This meta-analysis explores milestone rate, a proposed intermediate end point for immunotherapy trials among patients with non–small cell lung cancer.
Key Points
Question
What early end points should be explored to test new drugs in clinical trials for lung cancer?
Findings
This updated meta-analysis analyzed milestone rates (eg, 12-month survival) in 25 trials submitted to the US Food and Drug Administration that included 20 013 patients with advanced lung cancer. A moderate association was found between some milestones, such as overall survival rate at 12 months and overall survival hazard ratio.
Meaning
Milestone rates such as overall survival at 12 months could be studied as a potential early end point to measure or describe drug activity in immunotherapy trials in lung cancer.
Abstract
Importance
Novel intermediate end points may be useful to detect signals of early activity and prioritize new therapies to treat patients with advanced malignant neoplasms, including metastatic non–small cell lung cancer (mNSCLC).
Objective
To explore milestone rate, a proposed intermediate end point for immunotherapy trials.
Data Sources
Trials submitted to the US Food and Drug Administration with more than 150 patients and in which the intention-to-treat population was assessed were identified.
Study Selection
An initial meta-analysis identified 14 randomized clinical trials for treatment of mNSCLC with active controls submitted to the US Food and Drug Administration from January 1, 2003, through December 31, 2013. An additional 11 randomized clinical trials submitted from January 1, 2014, through December 31, 2016 were included.
Data Extraction and Synthesis
Two investigators abstracted data and pooled data to compare trial-level milestone ratios with conventional end points.
Main Outcomes and Measures
Trial-level milestone ratios for milestone rates were calculated for overall response rate (ORR) within 6 months, 9-month progression-free survival (PFS), 9-month overall survival (OS), and 12-month OS. A weighted linear regression model evaluated associations between milestone ratios and hazard ratios (HRs). Experimental and control arms of trials testing immunotherapy, targeted therapy, and other trials were pooled to compare Kaplan-Meier survival estimates in the 3 therapeutic classes.
Results
A total of 20 013 unique patients (65.4% male and 34.6% female; mean age, 60 [range, 18-92] years) with advanced lung cancer were identified in 25 unique trials. A moderate association was observed between 12-month OS milestone ratio and OS HR (R2 = 0.80; 95% CI, 0.63-0.91) and 9-month OS milestone ratio and OS HR (R2 = 0.67; 95% CI, 0.49-0.82). No associations were observed between 9-month PFS milestone ratio and OS HR (R2 = 0.19; 95% CI, 0.03-0.49) or 6-month ORR and OS HR (R2 = 0.05; 95% CI, 0.0001-0.31). The aggregated Kaplan-Meier analysis of immunotherapy trials vs chemotherapy revealed an OS HR of 0.69 (95% CI, 0.63-0.75) and PFS HR of 0.82 (95% CI, 0.76-0.89). Targeted therapy trials vs chemotherapy had an OS HR of 0.98 (95% CI, 0.80-1.19) and PFS HR of 0.48 (95% CI, 0.42-0.56).
Conclusions and Relevance
This analysis of milestone rates suggests a moderate association between OS milestones at 12 or 9 months and OS HR but not 9-month PFS or 6-month ORR milestones and OS HR. Although OS at 12 months had the strongest association with OS HR, it may not be the optimal time for future trials, which will increasingly have immunotherapy as the control, deploy new biomarker-enrichment strategies, and likely enroll patients with longer survival. Milestone rates may be useful as a complementary tool to summarize or interpret trial results or as a secondary end point in exploratory studies.
Introduction
In the past decade, through improved understanding of the molecular and immunologic underpinnings of cancer, new targeted therapies and immunotherapies are available to treat patients with metastatic non–small cell lung cancer (mNSCLC). Approved targeted therapies for patients with oncogenic driver mutations in EGFR (OMIM 131550), ALK (OMIM 105590), and ROS1 (OMIM 165020) result in large overall response rates (ORRs) and progression-free survival (PFS) gains over chemotherapy. In 2015 and 2016, the US Food and Drug Administration (FDA) approved 3 immune checkpoint inhibitors (anti–PD-1 [anti–programmed cell death 1] and anti–PD-L1 [anti–programmed cell death 1 ligand 1] antibodies) for treatment of patients with mNSCLC after progression of disease during platinum-based doublet chemotherapy, and in late 2016, the FDA approved an immune checkpoint inhibitor for the first-line treatment of patients with mNSCLC and high tumor PD-L1 expression based on overall survival (OS) gains compared with chemotherapy.
