Key Points
Question
Does onartuzumab, a mesenchymal-epithelial transition (MET) antibody, improve efficacy outcomes in human epidermal growth factor receptor 2–negative, MET-positive gastroesophageal adenocarcinoma?
Findings
This randomized clinical trial included 562 patients (intent-to-treat population), of whom 214 had MET-positive tumors. The addition of onartuzumab to first-line fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) did not significantly improve overall survival, progression-free survival, or objective response rate of mFOLFOX6 plus placebo in the intent-to-treat or MET-positive populations.
Meaning
Further research is required to better understand which patient populations may potentially derive clinical benefits from MET-targeting agents.
This randomized clinical trial assesses whether the addition of onartuzumab, a MET antibody, to first-line fluorouracil, leucovorin, and oxaliplatin therapy improves efficacy outcomes in HER2-negative, MET-positive gastroesophageal adenocarcinoma.
Abstract
Importance
Dysregulation of the mesenchymal-epithelial transition (MET) signaling pathway is associated with poor prognosis in gastroesophageal adenocarcinoma (GEC). We report results of METGastric, a phase 3 trial of the MET inhibitor onartuzumab plus standard first-line chemotherapy for human epidermal growth factor receptor 2 (HER2)-negative, MET-positive, advanced GEC.
Objective
To determine whether the addition of onartuzumab to first-line fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) improves efficacy compared with mFOLFOX6 plus placebo in HER2-negative, MET-positive GEC.
Design, Setting, and Participants
Randomized, double-blind, multicenter trial conducted from November 2012 to March 2014. Patients were 18 years or older with an adenocarcinoma of the stomach or gastroesophageal junction with metastatic disease not amenable for curative therapy. Tumor samples were centrally tested for MET expression using Ventana anti-Total c-MET (SP44) rabbit monoclonal antibody, HER2 status, and Lauren histologic subtype. MET-positive tumors were defined as at least 50% of tumor cells showing weak, moderate, and/or strong staining intensity (MET 1+/2+/3+, respectively) by immunohistochemistry.
Interventions
Patients with HER2-negative, MET-positive GEC were enrolled and randomized 1:1 to receive mFOLFOX6 with or without onartuzumab (10 mg/kg).
Main Outcomes and Measures
Co–primary end points: overall survival in the intent-to-treat (ITT) population and in patients with MET 2+/3+ GEC. Secondary end points: progression-free survival (PFS), overall response rate (ORR), and safety.
Results
Enrollment was stopped early due to sponsor decision, which was agreed with an independent data monitoring committee. At the data cutoff (April 25, 2014) there were 562 patients in the ITT population (n = 283 placebo plus mFOLFOX6 [median age, 58 y; 65% male]; n = 279 onartuzumab plus mFOLFOX6 [median age, 60 y; 67% male]); 109 (38.5%) and 105 (37.6%) of the ITT population were MET 2+/3+, respectively. Addition of onartuzumab to mFOLFOX6 did not significantly improve OS, PFS, or ORR vs placebo plus mFOLFOX6 in the ITT (OS hazard ratio [HR], 0.82; 95% CI, 0.59-1.15; P = .24; PFS HR, 0.90; 95% CI, 0.71-1.16; P = .43; ORR, 46.1% vs 40.6%) or MET 2+/3+ populations (OS HR, 0.64; 95% CI, 0.40-1.03; P = .06; PFS HR, 0.79; 95% CI, 0.54-1.15; P = .22; ORR, 53.8% vs 44.6%). Safety was as expected for onartuzumab.
Conclusions and Relevance
Addition of onartuzumab to first-line mFOLFOX6 did not significantly improve clinical benefits in the ITT or MET 2+/3+ populations.
