Abstract
An 8-y-old female rhesus macaque (Macaca mulatta) presented for swelling of the left lower limb distal to the inguinal region and associated with the femoral artery. Physical and ultrasound examinations suggested an arteriovenous fistula combined with a pseudoaneurysm. After review of possible treatment options, we determined that open surgical repair was the best course of action. The pseudoaneurysm and arteriovenous fistula were surgically resected, and the macaque recovered without complication.
Abbreviations: AVF, arteriovenous fistulas; DUS, Doppler ultrasonography; UGCR, ultrasound-guided compression repair
A true aneurysm is defined as an abnormal vascular dilation due to a weakened vessel wall and contains all 3 layers of the normal arterial wall: tunica externa, media, and intima. A pseudoaneurysm, or a false aneurysm, differs in that a pseudoaneurysm is not bound by all 3 layers but is either contained by the media or adventitia or sometimes by only the local tissue surrounding the injured vessel.6,10,21,27,29 Pseudoaneurysms can present as multilobed structures or as a simple sac;27 the sac can be fibrous and devoid of endothelium or vascular wall structure.6,20 Pseudoaneurysms arise due to a disruption in the arterial wall, most commonly resulting as a complication of penetrating or blunt trauma, inflammation or infection, intravenous drug use, orthopedic procedures, or iatrogenically due to percutaneous arterial catheterizations.6,21,27
Pseudoaneurysms can be asymptomatic, or symptoms can include localized swelling and pain. In cases with a palpable mass, the mass may be pulsatile or contain a palpable thrill, and a distinctive systolic bruit can be heard directly above the pseudoaneurysm on auscultation when the lesion is associated with arteries of the extremities.6,19,21,27 Diagnostic methods for confirming pseudoaneurysms include angiography, Doppler ultrasonography (DUS), MRI, and CT.27 Color DUS is the ‘gold standard’ for diagnosis of pseudoaneurysms of the femoral artery6,11,19 and was used in the current case. Consistent findings of pseudoaneurysms on DUS consist of turbulent flow within a saccular structure and communication with the artery from which the pseudoaneurysm arises.27
Complications associated with pseudoaneurysms are persistent pain and swelling, skin necrosis, infection, compression of adjacent vessels and nerves and associated compression neuropathy, limb ischemia, diminished distal pulses, distal embolization, deep vein thrombosis, or rupture causing extensive hemorrhage.6,19,24,27,29,34 Current therapeutic options for the treatment of pseudoaneurysms include open surgical reduction, ultrasound-guided compression repair (UGCR), ultrasound-guided thrombin injections, and stent or coil embolization through an endovascular approach.21 Treatment options for pseudoaneurysms, including observation only (for small lesions), should consider the site of the pseudoaneurysm, the risk of rupture, and any comorbidities.27
Although rare, pseudoaneurysms and associated arteriovenous fistulas (AVF) have been reported in several cases and studies and can pose a serious threat when present in an extremity.4,7,9,13,18 One study evaluating UGCR for treatment of pseudoaneurysms following catheterization of the femoral artery in humans found that AVF were present with pseudoaneurysms in approximately 1.5% of the patients sampled, whereas another study reported a range of 0.02% to 9%.5,32 AVF are described as abnormal connections between an artery and a vein in which the capillary bed has been bypassed. AVF typically arise as a result of iatrogenic trauma due to percutaneous vascular access.4,10,15 Pseudoaneurysms and associated AVF typically result from penetrating injuries, blunt trauma, sports activities, or orthopedic injuries.15 AVF can result in the formation of pseudoaneurysms due to puncture of the AVF or by turbulent blood flow and endothelial damage caused by the AVF.4,20 Typically AVF are identified clinically by a pathognomonic machinery murmur and palpable thrill associated with the defect.13,21
An adult female rhesus macaque presented with a swelling just distal to the inguinal canal of her left lower limb. Preliminary diagnosis based on clinical signs suggested an AVF of the femoral artery and vein. DUS was used to evaluate the AVF and bloodflow to the limb. DUS revealed a probable pseudoaneurysm; initially the AVF was undetectable. An open surgical approach was used to correct the vascular abnormality, at which time an AVF was confirmed intraoperatively. The macaque recovered without complication.
