Key Points
Question
What are the incidence and prognosis of patients with breast cancer and brain metastases at diagnosis?
Findings
In this population-based cohort study, patients with triple-negative and hormone receptor–negative human epidermal growth factor receptor 2–positive subtypes and metastatic disease to any distant site harbored the highest likelihood (11.37% and 11.45%, respectively) of presenting with brain metastases at diagnosis of breast cancer. The prognosis of patients with triple-negative disease was poorest (median survival, 6 months).
Meaning
Future studies evaluating the utility of screening brain magnetic resonance imaging among patients at high risk of brain metastases may be warranted.
Abstract
Importance
Population-based estimates of the incidence and prognosis of brain metastases at diagnosis of breast cancer are lacking.
Objective
To characterize the incidence proportions and median survivals of patients with breast cancer and brain metastases at the time of cancer diagnosis.
Design, Setting, and Participants
Patients with breast cancer and brain metastases at the time of diagnosis were identified using the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute. Data were stratified by subtype, age, sex, and race. Multivariable logistic and Cox regression were performed to identify predictors of the presence of brain metastases at diagnosis and factors associated with all-cause mortality, respectively. For incidence, we identified a population-based sample of 238 726 adult patients diagnosed as having invasive breast cancer between 2010 and 2013 for whom the presence or absence of brain metastases at diagnosis was known. Patients diagnosed at autopsy or with an unknown follow-up were excluded from the survival analysis, leaving 231 684 patients in this cohort.
Main Outcomes and Measures
Incidence proportion and median survival of patients with brain metastases and newly diagnosed breast cancer.
Results
We identified 968 patients with brain metastases at the time of diagnosis of breast cancer, representing 0.41% of the entire cohort and 7.56% of the subset with metastatic disease to any site. A total of 57 were 18 to 40 years old, 423 were 41 to 60 years old, 425 were 61-80 years old, and 63 were older than 80 years. Ten were male and 958 were female. Incidence proportions were highest among patients with hormone receptor (HR)-negative human epidermal growth factor receptor 2 (HER2)-positive (1.1% among entire cohort, 11.5% among patients with metastatic disease to any distant site) and triple-negative (0.7% among entire cohort, 11.4% among patients with metastatic disease to any distant site) subtypes. Median survival among the entire cohort with brain metastases was 10.0 months. Patients with HR-positive HER2-positive subtype displayed the longest median survival (21.0 months); patients with triple-negative subtype had the shortest median survival (6.0 months).
Conclusions and Relevance
The findings of this study provides population-based estimates of the incidence and prognosis for patients with brain metastases at time of diagnosis of breast cancer. The findings lend support to consideration of screening imaging of the brain for patients with HER2-positive or triple-negative subtypes and extracranial metastases.
This population-based cohort study uses the SEER database to characterize the incidence proportion of brain metastases at the time of cancer diagnosis among patients with breast cancer on a population-based level.
Introduction
Brain metastases represent a significant cause of morbidity and mortality among patients with breast cancer. Robust population-based estimates relating to the incidence of brain metastases at diagnosis of breast cancer are lacking, in part due to diagnosis coding schema that cannot specifically capture the presence or absence of brain metastases. In autopsy studies, 15% to 35% of patients with breast cancer are found to have brain metastases, not all of which are clinically apparent prior to death. The risk of brain metastasis is subtype-dependent, and patients with human epidermal growth factor receptor 2 (HER2)-positive and triple-negative subtypes experience significantly higher rates of central nervous system (CNS) relapse than patients with hormone receptor (HR)-positive HER2-negative tumors. Based on a lack of proven benefit, current breast cancer screening guidelines, in patients with both localized and metastatic disease, do not recommend routine assessment or continued reassessment of brain metastases via imaging of the brain. Thus, most brain metastases in patients with breast cancer are detected because of neurologic symptoms, often necessitating interventions such as neurosurgical resection and/or whole brain radiation.
Population-level estimates for prognosis among patients with newly diagnosed breast cancer and brain metastases are also lacking. Data from single institution experiences and prospective studies have yielded varying results. Triple-negative subtype seems to be associated with a poorer prognosis. Other sociodemographic and clinical predictors of outcome, on a population level, have not been well characterized.
The purpose of this study was to use the Surveillance, Epidemiology, and End Results (SEER) database to characterize the incidence proportion of brain metastases at the time of cancer diagnosis among patients with breast cancer on a population-based level. We also sought to quantify survival estimates and to examine clinical and sociodemographic predictors of poorer survival among patients with breast cancer and brain metastases present at diagnosis of breast cancer.
Methods
The SEER database includes information on cancer incidence, treatment, and survival for approximately 30% of the US population. Information on the presence or absence of brain metastases at the time of initial cancer diagnosis was released in 2016 for patients diagnosed as having systemic malignant disease from 2010 to 2013. Within the SEER database, we identified 246 343 patients 18 years or older who were diagnosed as having a primary, invasive breast cancer between January 1, 2010, and December 31, 2013. Patients with carcinoma in situ were not included in the cohort. Patients for whom the presence or absence of brain metastases at diagnosis was unknown were excluded, leaving 238 726 patients in the final cohort for incidence analysis. Of these, 968 patients were diagnosed as having brain metastases. We subsequently removed patients who were diagnosed at autopsy or via death certificate, as well as patients who had an unknown follow-up, leaving 848 patients eligible for survival analysis. This study was approved by the institutional review board at Dana Farber Cancer Institute, and written informed consent was waived.
