Abstract
Eosinophils have been postulated to play a protective role against infection with respiratory syncytial virus (RSV), increase the severity of allergic asthma during respiratory viral infection, and drive vaccine-enhanced disease. To address these questions in the cotton rat model of RSV infection, we characterized cotton rat eosinophils by electron microscopy as well as by bronchoalveolar lavage and histology of lung sections. Using these methods, we demonstrated that eosinophils comprise approximately half of all cells in the bronchoalveolar lavage fluids from cotton rats. The function of these cells was comparable to that of eosinophils of other species. Ex vivo, eosinophils stimulated with calcium ionophores secreted eosinophil peroxidase. In vivo, treatment with house dust mite antigen increased eosinophil numbers in lung. Infection with Staphylococcus aureus lead to a marked increase in neutrophils without an increase in eosinophils, and eosinophil numbers were not influenced by infection with influenza virus or measles virus. Similarly, infection with RSV did not result in an increase in eosinophils. Lastly, RSV infection did not increase eosinophil recruitment into the lung after challenge with house dust mite antigen, but it did increase eosinophil recruitment into the lungs of cotton rats previously immunized with formalin-inactivated RSV vaccine, thus contributing to vaccine-enhanced disease.
Abbreviations: BAL, bronchoalveolar lavage; HDM, house dust mite; HEp2, human epithelial type 2, FI, formalin-inactivated; RSV, respiratory syncytial virus
Eosinophils are leukocytes of the granulocyte lineage and develop from CD34-expressing pluripotent progenitor cells in the bone marrow (see reference 48 for review). Typically, eosinophils are thought to play a role in the development of allergic diseases (including asthma) and in antiparasitic defense against, for example, helminths (see reference 1 for review). However, eosinophils may also play a role in the antiviral defense against various viruses, particularly human respiratory syncytial virus (RSV).7 Although RSV infects the upper respiratory tract in humans of all age groups,52 severe disease with involvement of the lower respiratory tract is common in infants and the elderly.52 Epidemiologic evidence links severe RSV infection and subsequent wheezing to the development of asthma, although some studies point to rhinovirus as being the main viral inducer of asthma development (see reference 49 for review). In humans, increased eosinophils and eosinophil granule proteins (eosinophil-derived neurotoxin and eosinophil cationic protein) have been found in patients with RSV bronchiolitis without17 or with20,32 mechanical ventilation and in infants with early primary infection.31,54 In individual patients, the presence of eosinophils was linked to increased RSV-associated induction of IL5.26 Support for the assumption that RSV-induced eosinophils contribute to respiratory disease is supplied by a clinical trial using a formalin-inactivated RSV (FI-RSV) vaccine candidate. Natural exposure to wild-type RSV during the first year after vaccination led to a severe, atypical (that is, vaccine-enhanced) disease in about 80% of unvaccinated persons that was lethal for 2 patients.11,16,25 The published autopsy report of these 2 patients mentioned marked influx of eosinophils into the lung.27 In addition, increased numbers of eosinophils were found in the blood of some children hospitalized with vaccine-enhanced RSV disease, suggesting that eosinophilia might be a hallmark of vaccine-enhanced disease.11 However, revisiting the original pathology reports of these 2 fatal cases and studying additional histologic slides instead revealed a predominance of neutrophils rather than eosinophils.43
Although eosinophils typically have been viewed as potentiators of respiratory disease, other evidence suggests that they may play a role in antiviral defense. In one study, the addition of eosinophils to a suspension of RSV viral particles reduced viral infectivity, and it was demonstrated that this reduction in viral activity was mainly due to the major eosinophil ribonuclease.15 In another study, the addition of eosinophils led to reduced spread of RSV infection in the human bronchial epithelial cell line BEAS-2B.53 Furthermore, mice with peripheral eosinophilia due to transgenic expression of IL5 cleared RSV infection faster than nontransgenic mice.40
Thus far, the studies in humans and mice demonstrate that the roles of eosinophils in RSV infection are complex and not fully understood. To further investigate this role, we chose cotton rats as an animal model because they are highly susceptible to RSV infection and have previously been used to study RSV pathogenesis and to test RSV vaccines and antivirals.5,18 The blood9 and lung19 of uninfected cotton rats show relatively high baseline levels of eosinophils: 5% to 10% and 25%, respectively. In addition, RSV infection in cotton rats has been observed to lead to an increase of eosinophils in the lung, as detected through analysis of bronchoalveolar lavage (BAL) fluid, during days 2 through 28 after infection.19 In contrast, when atypical disease was induced experimentally in cotton rats by immunization with FI-RSV and subsequent RSV challenge, the infiltrating leukocytes were classified as neutrophils, rather than eosinophils.24,43
Given these findings and data from several of our preliminary studies, we aimed to characterize eosinophils in cotton rats morphologically and functionally in response to an allergen (house dust mite [HDM]), bacteria (S. aureus), and viruses (measles virus, influenza A virus). In addition, we sought to determine the eosinophil response to RSV infection after concomitant application of RSV and an allergen and to RSV challenge after vaccination with a FI-RSV vaccine.
Materials and Methods
Animals.
Inbred cotton rats (Sigmodon hispidus) were obtained from Harlan Laboratories (now Envigo, Indianapolis, IN) and housed in standard polycarbonate cages within an ABSL2 barrier system. Corncob bedding (Cincinnati Lab Supply, Cincinnati, OH) was used for all experimental animals, unless stated otherwise. Environmental conditions were constant at 22 ± 2 °C, 30% to 70% relative humidity, and 12:12-h light:dark cycle. Cotton rats of both sexes between the ages of 5 to 10 wk were used. Animals were euthanized by using CO2 inhalation. All animal experiments were approved by the IACUC of The Ohio State University. Cotton rats were purchased SPF and maintained in colonies free of endo- and ectoparasites, mouse parvoviruses 1 and 2, minute virus of mice, mouse hepatitis virus, murine norovirus, Theiler murine encephalomyelitis virus, mouse rotavirus, Sendai virus, pneumonia virus of mice, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, and ectromelia virus according to quarterly health monitoring of sentinel (CD1) mice exposed to 100% pooled dirty bedding from colony animals at each cage change.
In one experiment, the effect of bedding conditions on eosinophil levels in the lungs was evaluated. Three different conditions were examined. The first condition was our standard bedding protocol: cotton rats were housed on shredded aspen bedding (Harlan) until they were 4 wk old, when they were transferred to corncob bedding (Bed-o'Cobs, The Andersons, Maumee, OH). In this first experiment, the animals were euthanized at 6 wk of age, 2 wk after being switched to the corncob bedding. For the second bedding condition, cotton rats were housed exclusively on corncob bedding. For the final bedding condition, cotton rats were housed exclusively on low-dust paper bedding (ALPHA-dri, Shepherd Specialty Papers, Watertown, TN), which is free from many potentially allergenic contaminating factors commonly found in corncob or shredded aspen bedding.
Infection.
Cotton rats were anesthetized by using isoflurane inhalation before being inoculated intranasally with virus, bacteria, or allergen diluted in PBS (final volume, 100 µL).
Viruses and bacteria.