Case Report
The subject described here was a 7-y-old female Indian-origin rhesus macaque (Macaca mulatta) that weighed 8 kg. She was born and raised at the AAALAC-accredited Tulane National Primate Research Center. All procedures were IACAC-approved and were in accordance with the Guide for the Care and Use of Laboratory Animals.16 The case subject lived in the facility's breeding colony until she was approximately 7 y old, when she was removed for veterinary care for wounding involving superficial lacerations to her right shoulder and other extremities. She was then housed in an ABSL2 facility in accordance with the Animal Welfare Act, the Guide, and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.1,16,23 All animal housing rooms were maintained on a 12:12-h light:dark cycle, with a relative humidity of 30% to 70% and a temperature of 18 to 29 °C. The macaque was fed a standard commercial NHP diet, with fruit offered 3 times weekly as part of the enrichment program. At the time of presentation, the macaque tested negative for simian retrovirus type D, simian T-lymphotropic virus type 1, and measles virus. All semiannual tuberculin skin tests were negative throughout her history. After all wounds had healed, she was assigned to a research protocol. She was inoculated with SHIV intrarectally in mid-January 2016. Blood was collected by femoral venipuncture on 3 occasions during the 73 d preceding presentation for the distal limb swelling.
In late January 2016, an estimated 5-cm2 swelling just below the level of the skin was observed in the proximal and medial aspect of the left lower limb. Bruising was present distal to the swelling. The swelling was examined ultrasonographically (Logiq Book, GE Healthcare, Waukesha, WI), and a vascular dilation (internal dimensions, 3.2 × 2.2 cm; Figure 1) was discovered. DUS was used to evaluate bloodflow within the dilation. Turbulence was identified within the dilation and demonstrated the ‘yin-yang’ pattern of blood flow; pseudoaneurysm or true aneurysm was diagnosed (Figure 2). The affected limb was slightly cool to touch, and neovascularization was present distal to the swelling. All vital signs—including oxygen saturation, blood pressure, and heart rate—were within normal parameters. Results of CBC analysis and chemistry panel were within normal limits. We decided to reassess the dilation in 1 wk , during which treatment options were evaluated. At the follow-up physical examination, the affected limb was moderately cool to touch. The bloodflow previously noted inside the dilation was not present, and only a laminar structure, diagnosed as a large thrombus, was visible (Figure 3).
Figure 1.
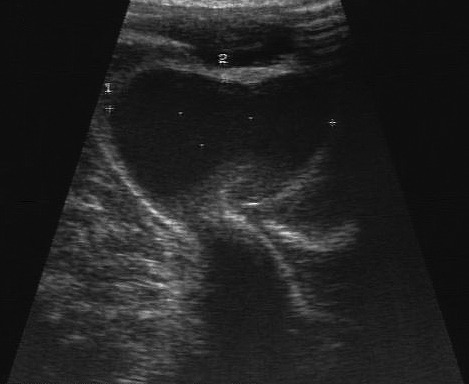
Ultrasound image measuring the internal diameter of the pseudoaneurysm.
Figure 2.
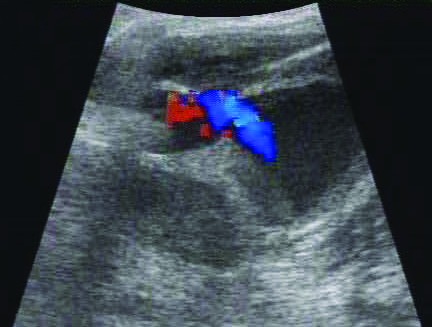
DUS image demonstrating the turbulent blood flow and yin–yang pattern typical of a pseudoaneurysm. Red indicates blood flow toward the tranducer, whereas blue indicates blood flow away from the transducer.
Figure 3.
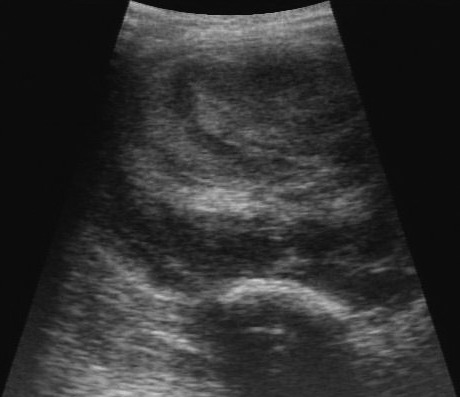
Ultrasound image of the concentric laminar pattern of the thrombus inside the pseudoaneurysm.
