This pooled data analysis evaluates ipsilateral breast tumor recurrence outcomes in women with ductal carcinoma in situ treated with vs without a radiotherapy boost after breast-conserving surgery and whole-breast radiotherapy.
Key Points
Question
Is the use of a radiotherapy boost to the tumor bed associated with improved local control in women with ductal carcinoma in situ treated with breast-conserving surgery and whole-breast radiotherapy?
Findings
In this analysis of 4131 patients, an a priori power analysis was used to estimate the sample size required to detect an outcome benefit of radiotherapy boost, and a pooled evaluation showed that a radiotherapy boost was associated with reduced in-breast tumor recurrence at 10 and 15 years. On multivariable analysis, the boost remained significantly associated with reduced in-breast tumor recurrence independent of age and tamoxifen citrate use.
Meaning
For patients with ductal carcinoma in situ and life expectancy of more than 10 to 15 years, the addition of a radiation boost dose may be considered to provide an incremental benefit in improving local control after whole-breast radiotherapy.
Abstract
Importance
The use of a radiotherapy (RT) boost to the tumor bed after whole-breast RT (WBRT) for ductal carcinoma in situ (DCIS) is largely extrapolated from invasive cancer data, but robust evidence specific to DCIS is lacking.
Objective
To compare ipsilateral breast tumor recurrence (IBTR) in women with DCIS treated with vs without the RT boost after breast-conserving surgery and WBRT.
Design, Setting, and Participants
This retrospective analysis pooled deidentified patient-level data from 10 academic institutions in the United States, Canada, and France from January 1, 1980, through December 31, 2010. All patients had newly diagnosed pure DCIS (no microinvasion), underwent breast-conserving surgery, and received WBRT with or without the boost with a minimum of 5 years of follow-up required for inclusion in the analysis. Given the limited events after WBRT, an a priori power analysis was conducted to estimate the DCIS sample size needed to detect the anticipated benefit of the boost. Data were uniformly recoded at the host institution and underwent primary and secondary reviews before analysis. Sample size calculations (ratio of patients who received the boost dose to those who did not, 2:1; α = .05; power = 80%) estimated that 2982 cases were needed to detect a difference of at least 3%. The final analysis included 4131 patients (2661 in the boost group and 1470 in the no-boost group) with a median follow-up of 9 years and media boost dose of 14 Gy. Data were collected from July 2011 through February 2014 and analyzed from March 2014 through August 2015.
Interventions
Radiotherapy boost vs no boost.
Main Outcomes and Measures
Ipsilateral breast tumor recurrence.
Results
The analysis included 4131 patients (median [SD] age, 56.1 [10.9] years; range, 24-88 years). Patients with positive margins, unknown estrogen receptor status, and comedo necrosis were more likely to have received an RT boost. For the entire cohort, the boost was significantly associated with lower IBTR (hazard ratio [HR], 0.73; 95% CI, 0.57-0.94; P = .01) and with IBTR-free survival (boost vs no-boost groups) of 97.1% (95% CI, 0.96-0.98) vs 96.3% (95% CI, 0.95-0.97) at 5 years, 94.1% (95% CI, 0.93-0.95) vs 92.5% (95% CI, 0.91-0.94) at 10 years, and 91.6% (95% CI, 0.90-0.93) vs 88.0% (95% CI, 0.85-0.91) at 15 years. On multivariable analysis accounting for confounding factors, the boost remained significantly associated with reduced IBTR (HR compared with no boost, 0.68; 95% CI, 0.50-0.91; P = .01) independent of age and tamoxifen citrate use.
Conclusions and Relevance
This patient-level analysis suggests that the RT boost confers a statistically significant benefit in decreasing IBTR across all DCIS age groups, similar to that seen in patients with invasive breast cancer. These findings suggest that a DCIS RT boost to the tumor bed could be considered to provide an added incremental benefit in decreasing IBTR after a shared discussion between the patient and her radiation oncologist.
Introduction
The incidence of ductal carcinoma in situ (DCIS) has steadily increased with widespread screening and advances in breast imaging. Despite its excellent prognosis relative to invasive breast cancer, DCIS remains a heterogeneous disease for which optimal treatment management is commonly debated. The use of whole-breast radiotherapy (WBRT) for DCIS is supported by level 1 data from randomized trials that consistently demonstrate an approximately 50% reduction in relative risk for ipsilateral breast tumor recurrence (IBTR), with very limited in-breast treatment failures with long-term follow-up. Because the benefit of WBRT is apparent in all DCIS subgroups, consistently defining subsets in which RT can be omitted remains a challenge. More recently, the use of WBRT in patients with higher-risk DCIS has been suggested to be associated with a statistically significant improvement in survival.
Thus, most patients with DCIS receive WBRT after local excision. A common practice after WBRT is delivery of an RT boost, whereby an additional 4 to 8 fractions of RT are directed to the tumor bed to allow for dose escalation to the region at highest risk for IBTR. The practice of boosting has been demonstrated to provide a modest but statistically significant reduction in IBTR risk for invasive cancers in all age groups. To date, no similar RT boost trials have been published for DCIS. Accordingly, the practice of using an RT boost in DCIS is largely extrapolated from the trials of invasive breast cancer.