Patients were stratified by breast cancer subtype: HR-positive HER2-negative, HR-positive HER2-positive, HR-negative HER2-positive, and triple-negative (HR-negative HER2-negative). Absolute numbers and incidence proportions were calculated for patients with breast cancer with brain metastases identified at diagnosis; incidence proportions were also calculated after stratification by age, race, and sex. Incidence proportion was defined as the number of breast cancer patients diagnosed as having brain metastases divided by the total number of patients with breast cancer. Incidence proportion was also computed among patients with metastatic disease to any distant site. Race/ethnicity was categorized as white, black, Hispanic, Asian American, or other according to the SEER database.
Multivariable logistic regression was used to determine whether age, race/ethnicity, and sex were associated with the presence of brain metastases at diagnosis; other variables in the model included marital status, insurance status, residence type (urban vs rural), education, median household income, breast cancer subtype, and the extent of systemic disease at diagnosis. Residence type, education level (ie, percentage of adults ≥25 years with a high school education), and median household income were determined at the county level by linkage to the 2003 US Department of Agriculture rural-urban continuum codes, 2000 US Census, and 2004 small area income and poverty estimates from the US Census, respectively. The presence of bone, lung, and liver metastases at diagnosis is available in the SEER database and was used to characterize the extent of systemic disease among patients in this study.
Survival estimates were obtained using the Kaplan-Meier method. Multivariable Cox regression was performed to identify covariates associated with increased all-cause mortality using the same variables as in the logistic regression model described herein. To assess breast cancer–specific mortality, we used Fine and Gray’s competing risk regression. The hazard function for patients with unknown subtype was nonproportional over time, and therefore we included the interaction between this covariate and time in the model. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc) with the exception of the competing risks analysis, which was performed in R (version 3.3.2; R Foundation) using the cmprsk package (version 2.2-72014).
Results
Incidence
Among the 238 726 patients with breast cancer analyzed for incidence, 67.9%, 9.4%, 4.1%, 10.6%, and 8.0% had HR-positive HER2-negative, HR-positive HER2-positive, HR-negative HER2-positive, triple-negative, and unknown subtypes, respectively. Among the cohort with metastatic disease to any site (n = 12 801), 51.6%, 13.3%, 7.2%. 11.9%, and 16.0% had HR-positive HER2-negative, HR-positive HER2-positive, HR-negative HER2-positive, triple-negative, and unknown subtypes, respectively. The number and incidence proportions of patients with breast cancer and identified brain metastases at diagnosis are provided in Table 1, as stratified by breast cancer subtype. Among the entire cohort, 968 patients presented with brain metastases, reflecting 0.41% of the entire study population and 7.56% of the subset with metastatic disease at any site. Incidence proportions were highest among patients with HR-negative HER2-positive (1.1% of the entire cohort, 11.5% of the metastatic subset) and triple-negative (0.7% of the entire cohort, 11.4% of the metastatic subset) subtypes.
Table 1. Incidence Proportion and Median Survival of Patients With Breast Cancer With Identified Brain Metastases at Diagnosis by Subtype.
| Subtype | Patients, No. | Incidence Proportion of Brain Metastases, % | Survival Among Patients With Brain Metastases, Median (IQR), mo | |||
|---|---|---|---|---|---|---|
| With Breast Cancer | With Metastatic Disease | With Brain Metastases | Among Entire Cohort | Among Subset With Metastatic Disease | ||
| HR+/HER2− | 162 078 | 6607 | 361 | 0.22 | 5.46 | 14.0 (4.0-34.0) |
| HR+/HER2+ | 22 376 | 1704 | 136 | 0.61 | 7.98 | 21.0 (6.0-NR) |
| HR−/HER2+ | 9719 | 926 | 106 | 1.09 | 11.45 | 10.0 (4.0-27.0) |
| Triple-negative | 25 362 | 1522 | 173 | 0.68 | 11.37 | 6.0 (2.0-13.0) |
| Unknown | 19 191 | 2042 | 192 | 1.00 | 9.40 | 6.0 (2.0-20.0) |
| All subtypes | 238 726 | 12 801 | 968 | 0.41 | 7.56 | 10.0 (3.0-30.0) |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IQR, interquartile range; NR, not reached. + Denotes positive; − denotes negative.