Stocks of RSV A/2 were grown in human epithelial type 2 (HEp2) cells in MEM supplemented with 2% fetal bovine serum. When infection yielded a cytopathic effect of approximately 80%, cells were scraped from the flask in the growth media. The cells were briefly frozen at –80 °C, thawed, and centrifuged at 1860 × g for 15 min at 4 °C to remove all large cellular debris. The supernatant was collected and placed on top a 15-mL 35% sucrose cushion and centrifuged at 15,000 rpm (that is, approximately 27,000 × g) in an SS 34 rotor (Sorvall, Thermo Fisher Scientific, Waltham, MA) for 5 h at 4 °C to pellet the virus. Virus pellets were resuspended in MEM containing 10% trehalose, and TCID50 was determined by titration on NY3 cells.19 Influenza virus A/Wuhan/359/95(H3N2) was grown on Madin–Darby canine kidney cells in MEM supplemented with 1% BSA and 5 µg/mL TPCK trypsin (Worthington Biochemical, Lakewood, NJ). Cells and supernatant were harvested when cytopathic effect reached approximately 80%. After 2 freeze–thaw cycles, cellular debris was pelleted by centrifugation at 1860 × g for 15 min at 4 °C, and the supernatant was frozen in small aliquots in liquid nitrogen. TCID50 was measured by titration on Madin–Darby canine kidney cells. Measles virus Bilthoven strain was grown on BJAB cells, and the TCID50 assay was performed on human Vero-CD150 cells as described.39Staphylococcus aureus serotype 5 (catalog no. 49521, American Type Culture Collection, Manassas, VA) was grown in tryptic soy broth to a density of OD600 0.9 and titered on tryptic soy agar.30 Each virus or bacterial stock was separated into aliquots and frozen in liquid nitrogen. Aliquots were thawed immediately prior to use and were only used once to prevent loss of titer due to repeated cycles of freeze-thaw.
Immunization with HDM antigen.
Cotton rats were sensitized by intraperitoneal injection of 100 µg HDM antigen (Dermatophagoides pteronyssinus), which was adsorbed to aluminum phosphate (AdjuPhos, Brenntag, Ballerup, Denmark) at a 1:1 (v/v) for 30 min at room temperature. Sensitization was followed by challenge 8 d later with 100 µg HDM intranasally in a volume of 100 µl PBS.
FI-RSV vaccine.
FI-RSV vaccine was prepared according to published procedures.27 In brief, RSV was grown on HEp2 cells. When cytopathic effect reached 80%, cells and medium were frozen. After thawing, samples were centrifuged 10 min at 300 × g, and the supernatant was collected. Formalin was added to achieve a final concentration of 1:4,000, mixtures were incubated for 72 h (37 °C), and samples centrifuged at 50,000 × g (Beckman L8-M ultracentrifuge with SW28 rotor) for 1 h at 4 °C. The pellet was diluted in PBS (1/25 of the original volume) and incubated with an equivalent volume of aluminum phosphate (Adju-phos, Brenntag, Ballerup, Denmark) for 1 h at room temperature. After centrifugation at 1,000 x g, the pellet was resuspended in MEM (1/4 of the original volume). Mock-infected HEp2 cells were harvested and prepared similarly to obtain a FI-mock vaccine, designated ‘FI-sup.’ For the ‘FI-spRSV’ vaccine, RSV was purified through a 30% sucrose cushion to remove some of the contaminating HEp2 cellular components from the virus stock, before formalin inactivation and adsorption to aluminum phosphate. Cotton rats were immunized twice with 200 µL of the appropriate preparation, on days 0 and 28. On day 49, animals were challenged intranasally with 105 TCID50 RSV A/2.
BAL collection and analysis.
To collect BAL fluid, animals were euthanized, the trachea was cannulated, and the lungs were lavaged with PBS supplemented with 1% protease-free BSA in a volume sufficient to fully inflate the lungs (1.5 to 2 mL). The lavage fluid was collected and kept on ice until processed (Comparative Pathology and Mouse Phenotyping Shared Resource, Ohio State University). Automated nucleated cell counts were performed by using a Forcyte veterinary hematology analyzer (Oxford Science, Oxford, United Kingdom), and differential cell counts from cytospin preparations were stained with either modified Wright Giemsa (Aerospray 7150, Wescor, Logan, UT) or Hansel (Lide Laboratories, New Prague, MN) stain and manually read by a medical technologist and specialist in hematology certified by the American Society for Clinical Pathology.
Histology and electron microscopy.
Lungs were inflated and fixed in freshly prepared 4% paraformaldehyde for 48 to 72 h, paraffin-embedded, cut into 5-µm sections, and treated with hematoxylin and eosin stains or Luna stain.37 For transmission electron microscopy, paraffin-embedded lung tissue was posttreated with OsO4 in toluene and acetone and reimbedded in Spurr resin. Lungs that had been flushed for BAL analysis were not used for histologic review.
Eosinophil peroxidase assay.
Eosinophil peroxidase release was measured as previously described.2 Briefly, cells from BAL were plated in 10-cm tissue culture dishes at 37 °C. After 2 h, macrophages had adhered to the plastic surface, and eosinophils were obtained from the supernatant with a purity of greater than 95% as confirmed by cytostains. Graded numbers of eosinophils were plated in a 96-well plate (Nunc) in a volume of 50 µL per well, in triplicate. After stimulation of eosinophils with 25 µM of the calcium ionophore A23187 (Sigma Aldrich, St Louis, MO) for 30 min at 37 °C, 100 µL O-phenylenediamine (0.4 mg/mL) was added directly to the wells. The reaction was terminated by adding 50 µL 2 N H2SO4, and color intensity was measured at a wavelength of 492 nm in an ELISA plate reader (Sunrise, Tecan Life Sciences, Zurich, Switzerland).
Statistical analysis.
Data are represented as means ± 1 SD. Statistical significance was determined by using multiple t tests, and the Holm–Sidak method was used to correct for multiple comparisons with α set at 0.05. Each cell type was analyzed individually, without assuming a consistent standard deviation. Data were analyzed by using Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA).
Results
Identification of cotton rat eosinophils.
The ultimate goal of our study was to define the cellular response to RSV infection by using the cotton rat model, given its unique permissiveness to RSV. However, cotton rat eosinophils are not well described in the literature. In addition, cell differential analyses often do not distinguish between eosinophils, basophils, and neutrophils but instead summarize all 3 cell types as polymorphonuclear granulocytes. To define cotton rat eosinophils, electron microscopy was used in conjunction with various staining methods using paraffin-embedded lung sections or cytospin preparations of BAL fluid.
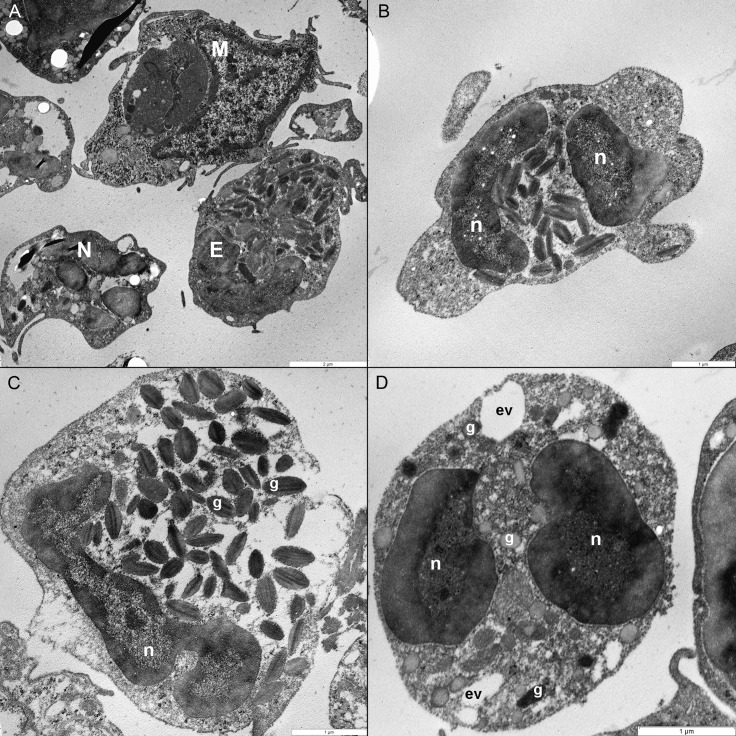
To obtain a visualization of cotton rat eosinophils on an ultrastructural level, we performed transmission electron microscopy on lung tissue. By using this method, eosinophils were readily identified by their often bilobed nucleus and the abundance of cytoplasmic granules containing an electron-dense crystalline core. The other predominant cell type observed, neutrophils, often exhibited a multilobed nucleus and a cytosol that was filled with granules lacking a crystalline core (Figure 1).
