This cohort study determines whether there is an association between biomarker levels in surgical drain fluid of patients with head and neck cancer and disease-free survival and cancer recurrence.
Key Points
Question
Are wound fluid biomarkers in patients with oral cavity or oropharyngeal cancer associated with cancer prognosis?
Findings
In this cohort study of 20 adults after surgical extirpation, assays of wound fluid biomarkers indicated that levels of matrix metalloproteinases 1 and 3 and soluble fms-like tyrosine kinase 1 were associated with recurrence; vascular endothelial growth factor isoform A, with nodal metastasis; and basic fibroblast growth factor, with lymphovascular invasion. No biomarkers assayed showed a statistically significant association with disease-free survival.
Meaning
Measurement of biomarkers in surgical drain fluid potentially represents a novel means of assessing cancer prognosis in this population.
Abstract
Importance
Survival rates for head and neck cancer have been relatively stable for several decades. Individualized prognostic indicators are needed to identify patients at risk for poorer outcomes.
Objective
To determine whether biomarker levels in surgical drain fluid of patients with head and neck cancer are associated with poor cancer outcomes.
Design, Setting, and Participants
This prospective cohort study enrolled patients with squamous cell carcinoma (SCC) of the oral cavity and oropharynx who required surgical treatment from April 1, 2011, to February 1, 2016, at a tertiary or academic care center. Twenty patients, including 14 with stage IV disease, had complete specimen collection. Differences in cytokine and MMP levels by disease outcomes were evaluated.
Interventions
Patients underwent surgical treatment with drain placement as dictated by the standard of care. Drain fluid samples were collected every 8 hours postoperatively until drains were removed because of clinical criteria. Levels of cytokines and matrix metalloproteinases (MMPs) were measured using electrochemiluminescent, patterned array, multiplex technology.
Main Outcomes and Measures
The primary clinical outcome measures were survival outcome and recurrence. The biomarkers measured included the cytokines basic fibroblastic growth factor, vascular endothelial growth factor isoform A, soluble fms-like tyrosine kinase-1 (sFlt-1), and placental growth factor (PIGF) and MMP-1, MMP-3, and MMP-9. Other clinical and pathologic cancer characteristics were recorded.
Results
In this cohort of 20 patients with SCC (15 men and 5 women; mean [SD] age, 63.5 [9.9] years), a significant association with recurrence was found for levels of MMP-1 (relative difference between groups, 2.78; 95% CI, 1.23-6.29), MMP-3 (relative difference between groups, 5.29; 95% CI, 2.14-13.05), and sFlt-1 (relative difference between groups, 3.75; 95% CI, 1.84-7.65). No biomarkers were associated with disease outcome. Vascular endothelial growth factor isoform A was associated with nodal metastasis (relative difference between groups, 1.98; 95% CI, 1.12-3.51), and basic fibroblastic growth factor was associated with lymphovascular invasion (relative difference between groups, 1.74; 95% CI, 1.02-2.97).
Conclusions and Relevance
In this pilot sample of patients with SCC of the oral cavity and oropharynx, MMP-1, MMP-3, and sFlt-1 levels in wound fluid were associated with poor clinical cancer outcomes in the form of recurrence. This finding is consistent with the literature of tumor microenvironment in saliva, serum, and tumor tissue biomarkers. To our knowledge, this report is the first of such findings in surgical drain fluid, an easily accessible means of cytokine measurement. Measurement of these biomarkers in surgical fluid potentially represents a novel means of assessing cancer prognosis in this population.
Introduction
Survival rates for squamous cell carcinoma (SCC) of the head and neck have been relatively stable for decades. Currently, clinicians make treatment decisions primarily by using standard clinical, pathologic, and radiologic variables. These indicators are collectively combined to create an individualized TNM and American Joint Committee on Cancer stage that informs treatment selection and prognosis. Surgeons and physicians treating these patients, however, have an immense interest in further individualizing therapy to allow for de-escalation of treatment in low-risk patients vs additional treatments in high-risk patients. Although the data regarding tumors positive for human papilloma virus suggest that a discrete disease variable can be used to recommend less intense therapy,1,2 other biomarkers are needed to better individualize care in this patient population.
The tumor environment offers rich possibilities for interrogating and understanding the behavior of head and neck cancers. During the past decade, this area has undergone intense investigation in SCC of the head and neck as well as multiple systemic cancers. Numerous candidate biomarkers have been evaluated in blood, tumor, and saliva specimens with hopes of elucidating disease pathways and predicting prognosis. Several classes of molecules of particular interest have been evaluated, including matrix metalloproteinases (MMPs), interleukins, proangiogenic and antiangiogenic factors, growth factors, and tumor factors.
Biomarkers found in the postsurgical wound are additionally interesting because they potentially represent not only the tumor itself but also the regulated stages of wound healing in the operative bed. Interrogation of the healing wound after surgery for head and neck cancer thus may offer a unique window into the inflammatory and immune tumor modulation that is of high interest to researchers working to understand tumor behavior. Surgical drain fluid from the postoperative wound is a logical specimen of interest to evaluate these complex pathways in hopes of identifying a prognostic biomarker. Furthermore, this specimen type is routinely clinically collected and discarded and, as such, adds no additional risk to the patient and little inconvenience to the investigator. We sought to evaluate surgical drain fluid to determine whether drain fluid cytokine levels in patients with head and neck cancer are associated with poor cancer outcomes.
