Abstract
Currently available animal models for delivery of drug capsules and pharmacokinetic testing are limited by either intersubject variability in gastric emptying time or the need to sedate animals when using targeted delivery methods of drug capsules. With the increasing development of large-molecule biologics, better in vivo models for testing the pharmacokinetics of capsule-delivered drugs are urgently needed. To this end, we made engineering modifications to an existing bovine surgical cannula device, successfully implanted this modified cannula into pigs, and delivered drug capsules directly to the proximal duodenum. In our porcine model, capsule insertion and serial blood samples were all acquired without the use of sedatives. Furthermore, we were able to maintain cannulated pigs for weekly pharmacokinetic testing for more than 18 mo, with minimal postoperative complications. This study demonstrates a novel and effective porcine model of sedation-free drug delivery and blood collection that eliminates inconsistencies associated with models that require either gastric emptying or animal sedation.
Abbreviation: VAP, vascular access port
In vivo models of drug delivery often involve testing of mice and rats,22 whose major benefits as an animal model includes their small size, short gestation time, wide availability of genetically modified models, and relatively low expense, compared with large animal models. Biologics accounted for 22% of the sales of large commercial pharmaceutical companies in 2013 and are estimated to comprise as much as 32% of sales in 2023.2 In the last decade, the increased development of biologics drugs has significantly increased the demand for large-molecule drug therapy testing. The high molecular mass of biopharmaceutical drugs causes challenges regarding enteric delivery of these products, particularly their reduced permeability across mucosal and cell membranes.14 To overcome these challenges, several novel drug delivery devices and capsules are emerging.14 A large animal model that accommodates enteric drug and capsule delivery for preclinical pharmacokinetic testing will allow scientists to test these novel delivery methods in vivo before moving to clinical trials. The porcine duodenal cannula drug delivery method we describe here offers a novel large animal model for pharmacokinetic testing and successfully overcomes several mechanical obstacles in other similar animal models.14
The benefit of cannulation drug delivery is 3-fold: it allows the delivered drug to bypass the stomach; it enables the targeting of a specific area of the intestine; and it accommodates the insertion of a large capsule or drug without the need for sedation or anesthesia. Targeted drug delivery to specific areas of the small intestine has been accomplished in rabbit and dog models through the use of a modified vascular access port surgically implanted into the intestine.11,13 Although these models allow for direct delivery to specific areas of the intestine, the small size of the port (5 French) limits delivery to liquids.11,13 Small intestinal cannulation has been described in pigs, goats, sheep, and cows for the sampling of gastrointestinal contents during nutrition studies.1,5,7,10,19 Publications specifically on cannulated pig models for the purpose of intestinal sampling date back to the 1970s. The surgical techniques described in these older publications include many of the same techniques that we used in the present study, with moderate modifications based on the updated surgical anesthetics, instruments, and plastics materials currently available.8,16 In our porcine duodenal cannulation model, we modified a cannulation technique used previously in nutritional studies1,21 so that we could instead deliver a solid (in the form of a capsule) and subsequently test drug absorption. The larger cannula of our model (internal diameter, 1.4 cm) compared with the 5-French catheter (internal diameter, 0.007 cm) used previously enabled us to deliver whole capsules into the small intestine of pigs.
Inserting a cannula directly into the duodenum eliminates inconsistencies associated with gastric emptying time and the ability of the capsule to pass through the pyloric sphincter. The physiology of digestion in swine is remarkably similar to that of humans,20 but due to the pig's anatomy, gastric emptying of large solids and capsules takes considerably longer than in humans.4 In comparison, gastric emptying of large-sized solids in the dog model is more similar to that in humans, but it still varies between subjects and, on average, occurs much faster than in humans making it a less-than-ideal model.4 In addition, a duodenal cannula enables us to insert the capsule into a pig's intestine without the use of sedation. Given that major side effects of sedation are decreased intestinal motility and secretion,18,21 our new method has a marked advantage over other methods, such as endoscopic placement of drugs or capsules, which can only be performed under general anesthesia in animal models.
Materials and Methods
Female Yucatan pigs (Sus scrofa; n = 7; weight, 35 to 50 kg) were used for this study. The pigs were housed and maintained according to the Guide for the Care and Use of Laboratory Animals9 in an AAALAC-accredited facility. The housing room was temperature- and humidity-controlled, with a 12:12-h light:dark cycle. The primary enclosure for each pig consisted of a pen a with solid floor, pine shavings, and an enrichment toy. The pigs had free access to water through an automatic sipper and were given 400 g of pelleted feed twice daily, along with 4 ounces of marshmallow or mixed dried fruit treats for training. Pig runs were cleaned daily by husbandry staff, and the animals were checked daily by veterinary technicians or veterinarians. Hay was withheld due to a concern for intestinal blockage at the site of the cannula. All procedures performed on the animals were approved by the Tufts University IACUC.
The pigs were fasted 12 h before cannula insertion surgery. The pigs were premedicated with ketamine (4 mg/kg IM), xylazine (4 mg/kg IM), and tiletamine–zolazepam (8 mg/kg IM) prior to endotracheal intubation. Once intubated, the animals were maintained under anesthesia with isoflurane gas (2% to 3%).
