Key Points
Question
Is proton beam radiotherapy (PBT) safe and effective in the long term to treat unresectable stage III non–small cell lung cancer (NSCLC)?
Findings
This open-label, single-group assignment phase 2 study evaluates survival, toxic effects, and patterns of treatment failure with concurrent chemotherapy and high-dose PBT in 64 patients with unresectable stage III NSCLC. This management was found to be efficacious with promising rates of grade 3 and above toxic effects as compared with historical photon therapy data.
Meaning
Concurrent chemotherapy and high-dose PBT may be an important option with which to definitively treat locally advanced NSCLC.
This open-label, single-group assignment phase 2 study reports 5-year results evaluating concurrent chemotherapy and high-dose proton beam radiotherapy to treat unresectable stage III non–small cell lung cancer.
Abstract
Importance
Proton beam radiotherapy (PBT) has the potential to reduce toxic effects in the definitive management of locally advanced non–small cell lung cancer (NSCLC), but long-term prospective data are lacking.
Objective
To report the final (5-year) results of a prospective study evaluating concurrent chemotherapy and high-dose PBT to treat unresectable stage III NSCLC.
Design, Setting, and Participants
In this open-label, single-group assignment study, with median follow-up of 27.3 months for all patients and 79.6 months for survivors, 64 patients were enrolled and analyzed; inclusion criteria were unresectable IIIA/IIIB histologically confirmed NSCLC, Karnofsky performance status 70 to 100, and 6-month prediagnosis weight loss of no more than 10%. Staging used positron emission tomography and/or computed tomography. Induction chemotherapy was allowed.
Interventions
Concurrent chemotherapy (carboplatin-paclitaxel) and passively scattered PBT (74-Gy relative biological effectiveness) in all patients.
Main Outcomes and Measures
Kaplan-Meier analysis of overall survival (OS), progression-free survival (PFS), actuarial distant metastasis, and locoregional recurrence. Patterns of treatment failure were categorized as local/regional or distant. Acute and late toxic effects were prospectively assigned using Common Terminology Criteria for Adverse Events, v3.0.
Results
Of 64 patients (22 [34%] female; median [range] age, 70 [37-78] years; stage IIIA, 30 [47%]; IIIB, 34 [53%]), 17 (27%) were alive at last follow-up. Median OS was 26.5 months (5-year OS, 29%; 95% CI, 18%-41%). Five-year PFS was 22% (95% CI, 12%-32%); 5-year actuarial distant metastasis and locoregional recurrence were 54% (n = 36) and 28% (n = 22), respectively. Treatment failures were largely (31 [48%] patients) distant, with low rates of crude local (10 [16%]) and regional (9 [14%]) recurrences. Rates of grade 2 and 3 acute esophagitis were 18 (28%) and 5 (8%), respectively. Acute grade 2 pneumonitis occurred in 1 (2%) patient. Late toxic effects were uncommon: 1 (2%) patient developed an esophageal stricture (grade 2) and 1 (2%) grade 4 esophagitis. Late grades 2 and 3 pneumonitis occurred in 10 (16%) and 8 (12%), respectively. Two (3%) patients developed a bronchial stricture (grade 2), and 1 (2%) a grade 4 bronchial fistula. There were no acute or late grade 5 toxic effects.
Conclusions and Relevance
Concurrent chemotherapy and PBT to treat unresectable NSCLC afford promising clinical outcomes and rates of toxic effects compared with historical photon therapy data. Further optimization of proton therapy, particularly intensity-modulated proton therapy, is still needed.
Introduction
Non–small cell lung cancer (NSCLC) is a major health burden worldwide; importantly, both the disease—as well as its treatment—can cause symptomatic deterioration. In unresectable locally advanced disease, concurrent chemoradiotherapy (CRT) is recommended, but patients often find it difficult to tolerate because they frequently have comorbidities and suboptimal performance status.1,2
The use of proton beam therapy (PBT) is an attractive option with which to reduce toxic effects of CRT.3,4 The unique Bragg peak results in deposition of the highest dose within a targeted area with virtually no dose distally. Implications for lung cancer therapy include the ability to safely deliver the desired dose while maintaining low doses to cardiopulmonary structures, which may affect toxic effects, functional status, quality of life, and/or survival.
Retrospective analyses have demonstrated that PBT decreases the rates of severe pneumonitis and esophagitis as compared with 3-dimensional conformal radiotherapy (3DCRT) and intensity-modulated radiotherapy (IMRT), despite dose escalation from 60 to 74 Gy relative biological effectiveness (RBE).5,6 In addition, our institutional phase 2 study of concurrent chemotherapy and passively scattered PBT for unresectable stage III NSCLC yielded encouraging outcomes and toxicity profiles at 20-month follow-up.7 Herein, we present final results of a phase 2 open-label, single-group assignment study with long-term (median, 79.6 months) follow-up.