Methods
We performed a prospective cohort study at the University of Minnesota Medical Center, Minneapolis, a tertiary care center, from April 1, 2011, through February 1, 2016. Patients with head and neck cancer who were recommended to undergo open surgical treatment requiring drain placement were recruited and enrolled from our academic practice. Patients with upper aerodigestive tract SCC of the head and neck entered the cohort. This study was approved by the institutional review board of the University of Minnesota. All patients provided written informed consent.
Treatment decisions were made based on standard clinical criteria, including tumor conference evaluation. Patients were subsequently treated with surgery; all enrolled patients underwent a selective or modified radical neck dissection. At the completion of the surgery, standard 10-mm flat silicone surgical drains (Jackson Pratt; Cardinal Health) were placed as clinically indicated. All patients had at least 1 drain placed running medial to lateral and superior to inferior in the lateral neck. All surgical procedures were extirpative (none were completed for any other indication such as infection or hematoma). All patients received antibiotics by a standard perioperative protocol. All wounds were fresh surgical wounds with no evidence of wound infection at the time of drain fluid sample collection. Wound fluid samples were collected from all patients undergoing surgery beginning on postoperative day 1 at a protocol-directed time from surgery. Once collected, the fluid samples were stored using a standard protocol in a −80°C freezer.
Surgical drain fluid samples were collected postoperatively every 8 hours until drains met clinical criteria for removal. Every 8-hour period of sequential wound fluid collection was deemed to be a shift throughout this report. Surgical drain fluid was evaluated for a panel of biomarkers present in the tumor environment and/or healing wound by an investigator blinded to clinical outcome end points. Biomarkers were measured by electrochemiluminescent, patterned array, multiplex technology (Spector 6000; Meso Scale Diagnostics, LLC). Biomarkers were evaluated from fluid samples collected after the first postoperative 8-hour shift on the morning of postoperative day 1. Each fluid sample was evaluated in duplicate with standards according to assay protocol.
Fluid samples were evaluated for MMP-1, MMP-3, and MMP-9 levels. These proteolytic enzymes are known to degrade the extracellular matrix and are involved in tumor angiogenesis, cell growth and differentiation, cell migration, tumor invasion, and apoptosis.3,4 Matrix metalloproteinase levels were measured in their proactive and active forms in the assayed drain fluid. Wound fluid samples were also evaluated for several angiogenesis-related markers. Vascular endothelial growth factor isoform A (VEGFA) and placental growth factor (PIGF), both protein isoforms in the VEGF family, were both measured as factors promoting tumor and wound angiogenesis. Soluble fms-like tyrosine kinase 1 (sFlt-1), a VEGF antagonist or the soluble form of VEGF receptor-1, was also measured as an antiangiogenic indicator.5 Basic fibroblastic growth factor (bFGF or FGF2) was additionally assayed; this cytokine represents a basement membrane and vessel extracellular matrix component and plays an important role in healing and angiogenesis by the regulation of cell differentiation, migration, and proliferation.6 All of these factors were measured in their soluble forms in the drain fluid samples.
A subset of patients had additional biomarker analysis performed to evaluate for trends during the postoperative hospitalization. These patients represent a convenience sample of the enrolled population and had specimens available for 4 total 8-hour shifts. For these patients, biomarkers were measured at the first recorded shift and then additionally at the 3 subsequent shifts.
Clinical and pathologic characteristics were also recorded for all patients. Disease site, TNM status, and American Joint Committee on Cancer stage were recorded. Each patient underwent evaluation for comorbidity severity using the Adult Comorbidity Evaluation–27 instrument (range, 0 [none] to 3 [severe]).7 Prognostic cancer variables, including perineural invasion, lymphovascular invasion, and tumor differentiation, were documented. Evidence of recurrence was recorded; any occurrence of local, regional, or distant recurrent disease was documented as a recurrence even if it subsequently underwent successful treatment. Patients with follow-up of 1 year or longer were included.
For statistical analysis, the mean of 2 readings of each biomarker (each was measured in duplicate) was calculated for each patient. Data transformation was needed owing to nonnormal distribution of raw data. Analysis of the biomarkers was performed in the natural logarithmic scale, with 2-sample, 2-sided t tests assuming unequal group variances testing differences in cytokine levels at shift 1 by survival outcome, recurrence status, nodal metastasis, and lymphovascular invasion. The geometric means were obtained by exponentiating the mean in the log scale to convert back to the units of measurement in the original scale. The results were reported as the ratio of the geometric means together with the 95% CIs to estimate the relative difference between patients without the outcome compared with those with the outcome (eg, recurrence). In addition, a repeated-measures analysis was performed for the subset of patients with fluid samples collected during four 8-hour shifts to evaluate the main effects associated with disease outcomes and shifts and the interaction effect between them on the biomarkers. Figure 1 and Figure 2 include the geometric means and their corresponding 95% CIs. Statistical significance was defined as P < .05. All analyses were performed using SAS software (version 9.3; SAS Institute).