After reassessment, we determined that open surgical repair was the most appropriate treatment plan. The preanesthetic protocol consisted of acepromazine maleate (0.2 mg/kg SC; Vedco, St Joseph, MO) and glycopyrrolate (0.01 mg/kg SC; West-Ward Pharmaceuticals, Eatontown, NJ). Both drugs were administered approximately 30 min prior to induction of anesthesia. The animal was then anesthetized by using ketamine hydrochloride (10 mg/kg IM; KetaVed, Vedco). After anesthetic induction, the macaque was intubated and maintained on a mixture of isoflurane gas (concentration, 1% to 3%) and 100% oxygen. A skin incision was made on the medial aspect of the left lower limb extending from just distal to the inguinal canal to just proximal to the stifle. The subcutaneous tissue was bluntly dissected, and electrocautery was used for hemostasis, because a network of small-caliber blood vessels (neovascularization) was encountered in the subcutaneous tissue and deeper structures. The femoral artery and vein were isolated proximally and distally to the dilation, occluded and ligated by using 4-0 absorbable suture (Monocryl, Ethicon, Somerville, NJ) in simple ligation, and transected, leaving preexisting collateral circulation intact. An AVF just proximal to the dilation (Figure 4) was confirmed intraoperatively. Further blunt dissection was used to isolate the dilation, which was adhered to the surrounding musculature. When the dilation was approximately 95% isolated, it ruptured, revealing a large thrombus (Figure 5). The thrombus was removed, and the isolation and removal of the dilation was completed. The surgical area was irrigated and closed in multiple layers by using absorbable suture. Due to the neovascularization present in the subcutaneous tissue and deeper structures, hemorrhage occurred during the surgical procedure. Near the end of the surgical procedure, blood was collected for CBC analysis to evaluate the extent and effect of hemorrhage. The patient's Hct was 17.7% and Hgb was 5.2 g/dL, and a blood transfusion was ordered. An appropriate donor was located, and 150 mL of whole blood was administered to the patient, as described elsewhere.3 Sustained-release buprenorphine hydrochloride (1 mg/mL SC once; Buprenorphine SR, ZooPharm, Laramie, WY) was administered at the time of the procedure to provide analgesia postoperatively.
Figure 4.
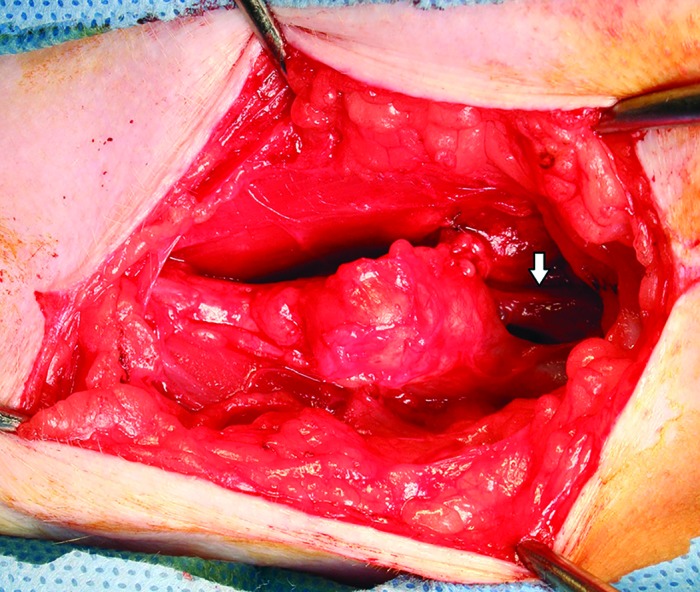
Dissection and isolation of the pseudoaneurysm. The arrow indicates the dilated and fragile femoral vein, which is seen in arteriovenous fistulas.
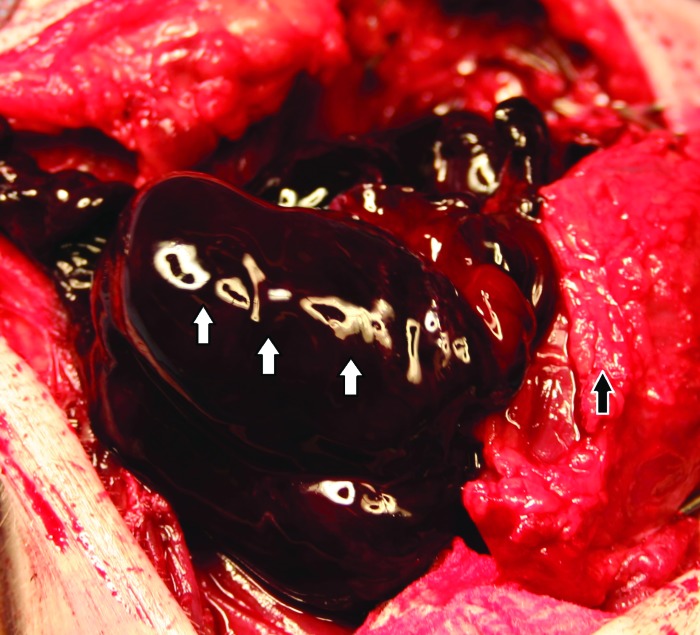
Figure 5.