Methods
Complete methodology for this study, which was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board and ethics committee (ClinicalTrials.gov identifier: NCT00495170), is described elsewhere.7 All patients signed informed consent.
Briefly, inclusion criteria for this study were inoperable and histologically and/or cytologically proven stage III NSCLC, including negative systemic workup with positron emission tomography–computed tomography (PET-CT) and brain computed tomography (CT) or magnetic resonance imaging. Other criteria included Karnofsky performance status of 70 to 100, medically fit to receive concurrent and/or induction chemotherapy, weight loss no more than 10% in the 6 months before diagnosis, and ability to provide informed consent. Patients were not enrolled in cases of prior chest radiation therapy and/or pregnancy. Those with prior and/or concomitant malignant neoplasms were also not enrolled, with the exception of curatively treated cervical carcinoma in situ, cutaneous basal cell carcinoma, or vesical superficial transitional cell carcinoma.
Passively scattered proton therapy planning first commenced with a 4-dimensional CT simulation scan, from which the reconstructed maximum-intensity projection image, as well as 10 breathing phases, was used to contour an internal gross tumor volume.7,8 This was followed by the addition of an 8-mm isotropic expansion to construct the internal clinical target volume and edited clinically, followed by a 5-mm expansion to form the planning target volume (PTV) for evaluation purposes. However, the actual PTV for proton planning was individualized per beam and designed on the basis of density, depth, energy, and other factors.7 The dose distribution was calculated using the average 4-dimensional CT images. Elective nodal irradiation was not performed in this study. Dose constraints to organs-at-risk have been previously described.6 Using a prescription dose of 74 Gy (RBE) for all patients (assuming RBE of 1.1), the PTV was required to be covered with at least 95% of this prescribed dose. Prior to PBT delivery, compensators and apertures were designed for the final treatment plan to control the depth and lateral portions of the passively scattered proton beams, respectively. Treatment for each fraction was set up using orthogonal kilovolt x-ray images.
Sixteen of 64 (25%) patients underwent adaptive replanning therapy 2 to 5 weeks after initial proton therapy (median, 4 weeks). The most common reason for adaptive replanning was tumor shrinkage and/or anatomical changes. Most adaptive replanning involved beam energy changes and/or compensator modification to improve target coverage and avoid normal tissue overdosing.
Patients received weekly infusions of carboplatin (area under the curve of 2 units) and paclitaxel (50 mg/m2) during PBT, in addition to induction and/or adjuvant chemotherapy at systemic doses as judged by the treating physician. Typically, carboplatin-paclitaxel were used for adjuvant chemotherapy; either carboplatin-paclitaxel or cisplatin-etoposide were used for induction chemotherapy. Antiemetics and intravenous hydration were given in all patients. Chemotherapy schedules could be modified at clinical discretion, or in cases of aberrant laboratory test values (resulting in a treatment break).
Follow-up was performed 6 weeks after CRT to assess adverse events (per Common Terminology Criteria for Adverse Events, version 3), with a complete interval history and physical examination, laboratory studies, and thoracic CT. Subsequent clinical follow-up was held every 3 months for the first 2 years, and every 6 months thereafter. Positron emission tomography and/or computed tomography was required during the first 2 to 6 months after therapy, and as clinically indicated thereafter. Acute toxic effects were defined as occurring within 90 days after last treatment and late toxic effects thereafter.
Enumeration of treatment failures was conducted on the basis of follow-up imaging schedules as detailed herein; these were defined according to radiologic features. In case of findings suspicious for recurrence, PET-CT was always done, but rebiopsy was not routinely performed. Local treatment failure was defined as tumor appearance within the PTV; the appearance of pathologically appearing lymph nodes on imaging outside the PTV was denoted as regional treatment failure. Recurrence beyond local and nodal sites was classified as distant failure.
The primary objective of the study was to improve overall survival (OS). We hypothesized that the median survival time would be increased from a baseline of 16 months1 to 24 months. Using the normal approximation, we calculated that 65 patients would be required to have an 80% chance of demonstrating improvement using a 1-sided test with significance level of .05. Statistics, using a significance level of α < .05, were performed with Stata/MP 14.2 statistical software. The primary end point, OS, was calculated using Kaplan-Meier methodology from the beginning of enrollment to date of death or last follow-up. Progression-free survival (PFS) was defined from enrollment to any treatment failure or death; other more specific end points were distant metastasis and locoregional recurrence rates. In the presence of competing risks (eg, death) when performing survival analyses for any recurrence, alternative cumulative-incidence competing risk methodology was used to overcome the overestimated probabilities of recurrences. Combined major late toxic effects were also analyzed using Kaplan-Meier methodology. Multivariate Cox proportional hazards modeling was used to examine predictors of OS when adjusting for each of the collected potential confounding variables.