The FDA also approved immune checkpoint inhibitors for the treatment of patients with advanced melanoma, squamous cell carcinoma of the head and neck, classic Hodgkin lymphoma, urothelial carcinoma, and renal cell carcinoma. The broad efficacy of the anti–PD-1 and anti–PD-L1 antibodies across various malignant neoplasms has led to unprecedented levels of research and development of these agents, along with development of novel immune-based targets.
Despite the progress and robust development of new immunotherapies and targeted therapies in mNSCLC and other cancers, additional intermediate end points may be needed to detect signals of early activity, prioritize combinations, and interpret exploratory study results. Response and progression by conventional Response Evaluation Criteria in Solid Tumors (RECIST) may not fully characterize the clinical benefit of the immune checkpoint inhibitors. For example, in the all-comer second-line mNSCLC studies of anti–PD-1 and anti–PD-L1 therapy vs docetaxel, the ORR was relatively modest (approximately 15%-20%), with no improvements in PFS, but the OS was demonstrably superior.
In addition, perhaps owing to the delayed effect of certain immunotherapies in some patients, the rare unconventional radiographic patterns (eg, immune cell infiltration of a tumor mimicking disease progression), and the heterogeneity of patient populations studied, the Kaplan-Meier curves of some clinical trials comparing immunotherapy with chemotherapy show nonproportionality and delayed separation. Thus, although most patients with mNSCLC do not appear to benefit from anti–PD-1 or anti–PD-L1 antibodies, a subset of these patients derives long-term benefit. This result may be analogous to anti–CTLA-4 therapy in metastatic melanoma, in which long-term follow-up of patients demonstrated a tail of the survival curve indicating that a subset of patients experiences long-term survival.
The unique patterns of response, progression, and survival with immune checkpoint inhibitors in mNSCLC and other cancers have renewed interest in exploring novel intermediate end points to gain an early signal of activity and assist in go/no-go decision making. Milestone analysis, which looks at survival at a given time point, such as at 12 months, has been proposed as a potential intermediate end point for immunotherapy clinical trials. Potential advantages of milestone analysis include its simplicity (it is time driven), it can capture effects beyond the median with delayed separation of the Kaplan-Meier curves (assuming the milestone time is beyond the median), and it may be agnostic to nonproportionality of survival curves. To further explore milestone analysis, we updated a pooled database of mNSCLC trials submitted to the FDA and compared milestone rates with more traditional oncology end points, including ORR, PFS, and OS.
Methods
Selection Criteria
Original Analysis
A previous analysis identified 14 randomized clinical active-control trials submitted to the FDA from January 1, 2003, through December 31, 2013, that evaluated treatments for advanced mNSCLC. Only trials with more than 150 patients and in which the intention-to-treat population was assessed were included. Associations among trial-level PFS hazard ratio (HR), OS HR, and ORR odds ratio were analyzed using weighted linear regression models.
Updated Analysis
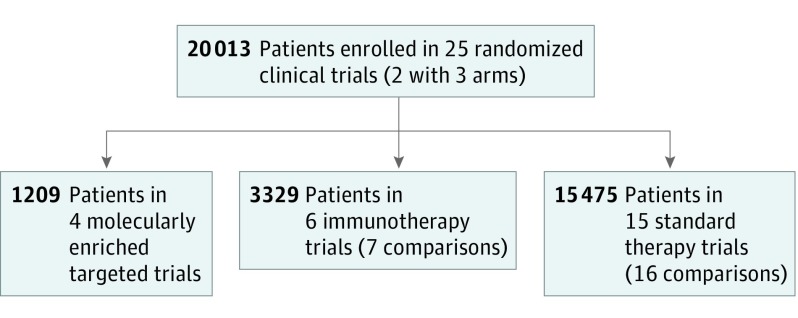
We added 11 randomized clinical trials submitted from January 1, 2014, through December 31, 2016, to the original pooled analysis for a total of 25 unique trials enrolling 20 013 unique patients (Table and Figure 1). Altogether, 4 trials were molecularly enriched for oncogene-driver mutations (EGFR mutation or ALK rearrangements) evaluating targeted therapy vs chemotherapy control, including 3 first-line trials and 1 in the second-line setting. Six trials tested immunotherapy vs chemotherapy control, including 5 that assessed immunotherapy vs docetaxel in the second-line setting and 1 that evaluated immunotherapy vs platinum-based doublet therapy in the front-line setting enriched for patients with high tumor PD-L1 expression. Fifteen trials tested various other therapies (nonmolecularly enriched targeted therapy or nonimmunotherapy) as head-to-head comparisons (n = 6) to standard of care or as an add-on to standard of care (n = 9), including 8 trials of first-line therapy and 7 trials of second-line therapy and beyond. Included in these 25 trials are a mix of positive studies (ie, leading to a new indication) and negative studies (ie, not leading to a new indication or noninferiority in design). No placebo-controlled studies were included.
