient study group and stratified across three age categories, 60–69 years, 70–79 years, and ≥80 years. The study sample's QALY losses and associated economic burden was then generalized to the overall susceptible US population 60 years of age and older using an average of literature‐derived prevalence rates of symptomatic vestibular vertigo (from which we expect our study patients were drawn),6, 7, 8, 10 relative‐prevalence weights by decade of age among US adults,1 and the size of the US population27 stratified by above age categories. Base case results were calculated for each age group using a 3% discount rate, a $50,000 WTP threshold, a weighted symptomatic vestibular loss prevalence of 8%, and an annual QoL decrease equivalent to the vestibular loss coefficient derived from the adjusted generalized linear model of overall HUI. Sensitivity analyses were performed by varying these four parameters.
Statistical analysis
Baseline demographic and medical history factors (Table 1) were characterized by mean and standard error for continuous variables and by frequency distributions and percentage of total for categorical variables. Respondents' overall health states were calculated using the prescribed methodology provided for the HUI3 instrument.16 Baseline differences in health utilities were explored using a multivariable generalized linear model, allowing for response variables that have both Gaussian and non‐Gaussian distributions. Covariates included demographic (age, gender, race, marital status) and clinical characteristics (history of hypertension, diabetes, stroke, smoking, hearing loss, and vision loss). STATA 13 (Stata Corp, College Station, TX) was used for all statistical analyses.
Table 1.
Quality of Life Attainment in Patients with Vestibular Loss by Demographic and Medical History Factors
| Vestibular Loss (n = 27) | General Population (n = 1265) | |||
|---|---|---|---|---|
| Characteristic | N (%) | HUI3 Mean (SE)a | N (%) | HUI3 Mean (SE)a |
| Gender | ||||
| Male | 10 (37.0) | 0.53 (0.35) | 489 (38.7) | 0.81 (0.25) |
| Female | 17 (63.0) | 0.44 (0.32) | 776 (61.3) | 0.77 (0.27) |
| Age | ||||
| 60–69 years | 6 (22.2) | 0.44 (0.41) | 580 (45.8) | 0.82 (0.24) |
| 70–79 years | 12 (44.4) | 0.54 (0.34) | 481 (38.0) | 0.79 (0.25) |
| ≥80 years | 9 (33.3) | 0.40 (0.28) | 204 (16.1) | 0.69 (0.30) |
| Race | ||||
| Non‐Hispanic White | 21 (80.8) | 0.41 (0.34) | 1000 (82.0) | 0.80 (0.25) |
| Non‐Hispanic Black | 4 (15.4) | 0.70 (0.21) | 93 (7.6) | 0.68 (0.32) |
| Hispanic | NA | NA | 61 (5.0) | 0.72 (0.30) |
| Asian | NA | NA | 21 (1.7) | 0.87 (0.21) |
| Other | 1 (3.8) | 0.82 (0.00) | 4 (3.6) | 0.74 (0.28) |
| Marital Status | ||||
| Single | NA | NA | 58 (4.6) | 0.77 (0.26) |
| Married | 2 (8.0) | 0.10 (0.28) | 606 (47.9) | 0.83 (0.24) |
| Widowed | 17 (68.0) | 0.53 (0.34) | 411 (32.5) | 0.73 (0.29) |
| Divorced | 6 (24.0) | 0.35 (0.23) | 149 (11.8) | 0.78 (0.25) |
| Unknown | NA | NA | 41 (3.2) | 0.84 (0.21) |
| History of Diabetes | ||||
| Yes | 8 (29.6) | 0.54 (0.32) | 177 (15.7) | 0.67 (0.30) |
| No | 19 (70.4) | 0.44 (0.34) | 953 (84.3) | 0.80 (0.25) |
| History of Hypertension | ||||
| Yes | 21 (77.8) | 0.44 (0.34) | 639 (50.7) | 0.76 (0.28) |
| No | 6 (22.2) | 0.57 (0.31) | 621 (49.3) | 0.82 (0.23) |
| History of Strokeb | ||||
| Yes | 3 (11.1) | 0.29 (0.17) | 26 (3.3) | 0.28 (0.27) |
| No | 24 (88.9) | 0.50 (0.34) | 758 (96.7) | 0.72 (0.28) |
| History of Smoking | ||||
| Yes | 15 (55.6) | 0.44 (0.38) | 651 (51.8) | 0.79 (0.26) |
| No | 12 (44.4) | 0.51 (0.25) | 606 (48.2) | 0.79 (0.26) |
| History of Hearing Lossc | ||||
| Yes | 18 (66.7) | 0.52 (0.27) | 122 (9.6) | 0.55 (0.29) |
| No | 9 (33.3) | 0.38 (0.42) | 1143 (90.4) | 0.81 (0.24) |
| History of Vision Lossd | ||||
| Yes | 11 (40.7) | 0.46 (0.33) | 1006 (88.8) | 0.77 (0.27) |
| No | 16 (59.3) | 0.48 (0.34) | 127 (11.2) | 0.78 (0.27) |
Health Utilities Index measured using Mark III transforms.