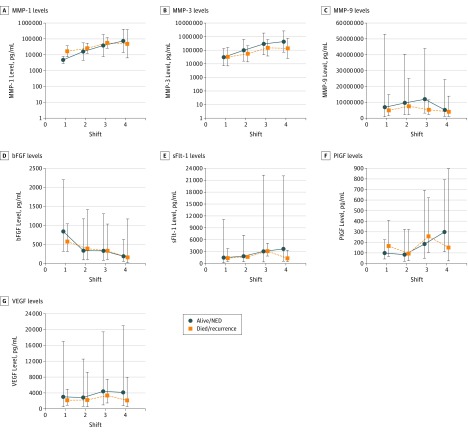
Figure 1. Change in Biomarker Levels Associated With Survival.
Graphs depict the geometric mean values of biomarkers by survival and 8-hour shift (every 8-hour period of sequential wound fluid collection was deemed to be a shift). None of the interaction assessments reached statistical significance for survival outcome. Error bars indicate 95% CIs. bFGF indicates basic fibroblastic growth factor; MMP, matrix metalloproteinase; NED, no evidence of disease; PIGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase 1; and VEGFA, vascular endothelial growth factor isoform A.
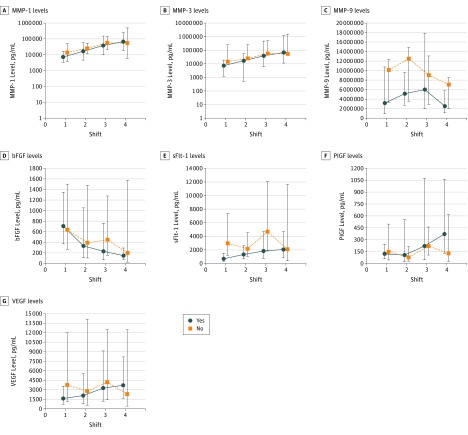
Figure 2. Change in Biomarker Levels Associated With Recurrence.
Graphs depict geometric mean values of biomarkers by presence (yes) or absence (no) of recurrence and 8-hour shift (every 8-hour period of sequential wound fluid collection was deemed to be a shift). Only the soluble fms-like tyrosine kinase 1 (sFlt-1) level had an interaction assessment that reached statistical significance for recurrence. Error bars indicate 95% CIs. bFGF indicates basic fibroblastic growth factor; MMP, matrix metalloproteinase; PIGF, placental growth factor; and VEGFA, vascular endothelial growth factor isoform A.
Results
Twenty patients (15 men and 5 women; mean [SD] age, 63.5 [9.9] years) were successfully enrolled and had complete specimen collection. Patient characteristics are seen in Table 1. T3 and T4 tumors were more frequent than T1 and T2 tumors. Eleven patients (55%) had N2 disease. No patient had distant metastases. By overall TNM stage, most patients had advanced disease, with 14 of 20 (70%) having stage IV cancer. The cohort included only patients with oral cavity or oropharynx primary tumors. Although patients with tumors in other anatomic tumor sites were recruited, none were enrolled. Tobacco and alcohol use were relatively well balanced among current, former, and never users. Most patients had mild overall comorbidity as assessed by the Adult Comorbidity Evaluation–27 score.
Table 1. Patient Characteristics.
| Characteristic |
No. (%) of
Patients (N = 20) |
|---|---|
| Sex | |
| Male | 15 (75) |
| Female | 5 (25) |
| Age, mean (SD), y | 63.5 (9.9) |
| T classification | |
| 0 | 1 (5) |
| 1 | 3 (15) |
| 2 | 5 (25) |
| 3 | 4 (20) |
| 4 | 7 (35) |
| N classification | |
| 0 | 7 (35) |
| 1 | 2 (10) |
| 2 | 11 (55) |
| 3 | 0 |
| M classification | |
| 0 | 19 (95) |
| Unknown | 1 (5) |
| TNM stage | |
| 0 | 1 (5) |
| II | 1 (5) |
| III | 4 (20) |
| IV | 14 (70) |
| Tumor site | |
| Oral cavity | 9 (45) |
| Oropharynx | 11 (55) |
| Tobacco use | |
| Never | 6 (30) |
| Former | 5 (25) |
| Current | 9 (45) |
| Alcohol use | |
| Never | 7 (35) |
| Former | 2 (10) |
| Current | 10 (50) |
| Unknown | 1 (5) |
| Adult Comorbidity Evaluation–27 score7a | |
| 0 | 4 (20) |
| 1 | 11 (55) |
| 2 | 4 (20) |
| 3 | 1 (5) |
0 indicates none; 1, mild; 2, moderate; and 3, severe.
We then evaluated for associations between wound fluid biomarker levels and the survival outcomes and recurrence (Table 2). We found no statistically significant association between any of the MMPs or cytokines and survival outcome. Patients with recurrence, however, had significantly lower levels of MMP-1 and MMP-3; the relative difference for MMP-1 was 2.78 (95% CI, 1.23-6.29) and for MMP-3 was 5.29 (95% CI, 2.14-13.05). We also found a statistically significant association between sFlt-1 levels and recurrence, with levels of the cytokine being lower in patients who experienced recurrence (relative difference, 3.75; 95% CI, 1.84-7.65).
