Key Points
Question
What is the activity of abiraterone acetate plus prednisone in patients with metastatic prostate cancer who have a poor prognosis with a suboptimal response to initial androgen deprivation therapy?
Findings
In this phase 2 study including 40 patients, 13% of the patients had a strong prostate-specific antigen response with combination abiraterone acetate plus prednisone therapy, and the median overall survival was longer than that of historical controls.
Meaning
Abiraterone acetate showed potential activity in this poor-prognosis setting; further testing of its upfront use in patients with metastatic prostate cancer should be considered.
Abstract
Importance
Men with metastatic prostate cancer who have a poor response to initial androgen-deprivation therapy (ADT), as reflected by a prostate-specific antigen (PSA) level higher than 4.0 ng/mL after 7 months of ADT, have a poor prognosis, based on historical controls.
Objective
To determine the efficacy of abiraterone acetate with prednisone in these high-risk patients with a suboptimal response to hormonal induction.
Design, Setting, and Participants
A phase 2 single-arm study was conducted through the National Clinical Trials Network–Southwest Oncology Group. Eligible patients had metastatic prostate cancer and a PSA level higher than 4.0 ng/mL between 6 and 12 months after starting ADT. The PSA level could be rising or falling at the time of enrollment, but had to be higher than 4.0 ng/mL. No previous chemotherapy or secondary hormonal therapies were allowed, except in patients receiving a standard, first-generation antiandrogen agent with a falling PSA level at the time of enrollment; this therapy was continued in this cohort. Abiraterone acetate, 1000 mg, once daily with prednisone, 5 mg, twice daily was administered to all participants. A total of 41 men were enrolled between the trial’s activation on August 9, 2011, and closure on August 1, 2013. Data analysis was conducted from March 21 to November 29, 2016.
Interventions
Abiraterone acetate, 1000 mg, once daily by mouth with prednisone, 5 mg, by mouth twice daily.
Main Outcomes and Measures
The primary end point was a PSA level of 0.2 ng/mL or lower within 12 months of starting abiraterone acetate plus prednisone. A partial response (PR) was a secondary end point, defined as a PSA level reduction to lower than 4.0 ng/mL but higher than 0.2 ng/mL.
Results
Of the 41 men enrolled, 1 did not receive any protocol treatment and was excluded from analysis. The median (range) age of the 40 participants was 66 (39-85) years. Five (13%) patients achieved a PSA level of 0.2 ng/mL or lower (95% CI, 4%-27%). Thirteen (33%) additional patients achieved a partial response, with a reduction in the PSA level to lower than 4.0 ng/mL but higher than 0.2 ng/mL. Sixteen (40%) patients had no PSA response and 6 (15%) were not assessable and assumed to be nonresponders. The median progression-free survival was 17.5 months (95% CI, 8.6-25.0 months) and the median overall survival was 25.8 months (95% CI, 15.7-25.8 months). There was 1 incident each of grade 4 adverse events of alanine aminotransferase level elevation and rectal hemorrhage. Eleven patients reported grade 3 adverse events.
Conclusions and Relevance
This study did not reach its prescribed level of 6 PSA responses of 0.2 ng/mL or lower, although 5 responses were observed. The overall survival and progression-free survival rates observed in this trial are encouraging compared with historical controls. The therapy was generally well tolerated, without any clear signal of any unexpected adverse effects.
This study evaluated the use of abiraterone acetate plus prednisone in men with metastatic prostate cancer.
Introduction
The natural course of metastatic, hormone-naive prostate cancer is variable. Men with a suboptimal or incomplete response to initial hormone induction therapy fare poorly with a short overall survival rate. Although recent reports have described a role for up-front docetaxel chemotherapy combined with androgen deprivation therapy (ADT) for metastatic, hormone-sensitive prostate cancer, the use of next-generation androgen therapy, such as abiraterone acetate plus prednisone, is not well studied in the upfront setting. The S1014 trial examines the role of abiraterone acetate plus prednisone in men with an inadequate response to hormone induction therapy.