Trial Registration
clinicaltrials.gov Identifier: NCT01662869
Introduction
Gastroesophageal adenocarcinoma (GEC), which comprises tumors of the gastroesophageal junction and the stomach, is the fifth most frequently diagnosed cancer worldwide and is the third-highest cause of cancer mortality. The median overall survival (OS) for patients with human epidermal growth factor receptor 2 (HER2)-negative metastatic GEC treated with chemotherapy is approximately 8 to 11 months in Europe and 10 to 16 months in Asia. For patients with unresectable, metastatic GEC, the main therapeutic option is doublet or triplet combination chemotherapy containing a platinum-fluoropyrimidine combination, such as the mFOLFOX6 regimen (fluorouracil, leucovorin calcium, and oxaliplatin). Chemotherapy plus trastuzumab is standard of care for patients with HER2-positive metastatic GEC. Other effective therapeutics for the treatment of gastric cancer in the refractory setting include chemotherapy such as taxanes and irinotecan hydrochloride, and the vascular endothelial growth factor receptor 2 inhibitor ramucirumab (in the second-line setting). Despite all efforts, prognosis for patients with HER2-negative GEC is poor, with a 5-year OS rate of less than 10%.
The hepatocyte growth factor (HGF) receptor that activates key oncogenic pathways through RAS, PI3K, and STAT3 plays an important role in tumorigenesis and is encoded by the mesenchymal-epithelial transition (MET) oncogene. Signaling through the HGF/MET pathway stimulates tissue repair and regeneration in normal tissue but can promote proliferation, survival, and metastasis in tumor cells. In GEC, dysregulation of the MET/HGF pathway is associated with poor prognosis and more aggressive disease, with MET activation stimulating tumor invasiveness. In a randomized phase 2 study in patients with advanced gastric or gastroesophageal junction cancer, nonsignificantly improved survival was observed with the anti-HGF monoclonal antibody, rilotumumab, plus epirubicin hydrochloride, cisplatin, and capecitabine vs placebo plus epirubicin hydrochloride, cisplatin, and capecitabine in patients with MET-positive tumors.
Onartuzumab is a recombinant, fully humanized, monovalent monoclonal antibody that binds with the extracellular domain of MET. By binding with MET, onartuzumab prevents MET from binding with HGF and restricts cellular signaling via the MET pathway. Results of a phase 2 study demonstrated that second-/third-line onartuzumab in combination with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib hydrochloride improved progression-free survival (PFS) and OS vs placebo plus erlotinib in patients with MET-positive (50% of tumor cells staining with an immunohistochemistry [IHC] intensity of 2+/3+) non–small-cell lung cancer (NSCLC).
To investigate whether onartuzumab has clinical efficacy in patients with HER2-negative, MET-positive GEC, METGastric was designed to determine whether the addition of onartuzumab to first-line mFOLFOX6 improves efficacy outcomes when compared with mFOLFOX6 plus placebo.
Methods
Study Design
METGastric (NCT01662869) (study protocol in Supplement 1) was a randomized, double-blind, multicenter, placebo-controlled, phase 3 study designed to evaluate the efficacy and safety of onartuzumab plus mFOLFOX6 vs placebo plus mFOLFOX6 in patients with metastatic HER2-negative and MET-positive GEC. Patients were enrolled between November 19, 2012, and March 7, 2014. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the local institutional review board/ethics committee at each participating site. All patients provided written informed consent.
Inclusion and Exclusion Criteria
Eligible patients were 18 years or older with an adenocarcinoma of the stomach or gastroesophageal junction with metastatic disease not amenable for curative therapy. Other eligibility criteria included Eastern Cooperative Oncology Group performance status of 0 or 1, measurable disease or nonmeasurable but evaluable disease, adequate organ function, and no previous treatment for metastatic disease. Only patients with HER2-negative, MET-positive (as assessed by IHC; score of 1+, 2+, or 3+) GEC were enrolled. Key exclusion criteria included a history of HER2-positive tumors, previous chemotherapy for locally advanced or metastatic gastric carcinoma, a significant history of cardiac disease, peripheral neuropathy, and/or active (significant or uncontrolled) gastrointestinal bleeding.