Figure 1.
Transmission electron micrographs of eosinophils in cotton rat lung tissue. (A) Eosinophils (E) are readily distinguishable from macrophages (M) and neutrophils (N). Eosinophils are discernible by (B) their bilobed nucleus (n) and (C) oval cytoplasmic granules (g) that contain a dark crystalloid body. Unlike in eosinophil granules, (D) neutrophil granules (g) do not contain a dark crystalloid body; this cell also contains empty vesicles (ev). Magnification, 15,000× (A), 25,000× (B), 30,000× (C), 40,000× (D).
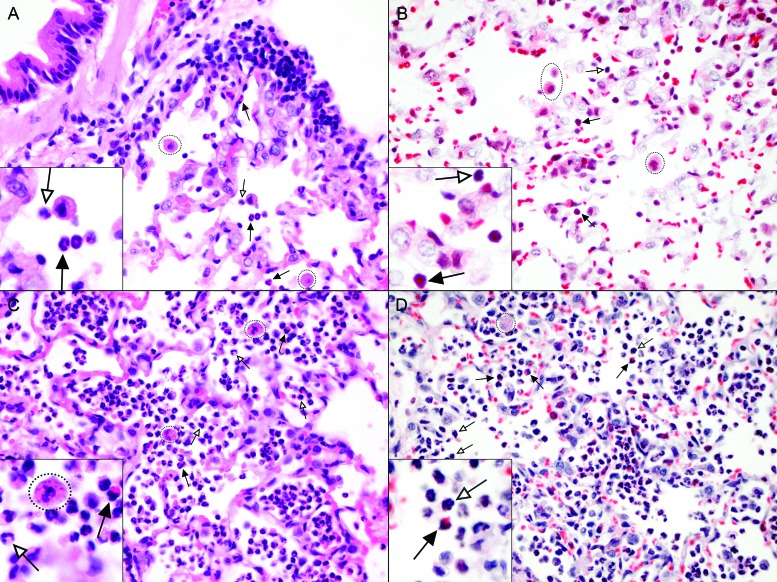
To identify an optimal method for the detection of cotton rat eosinophils on paraffin-embedded lung sections, we compared the standard hematoxylin and eosin with Luna stain, which has been used successfully to differentiate eosinophils from other polymorphonuclear granulocytes, including the less commonly encountered heterophils (or ‘pseudoeosinophils’).35 Heterophils are found in species such as rabbits, chickens, guinea pigs, and hamsters. They perform the same function as a neutrophil of other species, but unlike neutrophils, heterophils contain acidophilic (or ‘eosinophilic’) granules within their cytoplasm, causing them to easily be confused for eosinophils. In cotton rats, we did not detect heterophils by using either staining method. On paraffin-embedded lung sections, eosinophils clearly displayed large red granules in the cytoplasm regardless of the staining method (Figure 2). However, because Luna staining led to increased patchy background staining, hematoxylin and eosin was used to detect eosinophils in subsequent analyses.
Figure 2.
Standard staining with hematoxylin and eosin is better than Luna staining for visualization of cotton rat eosinophils in lung tissue. Paraffin-embedded lung sections from cotton rats (A and B) 4 d after infection with RSV or (C and D) 1 d after infection with S. aureus were stained with (A and C) hematoxylin and eosin or (B and D) Luna stain. Variable numbers of eosinophils with bilobed nuclei and prominent pink to red cytoplasmic granules (black arrows), neutrophils with segmented nuclei and less distinct cytoplasmic granules (white arrows), and alveolar macrophages (dotted circles), are present within alveoli. Macrophages in S. aureus infected cotton rats frequently contain cocci. Magnification, 60×; insets, 100×.
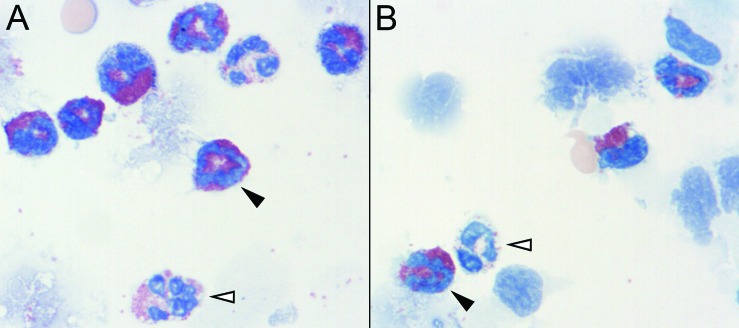
For the identification of eosinophils in BAL fluid, we compared the standard Wright–Giemsa stain with Hansel stain, which sometimes facilitates the differentiation of eosinophils from neutrophils. Wright–Giemsa staining worked well and enabled accurate cell differentiation, but we ultimately chose Hansel stain because it yielded greater color contrast between eosinophils and neutrophils in cotton rat BAL fluid, allowing for easier cell differentiation (Figure 3).
Figure 3.
Eosinophil detection in cotton rat BAL cytology is improved by using Hansel stain rather than Wright-Giemsa. In cytospin preparations of cells from cotton rat BAL, eosinophils (black arrowheads) are clearly distinguishable from neutrophils (white arrowheads) by using either staining method. However, (A) Wright–Giemsa also stains neutrophil cytoplasmic granules red. (B) In contrast, Hansel stain does not stain the cytoplasmic granules of cotton rat neutrophils as intensely as eosinophils, thereby enhancing the contrast between eosinophils and neutrophils for cell differentiation. Magnification, 100×.
Baseline levels of eosinophils in cotton rat blood and lungs.
We examined the percentage of eosinophils in blood and lungs of uninfected cotton rats. Hansel stain was used for differentiation of BAL samples, and cell counts were performed manually. In peripheral blood, the percentage of eosinophils ranged from 4.4% to 11.2%, with an average of 7.8% ± 3.4% of total blood leukocytes. In BAL fluid, the percentage of eosinophils ranged from 34% to 83% of total leukocytes with an average of 56.4% ± 12.5%. The analysis of the BAL fluid of 6 male and 8 female cotton rats did not reveal sex-specific differences (data not shown); this finding is consistent with a previous report of no sex-associated hematologic differences.42 For further analyses, data from male and female cotton rats were pooled. The percentage of eosinophils is much lower in humans and mice than in cotton rats.34 To rule out the possibility that eosinophils accumulate in cotton rat lung in response to allergens or dust in the bedding, we compared 3 types of bedding. Specifically cotton rats were housed from birth on traditional corncob bedding, low-dust paper bedding, or shredded aspen bedding; animals housed on aspen bedding were moved to corncob bedding after 4 wk (at time of weaning) according to our standard protocol. At 6 wk of age, all animals displayed similar numbers of eosinophils in their BAL fluid, regardless of the type of bedding used to house them (data not shown), This result suggests that the high percentage of eosinophils detected in the lungs of cotton rats is not due to an allergic response to excessively dusty or contaminated bedding.