The Southwest Oncology Group (SWOG) trial 9346 (NCT01309672) tested the use of continuous vs intermittent hormonal therapy in men with treatment-naive, metastatic prostate cancer (mPC). All men in S9346 were to receive 7 months of ADT, at which time those with a PSA level of 4.0 ng/mL or lower were randomized to continuous ADT vs intermittent therapy. In contrast, those with a PSA level higher than 4.0 ng/mL at month 7 (430 of 1395) were removed from the study owing to an inadequate PSA response to the induction hormone therapy. At the end of the 7-month induction period, the median survival was 75 months for those with a PSA level of 0.2 ng/mL or lower; in contrast, for those with a PSA level higher than 4.0 ng/mL, representing a very-high-risk mPC population, the median survival was 13 months.
Since the inception of S9346, treatment with abiraterone acetate plus prednisone or enzalutamide has become a first-line treatment for metastatic, castration-resistant prostate cancer (mCRPC). Abiraterone acetate plus prednisone is approved for use in men with mCRPC based on the positive results of 2 large, phase 3 randomized clinical trials. In the COU-301 trial, abiraterone acetate plus prednisone treatment in men with mCRPC previously treated with docetaxel chemotherapy was associated with an overall survival (OS) benefit (hazard ratio [HR], 0.65; P < .001) compared with prednisone alone. In the subsequent COU-302 study, abiraterone acetate plus prednisone vs prednisone plus placebo was investigated in patients with mCRPC not previously treated with docetaxel chemotherapy. There was also a substantial OS benefit in this prechemotherapy setting (HR, 0.75; P = .01).
The standard use of abiraterone acetate plus prednisone or enzalutamide in clinical practice is limited to mCRPC, and there is little information about its use to treat mPC in the hormone-sensitive or upfront setting. The use of upfront abiraterone acetate with ADT in hormone-sensitive PC was tested in a small randomized study in the neoadjuvant setting. Intraprostatic androgens were more effectively suppressed in the combined therapy group compared with the ADT-alone group, consistent with a more effective targeting of the androgen axis with this approach.
In S1014, men with a PSA level higher than 4.0 ng/mL after 6 to 12 months of ADT for mPC were treated with the addition of abiraterone acetate plus prednisone. Men in this study had either a declining PSA level (hormone-sensitive disease) or a rising PSA level (castration-resistant disease), but all had a PSA level higher than 4.0 ng/mL. The ability of abiraterone acetate plus prednisone to benefit men with an inadequate PSA response to initial ADT was assessed.
Methods
Patients
All patients were 18 years or older with a histologic or cytologic diagnosis of adenocarcinoma of the prostate and a Zubrod performance status of 0 to 2. All men had metastatic disease defined as (1) visceral metastases, (2) bone metastases, or (3) distant lymph node disease (eg, above the aortic bifurcation). Only patients with a suboptimal response to initial ADT were enrolled, defined as a PSA level higher than 4.0 ng/mL between 6 and 12 months after initiation of continuous ADT for mPC. Patients receiving standard antiandrogen therapy with a rising PSA level were required to undergo a formal washout period (eg, 6 weeks for bicalutamide) before enrollment. For patients with a declining PSA level, current antiandrogen therapy was continued. None of the patients had received prior cytotoxic chemotherapy, ketoconazole, sipuleucel-T, or radiopharmaceuticals for mPC. Adequate liver, kidney, and bone marrow function was required. A baseline testosterone level of less than 50 ng/dL (to convert to nanomoles per liter, multiply by 0.0347) was confirmed to ensure adequate and ongoing ADT.