Results
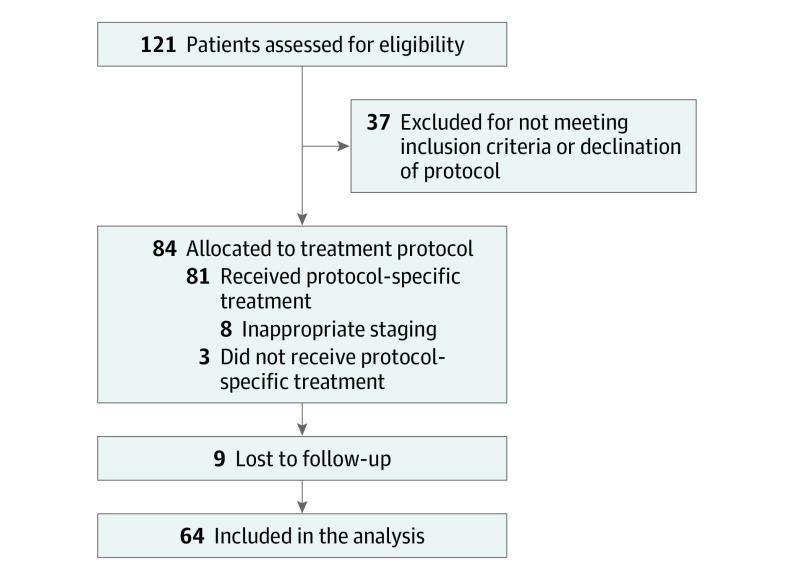
From 2009 to 2011, 121 patients were screened and 84 patients were enrolled on the protocol (Figure 1). Sixty-four patients were eligible for analysis; 20 patients were excluded due to inappropriate staging (n = 8), lack of follow-up information (n = 9), or not having received protocol-specific proton therapy (n = 3). Typically, most patients on protocol were not considered ideal for photon-based radiotherapy such as IMRT owing to extensive disease involvement as judged by the treating physicians and insurance reviewers, and/or likelihood of significant clinical improvement as compared with IMRT (as evaluated by both treating physicians and insurance companies). In addition, these patients needed to have insurance approval (except for self-payers), and Medicare for patients 65 years or older was the most common insurance. Table 1 displays clinical characteristics of the analyzed patients. Of note, 30 (47%) and 34 (53%) patients were stages IIIA and IIIB, respectively. The median Karnofsky performance status was 90 (range, 70-100). All patients received a course of concurrent CRT, and none required proton treatment breaks. Twenty (31%) and 18 (28%) patients received induction and adjuvant chemotherapy, respectively. Median follow-up was 27.3 (range, 2.7-111.5) months for all patients and 79.6 (range, 28.6-111.5) months for alive patients; by the last follow-up time, 47 (73%) patients had died.
Figure 1. CONSORT Diagram.
Table 1. Clinical Characteristics of the Study Population.
| Parameter | Value |
|---|---|
| Age, y, No. (%) | |
| ≤70 | 36 (56) |
| >70 | 28 (44) |
| Sex, No. (%) | |
| Male | 42 (66) |
| Female | 22 (34) |
| Histologic type, No. (%) | |
| Squamous cell carcinoma | 28 (44) |
| Adenocarcinoma | 25 (39) |
| Non–small cell lung cancer, not otherwise specified | 11 (17) |
| AJCC disease stage, No. (%) | |
| IIIA | 30 (47) |
| IIIB | 34 (53) |
| AJCC TNM categories, No. (%) | |
| T0-2 | 37 (58) |
| T3-4 | 27 (42) |
| N0-1 | 6 (9) |
| N2-3 | 58 (91) |
| Karnofsky performance status at diagnosis, median (range) | 90 (70-100) |
| Gross tumor volume, median (range), cm3 | 87.0 (4.1-753.2) |
| Chemotherapy | |
| Induction | |
| No. (%) | 20 (31) |
| No. of cycles, median (range) | 2 (1-6) |
| Concurrent | |
| No. (%) | 64 (100) |
| No. of cycles, median (range) | 7 (3-8) |
| Adjuvant | |
| No. (%) | 18 (28) |
| No. of cycles, median (range) | 2 (1-4) |
Abbreviation: AJCC, American Joint Committee on Cancer.