The pigs were positioned in left lateral recumbency. Skin over the surgical site was shaved and prepped with chlorhexidine and alcohol. A vascular access port (VAP) was placed in the right external jugular vein, with the port inserted under the skin over the right shoulder by using standard techniques.15
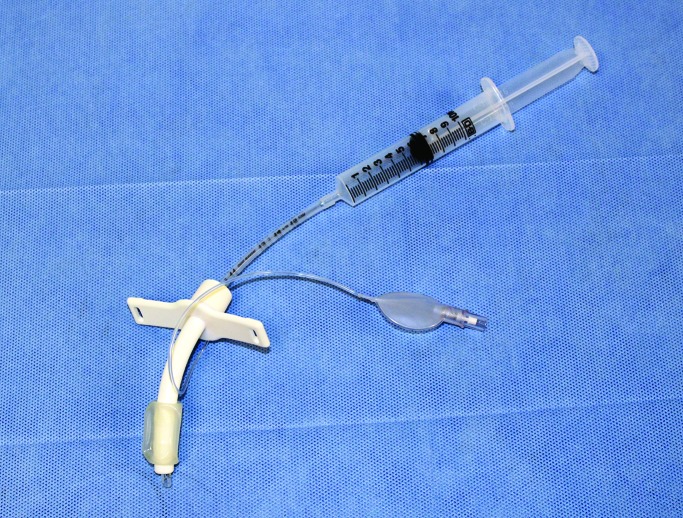
The duodenal cannula (ANKOM Technologies, Macedon, NY; Figure 1 A) underwent several modifications (Figure 1 B), which included shortening the intraintestinal piece of the cannula by cutting it to create a half pipe instead of a complete closed lumen pipe so that it would fit in the pig duodenum. In addition, the external plastic ring was modified from hard plastic to a silicone ring to reduce skin irritation. The external end of the plug was modified from a wing-nut design to a flat rubber top to prevent tearing of outer bandaging by the sharp edges on the wing nut. The cannula device was autoclaved prior surgical implantation.
Figure 1.
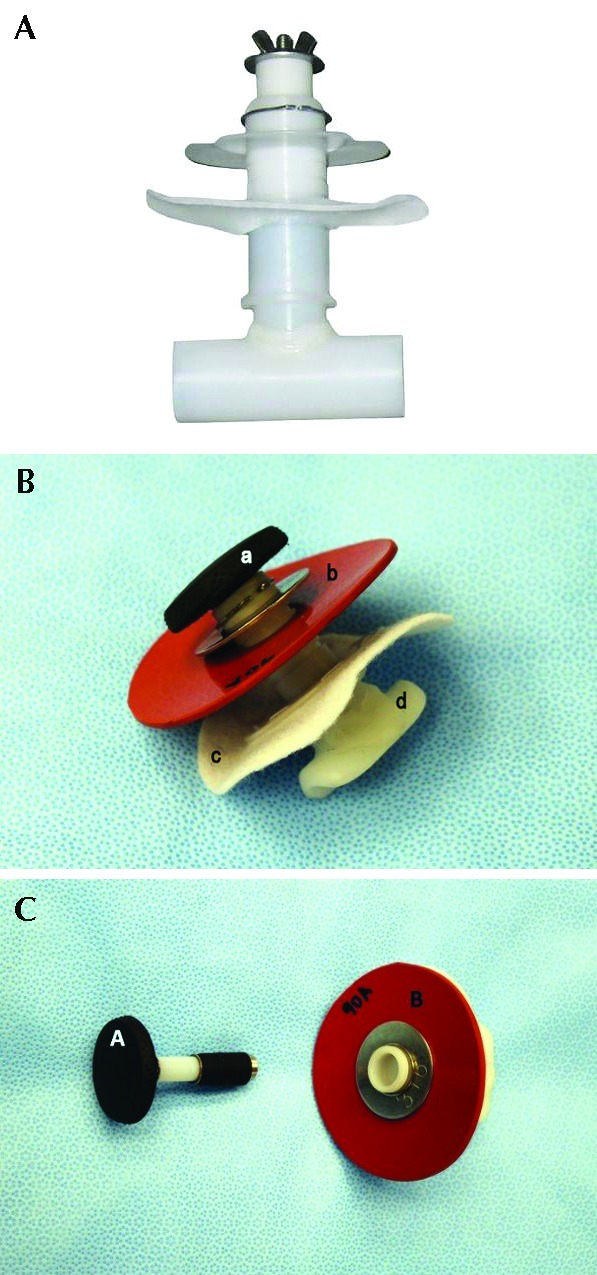
(A) Bovine intestinal cannula before modification. (B) Intestinal cannula used in the pigs after several modifications: open lumen design and reduction of the intraintestinal size of the cannula, modified silicone material for the outer ring instead of hard plastic, modified plug with flat top, and heavier c-clamp to secure the outer ring. Components of the duodenal cannula system include the plug (1) and outer silicone skin ring and metal washer (2) which are secured in place by the c-clamp. The inner fabric ring (3) sits inside the peritoneal wall to prevent the cannula from slipping out. The end of the T-shaped hard plastic insertion (4) sits inside the duodenum. (C) The plug (1) can be removed and reinserted into the plastic cannula to prevent intestinal leakage and allow direct access into the duodenum.
A 10-cm linear skin incision was made on the pig's right lateral flank 1cm caudal to the last rib by using a no. 10 scalpel blade (Figure 2). A no. 10 blade was used to incise through the internal oblique muscle tissue and create a paracostal laparotomy to expose the duodenum. The duodenum was located manually by following it from the gastric pylorus. A section of the duodenum 6 to 10 cm distal to the pylorus was located and the cannula placed over this area for size measurement. Vessel loops, made from soft flexible silicon, were placed around the duodenum to prevent it from slipping back into the abdomen. Sufficient laparotomy sponges were used to pack off the isolated intestinal segment to avoid contamination of the abdominal cavity.
Figure 2.
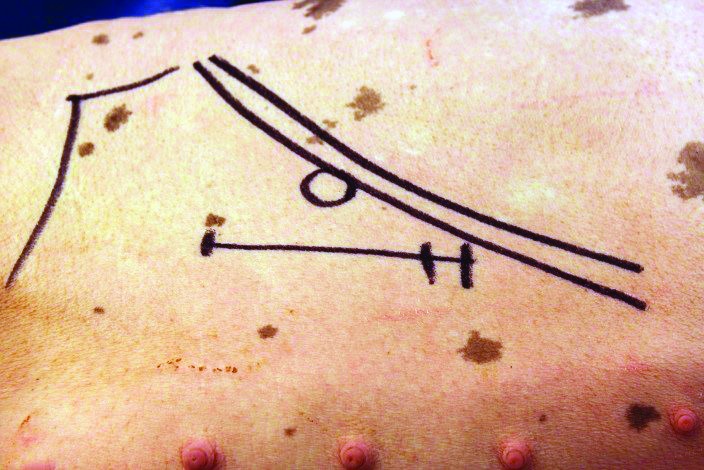
The caudal ribs and pelvis were palpated and marked to avoid these areas. The cannula exteriorization point has been marked with a circle just caudal to the last rib. The linear incision for access to duodenum is outlined just below.