Study Design and Treatment
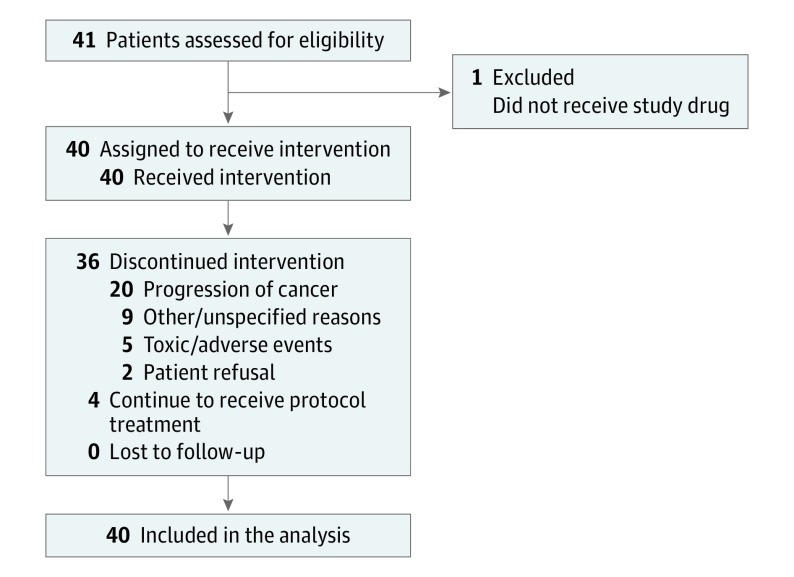
A single-arm, phase 2 study, S1014 was conducted by the SWOG. The diagram for S1014 is shown in Figure 1. All patients were given abiraterone acetate, 1000 mg, once daily by mouth with prednisone, 5 mg, twice daily by mouth. Abiraterone acetate was provided by the study. Dose modification was allowed and stipulated in the protocol for adverse events with a reduction of the abiraterone acetate dose to 750 or 500 mg/d; prednisone was not dose modified. Participants were removed from the protocol for progression of disease (PSA level rise alone was not sufficient), symptomatic deterioration, unacceptable toxic effects, patient withdrawal of consent, or delay in abiraterone acetate dosing of more than 4 weeks. Progression of disease was defined as unequivocal progression of disease, progressive disease as defined by RECIST guideline, version 1.1, progressive disease as defined by the Prostate Cancer Clinical Trials Working Group bone scan progression criteria, or death due to disease. Radiographic assessments were performed every 12 weeks. Survival was calculated from the date of registration to the date of death due to any cause. Patients without the end point of interest (PFS or OS) were censored at the date of the last known contact.
Figure 1. Study Flow.
Study accrual with patients receiving intervention, lost to follow-up, and included for statistical analysis.
The study was approved by the institutional review board of each participating institution. The subjects were recruited with IRB approval from these sites: University of Utah, Salt Lake City; University of Colorado, Aurora; Baylor College of Medicine; Wayne State University, Detroit, Michigan; Yale University; City of Hope National Medical Center, Duarte, California; Kaiser Permanente NCORP, Oakland California; Nevada Cancer Research Foundation NCORP; University of Southern California, Los Angeles; Wichita NCORP, Wichita, Kansas; Hawaii Minority Underserved NCORP, Honolulu, Hawaii; Henry Ford Hospital, Detroit, Michigan; University of Kansas, Lawrence, Kansas; Louisiana State University, in Shreveport/Gulf South Minority Underserved NCORP, New Orleans, Louisiana; Memorial Hospital Center, University of Colorado Health, Colorado Springs/Colorado, U of; Montana Cancer Consortium NCORP, Billings, Montana; Southeast Clinical Oncology Research Consortium Southeast CCC NCORP; University of Texas, San Antonio; SW Cancer & Res Ctr/San Antonio, U of TX; Tulane Medical/Cancer Center Minority-Based Community Clinical Oncology Program, Tulane University, New Orleans, Louisiana; University of Arkansas, Little Rock. Patients provided written informed consent and there was no financial compensation.
The primary end point in S1014 was the number of men achieving an undetectable PSA level, defined as a PSA level of 0.2 ng/mL or lower. Patients not responding in the first year were deemed nonresponders. A partial response (PR) was a secondary end point, defined as a PSA level reduction to lower than 4.0 ng/mL but higher than 0.2 ng/mL. Responses were required within 12 months of registration, and a 12-month landmark analysis, presented in this publication, was also performed for PSA responses. Other evaluations of PSA metrics were proscribed for descriptive analysis.
Statistical Analysis
The main objective of this study was to test whether this regimen has promise in terms of rates of achieving an undetectable PSA level (≤0.2 ng/mL), confirmed at least 4 weeks after initiation of therapy. If the proportion of men with an undetectable PSA level is 20% or greater, the regimen would be of further interest, whereas further testing would not be pursued if the proportion of men with an undetectable PSA level was 5% or less. Six or more participants with an undetectable PSA level of the total 38 eligible patients would indicate that continued study of this regimen is warranted. The design has a significance level (chance of falsely determining further study is warranted of a treatment with <5% response rate) of 5.0% and a power (probability of correctly identifying the agent as warranting further study for a likely response rate of >20%) of 90%.