Multiple challenges hinder the ability to demonstrate a similar significant benefit for the DCIS boost. These include the inherent heterogeneity of DCIS, the small number of IBTR events after excision and WBRT, and its long natural history, mandating large numbers of patients and lengthy follow-up. Furthermore, compared with invasive cancer, DCIS is associated with a lower incidence, delayed adoption of breast conservation therapy in some settings, a misconception that RT is not as effective for noninvasive cancer, and lack of awareness that local recurrence could lead to metastases and breast cancer−associated mortality. Thus, enrolling patients in DCIS trials remains a challenge. Several prospective randomized trials (BONBIS and BIG [Breast International Group] 3-07/TROG [Trans-Tasman Radiation Oncology Group] 07.01) are under way to assess use of RT boosts in the context of DCIS. Although these trials have completed accrual and are now in active follow-up, long-term results will likely not be available for a decade or longer.
This study evaluates IBTR outcomes in women with DCIS treated with vs without an RT boost after breast-conserving surgery and WBRT by using a large multi-institutional database that was assembled de novo. An a priori power analysis was conducted to estimate the sample size required to detect the anticipated benefit of the boost.
Methods
Data Set Assembly
Of 11 invited academic institutions across the United States, Canada, and France, 10 contributed to the final data set. The centers included the British Columbia Cancer Agency (Victoria, British Columbia, Canada), Dana Farber Cancer Institute (Boston, Massachusetts), Institut Curie (Paris, France), McGill University Health Center and University of Montreal (Montreal, Quebec, Canada), University of Texas MD Anderson Cancer Center (Houston), Rutgers School of Medicine (New Brunswick, New Jersey), University of Pennsylvania (Philadelphia), Oakland University William Beaumont School of Medicine (Royal Oak, Michigan), and Yale University School of Medicine (New Haven, Connecticut). Institutional review board approvals and data sharing agreements were obtained per each institution’s requirements. Because patient data were deidentified in these databases, the institutional review boards waived the need for informed consent.
To ensure the integrity of each data set combined into the master database, primary investigator(s) (all breast specialists and/or experts identified by individual institutions) verified uniformly compiled variables coded per protocol and cross-referenced patients’ medical records and pathology reports with protocol variables. Each data set was deidentified to maintain confidentiality and underwent secondary central review by the principal investigator (M.S.M.) before merging into the master database (Yale University). Missing data variables were returned to primary investigator(s) for additional record review.
Study Participants
The protocol inclusion criteria specified pure DCIS treated with breast-conserving surgery and WBRT (conventionally fractionated, 45.0-50.4 Gy in 25- to 28-Gy fractions; hypofractionated, 39.0-44.0 Gy in 13- to 16-Gy fractions) from January 1, 1980, through December 31, 2010, with at least 5 years of follow-up, with or without an electron or photon boost (10-16 Gy). (To convert Gy to rads, multiply by 100.) To account for variability in margin definitions across institutions, margins were quantified (ie, 1.0 mm, 2.0 mm). Exclusion criteria included brachytherapy boost, microinvasion (≤1.0 mm of invasive cancer), or accelerated partial breast irradiation.
Statistical Analysis
Data were collected from July 2011 through February 2014 and analyzed from March 2014 through August 2015. We conducted an a priori power analysis using the 10-year IBTR as the primary end point to estimate sample size, approximating a sample ratio of patients in the boost group to the no-boost group of 2:1, α significance level of .05, power of 80%, IBTR 10-year risk of 10% for the no-boost cohort, and a 3% difference between the boost and no-boost groups (10-year IBTR, 7% vs 10%). This estimation was based on a conservative underestimation of approximately 4% to 5% benefit derived from boost data for invasive cancer and 3% benefit from the ongoing BONBIS trial. With use of these variables, at least 2982 patients (1988 in the boost group and 994 in the no-boost group) would be required. The clinicopathologic features and use of RT boost were correlated using χ2 analysis. The primary outcome was IBTR, reported as IBTR-free survival (date of RT completion to IBTR or date last seen). Among patients who experienced IBTR, mastectomy was the standard local treatment. Univariate analysis (UVA) and multivariable analysis (MVA) of IBTR-free survival were performed using Cox proportional hazards regression modeling and incorporating prognostic features, including age, margin, grade, and tamoxifen citrate use. Patients were censored at the date of IBTR or the date of last follow-up. The missing-indicator method was used to handle missing data. The reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Statistical analysis was conducted by faculty in the Department of Biostatistics, Yale School of Public Health (Y.Z. and S.M.) by using SAS (version 9.3; SAS Software Inc).