Thrombus (white arrows) present in the capsule (black arrow) of the pseudoaneurysm.
On the day after surgery, the initial cageside exam found the macaque moving readily about the cage. In addition, CBC analysis showed that Hct had improved to 29.3% and Hgb to 9.2 g/dL. The physical exam revealed normal parameters of heart rate, mucous membrane color, capillary refill time, and respiratory rate. The incision in the left lower limb displayed no hemorrhage, discharge, or erythema, but slight edema was present. Overall the macaque was bright, alert, and responsive. The animal continued to recover without complication. Minimal weight loss (0.4 kg) occurred during the month immediately after surgery, but the animal had gained a total of 0.95 kg by approximately 2 mo postoperatively.
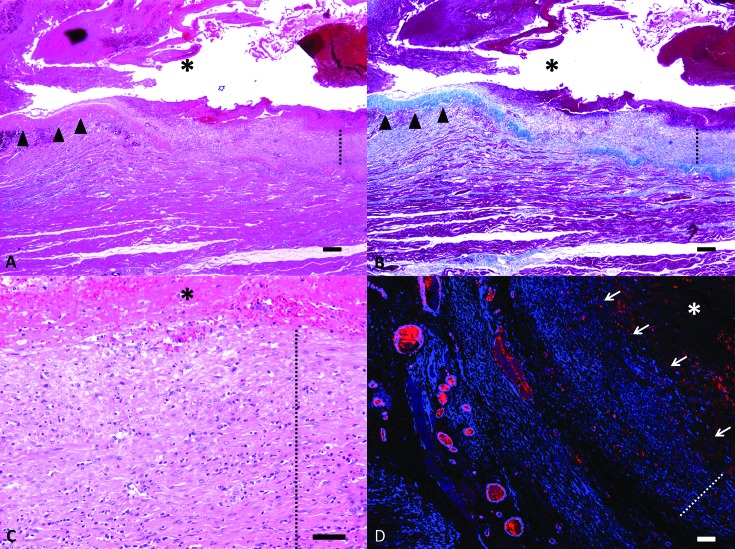
On microscopic analysis, sections of the vascular dilation contained a partially encapsulated, blood-filled cavity (hematoma). The capsule of the hematoma was segmentally composed of smooth muscle and dense collagen without an internal endothelial lining (Figure 6). Fibrosis surrounding the capsule variably extended into and isolated adjacent myocytes, which variably exhibited degeneration and regeneration. The capsule and adjacent skeletal muscle were multifocally infiltrated by lymphocytes, plasma cells, and histiocytes. Histiocytes often contained hemosiderin (Prussian blue stain).
Figure 6.
Microscopic findings. (A) The pseudoaneurysm is composed of a large blood-filled cavity (asterisk) that is surrounded by a capsule composed segmentally of dense collagen (arrowheads) and smooth muscle (dotted line). Hematoxylin and eosin stain; bar, 200 μm. (B) Masson trichrome staining highlights the capsular segments composed of collagen (blue, arrow heads) and smooth muscle (dotted line). (C) Magnified image illustrating the layering of the smooth muscle cells (dotted line), similar to normal tunica media, in segments of the capsule. Hematoxylin and eosin stain; bar, 50 μm. (D) Immunofluorescent staining for α-smooth muscle actin and von Willebrand factor highlight the smooth muscle within the wall of the capsule (dotted line) and the lack of an endothelial lining (arrows). Confocal microscopy; bar, 100 μm.
Double-label confocal microscopy for α-smooth muscle actin and von Willebrand factor disclosed that the wall of the capsule contained concentric layers of smooth muscle without an endothelial lining. The combined findings were consistent with a vascular pseudoaneurysm.