Procedures
Patients were randomized using a permutated block method in a 1:1 ratio to receive placebo plus mFOLFOX6 (oxaliplatin 85 mg/m2 intravenous [IV], day 1, fluorouracil 400 mg/m2 IV bolus followed by 2400 mg/m2 over 48 hours starting on day 1, leucovorin 400 mg/m2 IV, day 1) or onartuzumab (10 mg/kg IV on day 1, every 14 days) plus mFOLFOX6 for a maximum of 12 cycles (eFigure 1 in Supplement 2). Patients whose disease had not progressed after 12 cycles could continue treatment with either onartuzumab or placebo until disease progression, unacceptable toxic effects, or death. Stratification was performed by MET IHC expression status score (1+ vs 2+/3+), region (Asia-Pacific vs other), and prior gastrectomy (yes vs no). The first dose of study drug had to be administered within 3 days of randomization. No modifications of the onartuzumab/placebo dose were allowed. If onartuzumab/placebo was discontinued due to tolerability, patients could continue mFOLFOX6 treatment (if onartuzumab/placebo was discontinued during the first 12 cycles of study treatment) or fluorouracil/folinic acid if agreed on by the investigator and patient.
HER2 and MET Status
The provision of tumor samples (archival tissue block or 15 serial cut unstained slides) was mandatory. Tumor samples were tested centrally (Targos, Germany) to determine MET expression status (using the validated Ventana anti-Total c-MET [SP44] rabbit monoclonal antibody IHC assay), HER2 status (using the Ventana anti-HER2 [4B5] rabbit monoclonal antibody IHC assay), and the Lauren histologic subtype status. To be eligible for the study, patients were considered positive if their tumor samples demonstrated at least 50% of cells staining positive with an intensity of 1+ or above using the Ventana IUO assay system guidelines. A MET IHC-positive subgroup, for the purpose of testing the MET hypothesis, was defined as those patients with at least 50% of tumor cells showing moderate and/or strong staining intensity (MET 2+/3+).
Study End Points
The co-primary end points were OS in the intent-to-treat (ITT) population and in the subgroup of patients with MET 2+/3+ GEC. Secondary end points included PFS, overall response rate (ORR), disease control rate, duration of response, patient-reported outcomes, and safety.
Assessments
Tumor response and progression were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Evaluation of tumor response was documented every 6 weeks for the first 12 months. For patients who continued to receive study treatment and were followed up for more than 12 months, chest, abdomen, and pelvis computed tomography or magnetic resonance imaging scans were taken every 12 weeks. Overall survival was defined as the time from randomization to death from any cause. Progression-free survival was defined as the time from randomization to the first occurrence of disease progression, as determined by investigator-assessed RECIST v1.1 or death from any cause, whichever occurred first. Duration of response was defined as the time from first occurrence of a documented objective response to disease progression as determined by investigator-assessed RECIST v1.1, or death from any cause during the study. Disease control rate comprised complete response, partial response, and stable disease according to RECIST. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for AEs, version 4.0. An independent data monitoring committee (IDMC) monitored patient safety data approximately every 6 months during the course of the study.
Statistical Analysis
The study was designed to enroll approximately 800 patients and was powered to demonstrate an improvement of median OS from 9.0 to 12.3 months in the ITT population (hazard ratio [HR], 0.73) and 9 to 18 months in the MET 2+/3+ population (HR, 0.49).
The ITT population included all randomized patients, and the safety population included all randomized patients who received at least 1 dose of study treatment. Kaplan-Meier methodology was used to estimate median PFS and median OS for each treatment arm. A stratified Cox regression model was used to estimate HR and 95% confidence intervals of PFS and OS. The 2 treatment comparisons of OS were based on a stratified log-rank test at 1-sided nominal significance level of .00577 for the MET 2+/3+ subgroup and a 1-sided nominal significance level of .02 for the ITT population, using the stratification factors specified.
Results
Patients
Enrollment was stopped early due to sponsor decision, which was agreed with the IDMC, due to a lack of efficacy in a phase 2 trial also assessing mFOLFOX6 plus onartuzumab (NCT01590719). Patients were given the option of continuing treatment with onartuzumab after enrollment was stopped. The rationale to conduct concurrent phase 2/3 studies was to use data from the phase 2 study to inform the final MET IHC cutoff to be applied to the phase 3 study, and was endorsed by both the US Food and Drug Administration and the European Medicines Agency.