Results
The final cohort consisted of 4131 patients with DCIS (2661 in the boost group and 1470 in the no-boost group; median [SD] age, 56.1 [10.9] years; range, 24-88 years), exceeding the sample size estimation by 39%. For this cohort, median follow-up was 9 years and the median boost dose was 14 Gy. The median year of recruitment for the study cohort was 2001 (range, 1980-2010). The age distribution was wide, with 1301 (31.5%) diagnosed at younger than 50 years and 503 (12.2%) at 70 years or older. Based on the National Surgical Adjuvant Breast Project (NSABP) B-17 definition of a negative margin (no ink on tumor), the final margin status was reported as positive (ink on tumor) in 168 patients (4.1%), as unknown in 352 (8.5%), and categorically in varying degrees of negative, with 3026 (73.3%) reporting negative margin widths of at least 2 mm. Nearly two-thirds of the patients were classified with an intermediate or high grade (grade II or III component, 2817 [68.2%]), and only 620 (15.0%) were reported as unknown. The median age at diagnosis was 56.2 years for the no-boost group and 56.0 years for the boost group. We found no difference in the distribution of boost and no-boost cohorts with respect to age (≥50 years, 1801 [67.7%] and 1029 [70.0%], respectively) or grade (grades II and III, 1842 [69.2%] and 975 [66.3%], respectively; P > .05 for all), but the 2 cohorts differed in positive margin status (boost cohort, 124 [4.7%]; no-boost cohort, 44 [3.0%]; P < .001), presence of comedo necrosis (boost cohort, 1031 [38.7%]; no-boost cohort, 270 [18.4%]; P = .03), and positive estrogen receptor (ER) status (boost cohort, 955 [35.9%]; no-boost cohort, 287 [19.5%]; P = .04). Furthermore, although differences in the proportion receiving the RT boost were balanced when analyzed by tumor size of no more than 1.0 cm (1051 [39.5%]) vs greater than 1.0 cm (1051 [38.1%]; P = .08), difference in size was significant using the larger size criteria of no more than 2.0 cm (1692 [63.6%]) vs greater than 2.0 cm (374 [14.1%]; P = .006). These patient characteristics are summarized in Table 1.
Table 1. Distribution of Patients by Characteristics and Delivery of Radiotherapy Boost.
| Characteristic | No. (%) of Patientsa | P Valueb | ||
|---|---|---|---|---|
| All (N = 4131) |
No-Boost Group (n = 1470) |
Boost Group (n = 2661) |
||
| Age, y | ||||
| Median | 56.1 | 56.2 | 56.0 | NA |
| SD | 10.9 | 10.7 | 11.0 | |
| Age, y | ||||
| <50 | 1301 (31.5) | 441 (30.0) | 860 (32.3) | .12 |
| ≥50 | 2830 (68.5) | 1029 (70.0) | 1801 (67.7) | |
| DCIS grade | ||||
| I | 694 (16.8) | 250 (17.0) | 444 (16.7) | .48 |
| II or III | 2817 (68.2) | 975 (66.3) | 1842 (69.2) | |
| Unknown | 620 (15.0) | 245 (16.7) | 375 (14.1) | |
| Tumor size, cm | ||||
| ≤1.0 | 1659 (40.2) | 608 (41.4) | 1051 (39.5) | .08 |
| >1.0 | 1680 (40.7) | 665 (45.2) | 1015 (38.1) | |
| ≤2.0 | 2685 (65.0) | 993 (67.6) | 1692 (63.6) | .006 |
| >2.0 | 654 (15.8) | 280 (19.0) | 374 (14.1) | |
| Unknown | 792 (19.2) | 197 (13.4) | 595 (22.4) | |
| NSABP-defined margin status | ||||
| Positive, ink on tumor | 168 (4.1) | 44 (3.0) | 124 (4.7) | <.001 |
| Negative, no ink on tumor | 3611 (87.4) | 1285 (87.4) | 2326 (87.4) | |
| Unknown | 352 (8.5) | 141 (9.6) | 211 (7.9) | |
| SSO/ASTRO/ASCO–defined margin status | ||||
| Positive, <2 mm | 686 (16.6) | 189 (12.9) | 497 (18.7) | <.001 |
| Negative, ≥2 mm | 3026 (73.3) | 1131 (76.9) | 1895 (71.2) | |
| Unknown | 419 (10.1) | 150 (10.2) | 269 (10.1) | |
| Comedo necrosis | ||||
| Absent | 1066 (25.8) | 262 (17.8) | 804 (30.2) | .03 |
| Present | 1301 (31.5) | 270 (18.4) | 1031 (38.7) | |
| Unknown | 1764 (42.7) | 938 (63.8) | 826 (31.0) | |
| Estrogen receptor status | ||||
| Negative | 296 (7.2) | 85 (5.8) | 211 (7.9) | .04 |
| Positive | 1242 (30.1) | 287 (19.5) | 955 (35.9) | |
| Unknown | 2593 (62.8) | 1098 (74.7) | 1495 (56.2) | |
| Tamoxifen citrate use | ||||
| Yes | 1073 (26.0) | 271 (18.4) | 802 (30.1) | <.001 |
| No | 2631 (63.7) | 1111 (75.6) | 1520 (57.1) | |
| Unknown | 427 (10.3) | 88 (6.0) | 339 (12.7) | |
Abbreviations: DCIS, ductal carcinoma in situ; NA, not applicable; NSABP, National Surgical Adjuvant Breast Project; SSO/ASTRO/ASCO, Society of Surgical Oncology/American Society of Radiation Oncology/American Society of Clinical Oncology.