On multivariable logistic regression (Table 2) among patients with metastatic cancer, age 41 to 60 years (vs age 18-40 years; odds ratio [OR], 1.41; 95% CI, 1.05-1.88; P = .02) and age 61 to 80 years (vs age 18-40 years; OR, 1.40; 95% CI, 1.04-1.88; P = .03), metastatic disease to 2 extracranial sites (vs 0 or 1 site; OR, 1.65; 95% CI, 1.41-1.94; P < .001) or 3 extracranial sites (vs 0 or 1 site; OR, 3.40; 95% CI, 2.76-4.18; P < .001), and HR-positive HER2-positive (vs HR-positive HER2-negative subtype; OR, 1.41; 95% CI, 1.14-1.73; P = .001), HR-negative HER2-positive (vs HR-positive HER2-negative subtype; OR, 2.09; 95% CI, 1.66-2.64; P < .001), and triple-negative subtypes (vs HR-positive HER2-negative subtype; OR, 2.19; 95% CI, 1.81-2.66; P < .001) were associated with significantly greater odds of having brain metastases at diagnosis. Neither race nor income was associated with a risk of brain metastasis at diagnosis in the multivariable model. Insured status was associated with marginally lower odds of brain metastasis at diagnosis (OR, 0.77; 95% CI, 0.58-1.00; P = .05). Significant results are presented in Table 2.
Table 2. Multivariable Logistic Regression for the Presence of Brain Metastases at Diagnosis of Breast Cancera.
| Variable | Patients, No. | Among Entire Cohort | Among Subset With Metastatic Disease | |||
|---|---|---|---|---|---|---|
| Patients (n = 238 726) |
With Brain Metastases (n = 968) |
OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age at diagnosis, yb | ||||||
| 18-40 | 13 564 | 57 | 1 [Reference] | NA | 1 [Reference] | NA |
| 41-60 | 98 184 | 423 | NA | NA | 1.41 (1.05-1.88) | .02 |
| 61-80 | 103 700 | 425 | NA | NA | 1.40 (1.04-1.88) | .03 |
| >80 | 23 265 | 63 | NA | NA | NA | NA |
| Sex | ||||||
| Male | 1854 | 10 | 1 [Reference] | NA | 1 [Reference] | NA |
| Female | 236 872 | 958 | NA | NA | NA | NA |
| Raceb | ||||||
| White | 165 808 | 603 | 1 [Reference] | NA | 1 [Reference] | NA |
| Black | 25 914 | 187 | 1.27 (1.06-1.53) | .01 | NA | NA |
| Hispanic | 25 359 | 121 | NA | NA | NA | NA |
| Asian | 19 043 | 53 | NA | NA | NA | NA |
| Other | 1688 | 4 | NA | NA | NA | NA |
| Marital status | ||||||
| Unmarried | 97 199 | 526 | 1 [Reference] | NA | 1 [Reference] | NA |
| Married | 127 644 | 389 | 0.73 (0.63-0.84) | <.001 | NA | NA |
| Unknown | 13 883 | 53 | NA | NA | NA | NA |
| Median household income (per $10 000 annual increase) | 238 726 | 968 | NA | NA | NA | NA |
| High school education (per 10% increase) | 238 726 | 968 | NA | NA | NA | NA |
| Insurance status | ||||||
| Uninsured | 4446 | 68 | 1 [Reference] | NA | 1 [Reference] | NA |
| Insured | 229 369 | 876 | 0.60 (0.45-0.79) | <.001 | 0.77 (0.58-1.00) | .05 |
| Unknown | 4911 | 24 | NA | NA | NA | NA |
| Urban | ||||||
| Rural | 25 454 | 117 | 1 [Reference] | NA | 1 [Reference] | NA |
| Urban | 212 934 | 851 | NA | NA | NA | NA |
| Extracranial metastatic sites to bone, lung, and liver, No. | ||||||
| 0 or 1 | 234 640 | 502 | 1 [Reference] | NA | 1 [Reference] | NA |
| 2 | 2755 | 252 | 37.35 (31.78-43.90) | <.001 | 1.65 (1.41-1.94) | <.001 |
| All 3 | 777 | 139 | 75.12 (60.82-92.80) | <.001 | 3.40 (2.76-4.18) | <.001 |
| Unknown | 554 | 75 | 57.17 (43.84-74.56) | <.001 | 3.90 (2.98-5.12) | <.001 |
| Subtype | ||||||
| HR+/HER2− | 162 078 | 361 | 1 [Reference] | NA | 1 [Reference] | NA |
| HR+/HER2+ | 22 376 | 136 | 1.92 (1.57-2.37) | <.001 | 1.41 (1.14-1.73) | .001 |
| HR−/HER2+ | 9719 | 106 | 3.25 (2.57-4.09) | <.001 | 2.09 (1.66-2.64) | <.001 |
| Triple-negative | 25 362 | 173 | 2.61 (2.16-3.15) | <.001 | 2.19 (1.81-2.66) | <.001 |
| Unknown | 19 191 | 192 | 2.68 (2.22-3.24) | <.001 | 1.74 (1.44-2.10) | <.001 |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; OR, odds ratio. + Denotes positive; − denotes negative.
Only significant results presented (P < .05).
Unknown age and unknown race removed from model owing to nonconvergence (n = 920).