A sample size of 38 eligible patients was deemed to be sufficient to estimate the PSA undetectable and PSA level normalization rates, progression-free survival (PFS) and overall survival (OS) at a specified time point, and the probability of a particular adverse event within ±16% (95% CI). Any adverse effects occurring with at least a 5% probability are likely to be seen at least once (83%).
The 20% alternative hypothesis is based on the observation that approximately 20% of patients with CRPC treated with abiraterone acetate plus prednisone were observed to have a greater than 90% PSA level reduction, indicating that significant PSA responses may be expected in 20% of the patients with traditional CRPC. The trial was designed with 90% power to correctly identify the agent as warranting further study for a likely response rate of greater than 20% with a 1-sided significance level of 0.05. Data analysis for this trial was conducted from March 21 to November 29, 2016, using SAS, version 9.4 (SAS Institute Inc).
Results
Forty-one men were enrolled between the trial’s activation on August 9, 2011, and closure on August 1, 2013. One individual was not evaluable because he did not receive protocol treatment and therefore was not included in the analysis. Patient characteristics are reported in Table 1. The median PSA level at study entry was 23.6 ng/mL (range, 11.7-64.4 ng/mL). Thirty of the 40 (75%) evaluable patients had high-grade disease with a Gleason score of 8 to 10 and 6 (15%) of the men had visceral metastatic disease. Thirty-four (85%) patients had a rising PSA level and 6 (15%) patients had a stable or falling PSA level. Patients with an increase in the PSA level of more than 0.1 ng/mL from nadir were assessed as having a rising PSA level at study entry.
Table 1. Patient Characteristics.
| Characteristic | Abiraterone Acetate Plus Prednisone (n = 40) |
|---|---|
| Age, median, (range), y | 66 (39-85) |
| Testosterone, median (range), ng/dL | 12.8 (3.0-50.0) |
| PSA at entry, median (IQR), ng/mL | 23.6 (11.7-64.4) |
| Hispanic, No. (%) | |
| Yes | 4 (10) |
| No, unknown | 36 (90) |
| Race, No. (%) | |
| White | 27 (68) |
| Black | 10 (25) |
| Asian | 2 (5) |
| Native American | 1 (3) |
| SWOG PS, No. (%) | |
| 0-1 | 39 (98) |
| 2 | 1 (3) |
| Gleason score, No. (%) | |
| 2-6 | 3 (8) |
| 7 | 7 (18) |
| 8-10 | 30 (75) |
| Metastasis, No. (%) | |
| Bone | 37 (93) |
| Lymph node | 8 (20) |
| Visceral | 6 (15) |
| Prostate RT, No. (%) | 5 (13) |
| Prostatectomy, No. (%) | 5 (13) |
| Antiandrogen use, No. (%) | 35 (88) |
| Time from antiandrogen to registration, median (range), da | 74 (10-371) |
| PSA status at registration, No. (%) | |
| Rising level | 34 (85) |
| Stable/falling level | 6 (15) |
Abbreviations: IQR, interquartile range; PS, performance status; PSA, prostate-specific antigen; RT, radiotherapy; SWOG, Southwest Oncology Group.
Data available for 34 patients.