Percentages have been rounded and may not total 100.
Calculated using the χ2 test.
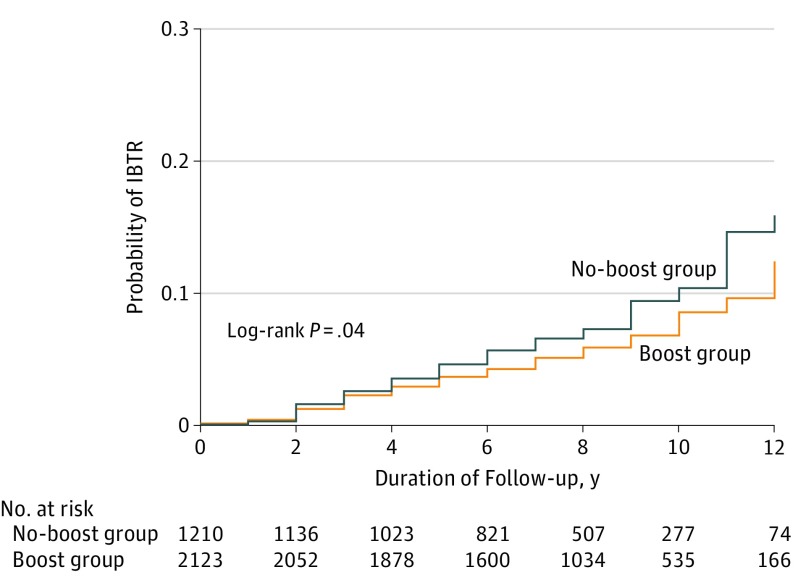
For the entire cohort, 253 IBTR events (6.1%) occurred, of which 118 (46.6%) were invasive, resulting in IBTR-free survival of 96.8% at 5 years, 93.6% at 10 years, and 90.4% at 15 years. When stratifying patients by receipt of the RT boost, the IBTR-free survival for the boost vs no-boost groups was 97.1% (95% CI, 0.96-0.98) vs 96.3% (95% CI, 0.95-0.97) at 5 years; 94.1% (95% CI, 0.93-0.95) vs 92.5% (95% CI, 0.91-0.94) at 10 years, and 91.6% (95% CI, 0.90-0.93) vs 88% (95% CI, 0.85-0.91) at 15 years (log-rank test, P = .04) (Figure 1).
Figure 1. Comparison of Ipsilateral Breast Tumor Recurrence (IBTR) by Treatment Group.
Patients were stratified by those who received a radiotherapy boost (boost group) vs those who did not (no-boost group).
On univariate analysis, the use of the RT boost was associated with reduced IBTR compared with the no-boost group (hazard ratio [HR], 0.73; 95% CI, 0.57-0.94; P = .01). Analysis of age as a dichotomous variable consistently demonstrated that increased age was significantly associated with lower IBTR (HR for ≥50 vs 50 years, 0.54; 95% CI, 0.41-0.71; P < .001; HR for ≥60 vs <60 years, 0.61; 95% CI, 0.46-0.81; P < .001; and HR for ≥70 vs <70 years, 0.57; 95% CI, 0.35-0.93; P = .02). Additional characteristics associated with lower IBTR were grade I (HR vs grade II or III, 1.63; 95% CI, 1.09-2.45; P = .02); ER positive (HR vs ER negative, 0.49; 95% CI, 0.29-0.82; P = .007), negative margins (HR vs positive margins, 1.64; 95% CI, 0.94-2.70; P = .07; and HR vs unknown, 2.35; 95% CI, 1.68-3.10; P < .001), and tamoxifen use (HR vs no tamoxifen, 0.52; 95% CI, 0.36-0.75; P < .001). To account for these confounding factors and adjust for potential treatment biases influenced by differences in clinicopathologic features, the significant variables from the univariate analysis were incorporated into the multivariable model. On multivariable analysis, the RT boost remained independently associated with decreasing IBTR when accounting for these potential confounders (P = .01) (Table 2). Other variables that remained significant on multivariable analysis included grade, necrosis, margins, age (<50 vs ≥50 years), tumor size (as a continuous variable), and tamoxifen use. In addition, the interactions between age and boost (HR for age ≥50 years, 0.64; 95% CI, 0.45-0.92; HR for age <50 years, 0.77; 95% CI, 0.53-1.11; P = .46) and between margin status and boost (HR for negative margin status, HR: 0.55; 95% CI, 0.41-0.76; HR for positive margin status, 0.93; 95% CI, 0.30-2.93; P = .31) were independently tested using log-rank multivariable ratio tests and were not found to be statistically significant.