Survival
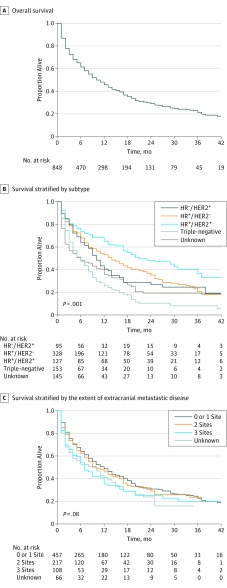
Median survival among patients in the survival cohort with breast cancer and identified brain metastases at diagnosis who were not diagnosed at autopsy or via death certificate and who had a defined period of follow-up (n = 848), as stratified by subtype, is presented in Table 1. The median survival among the entire cohort was 10.0 months, with patients with HR-positive HER2-positive subtype experiencing the longest median survival (21.0 months) and patients with triple-negative subtype experiencing the shortest median survival (6.0 months). Survival estimates overall (Figure, A) and as stratified by subtype (Figure, B) and by extent of extracranial metastatic disease (Figure, C) are graphically displayed in the Figure.
Figure. Overall Survival Among Patients With Breast Cancer and Brain Metastases at Diagnosis.
Extent of extracranial metastatic disease is classified by the number of metastatic sites to the bone, lung, or liver. HER2 denotes human epidermal growth factor receptor 2; HR, hormone receptor. + Denotes positive; − denotes negative.
On multivariable Cox regression (Table 3) for all-cause mortality among patients with brain metastases at diagnosis, age 41 to 60 years (vs age 18-40 years; hazard ratio, 1.70; 95% CI, 1.08-2.66; P = .02), age 61 to 80 years (vs age 18-40 years; hazard ratio, 2.28; 95% CI, 1.46-3.56; P < .001), and age greater than 80 years (vs age 18-40 years; hazard ratio, 2.45; 95% CI, 1.52-4.25; P = .002), black race (vs white; hazard ratio, 1.34; 95% CI, 1.06-1.69; P = .01), unmarried social status (vs married social status; hazard ratio, 1.21; 95% CI, 1.01-1.47; P = .04), metastatic disease to 3 extracranial sites (vs 0 or 1 site; hazard ratio, 1.42; 95% CI, 1.08-1.85; P = .01), and triple-negative subtype (vs HR-positive HER2-negative subtype; hazard ratio, 1.98; 95% CI, 1.56-2.50; P < .001) were significantly associated with an increased all-cause mortality. HR-positive HER2-positive subtype was significantly associated with a decreased all-cause mortality (vs HR-positive HER2-negative subtype; hazard ratio, 0.71; 95% CI, 0.53-0.95; P = .02). Breast cancer–specific mortality among patients with breast cancer and brain metastases at diagnosis is also presented in Table 3. Table 4 displays median survival by subtype as stratified by the extent of systemic disease. In general, survival was poorer among patients with more extensive systemic disease at diagnosis. We also found that the presence of brain metastases at initial diagnosis was associated with shorter survival time compared with patients presenting with de novo metastatic disease without baseline brain involvement (Table 4).
Table 3. Multivariable Cox Regression for All-Cause Mortality and Breast Cancer–Specific Mortality Among Patients With Brain Metastasesa.
| Variable | Patients, No. | All-Cause Mortality | Breast Cancer–Specific Mortality | |||
|---|---|---|---|---|---|---|
| Patients (n = 231 684) |
With Brain Metastases (n = 968) |
Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age at diagnosis, yb | ||||||
| 18-40 | 13 564 | 57 | 1 [Reference] | NA | 1 [Reference] | NA |
| 41-60 | 98 184 | 423 | 1.70 (1.08-2.66) | .02 | NA | NA |
| 61-80 | 103 700 | 425 | 2.28 (1.46-3.56) | <.001 | 1.72 (1.11-2.68) | .02 |
| >80 | 23 265 | 63 | 2.45 (1.41-4.25) | .002 | NA | NA |
| Sex | ||||||
| Male | 1854 | 10 | 1 [Reference] | NA | 1 [Reference] | NA |
| Female | 236 872 | 958 | NA | NA | 0.42 (0.19-0.94) | .03 |
| Raceb | ||||||
| White | 165 808 | 603 | 1 [Reference] | NA | 1 [Reference] | NA |
| Black | 25 914 | 187 | 1.34 (1.06-1.69) | .01 | 1.38 (1.09-1.74) | .008 |
| Hispanic | 25 359 | 121 | NA | NA | NA | NA |
| Asian | 19 043 | 53 | NA | NA | NA | NA |
| Other | 1688 | 4 | NA | NA | NA | NA |
| Marital status | ||||||
| Unmarried | 97 199 | 526 | 1 [Reference] | NA | 1 [Reference] | NA |
| Married | 127 644 | 389 | 0.82 (0.68-1.00) | .04 | 0.72 (0.59-0.89) | .002 |
| Unknown | 13 883 | 53 | NA | NA | NA | NA |
| Median household income (per $10 000 annual increase) | 238 726 | 968 | NA | NA | NA | NA |
| High school education (per 10% increase) | 238 726 | 968 | NA | NA | NA | NA |
| Insurance status | ||||||
| Uninsured | 4446 | 68 | 1 [Reference] | NA | 1 [Reference] | NA |
| Insured | 229 369 | 876 | NA | NA | NA | NA |
| Unknown | 4911 | 24 | NA | NA | NA | NA |
| Urban | ||||||
| Rural | 25 454 | 117 | 1 [Reference] | NA | 1 [Reference] | NA |
| Urban | 212 934 | 851 | NA | NA | NA | NA |
| Extracranial metastatic sites to bone, lung, and liver, No. | ||||||
| 0 or 1 | 234 640 | 502 | 1 [Reference] | NA | 1 [Reference] | NA |
| 2 | 2755 | 252 | NA | NA | NA | NA |
| All 3 | 777 | 139 | 1.42 (1.08-1.85) | .01 | NA | NA |
| Unknown | 554 | 75 | NA | NA | NA | NA |
| Subtypeb | ||||||
| HR+/HER2− | 162 078 | 361 | 1 [Reference] | NA | 1 [Reference] | NA |
| HR+/HER2+ | 22 376 | 136 | 0.71 (0.53-0.95) | .02 | NA | NA |
| HR−/HER2+ | 9719 | 106 | NA | NA | NA | NA |
| Triple-negative | 25 362 | 173 | 1.98 (1.56-2.50) | <.001 | 1.69 (1.33-2.14) | <.001 |
| Unknown | 19 191 | 192 | 2.42 (1.69-3.47) | <.001 | 2.22 (1.39-0.74) | <.001 |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor. + Denotes positive; − denotes negative.