Efficacy
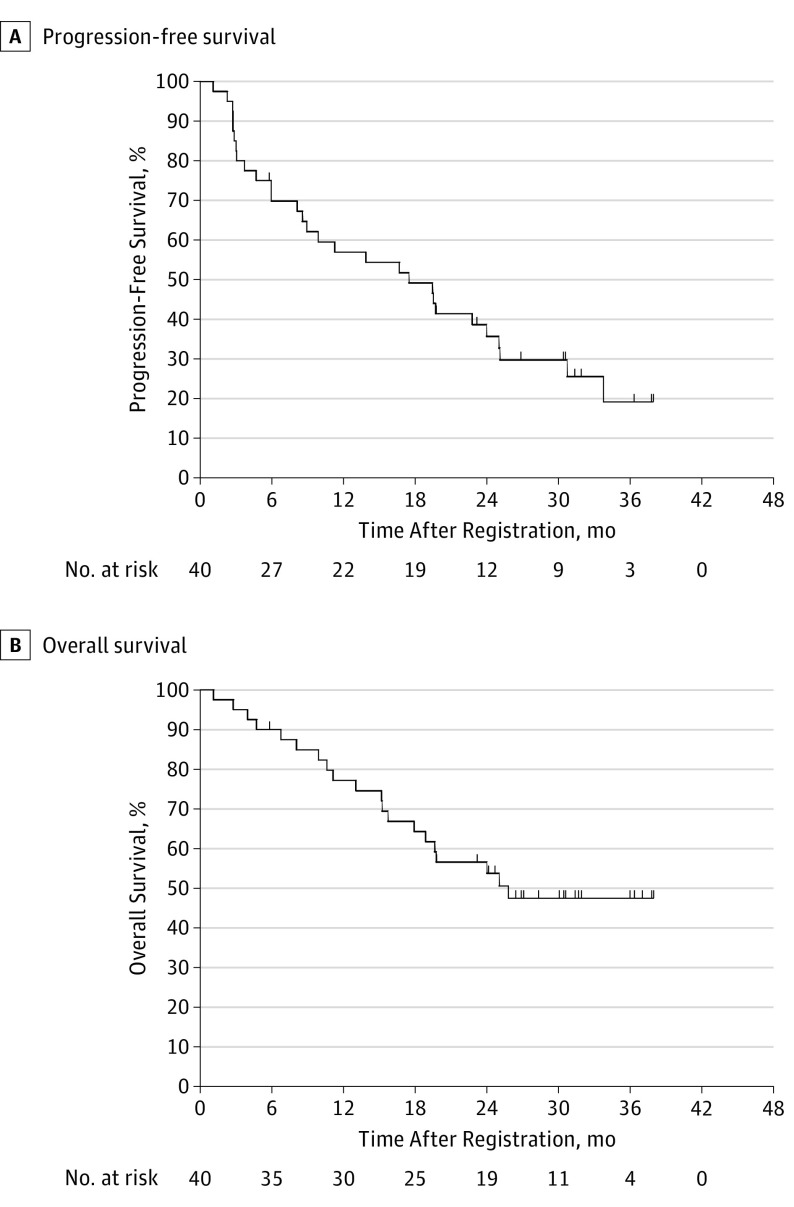
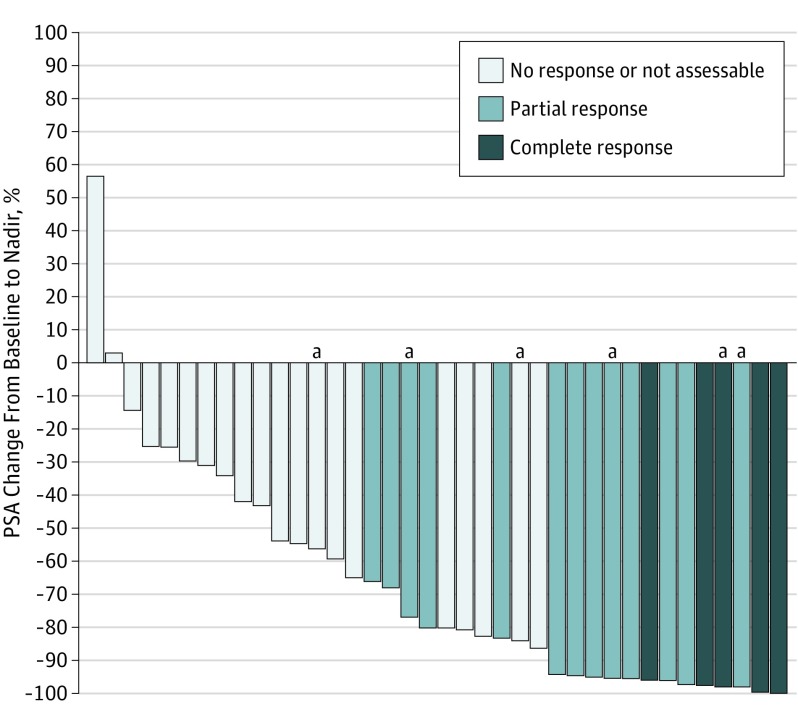
Although the study was overaccrued by 2 patients, the same threshold of PSA response activity specified in the Statistical Analysis subsection section of the Methods section for 38 patients also held for 40 patients. A nadir PSA level of 0.2 ng/mL or lower was obtained in 5 (13%) patients (95% CI, 4%-27%). Thirteen (33%) additional patients achieved PSA level normalization with a PSA level lower than 4.0 ng/mL, which was confirmed at least 4 weeks later, but had a nadir PSA level higher than 0.2 ng/mL. Of these, 8 (62%) men had ongoing PSA level normalization at a 12-month time point of assessment, whereas all of those with a PSA level decrease to 0.2 ng/mL or lower had that value maintained 12 months. Sixteen (40%) patients had no PSA response and 6 (15%) individuals were not assessable and assumed to be nonresponders (Table 2). The median PFS was 17.5 months (95% CI, 8.6-25.0 months) (Figure 2A) based on radiographic (not biochemical) progression. The median OS was 25.8 months (95% CI, 15.7-25.8 months) (Figure 2B). Of the 40 evaluable patients, 34 (85%) had a rising PSA level at study entry and 6 (15%) had a stable or falling PSA level (Table 2). The median PSA level at registration for men with a complete response was 11.5 ng/mL (interquartile range [IQR], 7.1-13.4 ng/mL) compared with 16.3 ng/mL (IQR, 10.4-20.8 ng/mL) in the PSA level normalization group and 41 (IQR, 19.9-265.8 ng/mL) in the no-response group. The best maximum relative PSA level reduction for each patient is shown in Figure 3. The results of a Wilcoxon rank-sum test yielded no evidence of an association between baseline testosterone levels (nanograms per deciliter) and response to abiraterone (testosterone levels for combined CR/PR patients: median, 10.8; range, 3-40 ng/dL; P = .54). Patient characteristics included PSA levels and testosterone levels at registration; the duration of ADT before registration is shown by PSA response group in Table 3.
Table 2. PSA Best Responses Within First 12 Months After Registrationa.
| Characteristic | PSA Status at Registration (n = 40) |