Discussion
The prevalence of pseudoaneurysms in the human population has increased due to the increased use of therapeutic vascular procedures, thereby making the management of pseudoaneurysms important.6,22,27 Pseudoaneurysms, as well as other vascular abnormalities, have also been reported in NHP and can be the result of vascular access procedures including venipuncture.26,30,31 The prevalence of pseudoaneurysms in humans after catheterization procedures using large-bore sheaths was reported to be 7.7%.27 Color DUS is the gold standard for diagnosis of pseudoaneurysms of the femoral artery.6,11,19 One study indicated that duplex ultrasonography was 94% sensitive and 97% specific for detecting post-catheterization pseudoaneurysms, whereas another demonstrated 94% sensitivity and 97% specificity for pseudoaneurysms particular to the femoral artery.6,27
The main characteristics of pseudoaneurysms on DUS include a ‘neck’ that communicates with an artery and that displays a reversal of bloodflow during diastole. Bidirectional bloodflow due to the swirling of blood within the pseudoaneurysm typically shows a yin–yang pattern on color flow imaging; however, this pattern can also be seen with a true aneurysm.15,27 The more definitive indication of a pseudoaneurysm on DUS is a ‘to and fro’ pattern, which demonstrates bloodflow entering the pseudoaneurysm during systole and exiting on diastole.15,25,27,29 DUS enables characterization of the size, shape (single or multilobed), and velocity within the neck and sac of the pseudoaneurysm.15,24,27,29 After thrombus formation, ultrasonography can demonstrate concentric layers of fibrin thrombus within the pseudoaneurysm, as occurred in the case we presented.27 DUS characteristics of an AVF include visualization of communication between the artery and vein and indicative of high-velocity blood flow, high-velocity arterialized waveform in the draining vein, a junction of low and high resistance bloodflow in the supplying artery, turbulent blood flow, and venous dilation.15,25
We obtained precise measurements of the interior of the pseudoaneurysm, found that the pseudoaneurysm was bilobed, and documented multidirectional bloodflow on DUS in the typical yin–yang pattern. We were unable to detect the neck of the pseudoaneurysm or the fistula between the femoral artery and vein on the initial ultrasound examination, potentially because of the large size of the pseudoaneurysm, the close proximity of pseudoaneurysm and artery, or the fibrous capsule surrounding the pseudoaneurysm.
The size of the pseudoaneurysm most often dictates the treatment approach. Pseudoaneurysms with a diameter of <1.8 to 3 cm and volume of <6 cm3 can be treated conservatively, through observation only, provided that the patient is not on anticoagulants, because smaller pseudoaneurysms can undergo spontaneous thrombosis.6,18,19,21,24,27,29 Patients treated conservatively within these parameters have a spontaneous resolution rate of 87% to 93% within 3 mo.19,29 Even though small pseudoaneurysms often resolve spontaneously, follow-up ultrasound examinations and restriction of patient activity should be required.19 The pseudoaneurysm in our macaque had a diameter of 3.2 cm and a volume greater than 6 cm3, thus indicating treatment options other than conservative therapy. Another factor that we considered was the requirement for decreased patient activity with conservative therapy: naturally reducing the amount of activity of NHP is quite difficult. Although the pseudoaneurysm in our patient was thrombosed on follow-up ultrasound examination, its presence in conjunction with an AVF prompted intervention.
Since 1991, the repair of pseudoaneurysms has shifted toward noninvasive techniques, and UGCR has become the treatment of choice for pseudoaneurysms of the extremities; however, contraindications for this method include large pseudoaneurysms, the presence of large hematomas at the site with overlying dermal ischemia, limb ischemia, compartment syndrome, and the presence of prosthetic grafts.6,12,24,27 In addition, compression can be painful for the patient. Typically, only superficial pseudoaneurysms of the extremities can be corrected by using UGCR.24,27 UGCR is performed by applying pressure to the neck of the pseudoaneurysm, thereby stopping bloodflow into and out of the pseudoaneurysm but still allowing arterial perfusion to the extremity. During compression, absence of blood flow to the pseudoaneurysm should be apparent on DUS, and compression should remain until thrombosis is evident, usually requiring 10 to 45 min. Compression of the pseudoaneurysm itself can be performed when the neck cannot be compressed.6,12,24,27,28 Multiple cycles of compression may be required to achieve resolution.12,19 Typically bed rest is recommended after UGCR.11,12,25,28 Success of UGCR is inversely related to the size of the pseudoaneurysm.5,17,33 Success rates for pseudoaneurysms confined to the femoral artery, as present in the macaque presented here, range from 66% to 90%, depending on the study.6,24 Another study found that 100% of uncomplicated pseudoaneurysms could be resolved with UGCR provided that the pseudoaneurysm was <2 cm in diameter. The success rate for resolution of pseudoaneurysms by using UGCR decreases in multilobed pseudoaneurysms.5 Another very important factor when considering UGCR is the presence of an AVF. One study demonstrated that the presence of an AVF significantly decreased the success of UGCR, and only half of the patients with both a pseudoaneurysm and AVF were treated nonoperatively.17 The same study17 also reported that the presence of an AVF with a pseudoaneurysm significantly predicted pseudoaneurysm recurrence. Other studies have demonstrated similar results, indicating that the presence of an AVF in conjunction with a pseudoaneurysm increases failure rates.33 In addition, some studies have shown that, when both pseudoaneurysms and AVF are present, the pseudoaneurysm can be resolved by using UGCR, but the AVF persists.8,12,25 UGCR has proven to have a poor success rate in patients with AVF only;12,25,28 this difficulty may be related to the relatively short communication between the artery and vein and the difference in pressure between them.25 Rare complications have been reported with UGCR, including venous thrombosis, skin necrosis, pseudoaneurysm rupture, and local arterial thrombosis and distal embolization due to the pressure applied during the procedure.27