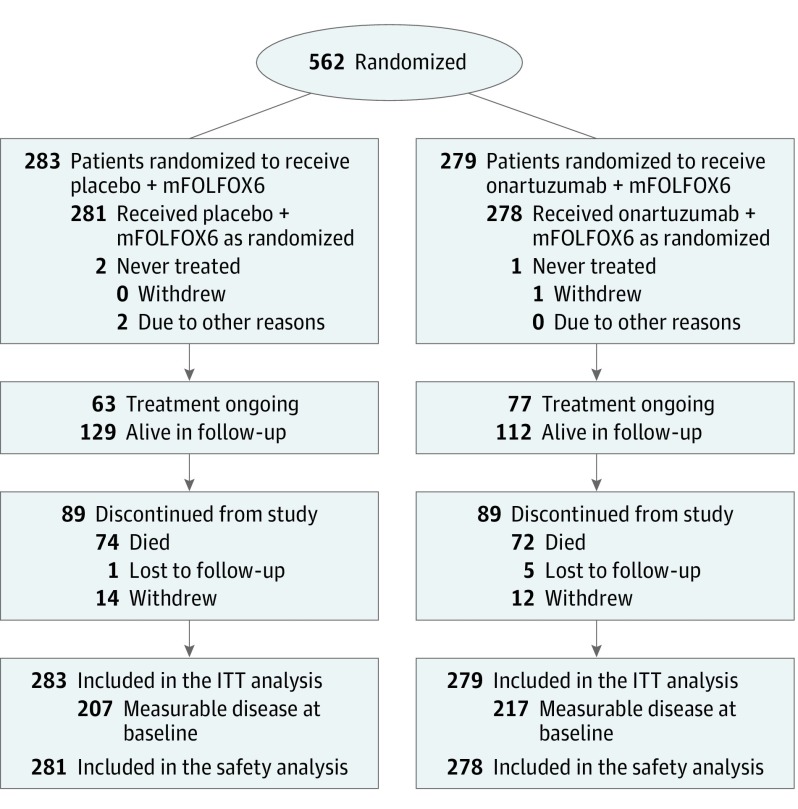
At the data cutoff (April 25, 2014), the ITT population comprised 562 patients, of whom 283 received placebo plus mFOLFOX6 and 279 were allocated to onartuzumab plus mFOLFOX6 (Figure 1). Baseline characteristics between treatment arms were balanced (eTable 1 in Supplement 2). In total, there were 109 (38.5%) and 105 (37.6%) patients with MET 2+/3+ GEC in the placebo plus mFOLFOX6 and onartuzumab plus mFOLFOX6 groups, respectively.
Figure 1. Consolidated Standards of Reporting Trials Diagram.
Safety population indicates all randomized patients who received at least 1 dose of study treatment; intent-to-treat (ITT) population, all randomized patients. mFOLFOX6 indicates fluorouracil, leucovorin, and oxaliplatin.