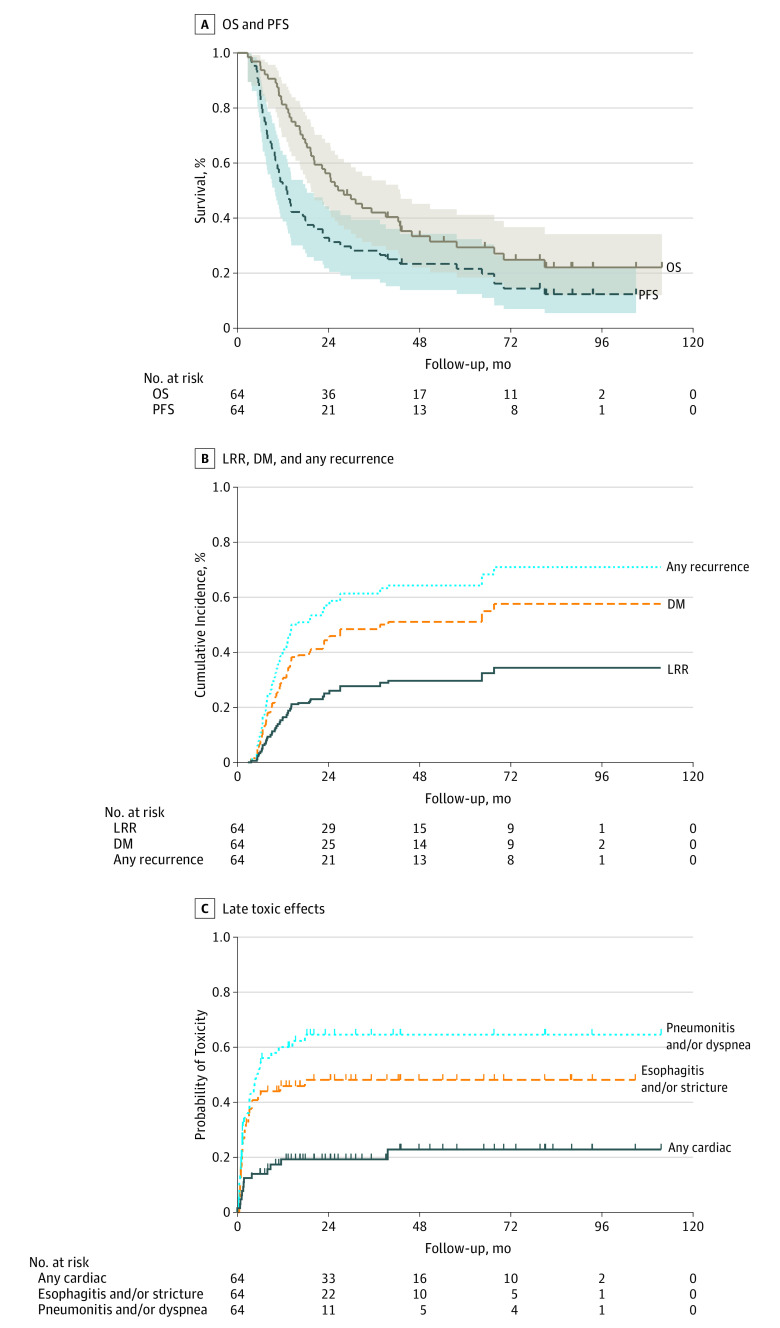
Figure 2A illustrates Kaplan-Meier survival analysis. Median OS was 26.5 months, and the corresponding OS at 5 years was 29% (95% CI, 18%-41%). Median PFS was 12.9 months, with a 5-year PFS of 22% (95% CI, 12%-32%).
Figure 2. Kaplan-Meier Curves .
A, Overall survival (OS) and progression-free survival (PFS). Shading indicates 95% confidence interval. B, Rates of locoregional recurrence (LRR), distant metastasis (DM), and any recurrence using death as a competing risk. C, Combined major late toxic effects rate using Kaplan-Meier methodology.
Patterns of treatment failure analysis are given in the eTable in the Supplement. In sum, 39 patients experienced relapse in a total of 50 sites (local, regional, or distant). The dominant mode of treatment failure was distant, corresponding to 48% (31 of 64 patients) and 62% (31 of 50) of all recurrences. Almost all distant treatment failures were not accompanied by local or regional relapses. The crude overall incidence of local and regional recurrences was 10 (16%) and 9 (14%) patients, respectively. Isolated local and regional relapses were uncommon (4 [6%] and 2 [3%] patients, respectively). Five-year actuarial distant metastasis, locoregional recurrences, and any recurrence were 54% (95% CI, 40%-68%), 28% (95% CI, 18%-43%), and 64% (95% CI, 51%-76%). Figure 2B shows rates of locoregional, distant metastatic, and any recurrence using death as a competing risk.
On multivariate analysis, there were 4 parameters independently correlative for poor OS: Karnofsky performance status 70 to 80 vs 90 to 100 (hazard ratio [HR], 2.48; 95% CI, 1.33-4.65; P = .004), stage IIIB vs IIIA (HR, 2.04; 95% CI, 1.09-3.83; P = .03), tumor location in left lung or right lower lobe vs right middle and/or right upper lobe (HR, 1.90; 95% CI, 1.03-3.50; P = .04), and pretreatment tumor size of greater than 7 cm vs 7 cm or less (HR, 2.39; 95% CI, 1.07-5.31; P = .03).