481 participants were missing data about a history of stroke, given how the variable was coded, see text for details.
History of hearing loss assessed by average pure‐tone hearing threshold (>25 dB) in the vestibular loss group and by participant response to presence of self‐reported or diagnosed hearing problems in the Joint Canada/United States Survey of Health
History of vision loss assessed by presence of ophthalmologic comorbidities in the vestibular loss group and by participant response to presence of self‐reported or diagnosed vision problems in the Joint Canada/United States Survey of Health
HUI3, Health Utilities Index Mark III; NA, not applicable; SE, standard error
RESULTS
Demographic and clinical characteristics of the vestibular loss patient and general population groups are shown in Table 1. The mean age and age ranges for the study and control groups was 76.3 (60–87) and 71.4 (60–85) years, respectively. Patients in the study group in general had greater comorbidity relative to the general population, with higher prevalences of diabetes, hypertension, stroke, smoking, and hearing loss. For most demographic and all comorbidity factors, the patient population had a lower HUI3 mean score. All of the patients in the study group had physiologic evidence of vestibular hypofunction that was not attributable to a specific diagnosis other than age. There were 21 (78%) patients with bilateral vestibular loss (BVL) and 6 (22%) patients with unilateral vestibular loss (UVL) in the patient group (Table 2).
Table 2.
Characteristics of Subjects with Vestibular Loss.
| Participant | Age (years) | Gender | Bilaterala | Basis for Diagnosisb |
|---|---|---|---|---|
| 1 | 60 | Male | Yes | HIT abnormal AU, absent cVEMP AU |
| 2 | 64 | Female | Yes | HIT abnormal AU, absent cVEMP AS |
| 3 | 67 | Female | No | HIT abnormal AS |
| 4 | 67 | Male | No | HIT abnormal AS |
| 5 | 67 | Female | Yes | HIT abnormal AU, caloric weakness AS |
| 6 | 69 | Female | Yes | Caloric weakness AS, absent cVEMP AU |
| 7 | 70 | Female | No | HIT abnormal AS, absent cVEMP AD |
| 8 | 70 | Male | Yes | Rotatory chair testing abnormal AU |
| 9 | 71 | Female | Yes | Absent cVEMP AU |
| 10 | 72 | Female | Yes | HIT abnormal AU |
| 11 | 74 | Female | Yes | HIT abnormal AU |
| 12 | 74 | Female | Yes | HIT abnormal AU |
| 13 | 77 | Male | Yes | HIT abnormal AU, caloric weakness AS, absent cVEMP AS |
| 14 | 77 | Male | Yes | HIT abnormal AS, caloric weakness AS, absent cVEMP AD |
| 15 | 78 | Female | Yes | HIT abnormal AU, absent cVEMP AS |
| 16 | 78 | Female | Yes | HIT abnormal AU, no caloric response AS, absent cVEMP AU |
| 17 | 79 | Male | No | HIT abnormal AU |
| 18 | 79 | Male | Yes | Caloric weakness AU, absent cVEMP AU |
| 19 | 80 | Female | Yes | HIT abnormal AU |
| 20 | 83 | Male | No | HIT abnormal AS |
| 21 | 84 | Female | Yes | HIT abnormal AU |
| 22 | 84 | Female | Yes | HIT abnormal AU |
| 23 | 86 | Male | Yes | HIT abnormal AU, absent cVEMP AD |
| 24 | 87 | Female | Yes | HIT abnormal AU |
| 25 | 87 | Female | No | HIT abnormal AU, absent cVEMP AU |
| 26 | 87 | Male | Yes | HIT abnormal AU, absent cVEMP AU |
| 27 | 87 | Female | Yes | HIT abnormal AU |
| Overall | 76 | 63% Female | 78% Bilateral |
Bilateral vs unilateral vestibular loss.