Efficacy: ITT Population
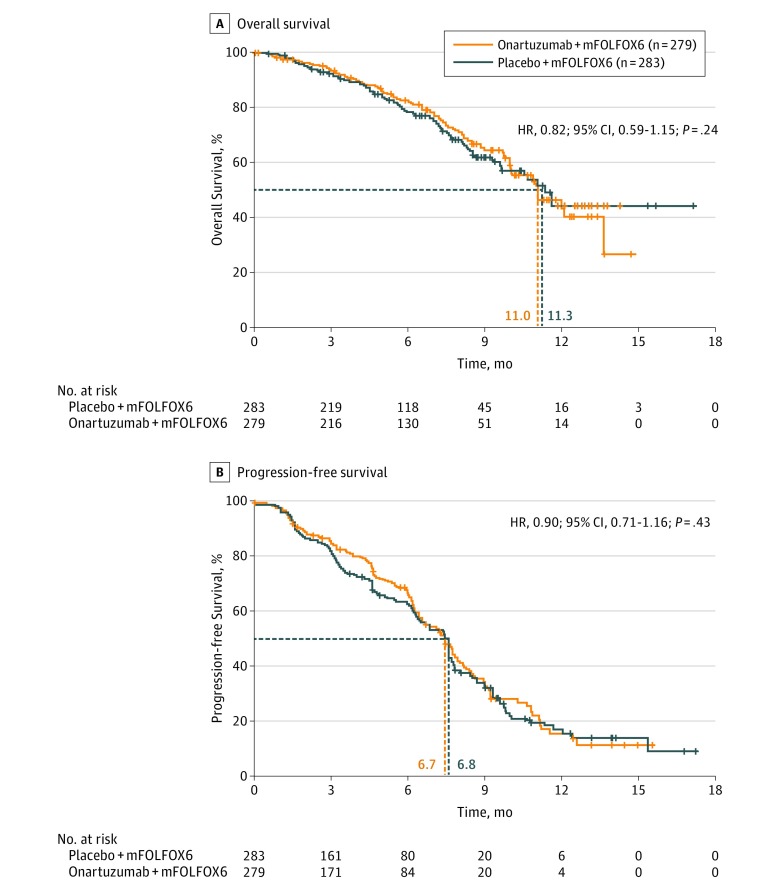
At the data cutoff, in the ITT population, 74 (26.1%) patients in the placebo plus mFOLFOX6 group and 72 (25.8%) patients in the onartuzumab plus mFOLFOX6 group had OS events (defined as death from any cause); 141 (49.8%) and 132 (47.3%) patients had PFS events in the placebo plus mFOLFOX6 and onartuzumab plus mFOLFOX6 groups, respectively. The addition of onartuzumab to mFOLFOX6 did not result in significant differences in OS or PFS compared with placebo plus mFOLFOX6 (Figure 2A and B). Median OS was 11.3 months for placebo plus mFOLFOX6 vs 11.0 months for onartuzumab plus mFOLFOX6 (HR, 0.82; 95% CI, 0.59-1.15; stratified P = .24), and median PFS was 6.8 vs 6.7 months (HR, 0.90; 95% CI, 0.71-1.16; stratified P = .43), respectively.
Figure 2. Kaplan-Meier Plots of Overall and Progression-Free Survival.
Median overall survival was 11.3 months for placebo plus fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) vs 11.0 months for onartuzumab plus mFOLFOX6, and median progression-free survival was 6.8 vs 6.7 months, respectively. Dashed lines indicate median survival, and HR, hazard ratio.
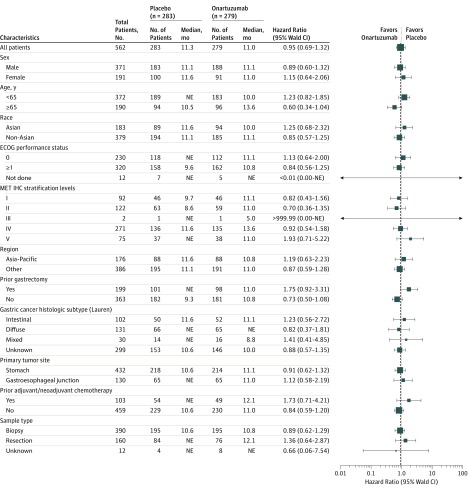
Subgroup analyses of OS in the ITT population showed no difference between onartuzumab and placebo in any subgroup (Figure 3). There was no difference in ORR between treatment arms in the ITT population for onartuzumab plus mFOLFOX6 compared with placebo plus mFOLFOX6 (100 patients [46.1%] vs 84 patients [40.6%]; P = .25) (Table 1). Four patients in each group achieved a complete response.
Figure 3. Subgroup Analysis of Overall Survival in the Intent-to-Treat Population.
Size of the data marker corresponds to the number of patients in each subgroup. ECOG indicates Eastern Cooperative Oncology Group; IHC, immunohistochemistry; MET, mesenchymal-epithelial transition; NE, not estimable.