A full-thickness linear incision (length, approximately 3 cm) on the antimesenteric surface of the duodenum was created by using a no. 11 scalpel blade. This incision was just large enough for insertion of the cannula. The cannula, which was unplugged, was inserted into the intestine. When manual insertion was insufficient, Debakey forceps were used to gently stretch the intestinal tissue over the cannula. A double pursestring suture was created with 0 to 3-0 multifilament polyester–polybutylate nonabsorbable suture, to secure the cannula in place. Both ties from the pursestring were cinched down to secure the cannula and then tied in place by using surgeon square knots (Figure 3). The cannula was then plugged, and the intestine visually checked for leaks by applying gentle pressure to the intestinal tissue adjacent to the cannula and watching for air or fluid leakage around the cannula and intestinal sutures.
Figure 3.
Cannula has been inserted into the duodenum and secured with a double pursestring suture. Laparotomy sponges are used to pack off the rest of the abdominal cavity, to prevent any leakage of intestinal contents into the peritoneal cavity. Care is taken to avoid tearing the intestine during insertion.
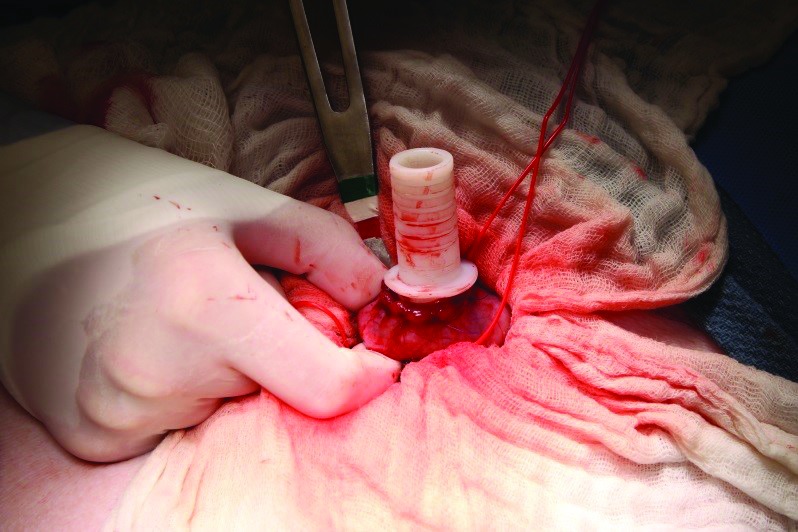
After the cannula was inserted into the intestine and secured with the double pursestring suture, the inner fabric ring was applied above the intestine but below the peritoneal membrane. A full-thickness incision was made through the skin, muscle, and peritoneum by using a 10-mm punch biopsy and no. 11 blade. Care was taken to position the cannula opening through the skin such as to minimize pulling and kinking of the duodenum. An insertion device was tunneled through the full-thickness opening and inserted into the open cannula. This device was then used to pull the cannula back out through the skin (Figure 4). The final step to secure the cannula in place by applying the outer silicone ring and washer above skin and affixing this apparatus by using a c-clamp (Figure 5). The tightest possible notch was used to secure the outer ring in place. The peritoneum, muscle, and skin layers were closed separately in a 4-layer closure for the lateral laparotomy incision.
Figure 4.

Insertion device used to pull the cannula through the muscle wall and skin after the cannula has been secured in the intestine by using a double pursestring suture. This device is placed into the tubular port of the cannula, where the plug goes. This insertion device is removed after surgical placement of the duodenal cannula is complete.
Figure 5.
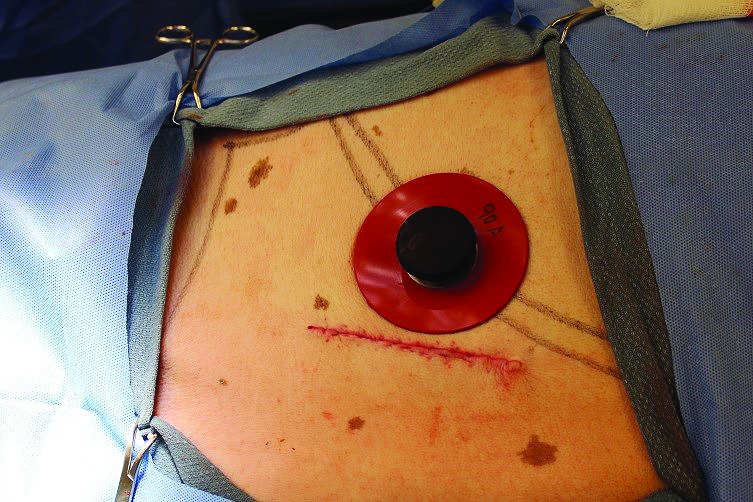
Immediate postoperative view of cannula insertion. The linear incision has been closed by using a subcuticular pattern. The external cannula sits just behind the last rib. The silicone ring and metal washer hold the cannula in place and allow enough flexibility to accommodate minor postoperative swelling.
Ampicillin (6 mg/kg IV) was given intraoperatively to prevent infection secondary to gastrointestinal surgery. Pain associated with cannula placement was alleviated with local bupivacaine on the day of surgery, a dermal fentanyl patch (25 μg/h) placed 24 h before surgery, and ketoprofen (3 mg/kg IM) at surgery and then once daily postoperatively as needed for a minimum of 3 d.