Acute toxic effects are enumerated in Table 2. Rates of grade 2, 3, and 4 esophagitis were 28% (n = 18), 8% (n = 5), and 0. Grades 2, 3, and 4 pneumonitis occurred in 2% (n = 1), 0, and 0 patients. Cardiac arrhythmia and ischemia occurred in 2 (3%) patients each. There were no acute or grade 5 adverse effects.
Table 2. Acute Toxic Effects Possibly, Probably, or Definitely Related to Protocol Treatmenta.
| Toxic Effect | Grade, No. (%) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Pulmonary | |||
| Lobar atelectasis | 0 | 2 (3) | 0 |
| Cough | 27 (42) | 2 (3) | 2 (3) |
| Dyspnea | 16 (25) | 10 (16) | 4 (6) |
| Hemoptysis | 0 | 1 (2) | 0 |
| Hoarseness | 0 | 1 (2) | 0 |
| Pleural effusion | 1 (2) | 2 (3) | 0 |
| Pneumonitis | 4 (6) | 1 (2) | 0 |
| Wheezing | 2 (3) | 0 | 0 |
| Gastrointestinal | |||
| Constipation | 2 (3) | 4 (6) | 0 |
| Diarrhea | 0 | 3 (5) | 0 |
| Dyspepsia | 1 (2) | 1 (2) | 0 |
| Dysphagia | 25 (39) | 7 (11) | 0 |
| Esophagitis | 1 (2) | 18 (28) | 5 (8) |
| Esophageal stricture | 0 | 0 | 1 (2) |
| Gastritis | 0 | 1 (2) | 0 |
| Nausea | 1 (2) | 7 (10) | 1 (2) |
| Odynophagia | 0 | 4 (6) | 0 |
| Vomiting | 0 | 2 (3) | 0 |
| Cardiac | |||
| Arrhythmia | 1 (2) | 1 (2) | 0 |
| Palpitations | 0 | 1 (2) | 0 |
| Tachycardia | 1 (2) | 2 (3) | 0 |
| Hematologic/electrolyte | |||
| Uremia | 0 | 0 | 0 |
| Blood urea nitrogen increase | 0 | 2 (3) | 0 |
| Elevated creatinine | 0 | 1 (2) | 0 |
| Anemia | 16 (25) | 10 (16) | 3 (5) |
| Hypocalcemia | 0 | 1 (2) | 0 |
| Hypomagnesemia | 0 | 1 (2) | 0 |
| Hyponatremia | 0 | 0 | 3 (5) |
| Hypotension | 0 | 0 | 1 (2) |
| Leukopeniab | 11 (17) | 30 (47) | 14 (22) |
| Lymphopenia | 0 | 0 | 0 |
| Neutropenia | 0 | 5 (8) | 3 (5) |
| Thrombocytopenia | 18 (28) | 5 (8) | 0 |
| General | |||
| Anorexia | 2 (3) | 5 (8) | 0 |
| Dehydration | 0 | 4 (6) | 4 (6) |
| Dermatitis | 24 (38) | 22 (34) | 5 (8) |
| Dizziness | 1 (2) | 1 (2) | 0 |
| Fatigue | 3 (5) | 12 (19) | 6 (9) |
| Fever | 0 | 3 (5) | 2 (3) |
| Hyperpigmentation | 0 | 3 (5) | 0 |
| Pain | 3 (5) | 9 (14) | 2 (3) |
| Pruritus | 0 | 0 | 1 (2) |
| Rash | 0 | 0 | 1 (2) |
| Sourness | 1 (2) | 0 | 0 |
| Weight loss | 12 (19) | 2 (3) | 3 (5) |
| Other | |||
| Alopecia | 1 (2) | 0 | 0 |
| Anxiety | 0 | 1 (2) | 0 |
| Candidiasis | 0 | 2 (3) | 1 (2) |
| Infection | 0 | 2 (3) | 1 (2) |
| Insomnia | 0 | 1 (2) | 0 |
| Muscle weakness | 0 | 1 (2) | 0 |
| Peripheral motor neuropathy | 0 | 2 (3) | 0 |
| Peripheral sensory neuropathy | 0 | 2 (3) | 0 |
No patients had grade 4 or 5 toxic effects, except as noted.
One patient (2%) had grade 4 toxic effect.