Table 2. Cox Proportional Hazards Regression Multivariable Analysis of Ipsilateral Breast Tumor Recurrence.
| Patient Characteristic | HR (95% CI) | P Value |
|---|---|---|
| Age, y | ||
| <50 | 1 [Reference] | NA |
| ≥50 | 0.54 (0.41-0.71) | <.01 |
| DCIS grade | ||
| I | 1 [Reference] | NA |
| II or III | 1.59 (1.02-2.46) | .04 |
| Unknown | 1.56 (0.93-2.59) | .09 |
| Tumor size (continuous variable) | 1.15 (1.02-1.29) | .03 |
| Margin status | ||
| Negative | 1 [Reference] | NA |
| Positivea | 1.72 (0.97-3.05) | .06 |
| Unknown | 1.65 (1.13-2.41) | .01 |
| Comedo necrosis | ||
| No | 1 [Reference] | NA |
| Yes | 1.12 (0.78-1.62) | .53 |
| Unknown | 0.51 (0.34-0.77) | <.01 |
| Estrogen receptor status | ||
| Negative | 1 [Reference] | NA |
| Positive | 0.7 (0.39-1.25) | .23 |
| Unknown | 0.85 (0.52-1.38) | .51 |
| Tamoxifen citrate use | ||
| No | 1 [Reference] | NA |
| Yes | 0.56 (0.36-0.87) | .01 |
| Unknown | 0.93 (0.53-1.63) | .81 |
| RT boost | ||
| No | 1 [Reference] | NA |
| Yes | 0.68 (0.5-0.91) | .01 |
Abbreviations: DCIS, ductal carcinoma in situ; HR, hazard ratio; NA, not applicable; RT, radiotherapy.
Indicates the National Surgical Adjuvant Breast Project B-17 definition of ink on tumor or 0-mm margin width. The log-rank ratio tested the interaction between age and boost (P = .46), which was not statistically significant.
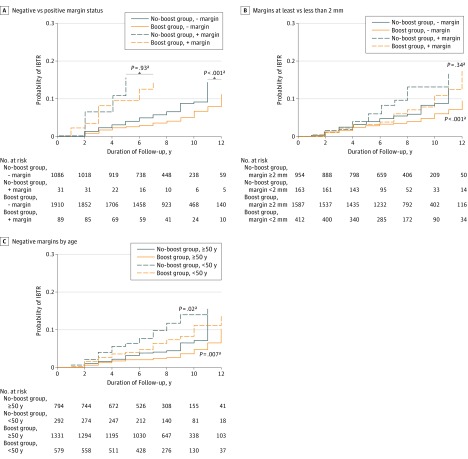
We also conducted an exploratory analysis of IBTR for the boost vs nonboost groups in subsets stratified by margin status, age thresholds, comedo necrosis, and ER status. We found no significant associations among IBTR, the RT boost, and the presence (or absence) of necrosis (HR for present, 0.71; 95% CI, 0.45-1.10; P = .13; HR for absent, 0.85; 95% CI, 0.46-1.56; P = .59) or ER status (HR for negative, 0.64; 95% CI, 0.21-1.88; P = .41; HR for positive, 0.78; 95% CI, 0.34-1.76; P = .54). Although the boost remained significantly beneficial with negative margins (HR, 0.54; 95% CI, 0.39-0.75; P < .001), it was not found to be significantly beneficial in any subsets of patients with positive margins (168 patients; HR for boost vs no boost, 0.95; 95% CI, 0.30-3.05; P = .93) (Figure 2A). Analysis of boost receipt in all patients with negative margins stratified by age (<50 vs ≥50 years) (Figure 2C) demonstrated the boost to be significant for decreasing IBTR across younger and older groups (HR for age <50 years, 0.54; 95% CI, 0.33-0.90; P = .02; HR for age ≥50 years, 0.56; 95% CI, 0.36-0.85; P = .007).
Figure 2. Radiotherapy (RT) Boost by Patient Characteristic.
Patients are compared between those who received an RT boost (boost group) vs those who did not (no-boost group), with additional stratification by characteristics. A, Negative (−) margin status indicates the National Surgical Adjuvant Breast Project B-17 definition of no ink on tumor; positive (+) margin status, ink on tumor. B, Margins were stratified using the Society of Surgical Oncology/American Society of Radiation Oncology definition of at least 2.0 vs less than 2.0 mm. The benefit of the RT boost in decreasing ipsilateral breast tumor recurrence (IBTR) is apparent in patients with margins of at least 2.0 mm but is not significant in patients with margins of less than 2.0 mm. C, Patients with negative margins were stratified by those younger than 50 years vs 50 years or older. IBTR estimates are significantly lower with the delivery of an RT boost relative to no boost.
aP value was calculated with control by confounders.
Last, owing to variations in definitions of negative margins for DCIS, this analysis was also conducted in its entirety using the recent Society of Surgical Oncology/American Society of Radiation Oncology/American Society of Clinical Oncology (SSO/ASTRO/ASCO) definition of at least 2 mm for a negative margin vs margins of less than 2 mm. The boost was significantly associated with reduced IBTR in the cohort with a negative margin of at least 2.0 mm (HR, 0.70; 95% CI, 0.54-0.91; P < .001) and not significant in the cohort with a margin of less than 2.0 mm (HR, 0.60; 95% CI, 0.24-1.50; P = .34) (Figure 2B). Boost use remained significant on univariate analysis (HR, 0.73; 95% CI, 0.57-0.94; P = .01) and multivariable analysis (HR, 0.70; 95% CI, 0.54-0.92; P = .01) irrespective of the different DCIS negative margin definition. The interaction between boost use and margin status using the SSO/ASTRO/ASCO definition was not found to be statistically significant by log-rank multivariable ratio test (HR for negative margin status, 0.54; 95% CI, 0.39-0.77; HR for positive margin status, 0.78; 95% CI, 0.42-1.48; P = .17).