Table 1. Response Rates in the Intent-to-Treat (ITT) and Mesenchymal-Epithelial Transition (MET) 2+/3+ Populations With Measurable Disease at Baseline.
| Response | ITT Population | MET 2+/3+ | ||||
|---|---|---|---|---|---|---|
| No. (%) | P Value | No. (%) | P Value | |||
| Placebo + mFOLFOX6 (n = 207) |
Onartuzumab + mFOLFOX6 (n = 217) |
Placebo + mFOLFOX6 (n = 92) |
Onartuzumab + mFOLFOX6 (n = 78) |
|||
| Overall response rate | 84 (40.6) | 100 (46.1) | .25 | 41 (44.6) | 42 (53.8) | .23 |
| Complete response | 4 (1.9) | 4 (1.8) | 0 | 3 (3.8) | ||
| Partial response | 80 (38.6) | 96 (44.2) | 41 (44.6) | 39 (50.0) | ||
| Stable disease | 69 (33.3) | 70 (32.3) | 25 (27.2) | 20 (25.6) | ||
| Progressive disease | 27 (13.0) | 26 (12.0) | 13 (14.1) | 9 (11.5) | ||
| Disease control rate | 153 (73.9) | 170 (78.3) | 66 (71.7) | 62 (79.5) | ||
Abbreviation:mFOLFOX6, fluorouracil, leucovorin, and oxaliplatin.
Efficacy: MET 2+/3+ Subgroup
MET IHC comprised both staining intensity and the percentage of MET-expressing tumor cells (eFigure 2 in Supplement 2). MET IHC–positive tumors were defined as those with at least 50% of tumor cells showing moderate and/or strong staining intensity (MET 2+/3+). Overall agreement between central laboratory pathologists was more than 95% (eTable 2 in Supplement 2).
In the MET 2+/3+ subgroup, 41 (37.6%) patients in the placebo plus mFOLFOX6 group and 35 (33.3%) of patients in the onartuzumab plus mFOLFOX6 group had OS events at the data cutoff. The addition of onartuzumab to mFOLFOX6 did not significantly improve efficacy outcomes compared with placebo in this subgroup (eFigure 3 in Supplement 2). Median OS was 9.7 months for placebo plus mFOLFOX6 vs 11.0 months for onartuzumab plus mFOLFOX6 (HR, 0.64; 95% CI, 0.40-1.03; stratified P = .06); median PFS was 5.7 vs 6.9 months (HR, 0.79; 95% CI, 0.54-1.15; stratified P = .22), respectively.
Efficacy: Post hoc Exploratory Subgroup Analyses
Post hoc exploratory subgroup analyses of OS by region and prior gastrectomy are shown in eFigure 4 in Supplement 2. In the MET 2+/3+ subgroup, non-Asian patients without prior gastrectomy (n = 125) seemed to derive a clinical benefit from onartuzumab plus mFOLFOX6 (HR, 0.51; 95% CI, 0.29-0.89). There was no difference in ORR between the treatment arms in the MET 2+/3+ subgroup for onartuzumab plus mFOLFOX6 compared with placebo plus mFOLFOX6 (42 patients [53.8%] vs 41 patients [44.6%]; P = .23) (Table 1). Three patients in the MET 2+/3+ subgroup achieved a complete response in the onartuzumab plus mFOLFOX6 group compared with none in the placebo plus mFOLFOX6 group.
Safety
Overall, 273 (97.5%) patients in the placebo plus mFOLFOX6 group and 274 (98.2%) patients in the onartuzumab plus mFOLFOX6 group experienced at least 1 AE. Serious AEs were more frequent with onartuzumab than with placebo (100 [35.8%] vs 91 [32.5%], respectively), but the difference between the treatment arms was not statistically significant (Table 2). All-grade AEs and grade 3 and above AEs are summarized in eTable 3 in Supplement 2. Grade 3 and above AEs more commonly observed with onartuzumab included neutropenia (98 [35.1%] vs 82 [29.3%]), hypoalbuminemia (16 [5.7%] vs 1 [0.4%]), peripheral edema (13 [4.7%] vs 1 [0.4%]), thrombocytopenia (12 [4.3%] vs 3 [1.1%]), pulmonary embolism (17 [6.1%] vs 10 [3.6%]), and gastric perforation (2 [0.7%] vs 0).