Only significant results presented (P < .05).
Unknown age and unknown race removed from model due to nonconvergence (n = 745); model includes interaction between unknown subtype and time.
Table 4. Median Survival of Breast Cancer Patients by Extent of Systemic Metastatic Disease.
| Subtype | Type of Metastasis | Survival, Median (IQR), mo | |
|---|---|---|---|
| Extracranial Systemic Disease Only | Extracranial Systemic Disease and Brain Metastases | ||
| HR+/HER2− | Lung | 42.0 (15.0-NR) | 18.0 (2.0-36.0) |
| Liver | 30.0 (10.0-NR) | 9.0 (2.0-18.0) | |
| Bone | 39.0 (21.0-NR) | 15.0 (6.0-31.0) | |
| 2 of 3 | 28.0 (11.0-NR) | 16.0 (5.0-37.0) | |
| All 3 | 18.0 (6.0-39.0) | 12.0 (4.0-NR) | |
| HR+/HER2+ | Lung | NR (21.0-NR) | 18.0 (10.0-NR) |
| Liver | NR (19.0-NR) | NR (NR-NR) | |
| Bone | 46.0 (25.0-NR) | 29.0 (12.0-NR) | |
| 2 of 3 | 34.0 (16.0-NR) | 17.0 (4.0-NR) | |
| All 3 | 24.0 (6.0-NR) | 12.0 (6.0-NR) | |
| HR−/HER2+ | Lung | 25.0 (14.0-NR) | 9.0 (4.0-17.0) |
| Liver | NR (12.0-NR) | 6.0 (4.0-6.0) | |
| Bone | NR (13.0-NR) | NR (8.0-NR) | |
| 2 of 3 | 28.0 (10.0-NR) | 9.0 (5.0-NR) | |
| All 3 | 19.0 (8.0-NR) | 11.0 (3.0-16.0) | |
| Triple-negative | Lung | 14.0 (5.0-29.0) | 7.0 (2.0-9.0) |
| Liver | 14.0 (5.0-27.0) | NR (3.0-NR) | |
| Bone | 14.0 (5.0-32.0) | 11.0 (4.0-15.0) | |
| 2 of 3 | 10.0 (5.0-17.0) | 5.0 (3.0-7.0) | |
| All 3 | 5.0 (2.0-13.0) | 5.0 (1.0-12.0) | |
| Unknown | Lung | 16.0 (4.0-38.0) | 9.0 (2.0-20.0) |
| Liver | 18.0 (4.0-NR) | 1.0 (1.0-9.0) | |
| Bone | 28.0 (8.0-47.0) | 12.0 (2.0-19.0) | |
| 2 of 3 | 15.0 (5.0-33.0) | 12.0 (1.0-18.0) | |
| All 3 | 12.0 (3.0-28.0) | 2.0 (1.0-7.0) | |
| All subtypes | Lung | 25.0 (10.0-NR) | 8.0 (2.0-19.0) |
| Liver | 29.0 (9.0-NR) | 9.0 (3.0-27.0) | |
| Bone | 37.0 (17.0-NR) | 14.0 (4.0-35.0) | |
| 2 of 3 | 24.0 (9.0-NR) | 10.0 (3.0-34.0) | |
| All 3 | 16.0 (5.0-35.0) | 7.0 (2.0-27.0) | |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IQR, interquartile range; NR, not reached. + Denotes positive; − denotes negative.
Discussion
In this study, we describe the incidence of identified brain metastases among patients with newly diagnosed breast cancer and characterize the subsequent survival of such patients. We found that the incidence proportion of brain metastases was highest among patients with HR-negative HER2-positive and triple-negative subtypes. Patients in the incidence cohort were likely diagnosed as a result of neurologic symptoms given that consensus guidelines for patients with breast cancer do not recommend screening imaging of the brain. As a result, the true incidence of brain metastases in patients with breast cancer is likely underestimated by the results in this study. Notably, median survival from diagnosis varied significantly by subtype, ranging from 6.0 months in patients with triple-negative breast cancer to 21.0 months in patients with HR-positive HER2-positive breast cancer. Because SEER data include approximately 30% of the United States population, the incidence proportions and median survivals we describe are highly generalizable and likely more reflective of the population experience compared with previously published data focused primarily on patients treated at academic cancer centers. In addition, we report for the first time, to our knowledge, population-based survival outcomes by tumor subtype among patients with brain metastases on initial breast cancer presentation.