Table 3 displays late toxic effects. Three (5%) patients experienced grade 2, 1 (2%) grade 3, and 1 (2%) grade 4 esophagitis. One patient (2%) developed an esophageal stricture (grade 2). Grades 2 and 3 pneumonitis occurred in 10 (16%) and 8 (12%), respectively. Two (3%) patients developed a bronchial stricture (grade 2), and 1 (2%) a grade 4 bronchial fistula. Grade 2 cardiac arrhythmia occurred in 4 (6%) patients, and 2 (3%) patients each developed grades 2 and 3 pericardial effusion. There were no late grade 5 adverse effects. Figure 2C shows combined major late toxic effects using Kaplan-Meier methodology.
Table 3. Late Toxic Effects Possibly, Probably, or Definitely Related to Protocol Treatmenta.
| Toxic Effect | Grade, No. (%) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Pulmonary | ||||
| Lobar atelectasis | 0 | 3 (5) | 0 | 0 |
| Lung atelectasis | 0 | 1 (2) | 0 | 0 |
| Cough | 2 (3) | 6 (9) | 0 | 0 |
| Dysphagia | 1 (2) | 0 | 0 | 0 |
| Dyspnea | 6 (9) | 6 (9) | 2 (3) | 1 (2) |
| Bronchial fistula | 0 | 0 | 0 | 1 (2) |
| Bronchial stricture | 0 | 2 (3) | 0 | 0 |
| Pleural effusion | 15 (23) | 6 (9) | 2 (3) | 0 |
| Pneumonitis | 2 (3) | 10 (16) | 8 (12) | 0 |
| Pulmonary fibrosis | 0 | 0 | 0 | 0 |
| Pulmonary hemoptysis | 1 (2) | 0 | 0 | 0 |
| Wheezing | 0 | 1 (2) | 0 | 0 |
| Gastrointestinal | ||||
| Esophagitis | 0 | 3 (5) | 1 (2) | 1 (2) |
| Esophageal stricture | 0 | 1 (2) | 0 | 0 |
| Nausea | 1 (2) | 1 (2) | 0 | 0 |
| Cardiac | ||||
| Arrhythmia | 0 | 4 (6) | 0 | 0 |
| Pericardial effusion | 0 | 2 (3) | 2 (3) | 0 |
| Tachycardia | 0 | 1 (2) | 0 | 0 |
| Hematologic/electrolyte | ||||
| Anemia | 2 (3) | 3 (5) | 1 (2) | 0 |
| Leukopenia | 1 (2) | 0 | 1 (2) | 0 |
| General | ||||
| Dehydration | 0 | 1 (2) | 0 | 0 |
| Dermatitis | 1 (2) | 0 | 1 (2) | 0 |
| Dizziness | 1 (2) | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 1 (2) | 0 |
| Weight loss | 3 (5) | 5 (8) | 1 (2) | 0 |
| Other | ||||
| Alopecia | 1 (2) | 0 | 0 | 0 |
| Peripheral motor neuropathy | 1 (2) | 0 | 0 | 0 |
No patients had grade 5 toxic effects.
Discussion
In light of encouraging previous data, it is imperative to provide long-term prospective experiences for the safety and efficacy of PBT as part of combined-modality management for locally advanced NSCLC. Herein, we demonstrate that concurrent PBT and chemotherapy was safe and efficacious from the long-term perspective, and hence justifies further prospective investigation.
There are several reflections from this analysis. First, the study was designed in 2004, when PET imaging had recently been approved for lung cancer staging; image quality was still poor and did not include a CT component in outside facilities, which could cause substantial understaging of the disease by modern standards. However, the observed median OS of 26.5 months was encouraging and in accord with our original statistical goal of 24 months. Local tumor control was equally noteworthy, and the observed patterns of treatment failure were largely expected. The low rate of regional (or, isolated regional) relapses provides further evidence in favor of omitting elective nodal irradiation; the overall low rate of local/regional recurrences indicates that current 4-dimensional CT–based planning, as well as compensator smearing parameters and range uncertainty margins, is adequate.7 However, it is naturally difficult to ascribe the low recurrence rates to therapy itself vs a potential contribution by the strong commitment to image guidance and/or adaptive replanning. In addition, the presence of learning curves for new technologies is inevitable, and we noted that many proton treatment plans in the present study could be further optimized. Next, regarding the multivariate analysis findings, an interesting finding was the effect of tumor location. Left-sided and right lower lobe disease displayed worse OS, which potentially could be related to their proximity to the heart (discussed subsequently in light of the Radiation Therapy Oncology Group [RTOG] 0617 study), although assessment of the precise dosimetry in that trial is ongoing and will be subsequently reported.