Discussion
Whole-breast radiation therapy delivered after breast-conserving surgery for DCIS consistently results in a decrease in IBTR of approximately 50% and is supported by multiple phase 3 trials with long-term follow-up. Although boosting the tumor bed is a common practice, a paucity of data supports its use in the setting of DCIS, and its rationale is extrapolated from invasive cancer trials. The largest trial among these is the European Organization for Research and Treatment of Cancer (EORTC) 22881 trial, which included 5569 patients, 5318 of whom had negative margins defined by local pathologic findings and who were randomized to a 16-Gy boost vs nonboost groups after WBRT. In the most recent 20-year follow-up report, the cumulative IBTR incidence was 12% with a boost vs 16.4% without a boost. Despite an absolute difference of only 4% at 20 years, the EORTC 22881 study highlights the importance and clinical value that incrementally small decreases in IBTR provide for the patient: at 10 years, mastectomy for recurrence was decreased by approximately 40% in patients who had received a boost (compared with those who did not). Most recently, a reanalysis of a subset of patients treated with long-term follow-up further elucidated that being younger and having high-grade invasive cancer were the most important risk factors for recurrence in this trial.
Given these benefits of the boost for invasive cancers, many radiation oncologists similarly use the RT boost in the context of DCIS, with a belief in its potential to similarly provide a small additional benefit in decreasing IBTR for DCIS and thus mitigate risks and emotional implications of recurrence and mastectomy after a DCIS diagnosis. In particular, given that approximately 50% of DCIS recurrences are invasive in nature, with the potential for regional and distant disease progression, any incremental benefit in decreasing IBTR likely has the potential to significantly affect individual patients. Thus, we are not entirely surprised that the benefits of the RT boost in DCIS appear to similarly parallel what has been demonstrated across all age groups and grades for invasive breast cancers: the estimated absolute benefit of 3.6% at 15 years (HR, 0.70; 95% CI, 0.54-0.92) for the use of a boost in DCIS is similar in magnitude to the most current update of the EORTC trial demonstrating an absolute benefit of 4.4% at 20 years (HR, 0.65; 95% CI, 0.52-0.81) for a boost in invasive cancer.
Our study differs from the current existing (retrospective) literature in that an a priori power estimation was conducted to estimate the total sample size and proportion of patients required to detect the estimated difference in IBTR between the boost and no-boost groups. Our threshold of at least 3% was a conservative underestimation of the approximately 4% to 5% benefit derived from the EORTC invasive-boost vs –no-boost trial and was derived from the 3% benefit anticipated for the ongoing BONBIS trial. With more than 4000 patients in the final analysis, this cohort exceeded the sample size power estimation by 39% with a median follow-up longer than that of most existing published studies. With these considerations, this study demonstrates the anticipated small but statistically significant benefit in decreasing long-term IBTR with a boost for DCIS of a similar degree experienced with invasive cancers. The use of a boost was significantly associated with decreasing IBTR across the entire cohort. In addition, this benefit was independent of tamoxifen use and was significant across all age groups, although the magnitude of the benefit appears to be greater in younger patients (similar to the boost data with invasive cancer). Of reassurance, the other variables significantly associated with IBTR on multivariable analysis (such as grade) are consistent with established prognostic factors in DCIS that have been previously described in the literature.
Defining what constitutes an adequate surgical margin in patients with DCIS has been controversial. In a meta-analysis of 4660 patients with DCIS treated with breast-conserving surgery and postoperative RT, Dunne et al reported that a 2-mm margin was superior to a margin of less than 2.0 mm. In the current analysis, the use of RT boost was significantly associated with decreased IBTR across the entire cohort but, on subset analysis, did not achieve significance in the positive margin subgroup irrespective of positive margin definition of ink on tumor or the SSO/ASTRO/ASCO definition of margins of less than 2.0 mm. Similar to other published negative studies of RT boost in DCIS, the lack of benefit with a boost in the subgroup with positive margins is likely related to the small sample size of this cohort, which represented less than 4% of the entire cohort and thus was substantially underpowered to detect a significant benefit. Thus, the results in the positive-margin cohort should be interpreted with caution.
The challenge in demonstrating a statistically significant benefit for the RT boost in DCIS is understandable given the excellent outcomes of DCIS with WBRT. To date, available data assessing the benefit of a boost in patients with DCIS are limited to single-institution, registry, and multiple-center retrospective reviews; the existing published studies are summarized in Table 3. Each study failing to demonstrate a significant benefit in the RT boost for DCIS can be rationalized by small cohort size (lack of statistical power), inadequate proportions of patients in the boost vs no-boost groups, limited follow-up, or a combination of these factors.