Cannulas were bandaged to reduce the leakage of intestinal contents and protect the cannulas from being dislodged during rubbing along the sides of the runs. Bandages were created by placing a thin foam dressing (Tegaderm, 3M, Maplewood, MN) over the cannula and securing in place with medical tape. Applying tincture coating (Compound Benzoin Tincture Swabstick, 4 in., Medex Supply, Passaic, NH) to the skin extended the life of the bandage by further securing the tape to the skin. After several months of bandage replacements as needed, we switched to using a fabric (nylon and cotton) reusable bandage that fit over the area of the cannula and was secured in place with hook-and-loop fasteners. This arrangement offered more breathability than the tape bandages and thus less irritation of the skin surrounding the cannula yet still provided protection from physical damage.
The pigs were given 2 wk to heal from implant surgery, after which we began weekly pharmacokinetic studies that included insertion of a drug capsule through the duodenal cannula followed by serial blood collection through the VAP at timed intervals to evaluate drug absorption (Figure 6). A modified tracheal tube was inserted behind the capsule, to flush the capsule into the intestine (Figure 7). In this particular study, a polymer capsule was used to deliver several biologics. The same method was used to insert a video capsule into the cannula to capture live images of intestinal mucosa as well as measure intestinal and capsular transit times. By inserting the capsule in unsedated pigs and then sedating (ketamine, 2 mg/kg IM; xylazine, 2 mg/kg; and tiletamine–zolazepam, 4 mg/kg IM; with isoflurane gas [1% to 2%] as needed) them 4 h later for fluoroscopic imaging, we were able to visualize the progression of the capsule through the intestine (Figure 8 B).
Figure 6.
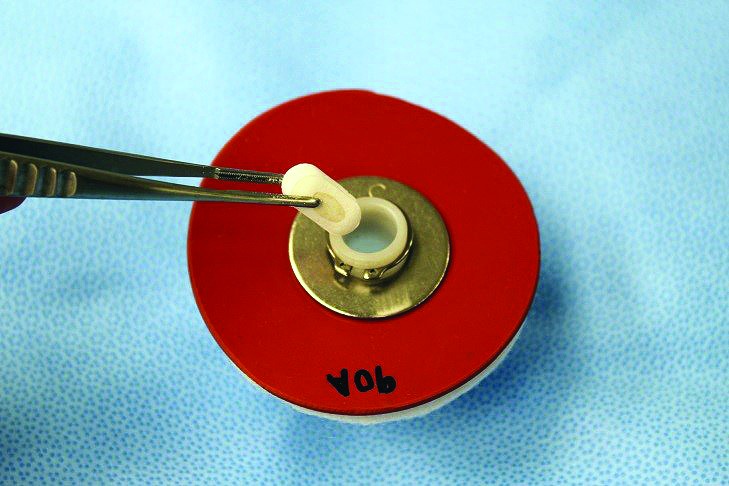
Demonstration of insertion of a size 0 capsule (outer diameter, 7.65 mm) into the cannula (inner diameter, 1.4 cm); the cannula is sufficiently wide to accommodate the insertion of large capsules.
Figure 7.
A modified (size 6) tracheostomy tube was used to flush 10 mL of saline into the cannula after capsule insertion, to ensure that the capsule reached the intestine and did not remain inside the cannula.
Figure 8.
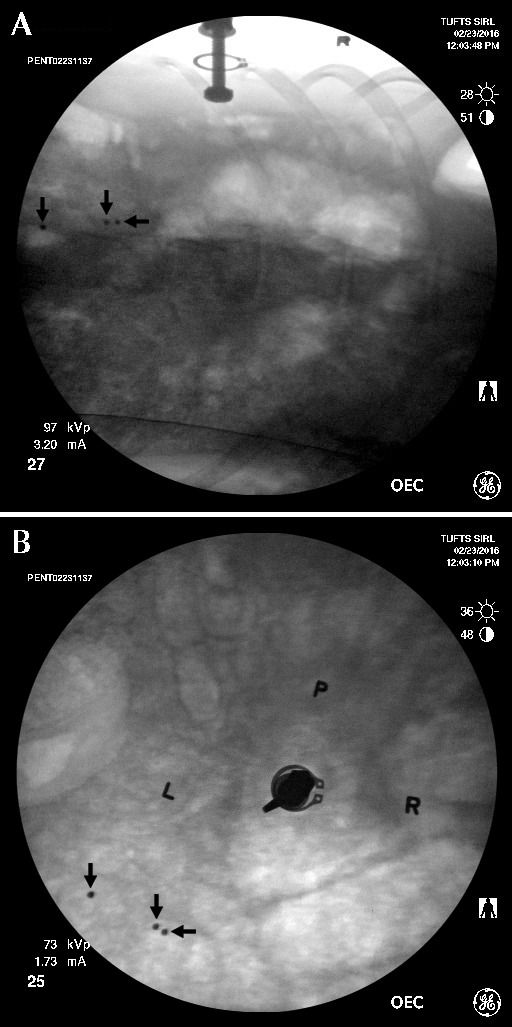
This 2-view fluoroscopic image was acquired 4 h after capsule insertion in an unanesthetized pig. The 3 radioopaque beads (arrows) inside the capsule can be seen; their separation indicates that the capsule has dissolved, thus releasing the beads. (A) Lateral view of duodenal cannula. (B) Dorsal view of duodenal cannula. L, left side of cannula; P, identification of individual pig (located dorsal to cannula); R, right side of cannula.