When comparing with our preliminary prospective data (median OS, 29.4 months),7 which had a median follow-up of 19.7 months, these data display that concurrent PBT and chemotherapy yielded comparable OS (26.5 months) in the long-term setting, as well as low overall rates of late adverse events. Another retrospective series of 134 patients with stage II to III NSCLC treated with concurrent chemotherapy and PBT (60-74 Gy [RBE]) and median 4.7-year follow-up demonstrated median OS of 30.4 months for patients with stage III disease (majority IIIA), as well as similar relapse patterns.6 Of 77 patients receiving 74 Gy (RBE), 3 (4%) developed grade 3 esophagitis, and another developed grade 4 esophageal stricture; 2 (3%) developed grade 3 pneumonitis. Next, a prematurely terminated phase 2 study from the University of Florida (n = 14) displayed a median OS of 33 months (majority of patients had stage IIIA disease); the PFS figure of 14 months is similar to that observed here.9 Our data are also consistent with preliminary reporting of the phase 2 randomized study to compare IMRT vs passive scattering proton therapy in locally advanced NSCLC.10 To this extent, it is important to note that in anatomically unfavorable circumstances, such as disease “wrapping” around critical structures, it is unlikely that passively scattered PBT will provide adequate conformality to completely prevent high doses from reaching various organs-at-risk due to lack of proximal conformality and limitations of 3-dimensional planning (eg, bronchi or esophagus, which may lead to strictures and/or fistulas).11 Instead, the investigation of other techniques such as intensity-modulated proton therapy (IMPT) may be most useful for anatomically challenging areas, and further prospective investigation is required in this realm.4
Outcomes and toxic effects in this study appear promising as compared with studies of 3DCRT and/or IMRT and concurrent chemotherapy, although those series evaluated a variety of stages and treatment methods.12,13 Median OS in the largest IMRT-chemotherapy series (n = 165, median 31.3-month follow-up for survivors, 16.5 months overall) was 21.6 months; 18% of the cohort developed grade 3 esophagitis. There was a 12% rate of grade 3 pneumonitis and 2 (1%) patients experienced grade 5 pneumonitis. Comparative figures for the RTOG 0617 trial will be presented subsequently. It is also noteworthy that IMRT produces fewer toxic effects than 3DCRT and has therefore been proposed to be the standard technique in this clinical setting.14 From a technological development point of view, IMRT should be more appropriately compared with IMPT (owing to similar planning features), and 3DCRT should be compared with passive scattering proton therapy (with similar image guidance and planning maturity). Prospective studies to address these issues are ongoing.
The role of dose escalation in stage III unresectable NSCLC has recently been addressed in the randomized RTOG 0617 trial (N = 544 patients) investigating receipt of 74 vs 60 Gy of photon-based radiotherapy.15 In addition to finding statistically inferior median OS in the high-dose arm (20.3 vs 28.7 months), factors independently associated with worse OS included heart V5 and V30 and planning target volume (PTV) size, but not radiotherapy technique (3DCRT vs IMRT). This is supported by a recent retrospective single-institutional analysis of 322 patients demonstrating that heart V50 and lung V5, among others, independently correlated with OS.16 A secondary analysis of RTOG 0617 has recently been performed to compare 3DCRT with IMRT.17 Despite the IMRT group having larger PTVs and PTV to normal lung ratios (and more frequent stage IIIB disease), outcomes between both cohorts were statistically similar. In addition, patients receiving IMRT received significantly less cardiac dose and experienced fewer cases of grade 3 or higher pneumonitis (3.5% vs 7.9%). In RTOG 0617, overall, grade 3 or higher pulmonary events occurred in 20% vs 19% for the 60 and 74 Gy cohorts, respectively; grade 3 or higher esophagitis was observed in 7% vs 21%. Corresponding figures in the present work were 12% and 11%, respectively.
Taking the results of RTOG 0617 and placing them in context with these data, the role of dose escalation is still controversial,18 in part because potential improvement in tumor control could be masked by treatment-related morbidity and mortality. Because dosimetric properties (but not treatment technique) seem to correlate with OS, it could be posited that advanced, highly conformal modalities in themselves may not directly affect outcomes but could do so secondarily by means of controlling cardiopulmonary doses. This notion must be further tested before definitive conclusions can be drawn, however. To this extent, the unique properties of PBT are thus noteworthy in being able to deliver high tumor doses while maintaining low cardiopulmonary doses, which theoretically could improve survival over photon-based therapy. In addition, it is well known that proton therapy significantly reduces the “low-dose bath” to normal structures as compared with photon therapy. Preliminary data indicate that this unique aspect of proton therapy might minimize immune suppression caused by scattering radiation and lead to higher lymphocyte counts.19 In addition, retrospective data supporting an OS benefit in patients treated with PBT vs photon therapy have recently been reported.20 As compared with RTOG 0617, the median OS and toxic effect rates of the present study seem favorable as compared with the 74-Gy arm therein.