Table. Summary of Trials Submitted.
| Source | Drug | Control (Trial Type)a | No. of Patients |
|---|---|---|---|
| Trials included in 2015 meta-analysis | |||
| Shaw et al, 2013 | Crizotinib | Pemetrexed (or docetaxel) (2L TT H-H) | 347 |
| Sequist et al, 2013 | Afatinib dimaleate | Cisplatin + pemetrexed (1L TT H-H) | 345 |
| Rosell et al, 2012 | Erlotinib hydrochloride | Cisplatin (carboplatin) + docetaxel (gemcitabine) (1L TT H-H) | 174 |
| Socinski et al, 2012 | Nab-paclitaxel + carboplatin | Carboplatin + paclitaxel (1L H-H) | 1052 |
| Lynch et al, 2010 | Cetuximab | Carboplatin + taxane (1L A-O) | 676 |
| Pirker et al, 2009 | Cetuximab | Cisplatin + vinorelbine tartrate (1L A-O) | 1125 |
| Natale et al, 2011 | Vandetanib | Erlotinib hydrochloride (2L H-H) | 1240 |
| de Boer et al, 2011 | Vandetanib | Pemetrexed (2L A-O) | 534 |
| Herbst et al, 2010 | Vandetanib | Docetaxel (2L A-O) | 1391 |
| Kim et al, 2008 | Gefitinib | Docetaxel (2L H-H) | 1466 |
| Reck et al, 2010 | Bevacizumabb | Cisplatin + gemcitabine (1L A-O) | 1043 |
| Sandler et al, 2006 | Bevacizumab | Carboplatin + paclitaxel (1L A-O) | 878 |
| Scagliotti et al, 2008 | Pemetrexed + cisplatin | Cisplatin + gemcitabine (1L H-H) | 1725 |
| Hanna et al, 2004 | Pemetrexed | Docetaxel (2L H-H) | 571 |
| Trials added since 2015 meta-analysis | |||
| Thatcher et al, 2015 | Necitumumab | Cisplatin + gemcitabine (1L A-O) | 1093 |
| Paz-Ares et al, 2015 | Necitumumab | Cisplatin + pemetrexed (1L A-O) | 633 |
| Garon et al, 2014 | Ramucirumab | Docetaxel (2L A-O) | 1253 |
| Soria et al, 2015 | Afatinib dimaleate | Erlotinib hydrochloride (2L H-H) | 795 |
| Borghaei et al, 2015 | Nivolumab | Docetaxel (2L IO H-H) | 582 |
| Brahmer et al, 2015 | Nivolumab | Docetaxel (2L IO H-H) | 272 |
| Solomon et al, 2014 | Crizotinib (IRC) | Cisplatin (carboplatin) + pemetrexed (1L TT H-H) | 343 |
| Herbst et al, 2016 | Pembrolizumabb | Docetaxel (2L IO H-H) | 1033 |
| Reck et al, 2016 | Pembrolizumab | Chemotherapy (1L IO H-H) | 305 |
| Fehrenbacher et al, 2016 | Atezolizumab | Docetaxel (2L IO H-H) | 287 |
| Rittmeyer et al, 2017 | Atezolizumab | Docetaxel (2L IO H-H) | 850 |
Abbreviations: A-O, add-on; H-H, head to head; IO, immunotherapy; TT, targeted therapy; 1L, first-line; 2L, second-line or beyond.
Alternative drugs are given in parentheses.
Indicates 3-arm trial with shared control.
Figure 1. Study Flowchart.
Eleven randomized clinical trials were added to the original analysis of 14 trials.
Outcome Measures
Traditional End Points
Overall survival was defined as the time from randomization to death. For patients alive at the data cutoff date, OS was censored at the last follow-up date. Progression-free survival was defined as the time from randomization to progression or death. Patients alive who had not experienced progression as of the analysis cutoff date were censored at the last disease assessment. In most of the trials, PFS was determined by RECIST. Overall response rate was defined as the proportion of patients who achieve a complete or partial response per RECIST or World Health Organization criteria. Patients with unevaluable or unknown response status were considered to be nonresponders. All analyses used the intention-to-treat population, defined as all randomized patients.