||
|---|---|---|---|
| Rising | Stable/Falling | Total | |
| Best PSA Response, No. (%) | |||
| None or NA | 20 (59) | 2 (33) | 22 (55) |
| PR | 10 (29) | 3 (50) | 13 (32) |
| CR | 4 (12) | 1 (17) | 5 (12) |
| Total | 34 | 6 | 40 |
| Response at 12 mo, No. (%) | |||
| None or NA | 25 (74) | 2 (33) | 27 (67) |
| PR | 5 (15) | 3 (50) | 8 (20) |
| CR | 4 (12) | 1 (17) | 5 (12) |
| Total | 34 | 6 | 40 |
Abbreviations: CR, complete response; NA, not applicable; PR, partial response; PSA, prostate-specific antigen.
Complete response, PSA level of 0.2 ng/mL or lower; PR, PSA level higher than 0.2 ng/mL but lower than 4.0 ng/mL.
Figure 2. Progression-Free and Overall Survival.
Eligible patients with follow-up as of March 7, 2016. There were 29 events in a median of 17.5 months for progression-free survival (A) and 20 events in a median of 25.8 months in overall survival (B). Events were defined as unequivocal progression of disease, progressive disease as defined by RECIST 1.1, progressive disease defined by the Prostate Cancer Working Group bone scan progression criteria, or death due to disease.
Figure 3. Change in Prostate-Specific Antigen (PSA) Levels From Baseline in 38 Patients.
Partial response was PSA level lower than 4.0 ng/mL but higher than 0.2 ng/mL and complete response was PSA level of 0.2 ng/mL or lower. Two patients did not have follow-up PSA levels determined and so are omitted from the figure.
aAt baseline, all PSA levels were rising except these, which were declining.
Table 3. Patient Characteristics by Best PSA Responsea.
| Characteristic | CR | PR | None or NA |
|---|---|---|---|
| Age, median (range), y | 64 (51-72) | 63 (55-79) | 71 (39-85) |
| Entry PSA, median (IQR), ng/dL | 11.5 (5.1-13.4) | 12.2 (7.4-20.8) | 50.6 (22.9-266.9) |
| Testosterone, median (range), ng/dL | 10 (3-36) | 17 (3-40) | 13.8 (3-50) |
| Days from ADT to registration, median (range) | 137 (13-308) | 116 (44-316) | 54 (10-371) |
Abbreviations: ADT, androgen deprivation therapy; CR, complete response; NA, not applicable; IQR, interquartile range; PR, partial response; PSA, prostate-specific antigen.