Table 2. Cytokine Values for All Patients by All Recurrence and Survival at 1 Yeara.
| Cytokine | Recurrence | Survival 1-y Follow-up | |||||
|---|---|---|---|---|---|---|---|
| Geometric Mean Value (95% CI) | Relative Difference (95% CI)b | Geometric Mean Value (95% CI) | Relative Difference (95% CI)c | ||||
| Overall (N = 20) |
Without
Outcome (n = 14) |
With Outcome (n = 6) |
Alive or
NED (n = 12) |
Death or
Disease (n = 8) |
|||
| MMP-1, pg/mL | 15 421 (9208-25 825) | 20 963 (10 837-40 549) | 7533 (4096-13 856) | 2.78 (1.23-6.29)d | 14 888 (6401-34 626) | 16 256 (8919-29 631) | 0.92 (0.35-2.41) |
| MMP-3, pg/mL | 51 684 (27 022-98 854) | 85 188 (39 461-183 903) | 16 105 (8690-29 849) | 5.29 (2.14-13.05)d | 60 220 (24 305-149 207) | 41 093 (12 957-130 327) | 1.47 (0.38-5.70) |
| MMP-9, pg/mL | 4 762 580 (3 079 260-7 366 844) | 5 527 718 (3 218 642-9 493 341) | 3 364 623 (1 306 368-8 665 771) | 1.64 (0.61-4.44) | 4 332 388 (2 359 729-7 954 128) | 5 489 927 (2 503 300-2 039 826) | 0.79 (0.31-1.98) |
| bFGF, pg/mL | 715 (553-923) | 724 (514-1021) | 693 (424-1133) | 1.05 (0.61-1.80) | 806 (560-1162) | 596 (398-893) | 1.35 (0.82-2.23) |
| sFlt-1, pg/mL | 1698 (1063-2712) | 2525 (1522-4189) | 673 (367-1236) | 3.75 (1.84-7.65)d | 1869 (954-3663) | 1471 (661-3271) | 1.27 (0.48-3.34) |
| PIGF, pg/mL | 148 (104-210) | 160 (99-261) | 122 (72-207) | 1.31 (0.69-2.51) | 139 (84-229) | 163 (88-301) | 0.85 (0.41-1.78) |
| VEGFA, pg/mL | 2559 (1870-3500) | 3056 (2113-4420) | 1691 (911-3136) | 1.81 (0.94-3.48) | 2723 (1781-4162) | 2331 (1291-4209) | 1.17 (0.59-2.29) |
Abbreviations: bFGF, basic fibroblastic growth factor; MMP, matrix metalloproteinase; NED, no evidence of disease; PIGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; VEGFA, vascular endothelial growth factor isoform A.
Includes 20 patients with 1-year follow-up.
Calculated as the ratio of those without recurrence to those with recurrence.
Calculated as the ratio of those alive or with NED to those who died or had recurrence.
Indicates with 95% certainty that the 2 geometric mean values are not equal.
We performed further analysis to investigate possible associations between biomarker levels and overall survival, nodal metastasis, and lymphovascular invasion (Table 3). Levels of VEGFA were associated with nodal metastasis, with lower levels measured in patients with nodal disease (relative difference, 1.98; 95% CI, 1.12-3.51). Significantly lower levels of bFGF were seen in patients with compared with patients without lymphovascular invasion (relative difference, 1.74; 95% CI, 1.02-2.97).
Table 3. Association of Cytokine Values With Nodal Metastasis and Lymphovascular Invasiona.
| Cytokine | Nodal Metastasis | Lymphovascular Invasion | ||||
|---|---|---|---|---|---|---|
| Geometric Mean Value (95% CI) | Relative Difference (95% CI)b | Geometric Mean Value (95% CI) | Relative Difference (95% CI)c | |||
| Negative (n = 7) |
Positive (n = 13) |
Absent (n = 13) |
Present (n = 7) |
|||
| MMP-1, pg/mL | 19 121 (9044-40 428) | 13 734 (6469-29 161) | 1.39 (0.53-3.68) | 12 812 (6946-23 631) | 21 755 (6807-69 525) | 0.59 (0.17-2.00) |
| MMP-3, pg/mL | 76 520 (17 657-331 622) | 41 840 (19 278-90 808) | 1.83 (0.39-8.58) | 54 944 (21 535-140 186) | 46 133 (16 397-129 800) | 1.19 (0.33-4.27) |
| MMP-9, pg/mL | 6 776 233 (2 688 452-17 079 469) | 3 939 227 (2 322 672-6 680 887) | 1.72 (0.64-4.62) | 4 956 766 (2 632 291-9 333 895) | 4 422 510 (2 231 543-8 764 607) | 1.12 (0.48-2.63) |
| bFGF, pg/mL | 894 (621-1285) | 634 (442-907) | 1.41 (0.88-2.25) | 867 (657-1145) | 499 (302-824) | 1.74 (1.02-2.97)d |
| sFlt-1, pg/mL | 2085 (767-5666) | 1520 (840-2750) | 1.37 (0.47-4.02) | 1624 (834-3164) | 1845 (841-4046) | 0.88 (0.34-2.26) |
| PIGF level, pg/mL | 176 (138-223) | 135 (78-234) | 1.30 (0.73-2.33) | 148 (99-222) | 147 (61-354) | 1.01 (0.41-2.50) |
| VEGFA, pg/mL | 3993 (2436-6545) | 2013 (1381-2935) | 1.98 (1.12-3.51)d | 2751 (1758-4305) | 2236 (1360-3676) | 1.23 (0.67-2.27) |
Abbreviations: bFGF, basic fibroblastic growth factor; MMP, matrix metalloproteinase; PIGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; VEGFA, vascular endothelial growth factor isoform A.