For unilateral caloric weakness, inter‐aural asymmetry > 20%, for bilateral caloric weakness, total slow phase velocity < 20 degrees/second.
AD, right ear; AS, left ear; AU, both ears; cVEMP, cervical vestibular‐evoked myogenic potential; HIT, head impulse testing
There was a 100% response rate for the HUI3 questions among patient respondents. Mean unadjusted HUI3 overall and domain‐specific scores of study sample versus general population controls are shown in Figure 1. The overall HUI score was 0.47 among patients (on a scale from −0.371 to 1 as described previously) compared to 0.79 among age‐matched general population controls. Statistically significant differences between patient and control groups were observed for overall score (p < 0.001), and for specific domains including vision (p < 0.002), hearing (p < 0.001), speech (p < 0.001), ambulation (p < 0.001), dexterity (p < 0.001), and emotion (p < 0.001) in unadjusted comparisons. Table 3 reports results of a multivariable generalized linear model analysis of variables associated with overall HUI3 score. After adjusting for gender, age, race, marital status, history of diabetes, hypertension, stroke, smoking, hearing loss, and vision loss, vestibular loss was responsible for a 0.15 decrease in overall health utility (p = 0.0105). Covariates significantly associated with a decreased overall HUI score included age ≥80 years (−0.07, p = 0.0253), non‐Hispanic Black race (−0.10, p = 0.0054), Hispanic race (−0.12, p = 0.0087), history of diabetes (−0.08, p = 0.0014), stroke (−0.36, p < 0.0001), vision loss (−0.07, p = 0.0075), and hearing loss (−0.18, p < 0.0001). A marital status of “married” correlated with a significantly increased overall HUI score (0.12, p = 0.0110).
Figure 1.
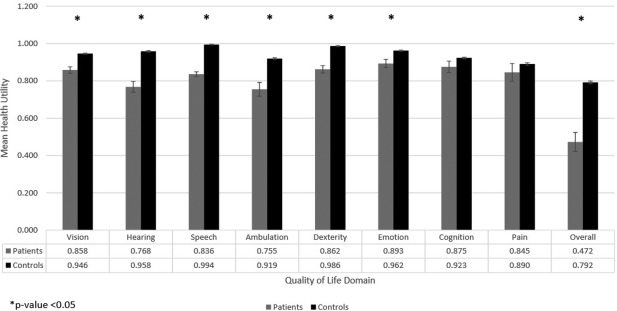
Mean unadjusted HUI3 domain and overall scores for patients with vestibular loss and the general population
Table 3.
Multivariable Adjusted Generalized Linear Model on the Association of Overall HUI3 Score with Vestibular Loss.
| Health Utilities Index Mark III Overall Scoreb | |||
|---|---|---|---|
| Variable | Coefficient | 95% Confidence Interval | P‐Value |
| Vestibular Loss | −0.15 | −0.26, −0.03 | 0.0105 |
| Female | −0.01 | −0.06, 0.04 | 0.7558 |
| Age | |||
| 65–69 years | refa | refa | refa |
| 70–79 years | −0.01 | −0.05, 0.04 | 0.8075 |
| ≥80 years | −0.07 | −0.13, −0.01 | 0.0253 |
| Race | |||
| Non‐Hispanic White | refa | refa | refa |
| Non‐Hispanic Black | −0.10 | −0.17, −0.03 | 0.0054 |
| Hispanic | −0.12 | −0.21, −0.03 | 0.0087 |
| Asian | 0.13 | −0.10, 0.37 | 0.2684 |
| Other | −0.03 | −0.14, 0.08 | 0.5602 |
| Marital Status | |||
| Single | refa | refa | refa |
| Married | 0.12 | 0.03, 0.22 | 0.0110 |
| Widowed | 0.07 | −0.03, 0.17 | 0.1729 |
| Divorced | 0.08 | −0.02, 0.19 | 0.1276 |
| Diabetes | −0.08 | −0.13, −0.03 | 0.0014 |
| Hypertension | −0.01 | −0.05, 0.03 | 0.7240 |
| Stroke | −0.36 | −0.47, −0.25 | <0.0001 |
| Smoking | −0.02 | −0.06, 0.03 | 0.4469 |
| Vision Lossc | −0.07 | −0.12, −0.02 | 0.0075 |
| Hearing Lossd | −0.18 | −0.24, −0.12 | <0.0001 |
Reference group
Health Utilities Index measured using Mark III transforms.