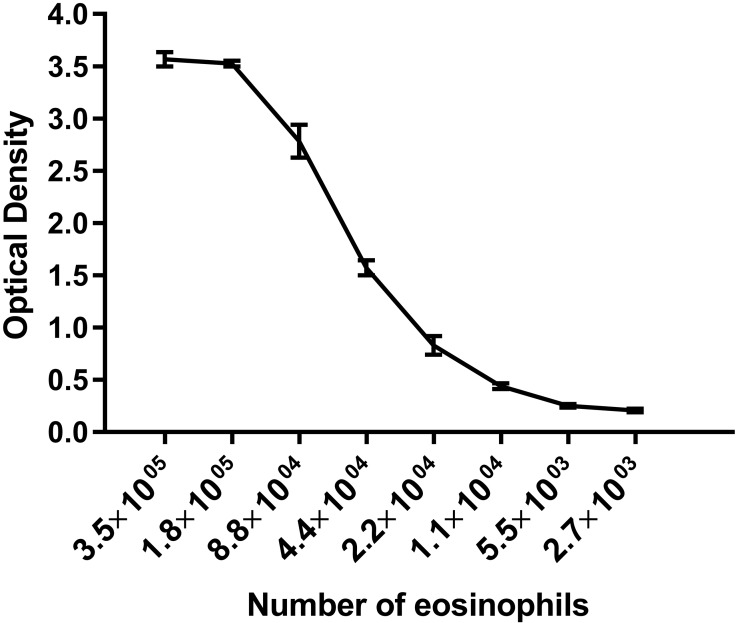
Another possible explanation for the high percentage of eosinophils in cotton rat lungs is that dysfunctional eosinophils accumulate in lung and undergo delayed replacement. In mice, the 50% replacement rate of eosinophils in the lung is 3 d.8 To investigate whether dysfunctional eosinophils accumulate in cotton rat lung, we tested the functionality of these cells by measuring the release of eosinophil peroxidase. On a per-cell basis, cotton rat lung eosinophils demonstrated similar peroxidase release as human eosinophils,2 indicating that cotton rat lung eosinophils are indeed functional (Figure 4). We then tested the response of cotton rat eosinophils toward allergens, bacteria, and viruses.
Figure 4.
Eosinophils from the lungs of cotton rats are functional. Purified cotton rat eosinophils stimulated with the calcium ionophore A23187. Eosinophil peroxidase activity detected by using O-phenylenediamine and measured by using optical density at 492 nm. Range of linearity for eosinophil peroxidase activity compared with numbers of eosinophils per well was similar between cotton rat eosinophils and published data for human eosinophils 36. Each data point represents a mean of at least 3 samples; error bars, 1 SD.
Cellular response of cotton rat lungs to allergen sensitization and challenge.
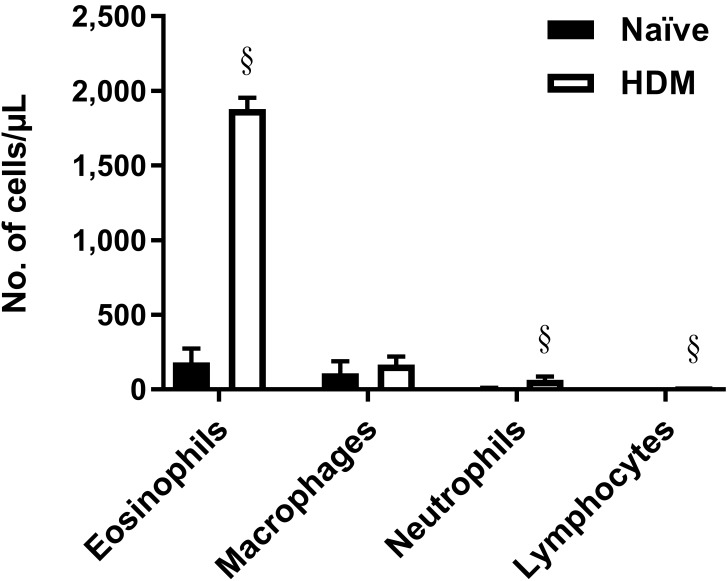
Typically, the number of eosinophils in lung increases in response to an allergenic stimulus. To ascertain whether a similar increase indeed occurs in cotton rat lung despite the high basal level of eosinophils, we modified current protocols for mice to develop a cotton rat model of allergic asthma by using the common clinical allergen HDM. After intraperitoneal sensitization with HDM adsorbed to alum and subsequent intranasal challenge with HDM alone, eosinophil numbers were greatly increased (adjusted P < 0.0001) in the BAL fluid of treated animals compared with naïve controls (Figure 5).
Figure 5.
Treatment with allergen increased the number of pulmonary eosinophils in cotton rats. Number of cells present in BAL collected from either naïve animals or animals sensitized and challenged with house dust mite (HDM) antigen. Animals were sensitized intraperitoneally on day 0 with 100 µg HDM absorbed to alum and then challenged intranasally with 100 µg HDM 8 d later. BAL fluid was collected 4 d after challenge. Bars represent mean values (n = 4 to 8 cotton rats per group); error bars, 1 SD. Values differ significantly (§,adjusted P < 0.0001; Welch t tests, Holm–Sidak correction) from that for naïve cotton rats.
Response of cotton rat lung to bacterial infection.
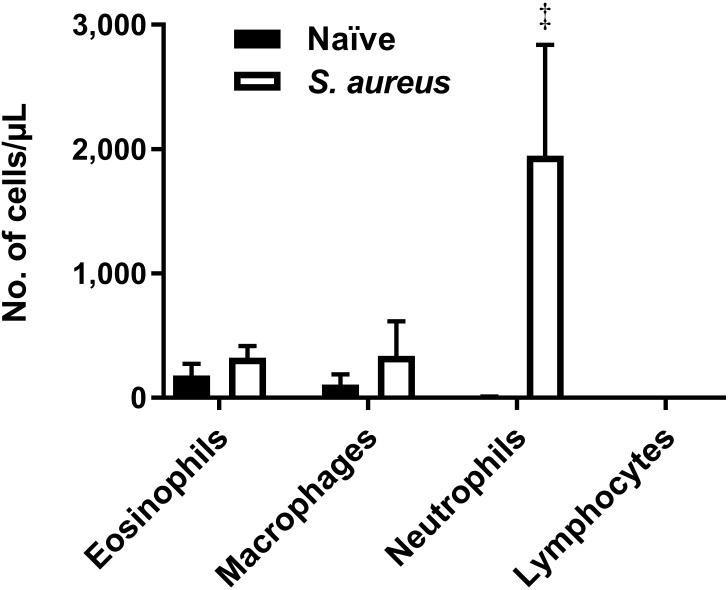
Neutrophil influx is a hallmark of bacterial infection of the lung. To examine the effect of bacterial infection on the cellular response in the lungs of the cotton rat, animals were challenged with 100 µL of 4 × 107 cfu S. aureus intranasally. Infection of the lungs with S. aureus had a pronounced effect on the number of neutrophils present in the lung. BAL taken from infected animals at 24 h after infection had more than a 500-fold increase in the number of neutrophils compared with naïve controls (adjusted P < 0.001; Figure 6), whereas the number of eosinophils in lung was not increased.