Includes 20 patients with 1-year follow-up.
Calculated as the ratio of those with negative to positive nodal metastasis findings.
Calculated as the ratio of those with absent to present lymphovascular invasion.
Indicates with 95% certainty that the 2 geometric mean values are not equal.
A repeated-measures analysis was performed on a subset of 10 patients who constituted a convenience sample of this small cohort and who had wound fluid samples collected during each of the first 4 postoperative shifts (Figure 1 and Figure 2). The cytokine levels assessed in this study were expected to change over time as the healing wound transitioned from the hemostatic to inflammatory to angiogenic phases of normal postoperative wound healing. Thus, this portion of the study was primarily designed to evaluate for interaction between outcome (survival or recurrence) and changes in values across shifts. We evaluated for interaction between disease outcome (survival and recurrence) and shift to determine whether changes in biomarker levels were different between patients who died or had recurrences and those who did not (Figures 1 and 2). Only one of these interaction assessments was statistically significant (Figure 2E), reflecting sFlt-1 levels and showing that the pattern of change over time was clearly different between the 2 groups (P = .02). Patients with recurrences had a gradual increase in levels from the first shift to the last, whereas those without recurrences had lower sFlt-1 levels at the last shift. In the remainder of the interaction assessments, some interesting changes were evident; however, none reached statistical significance. In Figure 1E and F, an interaction between the change in sFlt-1 and PIGF levels is revealed across shift and survival outcomes. In Figure 2F, a potential interaction between change in PIGF levels is suggested across shift and recurrence. Although these findings were not statistically significant, likely owing to inadequate power and small sample size, they represent areas of interest for future evaluation.
In this repeated-measures analysis (Figures 1 and 2), we also evaluated the biomarker levels for a main effect of survival (the mean across the 4 shifts), and none of the cytokine levels were significant. We performed a similar analysis to see whether the biomarker value was associated with recurrence. This association was statistically significant for MMP-3 (P = .04) (Figure 2B). We further evaluated the main effect for shift to see whether biomarker levels differed by shift, and we found this to be true in a statistically significant manner for MMP-1 (P = .001), MMP-3 (P < .001), MMP-9 (P = .047), bFGF (P = .005), sFlt-1 (P = .02), and PIGF (P = .04) (Figure 1B-D and F and Figure 2A-D and F). As mentioned above, this finding was expected because cytokine values normally change as part of the wound-healing cascade.
Discussion
Clinicians caring for patients with head and neck cancer seek improved ways to determine prognosis and individualize therapy. Biomarkers present in the tumor environment and healing wound represent attractive candidates for objective and predictive markers of anticipated cancer outcomes. Several scientists in the field of head and neck cancer and specialists with other systemic cancers have pursued the identification of such markers in tumor, saliva, and serum or blood specimens.3,6,8,9,10,11,12,13
Although this work is extremely promising for the development of a prognostic biomarker and for understanding the tumor microenvironment, certain drawbacks are associated with using these types of specimens. Tumor tissue provides the most information about prognosis from a histologic standpoint and likely represents the criterion standard as a source of candidate biomarkers. This tissue availability is limited, however, and often difficult to access. Tissue specimens beyond that needed for standard clinical indications may not be available or may be difficult to obtain routinely. Alternatively, saliva is easily accessible, but the specimens may not be universally representative of all types of head and neck cancers, simply because of the proximity to the tumor. Finally, blood or serum is likewise relatively easily accessible; however, these specimens may reflect the overall systemic response to cancer vs the local tumor environment.
For these reasons, postsurgical drain fluid is an attractive specimen candidate for evaluating the tumor environment and identifying a prognostic biomarker. First, drain fluid samples are collected as part of routine clinical care and are routinely discarded. As such, the collection of drain fluid samples adds no additional risk or inconvenience to the patient or the clinician. Furthermore, samples of this serosanguinous fluid are collected from the region surrounding the tumor and may be an easily accessible means by which to interrogate the tumor environment.
In this study, we found 3 biomarkers that were associated with recurrence in our population with head and neck cancer. Matrix metalloproteinases 1 and 3 were associated with recurrent disease in a statistically significant manner, and their levels were lower in patients who experienced recurrence. Likewise, MMP-3 was found to be associated with recurrence in our repeated-measures analysis. Matrix metalloproteinases 1 and 3 levels have been found to be associated with prognosis in other studies of cancer biomarkers. Significant basic and translational science work by Rosenthal and Matrisian4 has elucidated an important role for MMPs in head and neck cancer tumorigenesis, invasion, and metastasis. Fitting with our findings, several investigators3,14,15,16 have found MMP-1 and MMP-3 to be overexpressed or clinically associated with poor prognosis in head and neck cancer. Our data did not support a statistically significant association with MMP-9 and poor prognosis; other studies,8,16,17,18,19,20,21 however, have found that MMP-9 and MMP-2, the latter of which was not assayed herein, serve important roles in head and neck cancer.