History of vision loss assessed by presence of ophthalmologic comorbidities in the vestibular loss group and by participant response to presence of self‐reported or diagnosed vision problems in the Joint Canada/United States Survey of Health.
History of hearing loss assessed by average pure‐tone hearing threshold (>25 dB) in the vestibular loss group and by participant response to presence of self‐reported or diagnosed hearing problems in the Joint Canada/United States Survey of Health.
Table 4 presents results of multivariate generalized linear models on the association of vestibular loss with individual HUI domains, adjusted for the same variables as above. Statistically significant declines in domain‐specific health‐utility due to vestibular loss were observed with respect to vision (−0.11, p < 0.0001), speech (−0.15, p < 0.0001), dexterity (−0.13, p < 0.0001), and emotion (−0.07, p = 0.0065). Alternatively, vestibular loss was associated with a significant increase in domain‐specific health‐utility with respect to hearing (0.04, p = 0.0440).
Table 4.
Multivariable Adjusted Generalized Linear Models on the Association of Vestibular Dysfunction with Individual HUI3 Domains.
| Vestibular Dysfunction | |||
|---|---|---|---|
| Outcome Variablea | Coefficient | 95% Confidence Interval | P Value |
| Vision | −0.11 | −0.15, −0.07 | <0.0001 |
| Hearing | 0.04 | 0.00, 0.08 | 0.0440 |
| Speech | −0.15 | −0.18, −0.11 | <0.0001 |
| Ambulation | −0.08 | −0.17, 0.01 | 0.0672 |
| Dexterity | −0.13 | −0.19, −0.08 | <0.0001 |
| Emotion | −0.07 | −0.13, −0.02 | 0.0065 |
| Cognition | 0.01 | −0.06, 0.08 | 0.8041 |
| Pain | 0.05 | −0.06, 0.17 | 0.3783 |
HUI3, Health Utilities Index Mark III.
All models adjusted for age, gender, race, marital status, history of diabetes, hypertension, and stroke.
Lifetime QALY losses for the patient population were then calculated by discounting the adjusted 0.15 decrease in health utility (in QALY units) associated with vestibular loss from the above model across the remaining life‐expectancy of each study participant, at an annual discount rate of 3% (Table 5). This resulted in a total of 27.51 QALYs lost across the expected remaining lifetimes of the study population. This total number of QALYs was divided by the number of study patients (N = 27) yielding an average 1.30 lifetime QALYs lost per individual.
Table 5.
Expected Lifetime HUI Loss Associated with Vestibular Loss in Older Adults.
| Participant | Years to Life Expectancya | Expected Lifetime HUI Lossb (QALYs) |
|---|---|---|
| 1 | 21.4 | 2.26 |
| 2 | 21.0 | 2.23 |
| 3 | 18.6 | 1.94 |
| 4 | 18.6 | 0.69 |
| 5 | 16.1 | 1.80 |
| 6 | 18.6 | 2.03 |
| 7 | 16.3 | 1.82 |
| 8 | 14.1 | 1.61 |
| 9 | 15.6 | 1.75 |
| 10 | 14.9 | 1.68 |
| 11 | 13.5 | 1.55 |
| 12 | 13.5 | 1.54 |
| 13 | 10.9 | 1.63 |
| 14 | 9.7 | 1.13 |
| 15 | 10.8 | 1.26 |
| 16 | 10.8 | 1.26 |
| 17 | 8.6 | 1.01 |
| 18 | 8.6 | 0.97 |
| 19 | 10.2 | 1.19 |
| 20 | 6.7 | 0.90 |
| 21 | 7.4 | 0.86 |
| 22 | 7.4 | 0.86 |
| 23 | 5.4 | 0.61 |
| 24 | 6.0 | 0.69 |
| 25 | 5.0 | 0.42 |
| 26 | 6.0 | 0.69 |
| 27 | 6.0 | 0.69 |
| Total | 321.8 | 35.06 |
| Average | 11.9 | 1.30 |
QALYS, Quality‐Adjusted Life Years; HUI, Health Utilities Index
Derived using US Social Security Administration's Exact Age Actuarial Life Expectancy Tables stratified by gender, http://www.ssa.gov/oact/STATS/table4c6.html.