Endovascular management is a common method of managing pseudoaneurysms and includes the use of perfusion balloons, stents, coils, and thrombin injection.6,24,27 In human medicine, most pseudoaneurysms occur in patients with other morbidities, thereby making UGCR and percutaneous embolization the treatment of choice. However, some conditions mandate surgical repair: rapid expansion of the pseudoaneurysm; distal ischemia; neurologic deficits due to pressure from the pseudoaneurysm; infection; failure of previous percutaneous treatment; and compromised soft tissue viability.19,24 We considered several factors when evaluating therapeutic options to repair the combined pseudoaneurysm and AVF in this macaque. In particular, ultrasound-guided thrombin injection is contraindicated when an AVF accompanies a pseudoaneurysm due to the increased risk of systemic thrombosis and distal emboli into the venous circulation through the communication between the artery and vein;5,18,29 consequently we discounted this treatment for our patient.
Open surgical repair should be considered when infection, tissue ischemia, or an associated AVF is present.6,12 Surgical options for pseudoaneurysms include a resection and bypass procedure, simple arterial ligation when the artery is deemed expendable, and partial or complete organ removal.27 For infected pseudoaneurysms, simple ligation of the involved artery in extremities has been performed without any attempt at revascularization provided that distal bloodflow can be confirmed.2 One study involving 6 human patients, all of whom had infected pseudoaneurysms, were surgically corrected by simple ligation of the involved artery without revascularization, and none of the patients required amputation due to diminished blood flow to the affected limb.2 The patients in that study2 were chronic intravenous drug abusers, a feature that might have promoted collateral circulation due to previous arterial injuries stemming from the abuse. A second study14 involving 37 intravenous drug abusers who were diagnosed with infected pseudoaneurysms underwent ligation of the involved artery without revascularization; only one patient required amputation of the involved limb, and that patient had experienced 2 infected pseudoaneurysms in the same limb as well as an episode of distal ischemia due to an intraarterial injection. These 2 studies2,14 suggest that when collateral circulation is present and the distal vessels are patent, acute ligation of the artery involving the pseudoaneurysm is a safe and effective procedure.
The macaque case we presented here demonstrated the typical findings consistent with a pseudoaneurysm, including a pulsatile mass and a dilation near the involved artery that contained turbulent blood flow and displayed the yin–yang pattern on DUS. We discussed various treatment options and considered numerous factors. Because the pseudoaneurysm was quite large (exceeding the limitations of 3 cm in diameter and 6 cm3 in volume stipulated for conservative therapy) and because an accompanying AVF was present, therapeutic intervention was required. Given that the success rate of UGCR 1) is inversely proportional to the size of the pseudoaneurysm, 2) is decreased with multilobed pseudoaneurysms, and 3) requires restriction of activity (such as bed rest) and, most importantly, given that an AVF was present, we elected not to use this technique. In a previous study, a NHP with a pseudoaneurysm and AVF underwent UGCR, which resolved the pseudoaneurysm, but the AVF persisted.8 The studies discussed earlier that involved UGCR for human patients with concurrent pseudoaneurysms and AVF illustrate the difficulties in resolving AVF alone and in conjunction with a pseudoaneurysm. As previously mentioned, ultrasound-guided thrombin injection was contraindicated in our macaque, due to the presence of an AVF. The animal discussed here exhibited not only superficial collateral circulation distal to the pseudoaneurysm but also DUS-verified distal bloodflow. Given these features, coupled with the presence of the AVF, we elected surgical intervention, which ultimately included resection of the involved abnormal vasculature. We have managed numerous cases of femoral AVF in NHP and, in our experience, surgical resection of the damaged vasculature without prosthetic stents or grafts has proven a successful treatment option, provided that collateral circulation is present. When resecting damaged arterial and venous segments in cases of AVF with collateral circulation, we have not noted any lameness in the affected limb or seen a need for later amputation. The macaque described here did not display any postsurgical complications, including lameness. We feel that surgical resection without stents or grafts is a viable option for the repair of not only pseudoaneurysms of the extremities but also AVF in NHP.
