Abstract
This study maps the cellular response to the WEE1 inhibitor and explores the contributions of p53 to its effect.
We previously reported on the use of the high-throughput RNA interference kinome screen to identify genes required for the survival of p53 mutant head and neck squamous cell carcinoma (HNSCC) cells but not that of normal cells. We identified p53 synthetic lethal interactions with several G2/M checkpoint regulators, including WEE1 and CHK1, and found HNSCC killing by the WEE1 inhibitor AZD1775. During DNA damage, WEE1 initiates G2/M arrest via inhibition of cyclin-dependent kinase 1 to prevent mitosis and allow DNA repair. WEE1 also stabilizes DNA replication forks during S phase via a cyclin-dependent kinase 2 mechanism. Thus, WEE1 inhibition likely leads to unrestrained or premature mitosis and replication stress.
To more precisely map the cellular response to the WEE1 inhibitor and explore the contributions of p53 to its effect, we used flow cytometry and immunohistochemistry in HNSCC cell lines with defined p53 status. Understanding the mechanism of WEE1 inhibition and its dependence on p53 will help us optimize drug combination strategies for targeted therapy against p53-negative cancers.
Methods
To pinpoint p53-specific effects, we generated an isogenic cell line pair (UM-SCC-74A pBabe vector control and UM-SCC-74A p53 knockdown) by transducing a retroviral shp53 vector to the p53 wild-type parental line UM-SCC-74A (Figure 1A). Cells were treated with 500 nM of AZD1775 alone from 0 to 32 hours in a pulse chase experiment with 5-ethynyl-2-deoxyuridine (EdU) to label cells undergoing replication. Fixation and intracellular staining were performed to measure cell cycle, DNA damage, premature mitosis, and DNA content using antibodies for EdU, phosphorylated histone H2AX (γ-H2AX), histone H3S10P+ (HH3S10P+), and 4′,6-diamidino-2-phenylindole dye. Flow cytometry was performed on a BD Canto II system (BD Biosciences) with a minimum of 10 000 events captured. In conjunction, we performed immunofluorescence in situ against 4′,6-diamidino-2-phenylindole, EdU, and phosphorylated replication protein A (S4/S8). Phosphorylated replication protein A is a specific marker to quantify response to stalled and collapsed replication forks as a marker of replication-related stress. This study was approved by the institutional review board at the Fred Hutchinson Research Cancer Center.
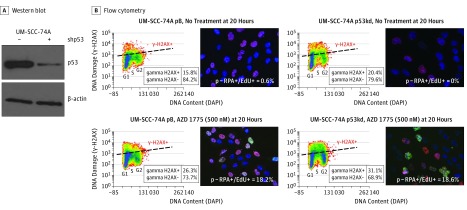
Figure 1. Markers of DNA Damage and Replication Stress After AZD1775 Therapy in a p53 Wild-Type vs Knockdown Isogenic Cell Line Model.
A, Western blot of UM-SCC-74A demonstrating knockdown of the p53 protein (UM-SCC-74A p53kd). B, Flow cytometry of UM-SCC-74A pBabe vector control (UMSCC-74A pB) and UM-SCC-74A p53kd showing staining of phosphorylated histone H2AX (γ-H2AX) with WEE1 inhibitor treatment (first columns); corresponding immunofluorescent staining of cells with phosphorylated replication protein A–positive (p-RPA+) (green) and 5-ethynyl-2-deoxyuridine–positive (EdU+) (red) (second columns). DAPI indicates 4′,6-diamidino-2-phenylindole.
Results
We observed that AZD1775 triggered DNA damage as measured by γ-H2AX, particularly in mid S phase of the cell cycle (Figure 1B). In addition, cells treated with AZD1775 stained double positive with EdU and phosphorylated replication protein A by immunofluorescence (Figure 1B). These 2 observations suggest that replication stress represents one specific mechanism of cellular response to WEE1 inhibition. Although there was increased overall DNA damage in p53-deficient lines, the percentage of cells positive for replication stress was equivalent in p53 functional and p53-deficient cell lines (18.2% vs 18.6%). The replication stress response induced by WEE1 inhibition represents a pathway independent of p53 status.