Figure 6.
Infection with S. aureus induces a neutrophilic response. Comparison of BAL cytology between naïve cotton rats and those infected with 4 × 107 cfu of S. aureus. BAL fluid was collected 24 h after infection. Bars represent mean values (n = 4–8 per group); error bars, 1 SD. Values differ significantly (‡, adjusted P < 0.001; Welch t tests, Holm–Sidak correction) from that for naïve cotton rats.
Eosinophil counts in cotton rat lung in response to infection with influenza A virus or measles virus.
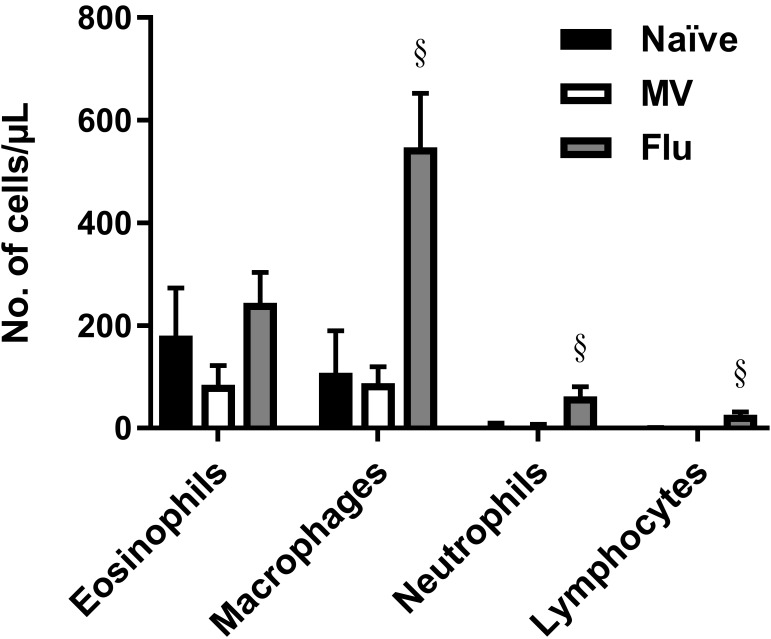
In humans and mice, infection with influenza A or measles virus does not increase the number of eosinophils in the lung. To determine the effect of virus infection on eosinophils in the lung of cotton rats, BAL fluid was collected at 4 d after infection from animals infected with influenza A virus or measles virus. Consistent with findings in other species, eosinophil levels in cotton rat lung did not increase after infection with either virus (Figure 7). Furthermore, measles virus infection did not significantly alter any cell population. Influenza A infection led to increases in neutrophils (adjusted P < 0.0001), macrophages (adjusted P < 0.0001), and lymphocytes (adjusted P < 0.0001), which correlates with our observations that influenza A virus is more cytotoxic for lung epithelial cells than measles virus (data not shown).
Figure 7.
Measles virus infection does not significantly alter BAL cellularity, and influenza infection primarily induces a macrophage response. BAL cytology of naïve animals was compared with that of cotton rats infected intranasally with 105 TCID50 measles virus (Bilthoven [MV]) or 106 TCID50 influenza A virus (Wuhan H3N2 [Flu]). BAL fluid was collected at 4 d after infection. Bars represent mean values (n = 4–8 cotton rats per group); error bar, 1 SD. Value differs significantly (§, adjusted P < 0.0001; Welch t tests, Holm–Sidak correction) from that for naïve cotton rats.
Eosinophil counts in cotton rat blood and lung in response to RSV infection.
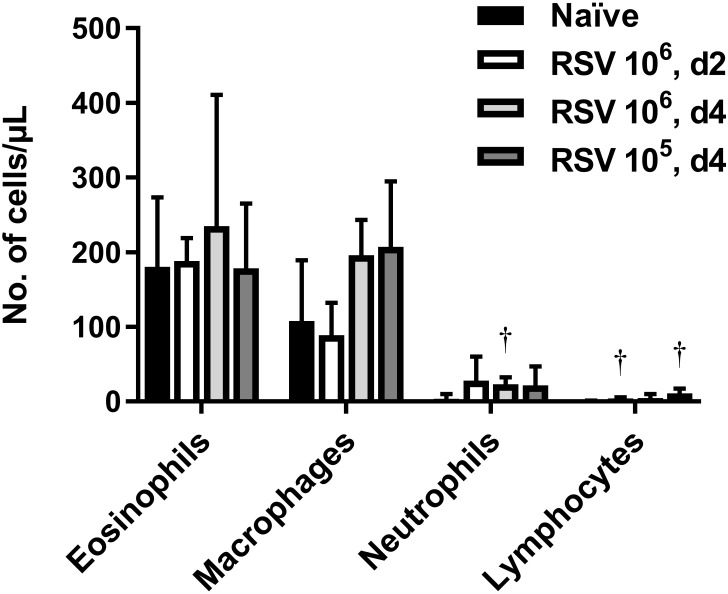
Increased numbers of eosinophils have sometimes been detected after RSV infection in the cotton rat lung, which supports a proposed antiviral role for eosinophils during RSV infection.19 To investigate the pulmonary eosinophil response to RSV infection, we infected cotton rats with RSV at a dose of 105 or 106 TCID50, and measured the number of eosinophils present in the blood and BAL fluid during the onset of viral replication (2 d after infection) and at peak viral replication (4 d after infection).44 However, RSV infection did not significantly alter eosinophil numbers in the blood (data not shown) or BAL fluid compared with those in naïve controls (Figure 8).
Figure 8.
Infection with RSV does not increase the number of eosinophils in the lungs. BAL cytology was compared 2 or 4 d after RSV infection (d2 or d4, respectively) of cotton rats that received 105 or 106 TCID50 RSV intranasally. Bars represent mean values (n = 4–8 cotton rats per group); error bar, 1 SD. Value differed significantly (†, adjusted P < 0.01; Welch t tests, Holm–Sidak correction) from that for naïve cotton rats.
Effect of RSV infection on pulmonary eosinophilia in a model of allergic asthma.
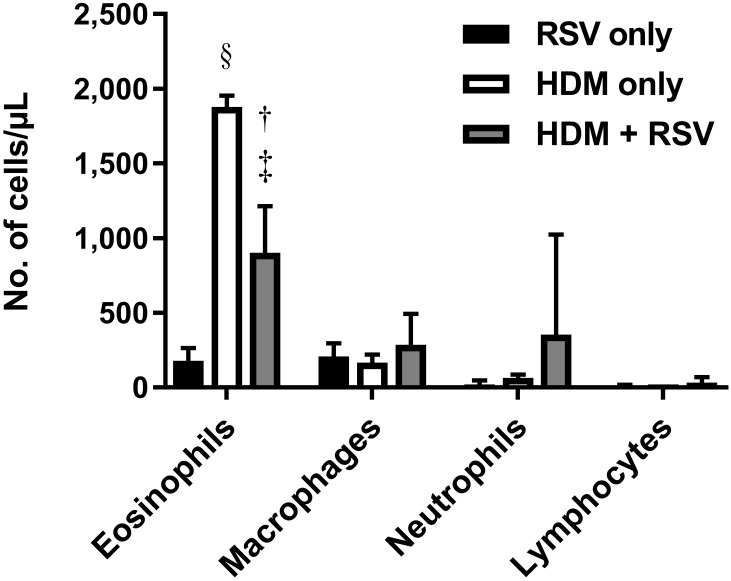
RSV is increasingly recognized as an important cause of asthma exacerbations in both children and adults.28,33,34 To examine whether RSV infection exacerbates pulmonary eosinophilia in a model of allergic asthma, we compared cotton rats sensitized and challenged with HDM to induce pulmonary eosinophilia with those that, in addition, were infected with RSV 1 d prior to HDM challenge (Figure 9). However, infection with RSV during an allergic asthma response actually decreased (adjusted P = 0.002) the number of eosinophils after HDM challenge, thus perhaps suggesting an immunosuppressive role for RSV.