Acknowledgments
This study was supported by NIH grant 1 R24 AI112350-01.
References
- 1.Animal Welfare Act as Amended 2013 7 USC §2131–2159. [Google Scholar]
- 2.Arora S, Weber MA, Fox CJ, Neville R, Lidor A, Sidawy AN. 2001. Common femoral artery ligation and local debridement: a safe treatment for infected femoral artery pseudoaneurysm. J Vasc Surg 33:990–993. [DOI] [PubMed] [Google Scholar]
- 3.Bohm RP, Jr, Gilbert MH. 2012. Emergency medicine and critical care for nonhuman primates, p 359–389. In: Abee CR, Mansfield K, Tardiff S, Morris T. Nonhuman primates in biomedical research: biology and management, 2nd ed. Waltham (MA) Academic Press. [Google Scholar]
- 4.Ching KC, McCluskey KM, Srinivasan A. 2014. Peroneal arteriovenous fistula and pseudoaneurysm: an unusual presentation. Case Rep Vasc Med 2014:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coley BD, Roberts AC, Fellmeth BD, Valji K, Bookstein JJ, Hye RJ. 1995. Postangiographic femoral artery pseudoaneurysms: further experience with US-guided compression repair. Radiology 194:307–311. [DOI] [PubMed] [Google Scholar]
- 6.Corriere MA, Guzman RJ. 2005. True and false aneurysms of the femoral artery. Semin Vasc Surg 18:216–223. [DOI] [PubMed] [Google Scholar]
- 7.Daniel HE, Firmin A, Angele PO, Esthelle MN, Freddy B, Bernadette NN. 2015. Giant pseudoaneurysm associated with arteriovenous fistula of the brachial and femoral arteries following gunshot wounds: report of 2 cases. Case Rep Vasc Med 2015:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daviau JS, Merton DA. 2010. Nonsurgical repair of a pseudoaneurysm in a cynomologous macaque (Macaca fascicularis). J Am Assoc Lab Anim Sci 49:647–651. [PMC free article] [PubMed] [Google Scholar]
- 9.DeMartino RR, Walsh TR, Powell RJ. 2016. Large traumatic thigh pseudoaneurysm with associated arteriovenous fistula. J Vasc Surg 63:1375. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger SJ, Feldman EC. 1995. Textbook of veterinary internal medicine. Philadelphia (PA): WB Saunders. [Google Scholar]
- 11.Feld R, Patton GM, Carabasi RA, Alexander A, Merton D, Needleman L. 1992. Treatment of iatrogenic femoral artery injuries with ultrasound-guided compression. J Vasc Surg 16:832–840. [DOI] [PubMed] [Google Scholar]
- 12.Fellmeth BD, Roberts AC, Bookstein JJ, Freischlag JA, Forsythe JR, Buckner NK, Hye RJ. 1991. Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology 178:671–675. [DOI] [PubMed] [Google Scholar]
- 13.Franz RW, Jump MA. 2009. Endovascular repair of posttraumatic, concomitant popliteal artery pseudoaneurysm and arteriovenous fistula. Int J Angiol 18:41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan JP, Leiberman DP, Pollock JG. 2000. Outcome after ligation of infected false femoral aneurysms in intravenous drug abusers. Eur J Vasc Endovasc Surg 19:158–161. [DOI] [PubMed] [Google Scholar]
- 15.Goksu E, Yuruktumen A, Kaya H. 2014. Traumatic pseudoaneurysm and arteriovenous fistula detected by bedside ultrasound. J Emerg Med 46:667–669. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Kumins NH, Landau DS, Montalvo J, Zasadzinski J, Wojciechowski J, Jovanovich BD, Dunn TB, Baraniewski H, Schuler JJ. 1998. Expanded indications for the treatment of postcatheterization femoral pseudoaneurysms with ultrasound-guided compression. Am J Surg 176:131–136. [DOI] [PubMed] [Google Scholar]
- 18.Lønnebakken MT, Gerdts E, Pedersen OM. 2012. Femoral pseudoaneurysm with a communicating arteriovenous fistula: a complication after percutaneous coronary intervention. Circulation 126:e161–e162. [DOI] [PubMed] [Google Scholar]