Barnholtz-Sloan et al previously reported on the incidence proportion of patients with multiple different cancers who were diagnosed as having brain metastases from 1973 to 2001 in the Metropolitan Detroit, Michigan, area. They found a cumulative incidence of identified brain metastases among patients with breast cancer (all stages at diagnosis) of 5.1%. Among patients with breast cancer with distant metastases to any systemic site, 14.2% developed brain metastases during their disease course. A strength of the study was the availability of information regarding the incidence of brain metastases that developed at any time over the disease course, as opposed to at first presentation, as in our study. However, tumor subtype was not available, and outcomes were not reported. Also, because the data were limited to only 3 counties near Detroit, the results may be less generalizable to the United States at large. Pelletier et al estimated the cumulative incidence over time of brain metastases among patients with breast cancer from a US commercial insurance claims database from 2002 to 2004. They found an incidence of 9.1% among the entire cohort. The study included only patients who made insurance claims, and results were not stratified by subtype. Similar population-based studies have been conducted outside the United States, finding cumulative incidence proportions ranging from 3.4% to 5.0% in the Netherlands, Germany, and Finland.
In contrast to most of the published experience on this topic, our work focuses on patients presenting with de novo metastatic breast cancer. Our results confirm and extend previous work describing the interaction between tumor subtype and the risk of brain metastases among patients initially presenting with stage I to III breast cancer. In a Canadian study of 3726 patients diagnosed as having early-stage breast cancer from 1986 to 1992 with a median follow-up time of 14.8 years, Kennecke et al reported cumulative incidence proportions of brain metastases for luminal A, HER2-positive and estrogen receptor–positive progesterone receptor–positive, HER2-positive, and nonbasal triple-negative subtypes of 2.2%, 7.9%, 14.3%, and 7.2%, respectively, among all patients. Among the patients who developed metastatic disease, the cumulative incidence proportions were 7.6%, 15.4%, 28.7%, and 22.0%, respectively. More contemporary studies have reported lifetime incidence proportions for the development of brain metastases among patients with HER2-positive and triple-negative metastatic breast cancer as high as 34% to 55% and 22% to 46%, respectively.
Current National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology, and European School of Oncology-Metastatic Breast Cancer guidelines for breast cancer do not include the use of routine brain screening of brain metastases in asymptomatic patients, based on a lack of demonstrated survival advantage in nonrandomized retrospective studies. During the time period analyzed (2010-2013), screening brain magnetic resonance imaging (MRI) for stage II-IV non–small-cell lung cancer was considered standard practice, based on NCCN guidelines. The recommendation of brain MRI at initial lung cancer diagnosis was added following the publication of various studies suggesting its potential benefit among selected patients with lung cancer. Kim et al found an incidence of brain metastases of 20.8% for the patients with NSCLC who were screened with brain MRI vs 4.6% for nonscreened patients. We found that 7.56% of patients with metastatic breast cancer to any site had brain metastases at diagnosis; presumably, these patients were symptomatic given the lack of routine screening in patients with breast cancer. When metastases are identified early, they are typically amenable to potentially less toxic approaches, such as stereotactic radiosurgery or use of systemic agents with intracranial penetration. Whether routine CNS staging in patients with metastatic breast cancer could reduce the need for neurosurgical resection, whole-brain radiation, or a higher neurologic death rate is not known at this time; our results support the need for further investigation of this very common clinical question.
Among the entire cohort, black patients (vs white patients) had significantly greater odds of having brain metastases at diagnosis (OR, 1.27; 95% CI, 1.06-1.53; P = .01), but this association was not seen among the cohort with metastatic disease to any distant site. This suggests but does not prove that breast cancers in black patients are likely being diagnosed at a later stage than in white patients. A greater cause for concern is that we found that black patients with brain metastases had worse overall median survival, with a significant hazard ratio of 1.34 (95% CI, 1.06-1.69; P = .01) despite adjustment for sociodemographic factors, insurance status, extent of systemic disease, and subtype. Further study evaluating potential explanations for this finding is warranted. Our group has previously found a significant increase in the hazard ratio (1.37 to 1.53; P interaction <.001) for all-cause mortality among black vs white patients with localized breast cancer from the 1988-1997 to 1998-2007 time periods, suggesting that outcomes in the black patients with breast cancer are worsening with time. It remains unclear if there is tumor biology, access to care, or another etiology is driving this trend, and further investigation is warranted.