To eliminate sedation and its side effects, we tried several methods of training and restraint to achieve multiple blood sampling in unsedated pigs and minimize distress to the animals and staff. The animals were clicker-trained by using positive reinforcement methods17 to accustom the pigs to handling and to get them to voluntarily enter into a sling restrainer. Topical lidocaine (2.5%)–prilocaine (2.5%) cream was applied 45 min prior to blood sampling to decrease pain associated with needle insertion into the VAP. To further decrease the pain associated with multiple needle sticks into the VAP, a Huber needle set (that is, a Huber needle, tube clip, and 3 in. of extension tubing) was fixed by using medical tape so that multiple blood samples could be collected after a single needle insertion. A chlorhexidine prep stick (2% chlorhexidine gluconate and 70% isopropyl alcohol) was used to cleanse the skin before any needle sticks, to help prevent infection. Heparin lock solution (5 mL, 100 U/mL) was used to flush the VAP after blood sampling to maintain patency between draws. After 3 to 4 wk of weekly blood draws, along with daily 15-min maintenance training sessions, we were able to place and secure Huber needle catheters in unsedated pigs inside their cages without the use of the restraint sling.
All pigs were euthanized by intravenous pentobarbital– phenytoin overdose (Beuthanasia-D, Merck Animal Health, Millsboro, DE) at study end point.
Results
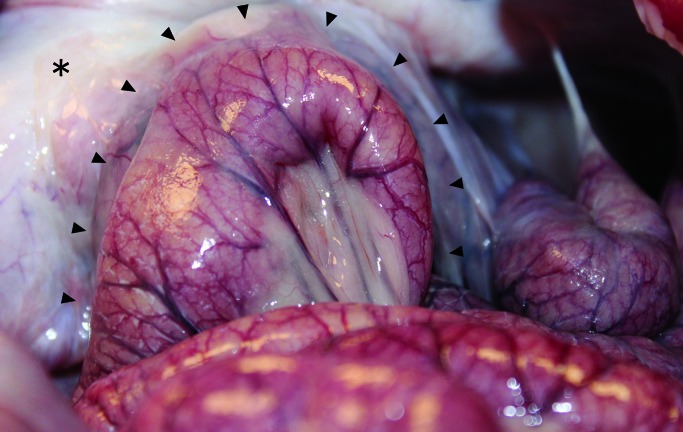
Duodenal cannula placement and long-term maintenance in our pig model was successful. Blood work results, fluoroscopic imaging, and video capsule monitoring confirmed that use of the cannula for capsule delivery led to capsule progression through the small intestines and subsequent drug absorption (Figure 8). Four of the cannulated pigs were necropsied at the experimental end point. All 4 pigs had abdominal adhesions associated with the fabric ring of the inner cannula (Figure 9). These adhesions created a seal between the cannulated intestine and the rest of the peritoneal cavity, thus helping to prevent the leaking of gastrointestinal contents into the peritoneum. Video capsule imaging supported the absence of obstructions and delayed intestinal transit due to these adhesions.
Figure 9.
Fibrous peritoneal adhesions (arrows) between the intestine and abdominal wall surrounding the duodenal cannula. A jejunal loop is adhered to the duodenum and peritoneal wall at the site of the duodenal cannula. No necrosis or obstruction was noted in the jejunal loop at the cannula site. *, Body wall.
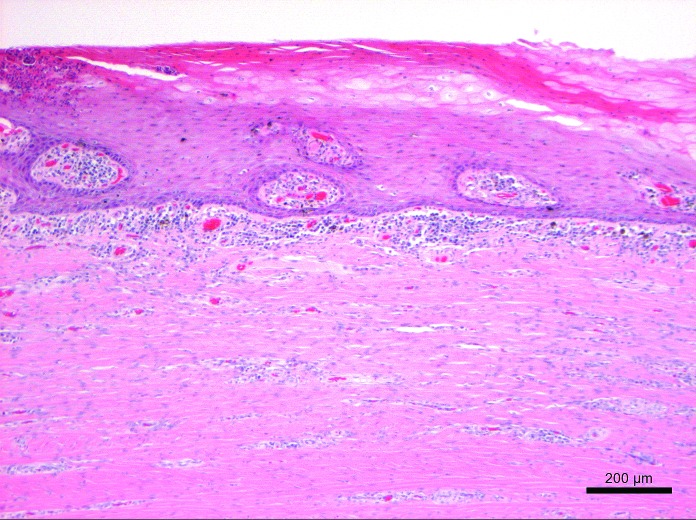
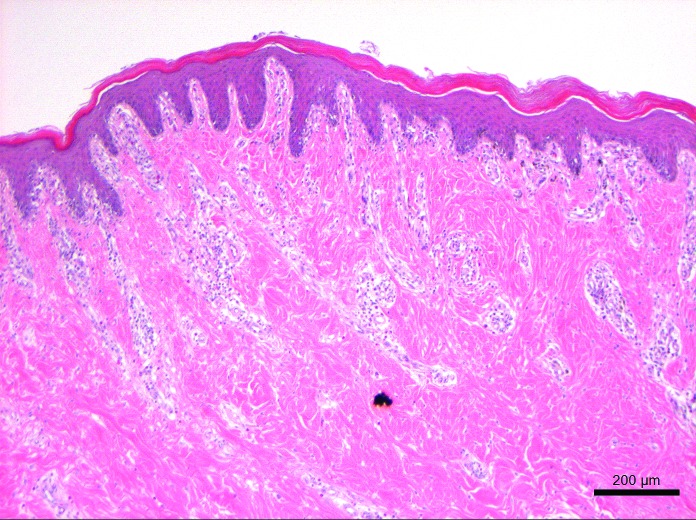
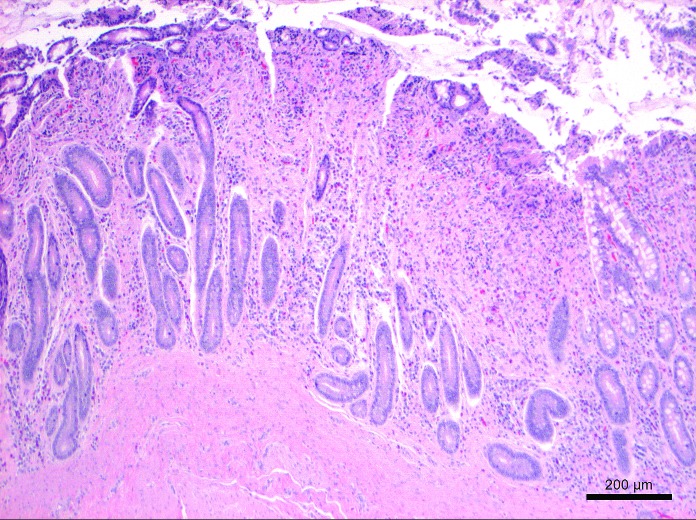
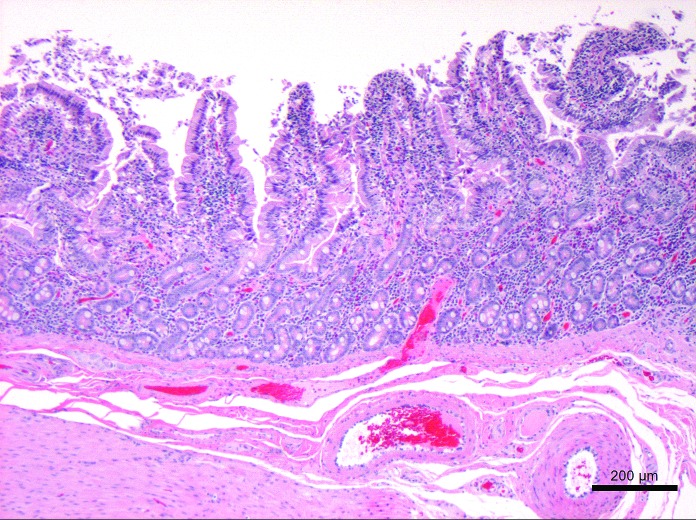
Representative tissues of skin at the cannulation site and intestine adjacent to the duodenal cannula were collected, fixed by immersion in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 um, and stained with hematoxylin and eosin. The tissue lining the tract of the cannula as it transited the body wall was lined by a thick layer of stratified squamous epithelium (Figure 10). The skin surrounding the external silicone ring was mildly thickened (Figure 11). Postmortem histology of the intestine directly adjacent to the internal portion of the duodenal cannula contained atrophied villi, fibrosis of the mucosa with loss of crypts, consistent with chronic rubbing of the mucosa by the cannula (Figure 12). Histology of the duodenum taken 6 cm distal to the cannulated intestine was normal (Figure 13), indicating that the presence of the indwelling cannula did not affect the integrity of duodenal mucosa distal to the cannulated portion of intestine (Figure 13). The histology and gross necropsy findings indicate a pig with a healthy intact cannula; this pig was euthanized due to external migration and failure of the VAP.