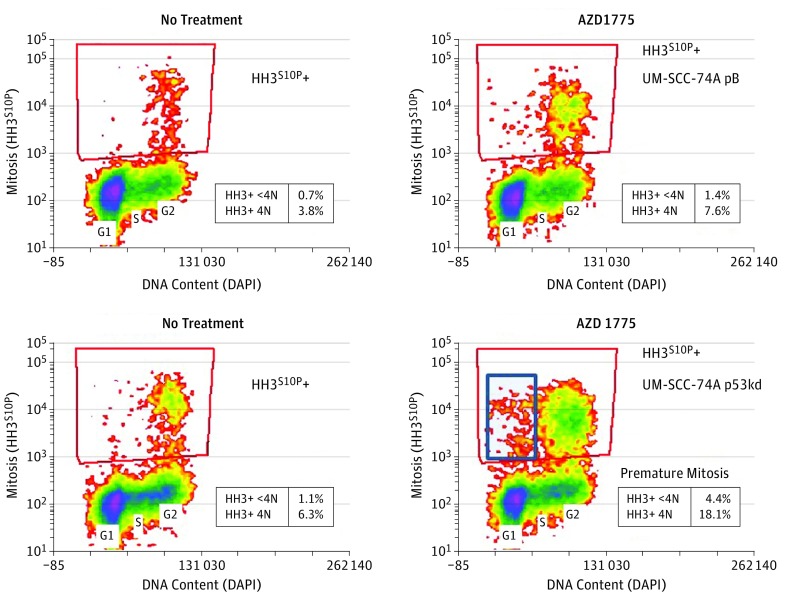
In later phases of the cell cycle, treatment with AZD1775 markedly (≥3-fold) upregulated mitosis (HH3S10P+), including premature mitosis (HH3S10P+, <4N DNA content) only in the UM-SCC74A p53-deficient line (Figure 2). This abrogation of the G2/M checkpoint by the WEE1 inhibitor represents a pathway to mitotic catastrophe that is specific to p53 status.
Figure 2. Flow Cytometry After 24-Hour Treatment With AZD1775.
Premature mitosis (histone H3S10P+ [HH3S10P+], <4N DNA content) is seen only in the p53-deficient line. DAPI indicates 4′,6-diamidino-2-phenylindole.
Discussion
Our data suggest that even as a single-agent AZD1775 is significantly more cytotoxic to p53 mutated than to p53 wild-type HNSCC cell lines. We characterize 2 separate mechanisms of action of AZD1775 in HNSCC cell lines: DNA replication stress and DNA damage independent of p53 status and abrogation of the G2/M checkpoint, which resulted in premature mitosis in p53-deficient lines. Elucidation of the mechanisms by which AZD1775 induces replication stress and abrogates the G2/M checkpoint is needed to maximize the effects of WEE1 therapy in p53-deficient HNSCC. We have already begun clinical translation of these findings, launching a phase 1 clinical trial with AZD1775 given neoadjuvantly to patients with HNSCC.
References
- 1.Moser R, Xu C, Kao M, et al. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin Cancer Res. 2014;20(16):4274-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarts M, Sharpe R, Garcia-Murillas I, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov. 2012;2(6):524-539. [DOI] [PubMed] [Google Scholar]
- 3.Domínguez-Kelly R, Martín Y, Koundrioukoff S, et al. Wee1 controls genomic stability during replication by regulating the Mus81-Eme1 endonuclease. J Cell Biol. 2011;194(4):567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirbu BM, Couch FB, Feigerle JT, Bhaskara S, Hiebert SW, Cortez D. Analysis of protein dynamics at active, stalled, and collapsed replication forks. Genes Dev. 2011;25(12):1320-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.clinicaltrials.gov WEE1 Inhibitor MK-1775, Docetaxel, and Cisplatin Before Surgery in Treating Patients With Borderline Resectable Stage III-IVB Squamous Cell Carcinoma of the Head and Neck. NCT02508246. https://clinicaltrials.gov/ct2/show/NCT02508246. Accessed January 25, 2016.