Using an annual HUI loss of 0.15 at a discount rate of 3% across the years to life expectancy
Assuming a $50,000/QALY WTP, the average lifetime economic burden of vestibular loss per affected older adult stratified into three age categories was $91,241, $71,698, and $38,363 for 60–69 years, 70–79 years, and ≥80 years of age, respectively (Table 6). When aggregated across the entire susceptible US population, these estimates resulted in a lifetime societal burden of $106 billion, $79 billion, and $41 billion across each of the above age groups, respectively. When combined across all three age groups, the total lifetime economic burden of vestibular loss per affected older individual was $64,929, resulting in an aggregate $227 billion societal burden of vestibular loss in older adults.
Table 6.
Economic Burden of Vestibular Loss in Older Adults and Sensitivity Analysis
| Symptomatic Vestibular Loss Prevalencea | Affected Populationb | Average QALYs Lostc | Population QALYs Lostd | $/QALYe | Individual Burdenf | Societal Burden (billion)g | |
|---|---|---|---|---|---|---|---|
| Base Case | |||||||
| 60–69 years | 0.07 | 1,164,166 | 1.82 | 2,124,388 | $50,000 | $91,241 | $106.22 |
| 70–79 years | 0.09 | 1,105,494 | 1.43 | 1,585,227 | $50,000 | $71,698 | $79.26 |
| >=80 years | 0.11 | 1,077,104 | 0.77 | 826,426 | $50,000 | $38,363 | $41.32 |
| Total | 0.08 | 3,346,764 | 1.30 | 4,346,050 | $50,000 | $64,929 | $226.80 |
| Base Estimate | Range of Estimate (Lowest to Highest) | 60–69 years (Base $91,241) | 70–79 years (Base $71,698) | >=80 years (Base $38,363) | Overall Individual Burden (Base $64,929) | Societal Burden (Base $226.80 billion) | |
|---|---|---|---|---|---|---|---|
| Sensitivity Analysis | |||||||
| Variables | |||||||
| Discount rate | 3% | 0–6 | $72,547‐$118,375 | $61,571‐$84,994 | $34,781‐$42,583 | $55,080‐$78,275 | $189.99‐$277.63 |
| Annual HUI Loss | 0.15 | 0.10‐0.20 | $65,827‐$131,654 | $52,382‐$104,764 | $30,576‐$61,151 | $48,101‐$96,202 | $167.47‐$334.95 |
| Average Prevalence | 0.08 | 0.07‐0.10a | Unchanged | Unchanged | Unchanged | Unchanged | $283.10‐$404.42 |
| $/QALY | $50,000 | $25,000‐$75,000e | $45,620‐$136,861 | $35,849‐$107,546 | $19,182‐$57,545 | $32,465‐$97,394 | $113.40‐$340.20 |
Abbreviations: QALY, Quality‐Adjusted Life Year
Using an average of literature‐derived prevalence of vestibular vertigo with relative age‐category weights from Agrawal et al (2009).
Derived using 2013 US Census data stratified by age; www.census.gov.
Using an annual health‐utility loss of 0.15 at a discount rate of 3% across the years to life expectancy in the vestibular loss study group
Product of discounted average QALYs lost and affected population
Using highly conservative Willingness‐to‐Pay (WTP) thresholds derived from Hirth et al. (2000).
Lifetime Economic Burden of vestibular loss per Affected Individual, product of Population QALYs Lost and $/QALY divided by Affected Population
Lifetime Societal Burden of vestibular loss, product of Population QALYs Lost and $/QALY
Sensitivity analyses for the lifetime economic burden of vestibular loss per affected older adult ranged from $32,465 to $97,394 and were most sensitive to changes in health utility loss and WTP thresholds (Table 6). Altering these parameters yielded a societal lifetime economic burden of vestibular loss ranging from $113 to $404 billion.