Figure 9.
RSV infection attenuates the eosinophil response after challenge with HDM allergen. BAL cytology of cotton rats infected with 105 TCID50 RSV intranasally (RSV only), treated with 2 doses of the allergen HDM (HDM only), or both treated with allergen and infected with RSV (HDM+RSV). Cotton rats that received HDM treatment were sensitized through intraperitoneal injection on day 0 and challenged intranasally on day 8. RSV infections were performed intranasally on day 7. BAL fluid was collected on day 11, at 4 d after RSV inoculation. Bars represent mean values (n = 4–8 cotton rats per group); error bar, 1 SD. Value differs significantly (‡, adjusted P < 0.001; §, adjusted P < 0.0001; Welch t test, Holm–Sidak correction) from that for the RSV-only group or (†, adjusted P < 0.01;Welch t test, Holm–Sidak correction) from the HDM-only group.
Effect of vaccination with FI-RSV vaccine and subsequent RSV challenge on pulmonary eosinophil counts.
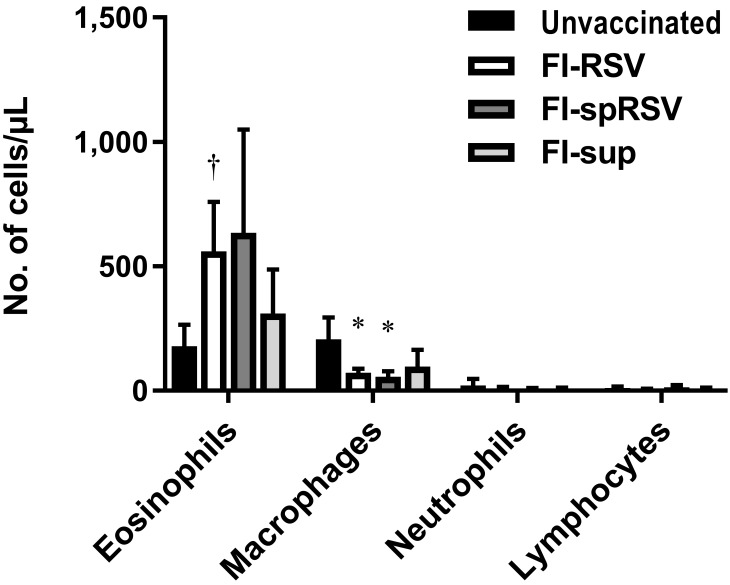
Immunization of mice and cotton rats with FI-RSV vaccines has been studied to determine the underlying mechanism of atypical disease after challenge with live virus. During these studies, an eosinophilic response occurred in mice.10,38 In cotton rats, polymorphonuclear granulocytic57 and neutrophilic responses without mention of eosinophils39,45 have been described. In addition, several studies have linked some of the effects of immunization with FI-RSV vaccines to contaminating factors including cellular proteins and fetal calf serum contained in the preparations of both vaccines and challenge viruses.4,41,51,56 To determine the effect of FI-RSV and nonviral antigens on the number of eosinophils in cotton rat lung, cotton rats were vaccinated with 1 of 2 different FI-RSV vaccine formulations or a formalin-inactivated mock vaccine before being challenged with live virus. We prepared the standard FI-RSV vaccine in the same way as the original vaccine so it contained both viral (that is, RSV) and nonviral (that is, cellular or fetal calf serum) antigens.43 The second vaccine, referred to as FI-spRSV, differed from the original vaccine in that the RSV was purified through a sucrose cushion before inactivation with formalin. This additional step served to remove many of the nonviral proteins that are carried over from growth in cell culture. The mock preparation (that is, FI-sup) was prepared from noninfected HEp2 cell cultures and therefore contained only nonviral antigens. In addition, we purified the live virus that was used to challenge all 4 groups through a sucrose cushion to reduce the amount of nonviral antigens present.51 After vaccination and challenge, cotton rats immunized with the FI-RSV vaccine had more eosinophils (adjusted P = 0.006) and fewer macrophages (adjusted P = 0.045) in their BAL fluid than did animals that received the challenge virus only (Figure 10). However, due to a large standard deviation, the difference in the number of eosinophils between the FI-spRSV group and the ‘challenge only’ group did not reach statistical significance (adjusted P = 0.051). Similar to those in the FI-RSV group, macrophage numbers in the FI-spRSV group were decreased (adjusted P = 0.035). No statistically significant difference occurred between any other groups.
Figure 10.
Vaccination with formalin-inactivated RSV induces an eosinophilic response after challenge with live virus. Cotton rats were immunized on days 0 and 28 with either formalin-inactivated RSV (FI-RSV), formalin-inactivated sucrose cushion-purified RSV (FI-spRSV), or a mock preparation of formalin-inactivated cell culture supernatant (FI-sup); control rats did not receive any vaccination, only RSV challenge (unvaccinated). All animals were challenged intranasally with 105 TCID50 RSV on day 49, and BAL fluid was collected on day 53. Bars represent mean values (n = 4–7 cotton rats per group); error bar, 1 SD. Value differs significantly (*, adjusted P < 0.05; †, adjusted P < 0.01; Welch t tests, Holm–Sidak correction) from that for unvaccinated group.
Discussion
The goal of our study was to explore whether RSV infection and eosinophilia were correlated, given the inconsistent data from humans and cotton rats. Perhaps the interaction of RSV and eosinophils is influenced by the severity of infection and other circumstances, such as allergen exposure. Because we aimed to use a cotton rat model to clarify the relationship between RSV and eosinophils, we were particularly interested in reports that described an increase in the percentage of eosinophils after RSV infection19 and those that did not.21,24,43 In the literature, photographs of tissue sections are often in black and white and at low magnification, thus complicating the characterization of eosinophils and the determination of the number of eosinophils. In the current study, we characterized eosinophils by using electron microscopy and Luna and Hansel stains. Of note, immunohistochemistry using antibodies directed against mouse eosinophil major basic protein has been unsuccessful for detecting cotton rat eosinophils. We attempted to use a rat antimouse monoclonal antibody to eosinophil major basic protein (product 14.7.4),13 but it was not cross-reactive.
In essence, the morphology of cotton rat eosinophils was the same as observed for mice and humans.48 Even the lung of uninfected cotton rats contained a high level of eosinophils (about half of all cells recovered in BAL fluid). Although most other species have low percentages of pulmonary eosinophils, cats and horses, like cotton rats, have high eosinophil numbers. Specifically, the BAL fluid of cats is 16% to 25% eosinophils in BAL fluid,36 and that of horses is as much as 35% eosinophils.14 In contrast, Brown Norway rats have fewer than 2% eosinophils in the BAL fluid from unsensitized lungs,55 and this figure is consistent with mouse data.7 However, the eosinophilic response varies widely between mouse strains. For example, sensitization with ovalbumin induced responses ranging from 0% to 90% eosinophils in BAL fluid.7 In mice, the majority of eosinophils reside in the small intestine. It has been suggested that this situation is not simply due to migratory patterns but also the provision of survival signals (GM-CSF, CCL11) in mouse intestine compared with lung.8 Cotton rat lungs may provide survival signals to eosinophils similar to those in the mouse intestine, thus perhaps explaining the high number of eosinophils present in cotton rat lung.