The process of tumor angiogenesis is important in head and neck cancers.22 As such, the proangiogenic and antiangiogenic factors assayed herein were found to be associated with cancer prognosis. Soluble tyrosine kinase inhibitor type 1 is the soluble form of VEGF receptor 1 and is thought to be a VEGF antagonist. Soluble tyrosine kinase inhibitor type 1 binds VEGFA and PIGF to block pathways of angiogenesis. In our study, sFlt-1 was found to be associated with recurrence, with lower values of this cytokine present in patients with recurrence. Although other groups appreciated this association in esophageal cancer,5,23 we did not find this association to be previously reported in head and neck cancer.
In addition to sFlt-1, other factors essential to angiogenesis were found to be associated with cancer characteristics in a significant manner. Levels of VEGFA were associated with incidence of nodal metastasis, and other groups have found a similar association between VEGFA and prognosis.22,23,24,25,26 In addition, in recent years, PIGF has been found to play an important role in tumor angiogenesis and progression by binding to VEGF receptor-1 and by augmenting the effects of VEGFA.5 We did not see a significant association between PIGF and recurrence or disease-free survival in our interaction assessments but found the suggestion of interaction in our repeated measures analysis. The curves for sFlt-1 and PIGF levels suggested that the change in value of these markers over time may be associated with head and neck cancer recurrence and disease-free survival. An association between PIGF levels and prognosis has been seen in other systemic cancers, including gastric, colorectal, breast, and lung,26,27,28,29,30,31 as well as in work by Cheng et al32,33 for SCC of the oral cavity in serum and tissue specimens. Additional serial study of these angiogenesis-related biomarkers in the postsurgical setting is indicated to evaluate these findings.
Finally, we found a statistically significant association between bFGF and lymphovascular invasion, with lower levels of the marker in patients with lymphovascular invasion. Basic fibroblastic growth factor plays a key role in angiogenesis and cellular invasion and has been evaluated for its role in head and neck SCC, thyroid carcinoma, and systemic malignant neoplasms. Expression of bFGF has been found to be present in tumor vs normal tissue in the head and neck34 and is thought to be a potential marker of prognosis in esophageal SCC.35 Other groups36,37,38 assayed bFGF in serum and urine samples, and likewise, some of these groups suggested that the biomarker may be a marker of poorer prognosis.
Strengths and Limitations
This study has several strengths. The study used a novel means of assessing prognosis in patients with head and neck cancer. In addition, the protocol used could be relatively easily transferred to clinical practice and thus has promise of actual utility in populations with head and neck cancer.
The present study also had several limitations. The sample size was very small, and as such, the study was performed in a pilot fashion. Thus, the work must be replicated in larger studies. Despite the small sample size, however, several significant associations were evident and suggest the value in further study. In addition, because this study was performed in a pilot fashion, several potentially relevant biomarkers of the tumor environment were not assayed, and additional study should include other cytokines and chemokines that are thought to influence prognosis and wound healing. Furthermore, multivariate analysis was not performed because the distribution of tumor prognostic factors (TNM classification and stage) among this small cohort of patients was prohibitive for this type of analysis. In future studies of the biomarkers of importance identified herein, a larger sample size will allow for the performance of univariate and multivariate analyses to control for other important prognostic factors associated with the outcomes evaluated. Those analyses, if confirmatory, would strengthen the present conclusions.
Finally, we recognize that healing wounds have inherent patient-to-patient variability. All patients in this study had surgical wounds from which fluid samples were collected beginning on postoperative day 1 after extirpative surgery, and a subset had wound fluid samples collected during the subsequent 8-hour shifts. All patients received a standard perioperative antibiotic treatment protocol, and none had evidence of wound infection. Thus, the wound fluid samples collected were obtained during the initial hemostatic, inflammatory, and proliferative phases of standard wound healing. These tightly regulated physiologic events involve a complex cascade of cellular migration; cytokine, chemokine, and protease release; and cellular and matrix deposition. Although wound fluid has been assessed in systemic sites such as the breast or in traumatic wounds, it has been studied minimally in the population with head and neck cancer. Candau-Alvarez and colleagues39 recently found that interleukin 1β and tumor necrosis factor in wound fluid samples collected on days 1 and 3 after neck dissection predicted surgical site infection, but we are not aware of other studies that have used biomarkers in postsurgical wound fluid to evaluate head and neck cancer prognosis. Thus, although a healing surgical wound is the site of considerable biological complexity, only through studying and reporting biomarker levels in patients with head and neck cancer will we determine norms for future study.