DISCUSSION
These analyses offer evidence of a strong association between vestibular loss and poor QoL outcomes in a sample of older adults with symptomatic and idiopathic vestibular loss seen in a Neurotology clinic. Only one study has previously published the QoL impact of vestibular impairment using the HUI3 survey.21 Although focused on a considerably younger patient population, the authors of that study reported mean HUI3 scores of 0.39 and 0.63 for BVL and UVL respondents, respectively. Given the predominance of BVL patients in our study population, our mean score of 0.47 corroborated these findings. A mean HUI3 score of 0.47 is classified as severe disability by HUI3 criteria28 and corresponds to a similar level of QoL impairment as present in individuals suffering from Parkinson's disease (0.45)29 or untreated osteoarthritis (0.46).30 Our study suggests that while vestibular loss may be overlooked as a benign chronic condition, it is associated with a pervasive negative impact on health‐related quality of life in older individuals.
Moreover, our results demonstrate that vestibular loss has a significant and independent impact on QoL after adjusting for a wide array of variables, including cardiovascular risk factors, vision loss, and hearing loss. The 0.15 decrease in mean adjusted HUI3 score associated with vestibular loss is the third largest QoL reduction observed in our study, behind stroke (−0.36) and clinically significant hearing impairment (−0.18). To further contextualize the magnitude of the QoL burden of vestibular loss, this impairment is equivalent to aging 27 years based on an average HUI3 decrease of 0.054 per decade of life.31
Individual HUI3 domain scores reveal that the QoL impact of vestibular loss in older adults occurred not only in the expected domains of “vision” and “ambulation,” but also in “speech,” “dexterity,” and “emotion” domains of health. Prior studies support a link between vestibular impairment and emotional health. Vestibular symptoms (specifically dizziness and vertigo) have been associated with social isolation, reduced autonomy, and difficulty performing activities of daily living in older adults, likely contributing to the emotional burden of these symptoms.7, 32, 33 Additionally, a recent epidemiologic study found that individuals with vestibular vertigo had a three‐fold increased odds of depressive symptoms, anxiety, and panic disorder than the general US population in adjusted analyses.34 An association between vestibular function and dexterity is supported by recent anatomic studies demonstrating vestibular inputs into central motor control centers such as the basal ganglia,35 and epidemiologic studies showing an association between vestibular function and fine motor tasks.36 The association between vestibular function and speech is more elusive, and may reflect the general link with motor control, and/or neural pathways that remain to be elucidated.
The overall low QoL attainment among older adults with vestibular loss carries significant individual and societal economic implications. Even without considering the economic consequences of reduced productivity (study participants were assumed to be out of the labor force) or health expenditures to treat their vestibular symptoms, vestibular loss was associated with an average $64,929 loss in individual welfare over an 11.9 year age life expectancy for our study population. The authors of a recent study also determined an estimated mean annual economic burden of $13,019 and $3531 for BVL and UVL patients, respectively.21 Although the patient population was different as described previously, these approximations support the average $5,456 annual loss in individual welfare determined in this report. The resultant $227 billion aggregate economic burden may represent an opportunity to considerably reduce health care costs through timely diagnosis and treatment of vestibular loss.37
Several limitations exist in this study, including the small sample size for the study group. The large effect size of the QoL reduction and statistical significance of the results, even after adjusting for a wide array of confounding variables, however, mitigates some of these concerns. Additionally, the use of cross‐sectional data in this analysis precludes causal inference and allows only for the determination of associations between QoL attainment and vestibular loss. Furthermore, relying on patient self‐reporting introduces a source of response bias due to variability in understanding of the questionnaires and the subjective nature of participants' symptoms. Although estimates of vestibular loss prevalence and incidence have been computed in several recent publications,6, 7, 8, 10 the absence of large‐scale, high‐quality epidemiological data that are based on objective, specific assessments of vestibular function makes it difficult to determine how well our study population represents the spectrum of health‐related quality of life among older individuals with symptomatic vestibular loss. This study population was also not a random sample, and therefore, the calculated lifetime societal burden of vestibular loss aggregated across the entire US population may not accurately represent the effects of vestibular loss in the general geriatric population. Finally, vestibular function was not measured in JCUSH, and our analyses assumed a zero prevalence, which if anything would have conservatively biased our results. Further limitations of the JCUSH data have been published previously.38
CONCLUSION
Our results demonstrate that age‐related vestibular loss among patients presenting to a Neurotology clinic was associated with a 0.15 reduction in HUI3 score, which corresponds to a loss of 1.30 quality‐adjusted life years. These data suggest that loss of vestibular function in older individuals can confer a significant decrement in quality of life, and is associated with substantial societal cost. Further studies are needed to measure the benefits of vestibular therapy from both the individual and societal perspective.