Milestone Rates and Milestone Ratios
In the updated pooled analysis, we calculated the following trial-level milestone rates using Kaplan-Meier estimates: 9-month PFS, 9-month OS, and 12-month OS. Overall response rate within the first 6 months for each trial was also calculated. We chose these particular milestones because they were near or beyond the median survival of most first- or second-line trials in mNSCLC but were at a time when the number of patients censored was not too high and the number of patients remaining at risk was not too low. From the milestone rates, we calculated milestone ratios. Milestone ratio is defined as the ratio of milestone rates between 2 treatment arms. As an example of the milestone ratio, if the estimated 9-month OS milestone rate is 50% in the experimental arm and 25% in the control arm, the milestone ratio is 2. We chose milestone ratios rather than absolute differences in milestone rates because the ratio is preferred when large variability in control arm milestone rates exists, as is the case with the studies included in the meta-analysis.
Statistical Analysis
Trial-Level Analysis
The associations between treatment effects on milestone rate and PFS and on milestone rate and OS were evaluated using weighted linear regression models, with analyses performed on a logarithmic scale and weights equal to the sample size of each randomized comparison. The coefficient of determination (R2) and the associated 95% CIs from the weighted linear regression model were used to measure the association between milestone rate and PFS and between milestone rate and OS by treatment effect. We presented treatment effects on PFS and OS using HRs estimated from Cox proportional hazards regression models and treatment effects on milestone rates using milestone ratios.
Patient-Level Pooled Analysis
For exploratory purposes, experimental and control arms for the targeted therapies, immunotherapies, and conventional therapies were pooled. Aggregated PFS and OS survival curves by treatment arm for each of these 3 therapeutic classes were explored using Kaplan-Meier estimates.
Results
The aggregated key baseline demographic and disease characteristics of the 20 013 unique patients enrolled in the 25 unique trials are listed in eTable 1 in the Supplement. Consistent with the previous analysis, the mean patient age was 60 (range, 18-92) years, and 80.2% of patients were enrolled outside the United States. Most of the patients were white (77.2%); 18.3% were Asian; and 2.1% were black. About two-thirds of patients (65.4%) were male, and 34.6% were female.
In the 25 trials, the median follow-up for PFS was 16 months (range, 8-31 months). The median follow-up for OS was 19 months (range, 10-32 months). In general, the highest rates of ORR within 6 months, 9-month PFS, and 12-month OS were observed in the 4 targeted therapy trials (eTable 2 in the Supplement). When pooling censoring patterns across all trials, rates of censoring were highest for 9-month PFS (median, 16%; range 4%-35%), followed by 12-month OS (median, 7%; range, 1%-45%) and 9-month OS (median, 5%; range, 0.7%-27%).
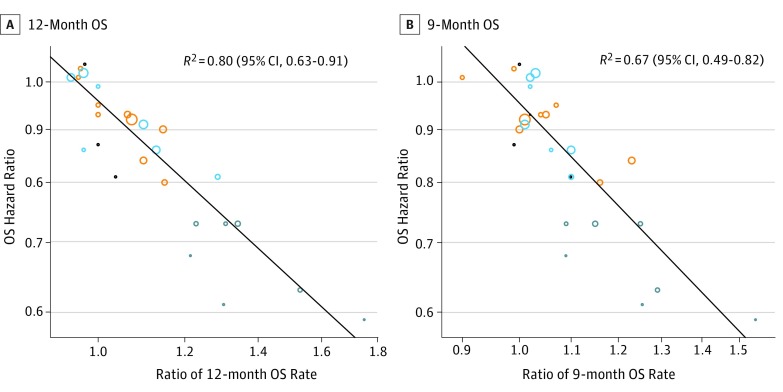
Figure 2 and eFigure 1 in the Supplement show the scatterplots of the treatment effects on the logarithmic scales, illustrating trial-level associations among the end points. As shown in Figure 2A, OS HR and 12-month OS milestone ratio were moderately associated (R2 = 0.80; 95% CI, 0.63-0.91). To assess whether any trial was more influential in the trial-level association between OS HR and 12-month OS milestone ratio, a leave-one-out cross-validation by excluding 1 trial at a time was performed. The median R2 from the cross-validation is 0.80 (range, 0.75-0.83). To assess the robustness of the findings from all 25 trials, we conducted 4 subgroup analyses, combining targeted and immunotherapies (11 comparisons), nontargeted therapies (16 comparisons), first-line studies (13 comparisons), and second-line studies (14 comparisons) (eFigure 2 in the Supplement). In this subgroup analysis, the association between OS HR and 12-month OS milestone ratio appeared to be stronger for the targeted plus immunotherapy studies (R2 = 0.78; 95% CI, 0.51-0.93) than for nontargeted studies (R2 = 0.69; 95% CI, 0.39-0.90). The association between OS HR and 12-month OS milestone ratio appeared to be stronger for second-line studies (R2 = 0.89; 95% CI, 0.72-0.98) than for first-line studies (R2 = 0.65; 95% CI, 0.20-0.91).