Table 3. Studies Reporting Outcomes of DCIS by Receipt of Boost vs No Boost.
| Source | Total No. of Patients (Patients Receiving Boost, %) | Median Follow-up, y | Effect of Boost on IBTR |
|---|---|---|---|
| Omlin et al, 2006 a | 316 (52.0) | 6.0 | Yes |
| Yerushalmi et al, 2006 |
75 (25.0) | 6.8 | No |
| Jiveliouk et al, 2009 | 107 (37.3) | 4.0 | No |
| Monteau et al, 2009b | 208 (71.0) | 7.4 | Yes |
| Wai et al, 2011 | 482 (29.8) | 9.3 | No |
| Rakovitch et al, 2013 | 1895 (29.6) | 10.0 | No |
| Meattini et al, 2013 | 389 (48.8) | 7.7 | Yes |
| Wong et al, 2012 | 220 (36.0) | 3.8 | Yes |
| Kim, et al 2014 | 728 (31.9) | 6.7 | No |
| Cutuli et al, 2016 | 819 (48.0) | 7.5 | No |
| Present study | 4131 (64.4) | 9.0 | Yes |
Abbreviations: DCIS, ductal carcinoma in situ; IBTR, ipsilateral breast tumor recurrence.
Included only patients with DCIS younger than 45 years.
Cohort included patients with DCIS with close and focally positive margins.
Limitations and Strengths
Although we wholly recognize that the retrospective nature of our study is a fundamental limitation, our collaborative effort attempted to improve on the quality of existing published data by only including data sets from large, academic institutions that underwent quality reviews by at least 2 different breast specialists (from contributing and receiving institutions) to ensure its integrity. We believe that the use of individual patient-level data from academic institutions with breast cancer expertise (radiation oncology and pathology), the review of each institutions’ data sets by assigned primary investigators, and the secondary central data review have ultimately provided a more valuable data set than that of a simple retrospective review or population-based data set, which have significant variability in contributions. Another limitation, the inherently nonrandomized selection of the boost vs no-boost groups, was believed to be at risk for confounding by clinicopathologic variables across institutions and physicians. To address this, the multivariable analytic regression model incorporated all variables that were significant on univariate analysis. Furthermore, although differences in delivery techniques are inevitable over time and across institutions and physicians, our current knowledge of the risks and benefits of breast RT suggest that any such differences should only affect toxicity (ie, fibrosis, telangiectasias) and not efficacy outcomes. Our protocol specified that patients included in the final analysis receive standardized doses and boost techniques and thus receive uniform treatment with regard to the study question concerning the benefit of an RT boost on IBTR. Published literature on the use of a boost (in similar median dose ranges) in addition to individual radiation oncologists’ clinical experiences with the use of a boost have already established the additional risk for fibrosis when an RT boost is delivered after WBRT.
Last, although the suboptimal reporting of clinical variables such as ER status and tamoxifen use is inherent to retrospective data collection, the proportions of missing data in our database are consistent (or better) with those of population-based data sets such as the Surveillance, Epidemiology, and End Results (SEER) program. To handle missing data, we assumed that the choice of delivering the RT boost (vs no boost) did not heavily depend on the missing variables and therefore chose the missing-indicator method, which is commonly used for large data set analysis, particularly because cancer registries such as SEER routinely include an unknown category. Notwithstanding these limitations, the present study represents the largest series, to our knowledge, to assess DCIS outcomes with and without an RT boost and demonstrates the significant decrease in IBTR with the use of the RT boost for DCIS of a scale similar to that demonstrated with the RT boost for invasive cancer.
Conclusions
With acknowledgment of the limitations of the retrospective study design, this study suggests that an RT boost for DCIS after WBRT is associated with a small but statistically significant benefit in decreasing long-term IBTR in magnitude similar to that experienced with invasive cancer boost. Given the small numbers of patients with positive margins in the present study, the effect of the RT boost for DCIS specifically for positive margins remains inconclusive. Ultimately, DCIS treatment decisions are complex and need to be tailored to the patient’s age, clinicopathologic features, tumor biology, individualized preferences, and anticipated longevity. For patients who have a life expectancy of more than 10 to 15 years and in whom WBRT is part of the treatment plan, the addition of an RT boost to the tumor bed should be considered to provide an added incremental benefit in decreasing IBTR.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61(6):409-418. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12(1):21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26(8):1247-1252. [DOI] [PubMed] [Google Scholar]
- 5.Bijker N, Meijnen P, Peterse JL, et al. ; EORTC Breast Cancer Cooperative Group; EORTC Radiotherapy Group . Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381-3387. [DOI] [PubMed] [Google Scholar]
- 6.Sagara Y, Freedman RA, Vaz-Luis I, et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: a population-based longitudinal cohort study. J Clin Oncol. 2016;34(11):1190-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25(22):3259-3265. [DOI] [PubMed] [Google Scholar]
- 8.Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15(3):963-968. [DOI] [PubMed] [Google Scholar]
- 9.Polgár C, Fodor J, Orosz Z, et al. Electron and high-dose-rate brachytherapy boost in the conservative treatment of stage I-II breast cancer first results of the randomized Budapest boost trial. Strahlenther Onkol. 2002;178(11):615-623. [DOI] [PubMed] [Google Scholar]
- 10.ClinicalTrials.gov Breast-Conserving Surgery and Whole-Breast Radiation Therapy With or Without Additional Radiation Therapy to the Tumor in Treating Women With Ductal Carcinoma in Situ. NCT00907868. https://clinicaltrials.gov/ct2/NCT00907868. Accessed August 2015.