Figure 10.
Histology of the tissue lining cannula tract through the abdominal wall at 18 mo after implantation. The tract was lined by hyperplastic epithelium contiguous with the epidermis of the skin. Hyperkeratosis, small pockets of intraepithelial neutrophils, and a mild superficial dermal mononuclear infiltrate are present. Hematoxylin and eosin stain.
Figure 11.
Histology of the normal sparsely haired skin adjacent to the external silicone ring of the duodenal cannula at 18 mo after implantation. Mild perivascular and superficial dermal mononuclear infiltrates are present. Hemotoxylin and eosin stain.
Figure 12.
Histology of the duodenal mucosa at the cannulation site at 18 mo after cannula implantation. This section contains atrophied villi and fibrosis of the mucosa with loss of crypts, consistent with chronic rubbing of the mucosa by the cannula. Hematoxylin and eosin stain.
Figure 13.
Histology of the normal duodenal intestinal mucosa 6 cm distal to the indwelling cannula at 18 mo after cannula implantation. Hematoxylin and eosin stain.
Two 35-kg female Yucatan pigs were used as an initial trial group for cannula implantation. The most challenging part of the procedure was the placement of the cannula in the intestine. Intestinal tearing occurred during the placement of the first cannula because of the tension that the cannula caused inside the duodenum. The tear was repaired by placing continuous sutures along the tear adjacent to the cannula. Given the size of capsule inserted through the cannula, decreasing the size of the cannula was not an option. In the second group of 2 pigs, we attempted to circumvent the problem regarding cannula fit by using slightly larger pigs (45 kg); this modification made the placement easier and eliminated tears, but the fit of the cannula inside the duodenum was still very tight. The pigs in the third group of 3 (60 kg) Yucatans did not have markedly larger intestinal diameters, and their increased abdominal fat made it more difficult to access and isolate the duodenum. Thus, according to our experience, 45-kg female Yucatan pigs are most appropriate for this device implantation model.
Additional postoperative complications included mild to severe external leaking of intestinal contents between the cannula and the skin or tissue layer (Table 1). Mild leakage was a quarter-sized area of gastrointestinal liquid that soaked through the bandage weekly. Moderate leakage consisted of a 2- to 4-cm soiled area that necessitated bandage changes every 2 to 3 d. Severe leakage was considerable leakage of contents daily that resulted in daily bandage changes and frequent skin irritation from the moisture. Skin irritations were managed with topical application of 1% silver sulfadiazine or 40% zinc oxide cream. Irritated areas of skin responded well to topical treatment and further improved with use of the fabric bandage. The pig that had the most severe periportal leakage from the cannula was left unbandaged, which (in addition to topical cream application) helped to alleviate the skin irritation. The cannula in this pig did not become dislodged in the absence of bandaging, but despite the cream application, after 5 mo, the skin around the port had become thinner, with areas of moderate necrosis. Owing to the compromised skin around the port, this pig was euthanized. In addition, 2 other pigs experienced moderate intermittent leakage around the duodenal port site, which caused repeated temporary skin irritations. The pigs rubbed at the irritated area but did not experience any systemic signs of illness, such as change in appetite, energy, or other behaviors. Application of topical cream appeared to reduce the rubbing behavior and alleviate potential pruritis associated with the irritation.