- 19.Middleton WD, Dasyam A, Teefey SA. 2005. Diagnosis and treatment of iatrogenic femoral artery pseudoaneurysms. Ultrasound Q 21:3–17. [PubMed] [Google Scholar]
- 20.Mudoni A, Cornacchiari M, Gallieni M, Guastoni C, McGrogan D, Logias F, Ferramosca E, Mereghetti M, Inston N. 2015. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J 8:363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naouli H, Jiber H, Bouarhroum A. 2015. False aneurysm of perforating branch of the deep femoral artery—report of 2 cases. Int J Surg Case Rep 14:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norwood MG, Lloyd GM, Moore S, Patel N, Panditi S, Sayers RD. 2004. The changing face of femoral artery false aneurysms. Eur J Vasc Endovasc Surg 27:385–388. [DOI] [PubMed] [Google Scholar]
- 23.Office of Laboratory Animal Welfare. [Internet] 2015. Public Health Service policy on humane care and use of laboratory animals. Washington (DC): US Department of Health and Human Services; [Cited 19 January 2018]. Available at: OLAW.nih.gov. [Google Scholar]
- 24.O'Sullivan GJ, Ray SA, Lewis JS, Lopez AJ, Powell BW, Moss AH, Dormandy JA, Belli AM, Buckenham TM. 1999. A review of alternative approaches in the management of iatrogenic femoral pseudoaneurysms. Ann R Coll Surg Engl 81:226–234. [PMC free article] [PubMed] [Google Scholar]
- 25.Paulson EK, Kliewer MA, Hertzberg BS, Tcheng JE, McCann RL, Bowie JD, Carroll BA. 1995. Ultrasonographically guided manual compression of femoral artery injuries. J Ultrasound Med 14:653–659. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg DP, Link DP, Prahalada S. 1983. Arteriovenous malformation in a rhesus monkey (Macaca mulatta). Lab Anim Sci 33:183–186. [PubMed] [Google Scholar]
- 27.Saad NEA, Saad WEA, Davies MG, Walderman DL, Fultz PJ, Rubens DJ. 2005. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics 25 Suppl_1:S173–S189. [DOI] [PubMed] [Google Scholar]
- 28.Schaub F, Theiss W, Heinz M, Zagel M, Schömig A. 1994. New aspects in ultrasound-guided compression repair of postcatheterization femoral artery injuries. Circulation 90:1861–1865. [DOI] [PubMed] [Google Scholar]
- 29.Shetty R, Lotun K. 2012. Treatment of an iatrogenic femoral artery pseudoaneurysm with concomitant arteriovenous fistula with percutaneous implantation of an amplatzer vascular plug. Catheter Cardiovasc Interv 81:E53–E57. [DOI] [PubMed] [Google Scholar]
- 30.Stetter MD, Wells SK, Kerstein MD, Soroyan M, Schwedler M. 1992. Femoral artery pseudoaneurysm in a monkey. J Am Vet Med Assoc 201:1091–1092. [PubMed] [Google Scholar]
- 31.Streett JW, Lord PF, Schwartz A. 1980. Iatrogenic arteriovenous fistula in a cynomologous macaque (Macaca fascicularis): a case report. Lab Anim Sci 30:1012–1015. [PubMed] [Google Scholar]
- 32.Thalhammer C, Kirchherr AS, Uhlich F, Waigand J, Gross CM. 2000. Postcatheterization pseudoaneurysms and arteriovenous fistulas: repair with percutaneous implantation of endovascular covered stents. Radiology 214:127–131. [DOI] [PubMed] [Google Scholar]
- 33.Waigand J, Uhlich F, Gross CM, Thalhammer C, Dietz R. 1999. Percutaneous treatment of pseudoaneurysms and arteriovenous fistulas after invasive vascular procedures. Catheter Cardiovasc Interv 47:157–164. [DOI] [PubMed] [Google Scholar]
- 34.Yu PT, Rice-Townsend S, Naheedy J, Almodavar H, Mooney DP. 2012. Delayed presentation of traumatic infrapopliteal arteriovenous fistula and pseudoaneurysm in a 10-y-old boy managed by coil embolization. J Pediatr Surg 47:e7–e10. [DOI] [PubMed] [Google Scholar]