Table 2. Overall Adverse Event (AE) Profile in the Safety Population.
| Parametera | No. (%) | |
|---|---|---|
| Placebo + mFOLFOX6 (n = 280) |
Onartuzumab + mFOLFOX6 (n = 279) |
|
| At least 1 AE | 273 (97.5) | 274 (98.2) |
| Deathsb | 73 (26.1) | 70 (25.1) |
| Serious AEs | 91 (32.5) | 100 (35.8) |
| AE leading to treatment withdrawal | 61 (21.8) | 87 (31.2) |
| Grade 3-5 AE at greatest intensity | 187 (66.8) | 192 (68.8) |
Abbreviation: mFOLFOX6, fluorouracil, leucovorin, and oxaliplatin.
Multiple occurrences of the same AE in 1 individual are counted only once. Note, 1 patient randomized to the placebo arm received onartuzumab during the first treatment cycle; data for this patient were included in the onartuzumab arm for analysis of safety.
Including death from disease progression.
Discussion
Further to discussion with the IDMC, enrollment into this trial was terminated prematurely. The addition of onartuzumab to first-line mFOLFOX6 did not improve OS, PFS, or ORR compared with mFOLFOX6 alone in patients with HER2-negative GEC, irrespective of MET expression status. These negative results are in line with other phase 2/3 trials that have reported similarly disappointing results with onartuzumab, including in triple-negative breast cancer, recurrent glioblastoma, and NSCLC after initial phase 2 data in second-line/third-line NSCLC were promising.
Several hypotheses have been advanced to explain the failure of onartuzumab, as well as other MET-directed drugs, in GEC and other solid tumors. One explanation is that MET overexpression might not be an appropriate target for onartuzumab in the majority of patients with GEC. Dysregulation of the MET pathway in GEC can arise from MET amplification, or rare MET mutations affecting the extracellular domain (involving the HGF binding domain), the tyrosine kinase domain (leading to a constitutive MET activation), or the juxtamembrane domain (causing a disruption in negative regulation of MET signaling). Targeting one of these alternative MET pathways may lead to improved clinical outcomes for patients with GEC. For example, because MET-amplified tumors are likely to signal primarily via ligand-independent mechanisms, a small molecule targeting the kinase domain could potentially prove beneficial in these cases.
An alternative hypothesis for the lack of efficacy with onartuzumab in GEC is that overexpression of HGF in the tumor cells or stroma may lead to autocrine or paracrine loop formation. In contrast to the situation of oncogene addiction, this inappropriate MET signaling is a consequence, rather than the cause, of the cell transformation, and targeting MET signaling in these tumors will not fundamentally affect tumor behavior or cancer outcomes. However, this does leave open the possibility that some tumors may still be dependent on sustained MET signaling for their growth and survival and may therefore be sensitive to MET blockade. Identification of these tumors would require an alternate biomarker to identify tumors that are driven by MET signaling. Other possible explanations for why MET inhibition alone may not yield an improvement in survival in GEC are the potential redundancy of signaling via the MET pathway, or because the MET IHC assay may not select appropriately for MET-driven tumors. Predictive biomarkers are sought that can more accurately identify patients who are most likely to gain clinical benefit from onartuzumab treatment.
Conclusions
Although METGastric failed to meet its co–primary end points, further research is required to better elucidate which patient populations may potentially derive clinical benefits from onartuzumab.