Table 1.
Summary of postoperative complications
| No. of pigs | Complication |
| 3 | Moderate periportal leaking from duodenal cannula |
| 2 | Severe skin irritation associated with the cannula leakage |
| 1 | Infected VAP (positive for Actinomyces) |
| 1 | C-ring failure requiring surgical repair of cannula |
| 1 | Unexpected anesthetic death from sedation |
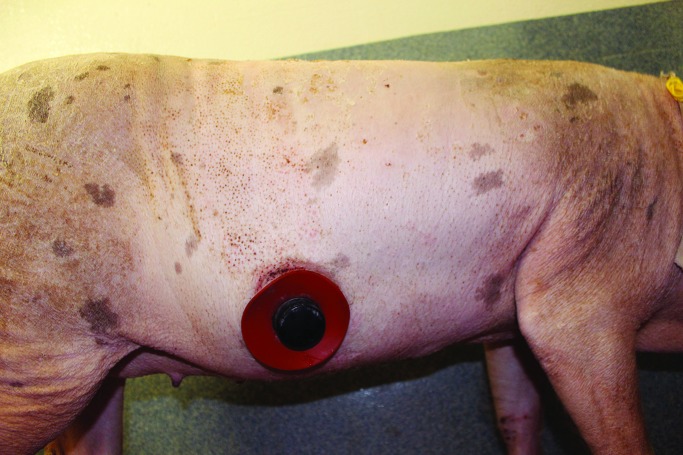
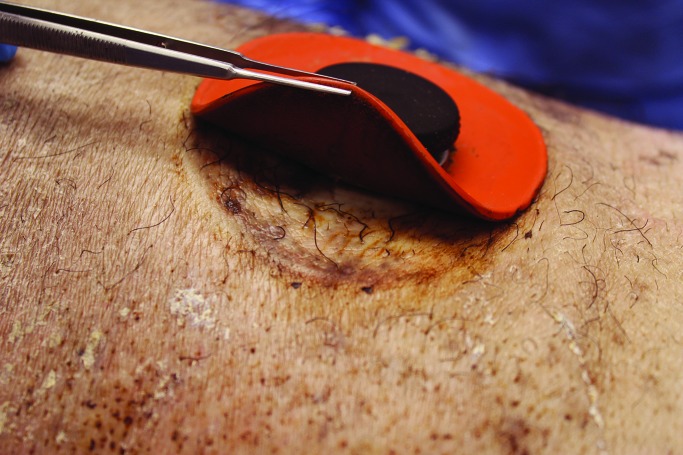
Our observation of natural pig behavior is that they frequently rub against the sides of their cages, even in the absence of an implanted cannula. For this reason, we constantly covered the cannula site with bandages. On several occasions when a bandage fell off, the cannula plug was completely dislodged as a result of the pig's rubbing against the cage, leading to leakage of intestinal contents and skin irritation. In these cases, skin irritation resolved after replacing the plug and rebandaging. Most of the time, the skin surrounding the cannula grossly appeared normal (Figure 14). In addition, the skin under the silicone outer ring typically appeared grossly normal in the absence of leakage (Figure 15).
Figure 14.
Duodenal cannula (fabric bandage removed) in a pig at 5 mo after implantation.
Figure 15.
Skin beneath the external silicone ring surrounding the duodenal cannula in a sedated pig at 18 mo after implantation.
After 4 mo, the VAP of one pig became infected and was no longer patent. The port was removed, and Actinomyces spp. was cultured from the area. A new port was inserted on the opposite side, and we were able to continue using this animal for further testing after treatment with ampicillin (6 mg/kg twice daily for 4 wk). Overall the VAP lasted longer than we expected: 6 of the 7 VAP remained patent and infection-free for more than 5 mo, with once-weekly saline flushes and heparin lock application.
Discussion
As the end result, this surgical model enabled us to implant a drug capsule directly into the duodenum of a pig and take serial blood samples without the use of sedation and with minimal stress to the animals and handlers. In addition, we exceeded our expectations by maintaining 6 of the 7 animals with minimal complications between 4 to 18 mo dependent on experimental end point. By getting repeated measurements from the same animals, we are able to decrease overall animal numbers, reduce overall animal costs, and use each pig as its own control.
Previously reported models of intestinal cannulation in pigs used stainless steel T-shaped cannulas and were focused on the use of the cannula to obtain samples from the intestinal tract.4,19 The relatively softer plastic material of the cannula we used here allowed us to modify it into a half-pipe shape, keeping the edges smooth to avoid damaging the intestinal mucosa (Figure 1 B). Although the full-pipe design has not been tested for the insertion of capsular drugs, we suspect that the capsule would get stuck inside the cannula and thus inconsistently reach the intestine after insertion.
Currently, preclinical pharmacokinetic testing of drugs can be done through a variety of in vivo and ex vivo methods, each of which has its pros and cons. Several factors influence drug absorption and toxicity, including mesenteric blood circulation, drug transporter proteins, intestinal metabolizing enzymes, microflora environment, and the flow rates and concentrations of intestinal secretions.3 The complexity of physiologic and anatomic parameters that influence drug absorption makes ex vivo testing especially challenging. To date, no ex vivo model completely replicates all physiologic parameters involved in pharmacokinetic testing, although models such as ‘organ on a chip’12 are coming closer and closer. Even with these advances in ex vivo technology, it is unlikely that the need for drug testing in animal models will be eliminated in the next several decades.
Animal models are commonly used for pharmacokinetic studies to test novel drug delivery methods, drug absorption rates, and toxicity studies. For capsule delivery drug studies, the most common models include endoscopic placement4 into the stomach or duodenum and oral delivery by pilling or hiding the capsule inside a treat.3 Similar to endoscopic placement or delivery through a 5-French port, providing a drug capsule through a surgically implanted cannula allows placement into a specific area of the gastrointestinal tract. The added benefit of drug delivery through a surgically placed cannula is that the drug capsule can be provided to an unsedated animal, which is not possible with endoscopic placement. The benefit of drug delivery without sedation is that the intestinal effects of sedation, mainly gastrointestinal stasis,18,21 are eliminated.
