Abstract
Importance
The combination of fluorouracil, oxaliplatin, and irinotecan plus bevacizumab (FOLFOXIRI-Bev) is an established and effective first-line chemotherapy regimen for metastatic colorectal cancer. However, resection rates of metastases and overall survival with this schedule have never been systematically evaluated in published studies including, but not limited to, the TRIBE (TRIplet plus BEvacizumab) trial.
Objective
To assess the clinical efficacy of FOLFOXIRI-Bev, including outcomes and rates of surgical conversions.
Data Sources
A systematic review was conducted in October 2016 in concordance with the PRISMA guidelines of PubMed, the Cochrane Central Register of Controlled Trials, SCOPUS, Web of Science, Google Scholar, CINAHL, Ovid, and EMBASE using the terms FOLFOXIRI and bevacizumab and (colorectal cancer).
Study Selection
Clinical trials, retrospective case series, and prospective case series that used FOLFOXIRI-Bev for the treatment of initially unresectable metastatic colorectal cancer in humans were included. Individual case reports and retrospective case series with fewer than 10 patients were excluded.
Data Extraction and Synthesis
Data were extracted independently by 2 reviewers on a predesigned, standardized form. Ultimately, data were aggregated to obtain the pooled effect size of efficacy, according to the random-effects model and weighted for the number of patients included in each trial.
Main Outcomes and Measures
Median overall survival and progression-free survival, overall response rates, and rates of R0 surgical conversions and overall surgical conversions.
Results
Eleven FOLFOXIRI-Bev studies published between 2010 and 2016 met the inclusion criteria and were pooled for analysis. The studies included 889 patients, with 877 patients clinically evaluable for overall response rates. The objective response rate to FOLFOXIRI-Bev was 69% (95% CI, 65%-72%; I2 = 25%). The rate of overall surgical conversions was 39.1% (95% CI, 26.9%-52.8%), and the rate of R0 surgical conversions was 28.1% (95% CI, 18.1%-40.8%). Median pooled overall survival was 30.2 months (95% CI, 26.5-33.7 months) in 6 trials with data available, and progression-free survival was 12.4 months (95% CI, 10.0-14.3 months) in 9 trials with data available. In meta-regression analysis, variables significantly associated with conversion surgery were disease limited to the liver and a higher median number of cycles (close to 12).
Conclusions and Relevance
For patients with surgically unresectable metastatic colorectal cancer, FOLFOXIRI-Bev is associated with a significant overall response rate. Such an effective regimen leads to a probability of surgical conversion of distant metastases approaching 40%, with more than one-fourth of patients having an R0 resection.
This systematic review and pooled analysis assesses the efficacy of FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer.
Key Points
Question
What is the efficacy of fluorouracil, oxaliplatin, and irinotecan plus bevacizumab (FOLFOXIRI-Bev) in terms of secondary surgical resections in patients with metastatic colorectal cancer?
Findings
In this systematic review and pooled analysis of 11 studies of FOLFOXIRI-Bev for metastatic colorectal cancer, the rate of overall surgical conversions was 39.1% and the rate of R0 surgical conversions was 28.1%; the objective response rate was 69%.
Meaning
In patients with unresectable metastatic colorectal cancer, FOLFOXIRI-Bev is associated with a significant objective response rate and a probability of surgical conversion of distant metastases approaching 40%. Overall, more than one-fourth of patients achieve an R0 resection.
Introduction
Colorectal cancer (CRC) is one the most frequently diagnosed malignant neoplasms worldwide and a leading cause of cancer-related death. Approximately 25% of patients present with liver metastases at the time of the first diagnosis, and up to 50% will further develop recurrence in the liver during their disease course. Surgical resection of metastases represents the only potentially curative strategy. In retrospective series, the 5-year survival rate of patients who undergo liver metastasectomy approaches 50%. However, only 20% to 30% of patients with metastatic CRC have a disease that is confined to the liver. Most of these patients have liver disease that is considered unresectable at presentation. Nevertheless, because of the availability of effective chemotherapy regimens and the development of innovative surgical techniques, an increasing number of patients whose disease is initially considered unresectable may find that their disease becomes resectable following treatment (a process known as conversion therapy). According to recent data, after conversion hepatectomy, patients can achieve survival rates similar to those of patients who underwent liver resection initially.
As demonstrated by Folprecht and colleagues, there is a strong correlation between response rates and liver resection rates among patients who have isolated liver involvement. In the metastatic setting, the addition of targeted agents to classic chemotherapy has been shown to significantly improve the objective response rate (ORR) and consequently increase the proportion of patients eligible for surgical resection.