Conclusions
Clinicians who treat head and neck cancer seek improved means by which to individualize prognosis estimates and treatment programs for patients. The tumor environment and surrounding postoperative wound serve as rich repositories for potential head and neck cancer biomarkers. Our study suggests that MMP-1, MMP-3, and sFlt-1 levels in postsurgical drain fluid are significantly associated with tumor recurrence. Furthermore, VEGFA levels were associated with nodal metastasis, and bFGF levels were associated with lymphovascular invasion. These findings must be further evaluated in larger studies, but our study used a novel means of evaluating head and neck cancer that, if replicated, has the potential for real-life clinical utility in the future.
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;9(6):665-673. [DOI] [PubMed] [Google Scholar]
- 3.Stott-Miller M, Houck JR, Lohavanichbutr P, et al. Tumor and salivary matrix metalloproteinase levels are strong diagnostic markers of oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2628-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal EL, Matrisian LM. Matrix metalloproteases in head and neck cancer. Head Neck. 2006;28(7):639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultze A, Ben Batalla I, Riethdorf S, et al. VEGFR-1 expression levels predict occurrence of disseminated tumor cells in the bone marrow of patients with esophageal carcinoma. Clin Exp Metastasis. 2012;29(8):879-887. [DOI] [PubMed] [Google Scholar]
- 6.Huang YQ, Li YD, Li GK, Jin Z, Ma J. The evaluation of basic fibroblast growth factor and fibroblastic growth factor receptor 1 levels in saliva and serum of patients with salivary gland tumor. DNA Cell Biol. 2012;31(4):520-523. [DOI] [PubMed] [Google Scholar]
- 7.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593-602. [DOI] [PubMed] [Google Scholar]
- 8.Lee KD, Lee HS, Jeon CH. Body fluid biomarkers for early detection of head and neck squamous cell carcinomas. Anticancer Res. 2011;31(4):1161-1167. [PubMed] [Google Scholar]
- 9.Colović Z, Pesutić-Pisac V, Poljak NK, Racić G, Cikojević D, Kontić M. Expression of matrix metalloproteinase-9 in patients with squamous cell carcinoma of the larynx. Coll Antropol. 2013;37(1):151-155. [PubMed] [Google Scholar]
- 10.Linkov F, Lisovich A, Yurkovetsky Z, et al. Early detection of head and neck cancer: development of a novel screening tool using multiplexed immunobead-based biomarker profiling. Cancer Epidemiol Biomarkers Prev. 2007;16(1):102-107. [DOI] [PubMed] [Google Scholar]
- 11.Zucker S, Lysik RM, Zarrabi MH, Moll U. M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res. 1993;53(1):140-146. [PubMed] [Google Scholar]
- 12.Garbisa S, Scagliotti G, Masiero L, et al. Correlation of serum metalloproteinase levels with lung cancer metastasis and response to therapy. Cancer Res. 1992;52(16):4548-4549. [PubMed] [Google Scholar]
- 13.Garzetti GG, Ciavattini A, Lucarini G, Goteri G, Romanini C, Biagini G. Increased serum 72 KDa metalloproteinase in serous ovarian tumors: comparison with CA 125. Anticancer Res. 1996;16(4A):2123-2127. [PubMed] [Google Scholar]
- 14.Tadbir AA, Purshahidi S, Ebrahimi H, et al. Serum level of MMP-3 in patients with oral squamous cell carcinoma: lack of association with clinico-pathological features. Asian Pac J Cancer Prev. 2012;13(9):4545-4548. [DOI] [PubMed] [Google Scholar]
- 15.Tao YS, Ma XY, Chai DM, et al. Overexpression of MMP-1 and VEGF-C is associated with a less favorable prognosis in esophageal squamous cell carcinoma. Onkologie. 2012;35(11):651-656. [DOI] [PubMed] [Google Scholar]
- 16.Wang WL, Chang WL, Yeh YC, et al. Concomitantly elevated serum matrix metalloproteinases 3 and 9 can predict survival of synchronous squamous cell carcinoma of the upper aero-digestive tract. Mol Carcinog. 2013;52(6):438-445. [DOI] [PubMed] [Google Scholar]
- 17.Virós D, Camacho M, Zarraonandia I, et al. Prognostic role of MMP-9 expression in head and neck carcinoma patients treated with radiotherapy or chemoradiotherapy. Oral Oncol. 2013;49(4):322-325. [DOI] [PubMed] [Google Scholar]
- 18.Fan HX, Li HX, Chen D, Gao ZX, Zheng JH. Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: relationships and prognostic implications. J Exp Clin Cancer Res. 2012;31:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elahi M, Rakhshan V, Ghasemian NT, Moshref M. Prognostic value of transforming growth factor beta 1 [TGF-β1] and matrix metalloproteinase 9 [MMP-9] in oral squamous cell carcinoma. Biomarkers. 2012;17(1):21-27. [DOI] [PubMed] [Google Scholar]
- 20.Singh RD, Nilayangode H, Patel JB, et al. Combined evaluation of matrix metalloproteinases and their inhibitors has better clinical utility in oral cancer. Int J Biol Markers. 2011;26(1):27-36. [DOI] [PubMed] [Google Scholar]