We found that median survival for patients with breast cancer with brain metastases varied significantly by subtype, with triple-negative breast cancer being associated with the poorest survival (median survival, 6.0 months). HR-positive HER2-positive patients had the longest survival (median survival, 21.0 months). Our data are consistent with some of the general trends reported in academic center–based retrospective studies. Importantly, given the potential referral bias and other biases that can be present in academic center–based series, we confirmed in a population-based sample of patients with newly diagnosed breast cancer and brain metastases that indeed, contrary to historical data, a median survival of nearly 2 years after diagnosis is now achieved in patients with HR-positive HER2-positive tumors. Our data support the importance of strategies to avoid long-term neurotoxic effects of CNS-directed treatments in such patients, as well as the importance of strategies to manage CNS progression events in patients with diagnosed brain metastases at the time of primary cancer diagnosis with relatively extended survival. We also found that the presence of brain metastases at initial diagnosis was associated shorter survival time compared with patients presenting with de novo metastatic disease without baseline brain involvement.
Limitations
Our study should be considered in the context of its limitations. First, we were able to describe only the presence vs absence of brain metastases at initial diagnosis. The SEER database does not provide information about disease recurrence or subsequent sites of disease involvement; thus, we were unable to comment on patients who developed brain metastases later in their disease course. Therefore, there may be some patients who subsequently developed brain metastases later in the disease course who would not be captured in our analysis. Future studies using alternative data sources should be carried out to address this important point. Second, because breast cancer screening guidelines do not recommend use of brain MRI for screening, many patients presenting with brain metastases at diagnosis were likely symptomatic. Therefore, we likely underestimated the actual rate of brain metastases in patients with newly diagnosed breast cancer. It is unclear from the data what the incidence might be for patients without neurologic symptoms. In addition, the threshold for obtaining imaging of the brain may have varied among different institutions and practitioners. Third, residence type, education level, and median household income were defined at a county level, not a patient level, possibly affecting the results of the logistic and Cox regressions. Fourth, information relating to comorbidities, performance status, and smoking status is not available in the SEER database. Finally, we cannot comment on the treatment that patients with brain metastases received given that such information is not recorded in the SEER database.
Conclusions
Despite these limitations, our study provides insight into the epidemiology of brain metastases in patients with newly diagnosed breast cancer in the United States. It lends support to consideration of studies evaluating the utility of screening MRI of the brain among patients at high risk of brain metastases, including those with HER2-positive or triple-negative disease and other systemic metastases. The degree to which earlier diagnosis may have an impact on important outcomes such as brain-directed neurosurgical or radiotherapeutic treatment, quality of life, and cost-effective care, warrants further investigation.
References
- 1.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175-180. [DOI] [PubMed] [Google Scholar]
- 2.Cummings MC, Simpson PT, Reid LE, et al. . Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma: autopsy study. Cancer. 1983;52(12):2349-2354. [DOI] [PubMed] [Google Scholar]
- 4.Kennecke H, Yerushalmi R, Woods R, et al. . Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271-3277. [DOI] [PubMed] [Google Scholar]
- 5.Lin NU, Vanderplas A, Hughes ME, et al. . Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaz-Luis I, Ottesen RA, Hughes ME, et al. . Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 2012;14(5):R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pestalozzi BC, Holmes E, de Azambuja E, et al. . CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244-248. [DOI] [PubMed] [Google Scholar]
- 8.Nam BH, Kim SY, Han HS, et al. . Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10(1):R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Network NCC. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 2. 2016. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 3, 2016.
- 10.Ramakrishna N, Temin S, Chandarlapaty S, et al. . Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso F, Costa A, Norton L, et al. ; European School of Oncology; European Society of Medical Oncology . ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489-502. [DOI] [PubMed] [Google Scholar]
- 12.Niwińska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys. 2010;77(4):1134-1139. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama H, Shirato H, Tago M, et al. . Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491. [DOI] [PubMed] [Google Scholar]
- 14.Chow L, Suen D, Ma KK, Kwong A. Identifying risk factors for brain metastasis in breast cancer patients: implication for a vigorous surveillance program. Asian J Surg. 2015;38(4):220-223. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara M, Mukai H, Nagai S, et al. . Retrospective analysis of risk factors for central nervous system metastases in operable breast cancer: effects of biologic subtype and Ki67 overexpression on survival. Oncology. 2013;84(3):135-140. [DOI] [PubMed] [Google Scholar]
- 16.Fokas E, Henzel M, Hamm K, Grund S, Engenhart-Cabillic R. Brain metastases in breast cancer: analysis of the role of HER2 status and treatment in the outcome of 94 patients. Tumori. 2012;98(6):768-774. [DOI] [PubMed] [Google Scholar]