We found that the function of pulmonary eosinophils in cotton rats is comparable to that of other species. After stimulation with a calcium ionophore, cotton rat eosinophils released eosinophil peroxidase in a similar manner to eosinophils purified from human blood.2 When we investigated the influence of external stimuli on the cotton rat eosinophilic response, we found that the amount of dust in various types of bedding did not influence the number of eosinophils. However, after sensitization and subsequent challenge with HDM, eosinophil numbers in the respiratory tract of cotton rats increased, similarly to what occurs in mouse lung.10,38 Again, comparable to data from mice50 and humans,29 cotton rats respond to pulmonary bacterial infection (S. aureus) via an influx of neutrophils, and in response to viral infections (influenza virus, measles virus, RSV), macrophages and neutrophils increase in number correlating with the severity of damage to the respiratory epithelium, but eosinophil levels are not increased in response to infection. In contrast to a recent study of RSV infection in cotton rats,19 we did not observe an increase in pulmonary eosinophils after infection with RSV. Our finding is consistent with a lack of IL5 induction during primary RSV infection in cotton rats; IL5 is an important cytokine for the regulation of eosinophil formation, maturation, recruitment, and survival.6 Because the methods used in the previous6,21 and our current studies are essentially the same (that is, same RSV strain and BAL collection method), it seems unlikely that the observed differences would be due to differences in methods or techniques. One possible explanation is that animals of the same strain but from different breeders respond differently to treatment. Breeder-specific differences in WBC, RBC, lymphocytes, monocytes, eosinophils, and other hematologic parameters have been demonstrated in Wistar rats of the same strain but from different breeders.23 When age, diet, and environmental factors were controlled for by allowing a 3- to 5-wk acclimation period in the same facility, hematologic data between Wistar rats from breeder 1 and Wistar rats from breeder 2 were still distinct, indicating that environmental influences and epigenetic factors may explain the observed differences. In addition, similar findings were obtained for lab-raised, inbred cotton rats compared with lab-raised wild-stock, although the differences between those 2 groups were more likely to have been genetic.46
In addition, we found that RSV infection reduced the number of eosinophils in cotton rat lung during an allergic reaction. This finding is consistent with those in mice, for which RSV infection reduced the eosinophilic response and airway hyperresponsiveness against ovalbumin by inducing the expression of KC (IL8 analog).3
RSV vaccine-enhanced disease has been described as a nonprotective, pathogenic antibody response elicited by inactivated RSV immunogens due to a lack of affinity maturation, which manifests in pulmonary eosinophilia in animal models.12 In the original reports about vaccine-enhanced disease, eosinophils were found in the lung and blood of affected patients.11,16,25,27 However, this finding has been questioned, and now neutrophilia is recognized as the predominant finding in vaccine-enhanced disease in humans.43 In the mouse model, vaccine-enhanced disease is characterized by the expression of IL4 and IL5 (see reference 38 for review). Similarly in cotton rats, IL4 and IL5 markedly increased after RSV infection of animals previously immunized with FI-RSV.6 In addition, IL13 is increased and acts in concert with IL5 to recruit eosinophils into the lung.42 The expression of both IL5 and IL13 in cotton rats is consistent with our finding that eosinophils are increased during FI-RSV vaccine-enhanced disease in this species, and these data are consistent with findings from the mouse model.
In summary, we have characterized cotton rat eosinophils both morphologically and functionally. We find that cotton rats have relatively high basal levels of eosinophils in blood and BAL fluid and respond normally to allergen, bacteria, and viruses. Taken together, our findings indicate that cotton rat eosinophils are not recruited to the lung in response to RSV infection, but eosinophils are increased in the vaccine-enhanced RSV disease model.
Acknowledgments
The Comparative Pathology and Mouse Phenotyping Shared Resource (Ohio State University) is supported in part by NIH grant P30 CA016058.
References
- 1.Acharya KR, Ackerman SJ. 2014. Eosinophil granule proteins: form and function. J Biol Chem 289:17406–17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamko DJ, Wu Y, Gleich GJ, Lacy P, Moqbel R. 2004. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods 291:101–108. [DOI] [PubMed] [Google Scholar]
- 3.Aeffner F, Davis IC. 2012. Respiratory syncytial virus reverses airway hyperresponsiveness to methacholine in ovalbumin-sensitized mice. PLoS One 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boelen A, Andeweg A, Kwakkel J, Lokhorst W, Bestebroer T, Dormans J, Kimman T. 2000. Both immunization with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine 19:982–991. [DOI] [PubMed] [Google Scholar]
- 5.Boukhvalova MS, Blanco JCG. 2013. The cotton rat Sigmodon hispidus model of respiratory syncytial virus infection. Curr Top Microbiol Immunol 372:347–358. [DOI] [PubMed] [Google Scholar]
- 6.Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. 2006. The TLR4 agonist monophosphoryl lipid A attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine 24:5027–5035. [DOI] [PubMed] [Google Scholar]
- 7.Brewer JP, Kisselgof AB, Martin TR. 1999. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med 160:1150–1156. [DOI] [PubMed] [Google Scholar]
- 8.Carlens J, Wahl B, Ballmaier M, Bulfone-Paus S, Forster R, Pabst O. 2009. Common γ-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J Immunol 183:5600–5607. [DOI] [PubMed] [Google Scholar]
- 9.Carsillo M, Klapproth K, Niewiesk S. 2009. Cytokine imbalance after measles virus infection but no correlation with immune suppression. J Virol 83:7244–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilow EM, Olson MR, Varga SM. 2007. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res 39:225–239. [DOI] [PubMed] [Google Scholar]
- 11.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 89:449–463. [DOI] [PubMed] [Google Scholar]
- 12.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. 2008. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 15:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denzler KL, Farmer SC, Crosby JR, Borchers M, Cieslewicz G, Larson KA, Cormier-Regard S, Lee NA, Lee JJ. 2000. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol 165:5509–5517. [DOI] [PubMed] [Google Scholar]
- 14.Depecker M, Richard EA, Pitel PH, Fortier G, Leleu C, Courouce-Malblanc A. 2014. Bronchoalveolar lavage fluid in Standardbred racehorses: influence of unilateral/bilateral profiles and cut-off values on lower airway disease diagnosis. Vet J 199:150–156. [DOI] [PubMed] [Google Scholar]
- 15.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. 1998. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis 177:1458–1464. [DOI] [PubMed] [Google Scholar]
- 16.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. 1969. Respiratory virus immunization. I. A field trial of 2 inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 89:435–448. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo R, Kimpen JL, Welliver RC, Ogra PL. 1992. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr 120:28–32. [DOI] [PubMed] [Google Scholar]