Funding: This work was supported in part by the National Institutes of Health [NIDCD K23 DC013056, NIDCD T32 DC000023].
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
BIBLIOGRAPHY
- 1. Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med 2009;169(10):938–944. [DOI] [PubMed] [Google Scholar]
- 2. Neuhauser HK, Radtke A, von Brevern M, Lezius F, Feldmann M, Lempert T. Burden of dizziness and vertigo in the community. Arch Intern Med 2008;168(19):2118–2124. [DOI] [PubMed] [Google Scholar]
- 3. Ward BK, Agrawal Y, Hoffman HJ, Carey JP, Della Santina CC. Prevalence and impact of bilateral vestibular hypofunction: results from the 2008 US National Health Interview Survey. JAMA Otolaryngol Head Neck Surg 2013;139(8):803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull 2010;96(1):5–21. [DOI] [PubMed] [Google Scholar]
- 5. Benecke H, Agus S, Kuessner D, Goodall G, Strupp M. The burden and impact of vertigo: findings from the revert patient registry. Front Neurol 2013;4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bittar RSM, Oiticica J, Bottino MA, Ganança FF, Dimitrov R. Population epidemiological study on the prevalence of dizziness in the city of São Paulo. Braz J Otorhinolaryngol 2013;79(6):688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gopinath B, McMahon C, Rochtchina E, Mitchell P. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol 2009;34(6):552–556. [DOI] [PubMed] [Google Scholar]
- 8. Lai Y‐T, Wang T‐C, Chuang L‐J, Chen M‐H, Wang P‐C. Epidemiology of Vertigo A National Survey. Otolaryngol Head Neck Surg 2011;145(1):110–116. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal NT, Bennett DA, Bienias JL, de Leon CFM, Morris MC, Evans DA. The prevalence of dizziness and its association with functional disability in a biracial community population. J Gerontol A Biol Sci Med Sci 2000;55(5):M288–M292. [DOI] [PubMed] [Google Scholar]
- 10. Neuhauser HK, von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology. 2005;65(6):898–904. [DOI] [PubMed] [Google Scholar]
- 11. Takano NA, Cavalli SS, Ganança MM, et al. Quality of life in elderly with dizziness. Braz J Otorhinolaryngol 2010;76(6):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weidt S, Bruehl AB, Straumann D, Hegemann SC, Krautstrunk G, Rufer M. Health‐related quality of life and emotional distress in patients with dizziness: a cross‐sectional approach to disentangle their relationship. BMC Health Serv Res 2014;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neuhauser H, Radtke A, Von Brevern M, et al. Migrainous vertigo prevalence and impact on quality of life. Neurology. 2006;67(6):1028–1033. [DOI] [PubMed] [Google Scholar]
- 14. Lasisi A, Gureje O. Disability and quality of life among community elderly with dizziness: report from the Ibadan Study of Ageing. J Laryngol Otol 2010;124(09):957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu LC, Hu HH, Wong WJ, Wang SJ, Luk YO, Chern CM. Quality of life in elderly patients with dizziness: analysis of the Short‐Form Health Survey in 197 patients. Acta Oto‐Laryngol 2005;125(1):55–59. [DOI] [PubMed] [Google Scholar]
- 16. Guinand N, Boselie F, Guyot J‐P, Kingma H. Quality of life of patients with bilateral vestibulopathy. Ann Otol Rhinol Laryngol 2012;121(7):471–477. [DOI] [PubMed] [Google Scholar]
- 17. World Population Ageing 2015 Highlights New York: United Nations; 2015. Available at: http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Highlights.pdf. Accessed October 9, 2017.