Complete response, PSA level of 0.2 ng/mL or lower; PR, PSA level higher than 0.2 ng/mL but lower than 4.0 ng/mL.
Treatment Administration and Safety
The median duration of therapy was 8.2 months (range, 1.4-37.8 months). A summary of the adverse events is included in the eTable in the Supplement, excluding adverse events deemed unlikely or not related to the study drug and also excluding adverse events with no grade 3 to 5 entries. No grade 5 adverse events were noted. One instance each of grade 4 alanine aminotransferase level increase and rectal hemorrhage were observed. The most common grade 3 adverse effects (2 incidences each) were hypertension, increased aspartate aminotransferase level, hyperglycemia, hypokalemia, and nausea and vomiting.
Discussion
Patients with mPC and a PSA level higher than 4.0 ng/mL after 7 months of ADT have a very poor prognosis, based on data from historical controls. In S1014, abiraterone acetate therapy was administered to these patients with the aim of reducing the PSA level to 0.2 ng/mL or lower, converting a hormone-induction failure to a traditional complete PSA response. Although encouraged by 5 such responses to the addition of abiraterone acetate, this result did not reach the protocol prespecified level of 6 responses.
The treatment paradigm for hormone-sensitive mPC has changed since the initiation of S1014, based on data from the Chemohormonal Therapy Vs Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) study. In CHAARTED, 790 men with hormone-sensitive mPC were randomized to ADT with or without 6 cycles of docetaxel chemotherapy, with a primary objective of improvement in the OS. As reported in 2015, there was a 13.6-month improvement in the OS with the addition of docetaxel, from 44.0 to 57.6 months (HR, 0.61; 95% CI, 0.47-0.80; P < .001). In this study, the rate of attaining a PSA level lower than 0.2 ng/mL at 12 months was 16.7% with ADT vs 27.7% with the combination. Therefore, the improvement in OS correlated with the increased attainment of a PSA level lower than 0.2 ng/mL in CHAARTED, further validating the relevance of this end point. The British Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) trial of hormone-sensitive mPC was also recently published. In patients with metastatic (M1) disease, the addition of 6 cycles of docetaxel to ADT was associated with an improvement in the median OS from 43 to 65 months (HR, 0.73; 95% CI, 0.59-0.89; P = .002).
With the success of upfront chemotherapy, the earlier application of more-potent hormonal approaches (eg, abiraterone acetate) in the disease process needs additional investigation. The S1014 trial gives a signal of activity in a subset of the very high-risk patients studied here. Although the findings were not significant, a larger study or perhaps a less rigorous end point, such as a reduction of the PSA level by 50%, may have yielded a different outcome. The OS of the patients in S1014 was 25.8 months, which compares favorably with historical controls, including the 13-month OS in the S9346 patients with failure to achieve a PSA level less than 4.0 ng/mL on which S1014’s entry criteria were modeled. As shown in Figure 2A, there is a small group of highly responsive patients with a prolonged PFS and ongoing response to abiraterone acetate plus prednisone therapy, with a median follow-up of nearly 3 years, despite the very high-risk nature of this group. In addition, there appears to be a “tail” on the OS curve, suggesting a subset of patients with prolonged survival despite their high-risk nature. With this small sample, it is difficult to identify any specific clinical characteristics to differentiate these patients and identify any predictive clinical characteristics in those who respond. It is also clear that there are additional agents approved by the US Food and Drug Administration for treatment of mCRPC available for the patients in S1014 that were not available at the time of the S9346 trial. It remains unknown whether high-risk patients with mPC, including those with rapid progression while undergoing ADT, should initially be treated with agents such as abiraterone acetate or enzalutamide vs cytotoxic chemotherapy with the development of mCRPC. The data from S1014 demonstrate that, despite their high-risk nature, patients in this trial demonstrated clinically relevant responses to abiraterone acetate, supporting a trial of a next-generation hormonal therapy, such as abiraterone acetate, in this setting before chemotherapy in select patients.