Figure 2. Scatterplots of Trial-Level Association Between Treatment Effects on Overall Survival (OS) Hazard Ratios (HRs) and Milestone Ratios.
Milestone ratio is calculated as the ratio of milestone rates between the experimental and control arms. A, Association between treatment effects on OS and 12-month OS rate. B, Association between treatment effects on OS and 9-month OS rate. Trials include targeted therapy trials (black), first-line nontargeted trials (orange), second-line nontargeted trials (light blue), and immunotherapy trials (dark blue).
The association between OS HR and 9-month OS milestone ratio was modest (R2 = 0.67; 95% CI, 0.49-0.82) (Figure 2B). No associations were observed between OS HR and 9-month PFS milestone ratio (R2 = 0.19; 95% CI, 0.03-0.49) or OS HR and 6-month ORR milestone ratio (R2 = 0.05; 95% CI, 0.0001-0.31), and modest association was observed between PFS HR and 9-month PFS milestone ratio (R2 = 0.62; 95% CI, 0.43-0.81) (eFigure 1 in the Supplement).
We found no association between treatment effect on OS as measured by HR and ORR odds ratio (R2 = 0.04; 95% CI, 0.0002-0.28) (eFigure 3A in the Supplement), which is consistent with the results reported in the previous analysis. The association between PFS as measured by HR and ORR as measured by odds ratio (R2 = 0.74; 95% CI, 0.55-0.88) (eFigure 3B in the Supplement) was moderate but weaker than in the prior analysis likely because of the addition of the 6 immunotherapy trials in which the association between ORR and PFS appeared to be weaker than with targeted therapy. The association between PFS HR and 6-month ORR milestone ratio was moderate (R2 = 0.70; 95% CI, 0.50-0.84) (eFigure 3C in the Supplement).
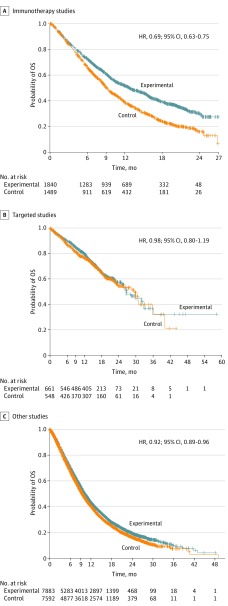
We pooled data from the 6 immunotherapy trials, the 4 targeted therapy trials, and the 15 conventional therapy trials and performed Kaplan-Meier analysis of treatment arm vs control. Each therapeutic class had different survival kinetics with respect to PFS and OS. Figure 3 depicts the OS curves. With the immunotherapy trials, the pooled OS curves appeared to separate after about 3 months and remain separated at 12, 18, and 24 months. The estimated median of OS for the immunotherapy- and chemotherapy-treated patients in this pooled analysis were 12 and 9 months, respectively (HR, 0.69; 95% CI, 0.63-0.75). In the targeted therapy trials, no separation of the OS curves between targeted therapy and control arms was discerned likely owing to high rates of crossover from the chemotherapy groups to the targeted therapy groups and the long postprogression survival time; the estimated median survival among those receiving targeted therapy was 27 months (HR, 0.98; 95% CI, 0.80-1.19). In the conventional therapy trials (a mix of positive and negative, first- and later-line, and superiority and noninferiority trials), no separation could be discerned between experimental and treatment arms and the estimated median survival was 9 months (HR, 0.92; 95% CI, 0.89-0.96).
Figure 3. Kaplan-Meier Overall Survival (OS) Estimates .
Overall survival was compared between experimental and control groups in immunotherapy, targeted, and other studies. HR indicates hazard ratio; +, censored.