Among the most effective treatment regimens in terms of ORR is the combination of fluorouracil, oxaliplatin, and irinotecan (FOLFOXIRI) with bevacizumab (FOLFOXIRI-Bev). This schedule yielded an 80% ORR among 30 patients with metastatic liver-limited disease (LLD), 40% of whom could undergo a curative (R0) resection. The subsequent phase 3 randomized TRIBE (TRIplet plus BEvacizumab) trial, which compared FOLFOXIRI-Bev with fluorouracil and irinotecan plus bevacizumab in a population of patients with unresectable metastatic CRC, showed a secondary curative R0 resection rate of 32% in the subgroup of patients with LLD. We therefore performed a systematic review and pooled analysis to assess the clinical efficacy of FOLFOXIRI-Bev, including long-term outcomes and rate of surgical conversion.
Methods
Search Strategy
The review was conducted according to the PRISMA guidelines for systematic reviews. We searched for available articles, both published and in abstract form, that evaluated the efficacy of FOLFOXIRI-Bev for the treatment of unresectable CRC. A literature search in PubMed, the Cochrane Central Register of Controlled Trials, SCOPUS, Web of Science, Google Scholar, CINAHL, Ovid, and EMBASE was performed in October 2016 using the following terms: FOLFOXIRI and bevacizumab and (colorectal cancer). A manual update of meeting abstracts presented at the 2016 European Society of Medical Oncology was also performed.
Study Selection and Data Extraction
The search was then narrowed down to include only prospective clinical trials, retrospective cohort series, or prospective cohort series that used FOLFOXIRI and bevacizumab for the treatment of unresectable CRC. Individual case reports and case series including fewer than 10 patients were excluded for the high risk of publication bias. Only articles written in English involving humans with metastatic unresectable CRC were included. After this initial selection process, the remaining titles and abstracts were screened for relevance independently by 2 of the 6 authors (G.T. and F.P.). Finally, full-text articles were reviewed for all studies that appeared to meet the inclusion criteria.
The primary end point was overall resection rate. Secondary end points were R0 resection rate, ORR, median progression-free survival (PFS), and overall survival (OS). Data were extracted independently by 2 reviewers (G.T. and F.P.) and entered into a standardized, predesigned Microsoft Excel form (Microsoft Corp). The following data were recorded: number of total patients, number of patients evaluable for response, dose and schedule, median cycles received, median OS, median PFS, ORR, rate of overall resections, and rate of R0 resections among all evaluable patients. Each reviewer also assessed the quality of reporting (Jadad scale for randomized clinical trials and Newcastle-Ottawa Scale for nonrandomized or retrospective studies).
Statistical Analysis
For dichotomous variables (resections and objective responses), we calculated raw proportions of events divided by the total number of clinically evaluable patients. Owing to the heterogeneity in study size and to the large variations in proportions, we calculated weighted pooled rates of events by the number of clinically evaluable patients, according to a random-effects model to account for heterogeneity. Median pooled weighted OS and PFS were calculated with descriptive statistics. Subgroup analyses were performed per type of publication (abstract vs full article), site of disease (LLD vs not LLD), and type of study (retrospective or not randomized vs randomized). Analyses were conducted using Comprehensive Meta Analysis, version 3.exe software (Biostat, Inc). A meta-regression analysis was also performed to investigate variables possibly associated with resection of metastases, including performance status, rate of LLD, median number of cycles, and median age.
An extensive search strategy was made to minimize the potential for publication bias. Graphical funnel plots were generated to visually assess a publication bias for primary end points. The Begg test and Egger funnel plots were performed to assess the funnel plot asymmetry.
Results
Literature Search
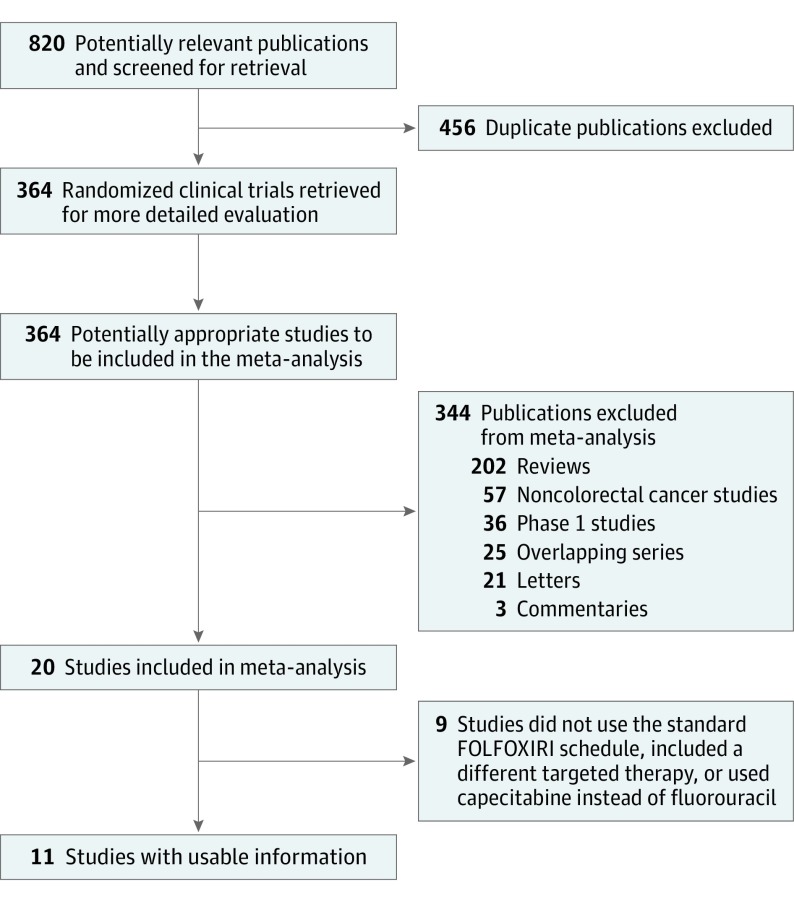
Overall, 820 records were identified according to the search strategy; 800 records were excluded after screening the titles and abstracts. Of the remaining 20 potentially relevant studies, 5 were excluded because of a lack of bevacizumab in combination with FOLFOXIRI, because of a different schedule of the 4 agents or the use of capecitabine instead of fluorouracil, or because of the association of chemotherapy with other targeted therapies. Ultimately, we enrolled 11 studies involving 889 patients with CRC. Figure 1 shows the studies’ selection process.