- 21.Liu CJ, Chang KW, Lin SC, Cheng HW. Presurgical serum levels of matrix metalloproteinase-9 and vascular endothelial growth factor in oral squamous cell carcinoma. Oral Oncol. 2009;45(10):920-925. [DOI] [PubMed] [Google Scholar]
- 22.Hsu HW, Wall NR, Hsueh CT, et al. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 2014;50(1):19-26. [DOI] [PubMed] [Google Scholar]
- 23.Kilic E, Schild SE, Thorns C, Bajrovic A, Rades D. Prognostic role of vascular endothelial growth factor and its receptor-1 in patients with esophageal cancer. Anticancer Res. 2014;34(9):5221-5226. [PubMed] [Google Scholar]
- 24.Chen M, Cai E, Huang J, Yu P, Li K. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1126-1134. [DOI] [PubMed] [Google Scholar]
- 25.Seibold ND, Schild SE, Bruchhage KL, Gebhard MP, Noack F, Rades D. Prognostic impact of VEGF and FLT-1 receptor expression in patients with locally advanced squamous cell carcinoma of the head and neck [published correction appears in Strahlenther Onkol. 2014;190(3):324]. Strahlenther Onkol. 2013;189(8):639-646. [DOI] [PubMed] [Google Scholar]
- 26.Pan J, Kong L, Lin S, Chen G, Chen Q, Lu JJ. The clinical significance of coexpression of cyclooxygenases-2, vascular endothelial growth factors, and epidermal growth factor receptor in nasopharyngeal carcinoma. Laryngoscope. 2008;118(11):1970-1975. [DOI] [PubMed] [Google Scholar]
- 27.Parr C, Watkins G, Boulton M, Cai J, Jiang WG. Placenta growth factor is over-expressed and has prognostic value in human breast cancer. Eur J Cancer. 2005;41(18):2819-2827. [DOI] [PubMed] [Google Scholar]
- 28.Wei SC, Tsao PN, Yu SC, et al. Placenta growth factor expression is correlated with survival of patients with colorectal cancer. Gut. 2005;54(5):666-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of Placenta growth factor (PlGF) in non-small cell lung cancer (NSCLC) and the clinical and prognostic significance. World J Surg Oncol. 2005;3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escudero-Esparza A, Martin TA, Davies ML, Jiang WG. PGF isoforms, PLGF-1 and PGF-2, in colorectal cancer and the prognostic significance. Cancer Genomics Proteomics. 2009;6(4):239-246. [PubMed] [Google Scholar]
- 31.Escudero-Esparza A, Martin TA, Douglas-Jones A, Mansel RE, Jiang WG. PGF isoforms, PLGF-1 and PGF-2 and the PGF receptor, neuropilin, in human breast cancer: prognostic significance. Oncol Rep. 2010;23(2):537-544. [PubMed] [Google Scholar]
- 32.Cheng SJ, Lee JJ, Kok SH, et al. Expression of placenta growth factor: an independent factor for prediction of progression and prognosis of oral cancer. Head Neck. 2010;32(10):1363-1369. [DOI] [PubMed] [Google Scholar]
- 33.Cheng SJ, Lee JJ, Cheng SL, et al. Increased serum placenta growth factor level is significantly associated with progression, recurrence and poor prognosis of oral squamous cell carcinoma. Oral Oncol. 2012;48(5):424-428. [DOI] [PubMed] [Google Scholar]
- 34.Gorugantula LM, Rees T, Plemons J, Chen HS, Cheng YS. Salivary basic fibroblast growth factor in patients with oral squamous cell carcinoma or oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura T, Ozawa S, Kitagawa Y, Shih CH, Ueda M, Kitajima M. Expression of basic fibroblast growth factor is associated with a good outcome in patients with squamous cell carcinoma of the esophagus. Oncol Rep. 2005;14(3):617-623. [PubMed] [Google Scholar]
- 36.Hase T, Kawashiri S, Tanaka A, et al. Correlation of basic fibroblast growth factor expression with the invasion and the prognosis of oral squamous cell carcinoma. J Oral Pathol Med. 2006;35(3):136-139. [DOI] [PubMed] [Google Scholar]
- 37.Homer JJ, Greenman J, Stafford ND. Circulating angiogenic cytokines as tumour markers and prognostic factors in head and neck squamous cell carcinoma. Clin Otolaryngol Allied Sci. 2002;27(1):32-37. [DOI] [PubMed] [Google Scholar]
- 38.Dietz A, Rudat V, Conradt C, Weidauer H, Ho A, Moehler T. Prognostic relevance of serum levels of the angiogenic peptide bFGF in advanced carcinoma of the head and neck treated by primary radiochemotherapy. Head Neck. 2000;22(7):666-673. [DOI] [PubMed] [Google Scholar]
- 39.Candau-Alvarez A, Gil-Campos M, De la Torre-Aguilar MJ, Llorente-Cantarero F, Lopez-Miranda J, Perez-Navero JL. Early modification in drainage of interleukin-1β and tumor necrosis factor-α best predicts surgical-site infection after cervical neck dissection for oral cancer. J Oral Maxillofac Surg. 2015;73(6):1189-1198. [DOI] [PubMed] [Google Scholar]
- 40.Kuropkat C, Plehn S, Herz U, Dünne AA, Renz H, Werner JA. Tumor marker potential of serum matrix metalloproteinases in patients with head and neck cancer. Anticancer Res. 2002;22(4):2221-2227. [PubMed] [Google Scholar]