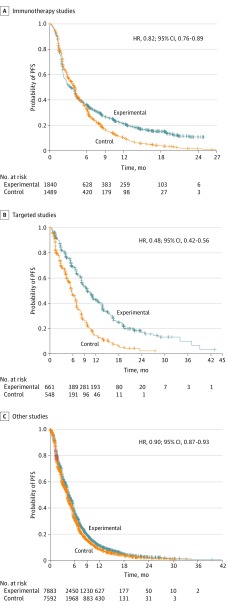
Figure 4 depicts the exploratory pooled PFS curves by therapeutic class. For the immunotherapy trials, the PFS curves separated at approximately 6 months and a fraction of patients were progression free at 12 and 18 months (HR, 0.82; 95% CI, 0.76-0.89). In contrast, in the targeted therapy trials, the PFS curves separated early, with a fraction of patients progression free at 12, 18, and 24 months (HR, 0.48; 95% CI, 0.42-0.56). In the standard-of-care studies (a heterogeneous mix of studies), no clear separation in the PFS curves could be discerned (HR, 0.90; 95% CI, 0.87- 0.93).
Figure 4. Kaplan-Meier Progression-Free Survival (PFS) Estimates.
Progression-free survival was compared between experimental and control groups in immunotherapy, targeted, and other studies. Other refers to a heterogeneous mix. HR indicates hazard ratio; +, censored.
Discussion
We explored milestone analysis for several reasons. First, traditional metrics of response and progression by RECIST may not be sufficient for signal finding or for prioritizing combinations of immunotherapies. For example, in all-comer second-line mNSCLC trials (not enriched based on presence of PD-L1 expression), the relatively modest ORR and absent PFS effects were not predictive of the survival gains achieved with anti–PD-1 and anti–PD-L1 agents vs docetaxel. Second, patients treated with immunotherapy agents such as anti–PD-1 and anti–PD-L1 therapies demonstrate unique patterns of response, progression, and survival. On a population level, these patterns can lead to delayed separation of survival curves beyond the median, nonproportional curves, and a subset of patients potentially deriving long-term benefit.
On a trial level, this exploratory analysis of milestone ratios suggests a moderate association between the OS milestone ratio at 12 months and OS HR (R2 = 0.80) and modest associations between the OS milestone ratio at 9 months and OS HR (R2 = 0.67), 6-month ORR odds ratio and PFS HR (R2 = 0.70), and the PFS milestone ratio at 9 months and PFS HR (R2 = 0.62). No associations were observed between the PFS milestone ratio at 9 months and OS HR or between the ORR milestone ratio at 6 months and OS HR. Therefore, in the present database of trials, the OS milestone ratio at 12 months had the strongest association with OS HR and could be explored prospectively as a secondary end point in mNSCLC trials or used to interpret or analyze study results for go/no-go decisions.
A specific milestone such as 12-month OS, while moderately associated with OS in our present database, may not be as strongly associated with OS HR in future trials. The optimal time point for milestone analysis may depend on the patient population studied (all-comer vs oncogene- or other marker-driven), the disease context (first-line vs later-line treatment), the therapeutic class of drug or drugs being studied (targeted vs immunotherapy vs cytotoxic chemotherapy or a combination thereof), the control arm being studied, and the magnitude of effect sought (incremental vs large or superiority vs noninferiority).
Use of the milestone rate as a clinical trial end point has several limitations, including the inability to account for the totality of the survival curve and the effect of censoring before the milestone time point. Milestones should not be thought of as conventional surrogate end points, given the significant overlap between a survival milestone and the ultimate clinical end point, OS HR. Milestone analyses may have advantages, which include predictability (being time driven rather than event driven) and simplicity of analysis. The outcome (such as survival) can be clinically meaningful, and relative and absolute differences in treatment effect can be assessed in the context of a randomized clinical trial. Furthermore, milestones (if measured at a mature time point) may be agnostic to whether the survival curves separate late or are nonproportional. Mature milestone rates may also capture subsets of patients who derive longer-term benefit.
The pooling of immunotherapy trials, targeted therapy trials, and conventional therapy trials to explore Kaplan-Meier estimates of OS and PFS showed that each therapeutic class had unique properties. In trials comparing immunotherapy with chemotherapy, there appeared to be delayed separation of PFS and OS curves, with improvement over chemotherapy, in OS (HR, 0.69; 95% CI, 0.63-0.75) and, to a lesser degree, PFS (HR, 0.82; 95% CI, 0.76-0.89). In trials comparing targeted therapy with chemotherapy, we found a large PFS effect (HR, 0.48; 95% CI, 0.42-0.56) but no effect in OS (HR, 0.98; 95% CI, 0.80-1.19) likely owing to high rates of crossover and/or long postprogression survival. The estimated median OS in the targeted therapy trials of approximately 27 months was more than double the median OS observed in the immunotherapy trials or other trials likely owing to the improved prognosis of patients with oncogene-driven tumors, such as EGFR and ALK, and to the therapeutic effect of these targeted agents.