By training of the pigs and using a VAP, serial blood draws after drug delivery are possible. Drawbacks of this model include the surgical challenge required to access the duodenum without causing excessive strain or torque on the intestine. In conclusion, our novel large animal model for drug delivery and pharmacokinetic testing can provide valuable preclinical information to support the development of new drugs and drug delivery methods such as those required to deliver large-molecule medications.
Acknowledgments
We thank Lily McLaughlin and Paige Crowley, who contributed outstanding technical assistance for this study. Thanks to our husbandry and other staff members (especially Abigail Follett, Claire Johnson, Jessica Kittel, and Avian Pace) who helped to train and provide enrichment for our pigs. Thanks to ANKOM engineers for working with our team on the successful cannula modifications. Thanks to Lauren Richey, DACVP, for necropsy and histology contributions.
References
- 1.Aguilar AA, Depeters EJ. 1988. Modification for cannulation of duodenum with flexible, T-shaped cannula in goats. Small Rumin Res. 1:73–79. [Google Scholar]
- 2.Antunes F, Andrade F, Ferreira D, Nielsen HM, Sarmento B. 2013. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr Drug Metab 14:4–20. [PubMed] [Google Scholar]
- 3.Aoyagi N, Ogata H, Kaniwa N, Uchiyama M, Yasuda Y, Tanioka Y. 1992. Gastric emptying of tablets and granules in humans, dogs, pigs, and stomach-emptying-controlled rabbits. J Pharm Sci 81:1170–1174. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, Mo S, Lee YL, Mazdiyasni H, Kogan L, Barman R, Cleveland C, Booth L, Bensel T, Minahan D, Hurowitz HM, Tai T, Daily J, Nikolic B, Wood L, Eckhoff PA, Langer R, Traverso G. 2016. Oral, ultralong-lasting drug delivery: application toward malaria elimination goals. Sci Transi Med 8:365ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilger RN, Sands JS, Ragland D, Adeola O. 2004. Digestibility of nitrogen and amino acids in soybean meal with added soyhulls. J Anim Sci 82:715–724. [DOI] [PubMed] [Google Scholar]
- 6.Economist 2015. Pharmaceuticals going large. [Cited 21 March 2017]. Available at: https://www.economist.com/news/business/21637387-wave-new-medicines-known-biologics-will-be-good-drugmakers-may-not-be-so-good
- 7.Hamilton CR, Dove CR, Zinn GM, Veum TL. 1985. Simultaneous cecostomy and ileal cannulation with a modified flexible T cannula in gilts. Am J Vet Res 46:942–944. [PubMed] [Google Scholar]
- 8.Hennig U, Idzior B, Wunsche JB, Bock HD. 1980. [Fistulation technic for the digestive tract of swine for the examination of protein metabolism.] Arch Exp Veterinarmed 34:325–331. [Article in German]. [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 10.Komarek RJ. 1981. Intestinal cannulation of cattle and sheep with a T-shaped cannula designed for total digesta collection without externalizing digesta flow. J Anim Sci 53:796–802. [DOI] [PubMed] [Google Scholar]
- 11.Kunta JR, Perry BA, Sutyak JP, Sinko PJ. 2001. Development of a novel intestinal and vascular access port (IVAP) rabbit model to study regiospecific oral absorption pharmacokinetics. Comp Med 51:349–356. [PubMed] [Google Scholar]
- 12.Lee H, Kim DS, Ha SK, Choi I, Lee JM, Sung JH. 2017. A pumpless multiorgan-on-a-chip (MOC) combined with a pharmacokinetic–pharmacodynamic (PK–PD) model. Biotechnol Bioeng 114:432–443. [DOI] [PubMed] [Google Scholar]
- 13.Meunier LD, Kissinger JT, Marcello J, Nichols AJ, Smith PL. 1993. A chronic access port model for direct delivery of drugs into the intestine of conscious dogs. Lab Anim Sci 43:466–470. [PubMed] [Google Scholar]
- 14.Mitragotri S, Burke PA, Langer R. 2014. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 13:655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moroni M, Coolbaugh TV, Mitchell JM, Lombardini E, Moccia KD, Shelton LJ, Nagy V, Whitnall MH. 2011. Vascular access port implantation and serial blood sampling in a Gottingen minipig (Sus scrofa domestica) model of acute radiation injury. J Am Assoc Lab Anim Sci 50:65–72. [PMC free article] [PubMed] [Google Scholar]
- 16.Noakes DE, Cranwell PD. 1977. Some experimental surgical techniques on the alimentary tract of young pigs. Res Vet Sci 22:243–250. [PubMed] [Google Scholar]
- 17.Pryor K. 2009. Reaching the animal mind. New York (NY): Sunshine Books. [Google Scholar]
- 18.Schnoor J, Unger JK, Kuepper T, Bode B, Hofeditz A, Silny J, Rossaint R. 2005. Effects of propofol and fentanyl on duodenal motility activity in pigs. Can Vet J 46:995–1001. [PMC free article] [PubMed] [Google Scholar]
- 19.Stein HH. [Internet]. 2017. Research procedures—cannula. University of Illinois. [Cited 31 August 2016]. Available from: http://nutrition.ansci.illinois.edu/research-procedures
- 20.Swindle MM, Smith AC. 2016. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques, 3rd ed. Boca Raton (FL): CRC Press. [Google Scholar]
- 21.Wright AB, McKelvey GM, Wood AKW, Post EJ. 1999. Effects of promethazine on porcine gastroduodenal function: a sonographic study. Ultrasound Med Biol 25:241–247. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Luo G, Ding X, Lu C. 2012. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm Sin B 2:549–561. [Google Scholar]