- 11.ClinicalTrials.gov Radiation Doses and Fractionation Schedules in Non-low Risk Ductal Carcinoma In Situ (DCIS) of the Breast (DCIS). NCT00470236. https://clinicaltrials.gov/ct2/NCT00470236. Accessed August 2015.
- 12.Azria D, Cowen D, Bourgier C, et al. Phase III randomized French multicentric study to evaluate the impact of a localized 16-Gy boost after conservative surgery and a 50-Gy whole-breast irradiation in breast ductal carcinoma in situ (the BONBIS trial). J Clin Oncol. 2011;29(15)(Suppl): Abstract TPS131. [Google Scholar]
- 13.ClinicalTrials.gov. Radiation doses and fractionation schedules in non-low risk ductal carcinoma in situ (DCIS) of the breast. NCT00472023. https://clinicaltrials.gov/ct2/show/NCT00470236. Accessed September 9, 2016.
- 14.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27(10):1615-1620. [DOI] [PubMed] [Google Scholar]
- 15.Groenwold RHH, White IR, Donders AR, Carpenter JR, Altman DG, Moons KG. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184(11):1265-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016;23(12):3801-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartelink H, Maingon P, Poortmans P, et al. ; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups . Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16(1):47-56. [DOI] [PubMed] [Google Scholar]
- 18.Vrieling C, van Werkhoven E, Maingon P, et al. ; European Organisation for Research and Treatment of Cancer, Radiation Oncology and Breast Cancer Groups . Prognostic factors for local control in breast cancer after long-term follow-up in the eortc boost vs no boost trial: a randomized clinical trial. JAMA Oncol. 2017;3(1):42-48. [DOI] [PubMed] [Google Scholar]
- 19.Ringberg A, Idvall I, Fernö M, et al. Ipsilateral local recurrence in relation to therapy and morphological characteristics in patients with ductal carcinoma in situ of the breast. Eur J Surg Oncol. 2000;26(5):444-451. [DOI] [PubMed] [Google Scholar]
- 20.Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186(4):337-343. [DOI] [PubMed] [Google Scholar]
- 21.Omlin A, Amichetti M, Azria D, et al. Boost radiotherapy in young women with ductal carcinoma in situ: a multicentre, retrospective study of the Rare Cancer Network. Lancet Oncol. 2006;7(8):652-656. [DOI] [PubMed] [Google Scholar]
- 22.Yerushalmi R, Sulkes A, Mishaeli M, et al. Radiation treatment for ductal carcinoma in situ (DCIS): is a boost to the tumor bed necessary? Neoplasma. 2006;53(6):507-510. [PubMed] [Google Scholar]
- 23.Jiveliouk I, Corn B, Inbar M, Merimsky O. Ductal carcinoma in situ of the breast in Israeli women treated by breast-conserving surgery followed by radiation therapy. Oncology. 2009;76(1):30-35. [DOI] [PubMed] [Google Scholar]
- 24.Monteau A, Sigal-Zafrani B, Kirova YM, et al. Ductal carcinoma in situ of the breast with close or focally involved margins following breast-conserving surgery: treatment with reexcision or radiotherapy with increased dosage. Int J Radiat Oncol Biol Phys.2009;75(4):1021-1028. [DOI] [PubMed] [Google Scholar]
- 25.Wai ES, Lesperance ML, Alexander CS, et al. Effect of radiotherapy boost and hypofractionation on outcomes in ductal carcinoma in situ. Cancer. 2011;117(1):54-62. [DOI] [PubMed] [Google Scholar]
- 26.Rakovitch E, Narod SA, Nofech-Moses S, et al. Impact of boost radiation in the treatment of ductal carcinoma in situ: a population-based analysis. Int J Radiat Oncol Biol Phys. 2013;86(3):491-497. [DOI] [PubMed] [Google Scholar]
- 27.Meattini I, Livi L, Franceschini D, et al. Role of radiotherapy boost in women with ductal carcinoma in situ: a single-center experience in a series of 389 patients. Eur J Surg Oncol. 2013;39(6):613-618. [DOI] [PubMed] [Google Scholar]
- 28.Wong P, Lambert C, Agnihotram RV, David M, Duclos M, Freeman CR. Ductal carcinoma in situ: the influence of the radiotherapy boost on local control. Int J Radiat Oncol Biol Phys. 2012;82(2):e153-e158. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Choi DH, Park W, et al. Influence of boost radiotherapy in patients with ductal carcinoma in situ breast cancer: a multicenter, retrospective study in Korea (KROG 11-04). Breast Cancer Res Treat. 2014;146(2):341-345. [DOI] [PubMed] [Google Scholar]
- 30.Cutuli B, Wiezzane N, Palumbo I, et al. Breast-conserving treatment for ductal carcinoma in situ: impact of boost and tamoxifen on local recurrences. Cancer Radiother. 2016;20(4):292-298. [DOI] [PubMed] [Google Scholar]