Limitations
Some limitations are inherent to the pooling of the trials by therapeutic class, including a relatively heterogeneous mix of different study designs and patient populations included in each group, as well as different drug properties in each class. The pooled analysis indicates unique properties of OS and PFS curves with the different therapeutic classes of drugs compared with chemotherapy, which may be useful for a prospective clinical trial design. Furthermore, median estimates and milestone estimates may differ if one studies a biomarker-enriched, oncogene-driven patient population vs an all-comer population.
Conclusions
We found moderate associations between the milestone rate of OS at 12 months and OS HR but no associations with other milestone rates, such as PFS at 9 months and OS HR. Although the milestone rate should not be used as a primary intermediate end point for a pivotal study, it could be prospectively incorporated as a secondary end point for future exploratory studies, particularly those testing immunotherapy combinations vs immunotherapy alone. For a primary end point in a randomized clinical trial using a time-to-event end point, the log-rank test is preferred to detect whether an effect exists. Milestone analysis could be used as a complementary tool to describe and analyze study results, along with more conventional measures such as ORR, duration of response, PFS, and OS, as well as other less established metrics such as tumor growth rates and depth of response. Whether our findings in mNSCLC can be generalized to other cancer types should also be explored. In addition, further research into other methods that potentially address nonproportionality should be investigated to better account for therapeutic effects on long-term survival that may be observed in immunotherapy trials.
eTable 1. Key Baseline Demographic and Disease Characteristics
eTable 2. Full Summary of Trials Analyzed
eFigure 1. Scatterplots of Trial-Level Association Between Treatment Effects on OS and PFS
eFigure 2. Scatterplots of Trial-Level Association Between Treatment Effects on OS
eFigure 3. Scatterplots of Trial-Level Association Between Treatment Effects on ORR
References
- 1.Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: erlotinib for the first-line treatment of metastatic non–small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5-e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21(11):2436-2439. [DOI] [PubMed] [Google Scholar]
- 4.Kazandjian D, Blumenthal GM, Yuan W, He K, Keegan P, Pazdur R. FDA Approval of gefitinib for the treatment of patients with metastatic egfr mutation-positive non–small cell lung cancer. Clin Cancer Res. 2016;22(6):1307-1312. [DOI] [PubMed] [Google Scholar]
- 5.Kazandjian D, Blumenthal GM, Luo L, et al. Benefit-risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive, metastatic non–small cell lung cancer. Oncologist. 2016;21(8):974-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkins E, Blumenthal GM, Chen H, et al. FDA approval: alectinib for the treatment of metastatic, ALK-positive non–small cell lung cancer following crizotinib. Clin Cancer Res. 2016;22(21):5171-5176. [DOI] [PubMed] [Google Scholar]
- 7.Khozin S, Weinstock C, Blumenthal GM, et al. Osimertinib for the treatment of metastatic epidermal growth factor T970M positive non–small cell lung cancer. Clin Cancer Res. 2016;23(9):2131-2135. [DOI] [PubMed] [Google Scholar]
- 8.Kazandjian D, Khozin S, Blumenthal G, et al. Benefit-risk summary of nivolumab for patients with metastatic squamous cell lung cancer after platinum-based chemotherapy: a report from the US Food and Drug Administration. JAMA Oncol. 2016;2(1):118-122. [DOI] [PubMed] [Google Scholar]
- 9.Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non–small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non–small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016;21(5):643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brawley L. With 20 agents, 803 trials, and 166,736 patient slots, is pharma investing too heavily in PD-1 drug development? Cancer Lett. 2016;42(37). http://cancerletter.com/articles/20161007_1/. Accessed May 8, 2017. [Google Scholar]
- 12.Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15(8):457-472. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412-7420. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR Study Group . Atezolizumab versus docetaxel for patients with previously treated non–small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. [DOI] [PubMed] [Google Scholar]
- 18.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoos A, Wolchok JD, Humphrey RW, Hodi FS. CCR 20th anniversary commentary: immune-related response criteria—capturing clinical activity in immuno-oncology. Clin Cancer Res. 2015;21(22):4989-4991. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research Guidance for Industry: Clinical Considerations for Therapeutic Cancer Vaccines. October 2011. https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm278673.pdf. Accessed May 8, 2017.
- 21.Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33(10):1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen TT. Milestone survival: a potential intermediate endpoint for immune checkpoint inhibitors. Jnl Natl Cancer Inst. 2015;107(9):pii: djv156. [DOI] [PMC free article] [PubMed]
- 23.Hoos A, Topalian S, Chen TT, et al. Facilitating the Development of Immunotherapies: Intermediate Endpoints for Immune Checkpoint Modulators. Issue Brief Conference on Clinical Cancer Research, November 2013. http://www.focr.org/sites/default/files/Immunotx%20%20final%2011%204.pdf. Accessed May 8, 2017.