- 18.Green MG, Huey D, Niewiesk S. 2013. The cotton rat (Sigmodon hispidus) as an animal model for respiratory tract infections with human pathogens. Lab Anim (NY) 42:170–176. [DOI] [PubMed] [Google Scholar]
- 19.Grieves JL, Yin Z, Durbin RK, Durbin JE. 2015. Acute and chronic airway disease after human respiratory syncytial virus infection in cotton rats (Sigmodon hispidus). Comp Med 65:315–326. [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. 1999. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med 159:1918–1924. [DOI] [PubMed] [Google Scholar]
- 21.Hwang HS, Lee YT, Kim KH, Park S, Kwon YM, Lee Y, Ko EJ, Jung YJ, Lee JS, Kim YJ, Lee YN, Kim MC, Cho M, Kang SM. 2016. Combined virus-like particle and fusion protein-encoding DNA vaccination of cotton rats induces protection against respiratory syncytial virus without causing vaccine-enhanced disease. Virology 494:215–224. [DOI] [PubMed] [Google Scholar]
- 22.Jartti T, Gern JE. 2017. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol 140:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kampfmann I, Bauer N, Johannes S, Moritz A. 2012. Differences in hematologic variables in rats of the same strain but different origin. Vet Clin Pathol 41:228–234. [DOI] [PubMed] [Google Scholar]
- 24.Kamphuis T, Stegmann T, Meijerhof T, Wilschut J, de Haan A. 2013. A virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A provides protection against viral challenge without priming for enhanced disease in cotton rats. Influenza Other Respir Viruses 7:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. [DOI] [PubMed] [Google Scholar]
- 26.Kim CK, Kim SW, Park CS, Kim BI, Kang H, Koh YY. 2003. Bronchoalveolar lavage cytokine profiles in acute asthma and acute bronchiolitis. J Allergy Clin Immunol 112:64–71. [DOI] [PubMed] [Google Scholar]
- 27.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. [DOI] [PubMed] [Google Scholar]
- 28.Knudson CJ, Varga SM. 2015. The relationship between respiratory syncytial virus and asthma. Vet Pathol 52:97–106. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi SD, Malachowa N, DeLeo FR. 2015. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol 185:1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokai-Kun JF, Walsh SM, Chanturiya T, Mond JJ. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob Agents Chemother 47: 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristjánsson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, Jonsdottir I. 2005. Respiratory syncytial virus and other respiratory viruses during the first 3 mo of life promote a local Th2-like response. J Allergy Clin Immunol 116:805–811. [DOI] [PubMed] [Google Scholar]
- 32.Kristjánsson S, Wennergren D, Eriksson B, Thorarinsdottir H, Wennergren G. 2006. U-EPX levels and wheezing in infants and young children with and without RSV bronchiolitis. Respir Med 100:878–883. [DOI] [PubMed] [Google Scholar]
- 33.Kurai D, Saraya T, Ishii H, Takizawa H. 2013. Virus-induced exacerbations in asthma and COPD. Front Microbiol 4:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacy P, Rosenberg HF, Walsh GM. 2014. Eosinophil overview: structure, biological properties, and key functions. Methods Mol Biol 1178:1–12. PubMed 10.1007/978-1-493910016-8_1 [DOI] [PubMed] [Google Scholar]
- 35.Marshall KL. 2008. Rabbit hematology. Vet Clin North Am Exot Anim Pract 11:551–567 [vii.]. [DOI] [PubMed] [Google Scholar]
- 36.McCullough S, Brinson J. 1999. Collection and interpretation of respiratory cytology. Clin Tech Small Anim Pract 14:220–226. [DOI] [PubMed] [Google Scholar]
- 37.Meyerholz DK, Griffin MA, Castilow EM, Varga SM. 2009. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol 37:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Openshaw PJ, Culley FJ, Olszewska W. 2001. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 20 Suppl 1: S27–S31. [DOI] [PubMed] [Google Scholar]
- 39.Pfeuffer J, Püschel K, Ter Meulen V, Schneider-Schaulies J, Niewiesk S. 2003. Extent of measles virus spread and immune suppression differentiates between wild-type and vaccine strains in the cotton rat model (Sigmodon hispidus). J Virol 77: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. 2007. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110:1578–1586. [DOI] [PubMed] [Google Scholar]
- 41.Piedra PA, Wyde PR, Castleman WL, Ambrose MW, Jewell AM, Speelman DJ, Hildreth SW. 1993. Enhanced pulmonary pathology associated with the use of formalin-inactivated respiratory syncytial virus vaccine in cotton rats is not a unique viral phenomenon. Vaccine 11:1415–1423. [DOI] [PubMed] [Google Scholar]
- 42.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. 2001. IL13 induces eosinophil recruitment into the lung by an IL5- and eotaxin-dependent mechanism. J Allergy Clin Immunol 108:594–601. [DOI] [PubMed] [Google Scholar]
- 43.Prince GA, Curtis SJ, Yim KC, Porter DD. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol 82:2881–2888. [DOI] [PubMed] [Google Scholar]
- 44.Prince GA, Jenson AB, Horswood RL, Camargo E, Chanock RM. 1978. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol 93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 45.Prince GA, Prieels JP, Slaoui M, Porter DD. 1999. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus). Lab Invest 79:1385–1392. [PubMed] [Google Scholar]
- 46.Robel GL, Lochmiller RL, McMurry ST, Qualls CW., Jr 1996. Environmental, age, and sex effects on cotton rat (Sigmodon hispidus) hematology. J Wildl Dis 32:390–394. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg HF, Dyer KD, Domachowske JB. 2009. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res 83:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg HF, Dyer KD, Foster PS. 2012. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi GA, Colin AA. 2015. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J 45:774–789. [DOI] [PubMed] [Google Scholar]
- 50.Sethi S, Chakraborty T. 2011. Role of TLR–NLR signaling and the associated cytokines involved in recruitment of neutrophils in murine models of Staphylococcus aureus infection. Virulence 2:316–328. [DOI] [PubMed] [Google Scholar]
- 51.Shaw CA, Galarneau JR, Bowenkamp KE, Swanson KA, Palmer GA, Palladino G, Markovits JE, Valiante NM, Dormitzer PR, Otten GR. 2013. The role of nonviral antigens in the cotton rat model of respiratory syncytial virus vaccine-enhanced disease. Vaccine 31:306–312. [DOI] [PubMed] [Google Scholar]
- 52.Siegrist CA, Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 9:185–194. [DOI] [PubMed] [Google Scholar]
- 53.Soukup JM, Becker S. 2003. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin Immunol 107:178–185. [DOI] [PubMed] [Google Scholar]
- 54.Sung RY, Hui SH, Wong CK, Lam CW, Yin J. 2001. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur J Pediatr 160:117–122. [DOI] [PubMed] [Google Scholar]
- 55.Tsuchiya K, Siddiqui S, Risse PA, Hirota N, Martin JG. 2012. The presence of LPS in OVA inhalations affects airway inflammation and AHR but not remodeling in a rodent model of asthma. Am J Physiol Lung Cell Mol Physiol 303:L54–L63. [DOI] [PubMed] [Google Scholar]
- 56.Vaux-Peretz F, Chapsal JM, Meignier B. 1992. Comparison of the ability of formalin-inactivated respiratory syncytial virus, immunopurified F, G, and N proteins and cell lysate to enhance pulmonary changes in Balb/c mice. Vaccine 10:113–118. [DOI] [PubMed] [Google Scholar]
- 57.Vaux-Peretz F, Meignier B. 1990. Comparison of lung histopathology and bronchoalveolar lavage cytology in mice and cotton rats infected with respiratory syncytial virus. Vaccine 8:543–548. [DOI] [PubMed] [Google Scholar]