Trial Protocol
eTable 1. Baseline Characteristics of the Intent-to-Treat Population
eTable 2. MET IHC Scoring Qualification of Central Laboratory Pathologists
eTable 3. All Grade and Grade ≥3 AEs
eFigure 1. Study design
eFigure 2. Met IHC scoring criteria and representative staining
eFigure 3. OS (A) and PFS (B) in the MET 2+/3+ subpopulation
eFigure 4. Overall Survival by MET status, region, and prior gastrectomy
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. [DOI] [PubMed] [Google Scholar]
- 2.Lordick F, Lorenzen S, Yamada Y, Ilson D. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer. 2014;17(2):213-225. [DOI] [PubMed] [Google Scholar]
- 3.Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33(16):1760-1769. [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. [DOI] [PubMed] [Google Scholar]
- 5.Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47(15):2306-2314. [DOI] [PubMed] [Google Scholar]
- 6.Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30(13):1513-1518. [DOI] [PubMed] [Google Scholar]
- 7.Ford HE, Marshall A, Bridgewater JA, et al. ; COUGAR-02 Investigators . Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15(1):78-86. [DOI] [PubMed] [Google Scholar]
- 8.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438-4444. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs CS, Tomasek J, Yong CJ, et al. ; REGARD Trial Investigators . Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31-39. [DOI] [PubMed] [Google Scholar]
- 10.Wilke H, Muro K, Van Cutsem E, et al. ; RAINBOW Study Group . Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235. [DOI] [PubMed] [Google Scholar]
- 11.Price TJ, Shapiro JD, Segelov E, et al. Management of advanced gastric cancer. Expert Rev Gastroenterol Hepatol. 2012;6(2):199-208. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Yao W, Newton RC, Scherle PA. Targeting the c-MET signaling pathway for cancer therapy. Expert Opin Investig Drugs. 2008;17(7):997-1011. [DOI] [PubMed] [Google Scholar]
- 13.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22(4):309-325. [DOI] [PubMed] [Google Scholar]
- 14.Blumenschein GR Jr, Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol. 2012;30(26):3287-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11(12):834-848. [DOI] [PubMed] [Google Scholar]
- 16.Zeng ZS, Weiser MR, Kuntz E, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265(2):258-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samamé Pérez-Vargas JC, Biondani P, Maggi C, et al. Role of cMET in the development and progression of colorectal cancer. Int J Mol Sci. 2013;14(9):18056-18077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15(9):1007-1018. [DOI] [PubMed] [Google Scholar]
- 19.Merchant M, Ma X, Maun HR, et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci U S A. 2013;110(32):E2987-E2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(32):4105-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeppen H, Yu W, Zha J, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib±onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res. 2014;20(17):4488-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah MA, Cho JY, Huat ITB, et al. Randomized phase II study of FOLFOX +/− MET inhibitor, onartuzumab (O), in advanced gastroesophageal adenocarcinoma (GEC). J Clin Oncol. 2015;33(suppl):abstr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diéras V, Campone M, Yardley DA, et al. Randomized, phase II, placebo-controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple-negative breast cancer. Ann Oncol. 2015;26(9):1904-1910. [DOI] [PubMed] [Google Scholar]
- 24.Cloughesy T, Finocchiaro G, Belda-Iniesta C, et al. Phase II study of onartuzumab plus bevacizumab vs placebo plus bevacizumab in patients with recurrent glioblastoma. Neuro Oncol. 2014;16:v81(suppl 5). [Google Scholar]
- 25.Spigel DR, Edelman MJ, O’Byrne K, et al. Onartuzumab plus erlotinib vs erlotinib in previously treated stage IIIb or IV NSCLC: results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol. 2014;32(suppl):abstr 8000. [DOI] [PubMed] [Google Scholar]
- 26.Pérol M. Negative results of METLung study: an opportunity to better understand the role of MET pathway in advanced NSCLC. Transl Lung Cancer Res. 2014;3(6):392-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89-103. [DOI] [PubMed] [Google Scholar]
- 28.Bachleitner-Hofmann T, Sun MY, Chen CT, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther. 2008;7(11):3499-3508. [DOI] [PubMed] [Google Scholar]
- 29.Corso S, Ghiso E, Cepero V, et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer. 2010;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics of the Intent-to-Treat Population
eTable 2. MET IHC Scoring Qualification of Central Laboratory Pathologists
eTable 3. All Grade and Grade ≥3 AEs
eFigure 1. Study design
eFigure 2. Met IHC scoring criteria and representative staining
eFigure 3. OS (A) and PFS (B) in the MET 2+/3+ subpopulation
eFigure 4. Overall Survival by MET status, region, and prior gastrectomy