- 18. Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Semenov YR, Yeh ST, Seshamani M, et al. Age‐dependent cost‐utility of pediatric cochlear implantation. Ear Hear 2013;34(4):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng AK, Rubin HR, Powe NR, Mellon NK, Francis HW, Niparko JK. Cost‐utility analysis of the cochlear implant in children. JAMA 2000;284(7):850–856. [DOI] [PubMed] [Google Scholar]
- 21. Sun DQ, Ward BK, Semenov YR, Carey JP, Della Santina CC. Bilateral vestibular deficiency: quality of life and economic implications. JAMA Otolaryngol Head Neck Surg 2014;140(6):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanmartin C, Berthelot J‐M, Ng E, et al. Comparing health and health care use in Canada and the United States. Health Aff (Millwood) 2006;25(4):1133–1142. [DOI] [PubMed] [Google Scholar]
- 23.Administration. USS. Actuarial Life Tables Stratified by Gender. 2010. Available at: http://www.ssa.gov/oact/STATS/table4c6.html. Accessed March 23, 2015.
- 24. Peacock B. The Appropriate Discount Rate for Social Policy Analysis: Discussion and Estimation. Washington, DC: US Department of the Interior, Office of Policy Analysis; 1995. [Google Scholar]
- 25. Lind RC. Reassessing the government's discount rate policy in light of new theory and data in a world economy with a high degree of capital mobility. J Environ Econ Manag 1990;18(2):S8–S28. [Google Scholar]
- 26. Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality‐adjusted life year in search of a standard. Med Decis Making 2000;20(3):332–342. [DOI] [PubMed] [Google Scholar]
- 27. UC Bureau . Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States. 2013. Available at: http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk. Accessed March 23, 2015.
- 28. Feng Y, Bernier J, McIntosh C, Orpana H. Validation of disability categories derived from Health Utilities Index Mark 3 scores. Health Rep 2009;20(2):43. [PubMed] [Google Scholar]
- 29. Kleiner‐Fisman G, Stern MB, Fisman DN. Health‐related quality of life in Parkinson disease: correlation between Health Utilities Index III and Unified Parkinson's Disease Rating Scale (UPDRS) in US male veterans. Health Qual Life Outcomes 2010;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torrance G, Raynauld J, Walker V, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan GF 20 into the treatment paradigm for patients with knee osteoarthritis (Part 2 of 2): economic results. Osteoarthritis Cartilage 2002;10(7):518–527. [DOI] [PubMed] [Google Scholar]
- 31. Grootendorst P, Feeny D, Furlong W. Health Utilities Index Mark 3: evidence of construct validity for stroke and arthritis in a population health survey. Med Care 2000;38(3):290–299. [DOI] [PubMed] [Google Scholar]
- 32. Mueller M, Strobl R, Jahn K, et al. Impact of vertigo and dizziness on self‐perceived participation and autonomy in older adults: results from the KORA‐Age study. Qual Life Res 2014;23(8):2301–2308. [DOI] [PubMed] [Google Scholar]
- 33. Holmes S, Padgham ND. Review of the burden of vertigo. J Clin Nurs 2011;20(19–20):2690–2701. [DOI] [PubMed] [Google Scholar]
- 34. Bigelow RT, Semenov YR, du Lac S, Hoffman HJ, Agrawal Y. Vestibular vertigo and comorbid cognitive and psychiatric impairment: the 2008 National Health Interview Survey. J Neurol Neurosurg Psychiatry. 2016;87(4):367–372. [DOI] [PubMed] [Google Scholar]
- 35. Stiles L, Smith PF. The vestibular–basal ganglia connection: Balancing motor control. Brain Res 2015;1597:180–188. [DOI] [PubMed] [Google Scholar]
- 36. Bigelow RT, Semenov YR, Trevino C, et al. Association between visuospatial ability and vestibular function in the baltimore longitudinal study of aging. J Am Geriatr Soc 2015;63(9):1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cowand JL, Wrisley DM, Walker M, Strasnick B, Jacobson JT. Efficacy of vestibular rehabilitation. Otolaryngol Head Neck Surg 1998;118(1):49–54. [DOI] [PubMed] [Google Scholar]
- 38. Sanmartin CA, Ng E, Blackwell D, Gentleman J, Martinez M, Simile C. Joint Canada/United States Survey of Health, 2002‐03. Statistics Canada, Health Analysis and Measurement Group; 2004.