The biologic mechanism of early resistance to ADT seen in the patients enrolled in S1014 is not clear. Since the initiation of S1014, the androgen receptor V7 (AR-V7) has been identified as a biomarker of response to abiraterone acetate and enzalutamide in patients with PC. This AR splice variant lacks the ligand binding domain, which is the target of these therapies and is constitutively active. In a study of 62 patients with mCRPC starting abiraterone acetate or enzalutamide therapy, the AR-V7–positive patients had a PSA response rate of 0% vs response rates of 53% and 68% with enzalutamide and abiraterone acetate treatment, respectively. AR-V7 does not appear to be a general marker of drug resistance and is not associated with complete resistance to docetaxel chemotherapy. The incidence of AR-V7 in patients with resistance to primary hormone induction therapy, including those treated in S1014, is not known. With ongoing technical advances in molecular testing, additional study of the biologic mechanisms contributing to the high-risk nature of these patients is needed.
The optimal upfront approach to treating hormone-sensitive mPC will be an area of ongoing investigation. With the CHAARTED and STAMPEDE data, the addition of docetaxel to ADT should be considered in chemotherapy-eligible men with hormone-sensitive mPC. Due to age, comorbidities, and patient preference, many men with hormone-sensitive mPC will not receive docetaxel. The upfront use of drugs such as abiraterone acetate or enzalutamide therapy in mPC, either in conjunction with chemotherapy or alone, needs further investigation. An ongoing trial of hormone-sensitive mPC, S1216 is comparing ADT plus orteronel (TAK-700) vs ADT plus bicalutamide. Orteronel is an experimental agent that acts as an androgen synthesis inhibitor of steroid 17α-monooxygenase (17,20 lyase) with possible antiandrogen activity as well. With the future clinical integration of AR-V7 testing, the ability to select AR-V7–negative patients who are most likely to benefit from testosterone-blocking agents in the upfront setting may also be possible. Data from the present study suggest activity with early abiraterone acetate therapy in this high-risk group of patients with mPC. This clinical trial also provides modern data on this high-risk group with primary ADT-refractory disease that may be used to inform additional study in this important patient subgroup.
Limitations
There are several limitations to this report. This was a single-arm intervention study, using a comparable subset of patients from the SWOG 9346 trial as a historical control. A larger, randomized study would provide a contemporary comparator to the intervention arm described here to better determine the benefit of abiraterone in this setting. The most appropriate PSA response surrogate in this hormone-induction failure group is also not known; the PSA CR level here (PSA<0.2 ng/mL), based on information from S9346, was ambitious, but a 50% PSA reduction, PSA normalization to 4 ng/mL, or other PSA reduction could also have been utilized. Biologic correlative studies were not performed in this study. The identification of biomarkers that predict which of these high-risk patients may respond to abiraterone acetate would also improve the clinical application of such an approach.
Conclusions
The S1014 clinical trial tested abiraterone acetate plus prednisone in patients with mPC who had a PSA level higher than 4.0 ng/mL after 6 to 12 months of ADT. There were 5 patients with a PSA level reduction to 0.2 ng/mL or lower, which is less than the 6 responses prespecified in the study for a positive result. However, the OS of patients in this study was 25.8 months and appears favorable compared with data in historical controls. Ongoing and future studies should evaluate the use of next-generation hormonal agents in the upfront setting and integration of such agents with chemotherapy for these patients.
eTable. Adverse Events
References
- 1.Sweeney CJ, Chen YH, Carducci M, et al. . Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain M, Tangen CM, Higano C, et al. ; Southwest Oncology Group Trial 9346 (INT-0162) . Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 2006;24(24):3984-3990. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. ; COU-AA-301 Investigators . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, et al. ; COU-AA-302 Investigators . Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taplin ME, Montgomery B, Logothetis CJ, et al. . Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32(33):3705-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oken MM, Creech RH, Tormey DC, et al. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Halabi S, Tannock I, et al. ; Prostate Cancer Clinical Trials Working Group . Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee . The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228-1242. [DOI] [PubMed] [Google Scholar]
- 11.Antonarakis ES, Lu C, Wang H, et al. . AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.clinicaltrials.gov. S1216, Phase III ADT+TAK-700 vs. ADT+Bicalutamide for Metastatic Prostate Cancer. NCT01809691. https://clinicaltrials.gov/ct2/show/NCT01809691 Updated February 23, 2017. Accessed February 23, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Adverse Events