- 24.Hellmann MD, Kris MG, Rudin CM. Medians and milestones in describing the path to cancer cures: telling “tails”. JAMA Oncol. 2016;2(2):167-168. [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33(9):1008-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394. [DOI] [PubMed] [Google Scholar]
- 27.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334. [DOI] [PubMed] [Google Scholar]
- 28.Rosell R, Carcereny E, Gervais R, et al. ; Spanish Lung Cancer Group; Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica . Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. [DOI] [PubMed] [Google Scholar]
- 29.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non–small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055-2062. [DOI] [PubMed] [Google Scholar]
- 30.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non–small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28(6):911-917. [DOI] [PubMed] [Google Scholar]
- 31.Pirker R, Pereira JR, Szczesna A, et al. ; FLEX Study Team . Cetuximab plus chemotherapy in patients with advanced non–small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525-1531. [DOI] [PubMed] [Google Scholar]
- 32.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non–small-cell lung cancer. J Clin Oncol. 2011;29(8):1059-1066. [DOI] [PubMed] [Google Scholar]
- 33.de Boer RH, Arrieta Ó, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non–small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29(8):1067-1074. [DOI] [PubMed] [Google Scholar]
- 34.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non–small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non–small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372(9652):1809-1818. [DOI] [PubMed] [Google Scholar]
- 36.Reck M, von Pawel J, Zatloukal P, et al. ; BO17704 Study Group . Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol. 2010;21(9):1804-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355(24):2542-2550. [DOI] [PubMed] [Google Scholar]
- 38.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non–small-cell lung cancer. J Clin Oncol. 2008;26(21):3543-3551. [DOI] [PubMed] [Google Scholar]
- 39.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non–small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589-1597. [DOI] [PubMed] [Google Scholar]
- 40.Thatcher N, Hirsch FR, Luft AV, et al. ; SQUIRE Investigators . Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non–small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774. [DOI] [PubMed] [Google Scholar]
- 41.Paz-Ares L, Mezger J, Ciuleanu TE, et al. ; INSPIRE investigators . Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non–small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol. 2015;16(3):328-337. [DOI] [PubMed] [Google Scholar]
- 42.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non–small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673. [DOI] [PubMed] [Google Scholar]
- 43.Soria JC, Felip E, Cobo M, et al. ; LUX-Lung 8 Investigators . Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897-907. [DOI] [PubMed] [Google Scholar]
- 44.Solomon BJ, Mok T, Kim DW, et al. ; PROFILE 1014 Investigators . First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167-2177. [DOI] [PubMed] [Google Scholar]
- 45.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. [DOI] [PubMed] [Google Scholar]
- 46.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 47.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines). J Natl Cancer Inst. 2000;92(3):205-216. [DOI] [PubMed] [Google Scholar]
- 48.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 49.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207-214. [DOI] [PubMed] [Google Scholar]
- 50.Wilkerson J, Abdallah K, Hugh-Jones C, et al. Estimation of tumour regression and growth rates during treatment in patients with advanced prostate cancer: a retrospective analysis. Lancet Oncol. 2017;18(1):143-154. [DOI] [PubMed] [Google Scholar]
- 51.Kazandjian DG, Blumenthal GM, Zhang L, et al. Exploratory responder analyses of greatest depth of response (DepOR) and survival in patients with metastatic non-small cell lung cancer (mNSCLC) treated with a targeted therapy or immunotherapy . J Clin Oncol. 34;2016(suppl):abstr 2590 http://meetinglibrary.asco.org/content/170479-176. Accessed May 8, 2017. [Google Scholar]
- 52.Chen TT. Predicting analysis times in randomized clinical trials with cancer immunotherapy. BMC Med Res Methodol. 2016;16:12. doi:10.1186/s12874-016-0117-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mick R, Chen TT. Statistical challenges in the design of late-stage cancer immunotherapy studies. Cancer Immunol Res. 2015;3(12):1292-1298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Key Baseline Demographic and Disease Characteristics
eTable 2. Full Summary of Trials Analyzed
eFigure 1. Scatterplots of Trial-Level Association Between Treatment Effects on OS and PFS
eFigure 2. Scatterplots of Trial-Level Association Between Treatment Effects on OS
eFigure 3. Scatterplots of Trial-Level Association Between Treatment Effects on ORR