Our results also have implications for future work delineating the cost-effectiveness of PBT for locally advanced NSCLC.21 It has been postulated that PBT is economically favorable for these cases, but the overall quality of evidence is low.22 Without long-term information on outcomes and toxic effects, however, cost-effectiveness is difficult to evaluate, and this prospective report should thus provide a notable building block for cost-effectiveness studies. Our demonstration of favorable OS, as well as low rates of adverse events as compared with photon-based CRT series, will likely play a role in its estimated cost-effectiveness because the known increase in PBT costs could likely be offset by cost reductions due to fewer clinically manifested toxic effects, particularly late toxic effects. The prospective finding of fewer patient-reported severe symptoms with PBT over 3DCRT or IMRT treatment portends an impact on quality-of-life data, which also play a role in cost-effectiveness.23
Limitations
This study is limited by several factors, including the use of nonstandardized induction and/or adjuvant chemotherapy, heterogeneity in the population regarding other salient clinical factors, as well as applicability based on the unresolved issues regarding RTOG 0617 data as discussed herein. Nevertheless, in light of these findings, the next major step to be taken will be through multi-institutional prospective trials, such as the PCG LUN005 and RTOG 1308 studies. The continued advancement of IMPT will also be crucial to address whether this should be the standard of care over passively scattered PBT for some or most if not all patients with locally advanced NSCLC.
Conclusions
This is the final report with long-term follow-up of a phase 2 open-label, single-group assignment study evaluating dose-escalated (74 Gy [RBE]) PBT and concurrent chemotherapy as part of definitive treatment for stage III unresectable NSCLC. Altogether, with median follow-up of 79.6 months for alive patients, we observed encouraging clinical outcomes and low rates of toxic effects in both the acute and late settings, comparing favorably with historical data using photon-based therapy, particularly regarding toxic effects. These results have implications for ongoing issues regarding the role of dose escalation in this population, further optimization of proton therapy such as IMPT, and cost-effectiveness.
eTable. Patterns of Failure of the Study Population
References
- 1.Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network . Non-Small Cell Lung Cancer. Version 1.2016. https://www.nccn.org/professionals/physician_gls/PDF/nsclc.pdf. Accessed January 2, 2017.
- 3.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1087-1096. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Jabbour SK, De Ruysscher D, et al. ; International Particle Therapy Co-operative Group Thoracic Subcommittee . Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):505-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117(13):3004-3013. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QN, Ly NB, Komaki R, et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115(3):367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117(20):4707-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, Zhang X, Chang JY, et al. 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys. 2007;67(3):906-914. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe BS, Henderson R, Pham D, et al. A phase 2 trial of concurrent chemotherapy and proton therapy for stage III non-small cell lung cancer: results and reflections following early closure of a single-institution study. Int J Radiat Oncol Biol Phys. 2016;95(1):517-522. [DOI] [PubMed] [Google Scholar]
- 10.Liao ZX, Lee JJ, Komaki R, et al. Bayesian randomized trial comparing intensity modulated radiation therapy vs passively scattered proton therapy for locally advanced non-small cell lung cancer. J Clin Oncol. 2016;34 (suppl):abstr 8500. [Google Scholar]
- 11.Chang JY, Li H, Zhu XR, et al. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2014;90(4):809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(1):94-102. [DOI] [PubMed] [Google Scholar]
- 13.Jiang ZQ, Yang K, Komaki R, et al. Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: the MD Anderson experience. Int J Radiat Oncol Biol Phys. 2012;83(1):332-339. [DOI] [PubMed] [Google Scholar]
- 14.Chang JY. Intensity-modulated radiotherapy, not 3 dimensional conformal, is the preferred technique for treating locally advanced lung cancer. Semin Radiat Oncol. 2015;25(2):110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(2):293-301. [DOI] [PubMed] [Google Scholar]
- 17.Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belderbos J, Walraven I, van Diessen J, Verheij M, de Ruysscher D. Radiotherapy dose and fractionation for stage III NSCLC. Lancet Oncol. 2015;16(4):e156-e157. [DOI] [PubMed] [Google Scholar]
- 19.Lin S. Comparison of protons (3D vs IMRT) vs protons in esophageal cancer. Paper presented at: Particle Therapy Co-Operative Group Annual Meeting; May 11, 2017; Yokohama, Japan. [Google Scholar]
- 20.Higgins KA, O’Connell K, Liu Y, et al. National Cancer Database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97(1):128-137. [DOI] [PubMed] [Google Scholar]
- 21.Verma V, Shah C, Rwigema JC, Solberg T, Zhu X, Simone CB II. Cost-comparativeness of proton versus photon therapy. Chin Clin Oncol. 2016;5(4):56. [DOI] [PubMed] [Google Scholar]
- 22.Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122(10):1483-1501. [DOI] [PubMed] [Google Scholar]
- 23.Wang XS, Shi Q, Williams LA, et al. Prospective study of patient-reported symptom burden in patients with non-small-cell lung cancer undergoing proton or photon chemoradiation therapy. J Pain Symptom Manage. 2016;51(5):832-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Patterns of Failure of the Study Population