- 17.Dawood S, Broglio K, Esteva FJ, et al. . Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20(4):621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawood S, Ueno NT, Valero V, et al. . Incidence of and survival following brain metastases among women with inflammatory breast cancer. Ann Oncol. 2010;21(12):2348-2355. [DOI] [PubMed] [Google Scholar]
- 19.Aversa C, Rossi V, Geuna E, et al. . Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5):623-628. [DOI] [PubMed] [Google Scholar]
- 20.Minisini AM, Moroso S, Gerratana L, et al. . Risk factors and survival outcomes in patients with brain metastases from breast cancer. Clin Exp Metastasis. 2013;30(8):951-956. [DOI] [PubMed] [Google Scholar]
- 21.Heitz F, Rochon J, Harter P, et al. . Cerebral metastases in metastatic breast cancer: disease-specific risk factors and survival. Ann Oncol. 2011;22(7):1571-1581. [DOI] [PubMed] [Google Scholar]
- 22.Swain SM, Baselga J, Miles D, et al. . Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubb CS, Jani A, Wu CC, et al. . Breast cancer subtype as a predictor for outcomes and control in the setting of brain metastases treated with stereotactic radiosurgery. J Neurooncol. 2016;127(1):103-110. [DOI] [PubMed] [Google Scholar]
- 24.Wiens AL, Martin SE, Bertsch EC, et al. . Luminal subtypes predict improved survival following central nervous system metastasis in patients with surgically managed metastatic breast carcinoma. Arch Pathol Lab Med. 2014;138(2):175-181. [DOI] [PubMed] [Google Scholar]
- 25.Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class, and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010;21(5):942-948. [DOI] [PubMed] [Google Scholar]
- 26.Honda Y, Aruga T, Yamashita T, et al. . Prolonged survival after diagnosis of brain metastasis from breast cancer: contributing factors and treatment implications. Jpn J Clin Oncol. 2015;45(8):713-718. [DOI] [PubMed] [Google Scholar]
- 27.Tarhan MO, Demir L, Somali I, et al. . The clinicopathological evaluation of the breast cancer patients with brain metastases: predictors of survival. Clin Exp Metastasis. 2013;30(2):201-213. [DOI] [PubMed] [Google Scholar]
- 28.Niikura N, Hayashi N, Masuda N, et al. . Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147(1):103-112. [DOI] [PubMed] [Google Scholar]
- 29.Surveillance E, Results E (SEER) Program Research Data (1973-2013), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission. http://www.seer.cancer.gov. Accessed August 15, 2016.
- 30.Aizer AA, Chen MH, McCarthy EP, et al. . Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aizer AA, Falit B, Mendu ML, et al. . Cancer-specific outcomes among young adults without health insurance. J Clin Oncol. 2014;32(19):2025-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Department of Agriculture Rural-Urban Continuum Codes http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. Accessed January 13, 2013.
- 33.US Census Bureau US Census 2000 Gateway. http://www.census.gov/main/www/cen2000.html. Accessed December 12, 2012.
- 34.US Census Bureau Small area income and poverty estimates. http://www.census.gov/did/www/saipe/data/statecounty/data/2004.html. Accessed December 12, 2012.
- 35.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 36.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872. [DOI] [PubMed] [Google Scholar]
- 37.Pelletier EM, Shim B, Goodman S, Amonkar MM. Epidemiology and economic burden of brain metastases among patients with primary breast cancer: results from a US claims data analysis. Breast Cancer Res Treat. 2008;108(2):297-305. [DOI] [PubMed] [Google Scholar]
- 38.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705. [DOI] [PubMed] [Google Scholar]
- 39.Vuong DA, Rades D, Vo SQ, Busse R. Extracranial metastatic patterns on occurrence of brain metastases. J Neurooncol. 2011;105(1):83-90. [DOI] [PubMed] [Google Scholar]
- 40.Sihto H, Lundin J, Lundin M, et al. . Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 2011;13(5):R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bendell JC, Domchek SM, Burstein HJ, et al. . Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972-2977. [DOI] [PubMed] [Google Scholar]
- 42.Clayton AJ, Danson S, Jolly S, et al. . Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91(4):639-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson EM, Najita JS, Sohl J, et al. . Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller KD, Weathers T, Haney LG, et al. . Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol. 2003;14(7):1072-1077. [DOI] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer (version 4.2016) [requires login]. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed October 3, 2016.
- 47.Kim SY, Kim JS, Park HS, et al. . Screening of brain metastasis with limited magnetic resonance imaging (MRI): clinical implications of using limited brain MRI during initial staging for non-small cell lung cancer patients. J Korean Med Sci. 2005;20(1):121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown PD, Jaeckle K, Ballman KV, et al. . Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metro G, Foglietta J, Russillo M, et al. . Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22(3):625-630. [DOI] [PubMed] [Google Scholar]
- 50.Soffietti R, Kocher M, Abacioglu UM, et al. . A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65-72. [DOI] [PubMed] [Google Scholar]
- 51.Hanzly M, Abbotoy D, Creighton T, et al. . Early identification of asymptomatic brain metastases from renal cell carcinoma. Clin Exp Metastasis. 2015;32(8):783-788. [DOI] [PubMed] [Google Scholar]
- 52.Aizer AA, Wilhite TJ, Chen MH, et al. . Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532-1539. [DOI] [PubMed] [Google Scholar]
- 53.Kuba S, Ishida M, Nakamura Y, et al. . Treatment and prognosis of breast cancer patients with brain metastases according to intrinsic subtype. Jpn J Clin Oncol. 2014;44(11):1025-1031. [DOI] [PubMed] [Google Scholar]
- 54.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. 2012;118(19):4652-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hines SL, Vallow LA, Tan WW, McNeil RB, Perez EA, Jain A. Clinical outcomes after a diagnosis of brain metastases in patients with estrogen- and/or human epidermal growth factor receptor 2-positive versus triple-negative breast cancer. Ann Oncol. 2008;19(9):1561-1565. [DOI] [PubMed] [Google Scholar]