Figure 1. Overview of Trials Search and Selection.
FOLFOXIRI indicates fluorouracil, oxaliplatin, and irinotecan.
Included Studies
The studies included were published between 2010 and 2016 and comprised 1 prospective cohort study, 2 retrospective cohort studies, 2 phase 2 studies, 1 phase 1-2 study, 4 randomized phase 2 studies, and 1 phase 3 study. Follow-up data were available for 6 studies, with a median follow-up period between 15.0 and 48.1 months. In all series except 1, the classical FOLFOXIRI regimen developed by the GONO group (Gruppo Oncologico del Nord Ovest) (without fluorouracil bolus on day 1 and 3200-mg/m2 continuous infusion for 48 hours plus 200 mg/m2 of folinic acid, 85 mg/m2 of oxaliplatin, and 160 mg/m2 of irinotecan on day 1) was used. The eTable in the Supplement summarizes the characteristics of the included studies and chemotherapeutic agents.
Patients’ Characteristics
Almost all patients included had metastases of CRC deemed unresectable at the time of study entry. Of these patients, 18% to 100% had LLD (only in 1 study was the rate of patients with LLD not reported). The median age of these patients was generally younger than 70 years (range, 56-63 years). The median number of cycles received ranged from 5 to 12 in 6 publications with such information available.
Pooled Overall and R0 Resection Rate
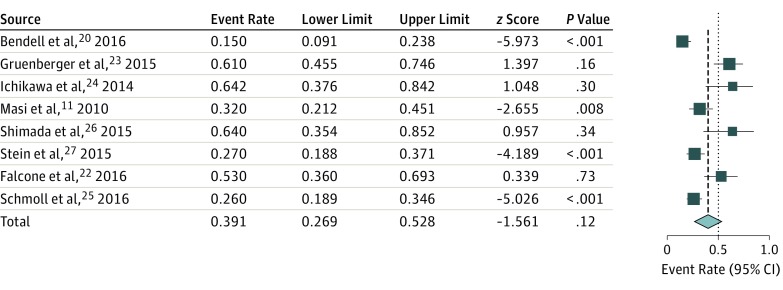
Overall rates of resection of metastases were available in 8 studies, and rates of R0 resections were available in 8 studies. The pooled rate of overall resections was 39.1% (95% CI, 26.9%-52.8%; I2 = 84.4% according to the random-effects model; Figure 2), and the pooled rate of R0 resections (all sites of surgical procedures) was 28.1% (95% CI, 18.1%-40.8%; I2 = 85.0%).
Figure 2. Pooled Analysis of Resection Rate of Liver Metastases.
The sizes of the data markers vary according to the number of patients in the study.
The pooled overall resection rate was similar when excluding series published in abstract form, at 38.8% (95% CI, 28.7%-59.2%; I2 = 85.4%). The rate of pooled overall resections was 36.1% (95% CI, 18.1%-59.1%; I2 = 91.0%) in randomized studies and 42.1% (95% CI, 26.2%-59.8%; I2 = 73.4%) in nonrandomized studies. By excluding the retrospective study by Shimada et al, which enrolled only 12 patients, the pooled overall resection rate was 36.6% (95% CI, 24.6%-50.5%). For patients with LLD, the overall rate of resections was 62.2% and the rate of R0 resections was 54.7%; in all patients who did not have LLD, the overall rate of resection was 28.7% and the rate of R0 resections was 16.9%. Meta-regression analysis showed that the only variables significantly associated with resection were the rate of LLD and a higher median number of cycles administered.
Overall Response Rate and Outcome (Median OS and PFS)
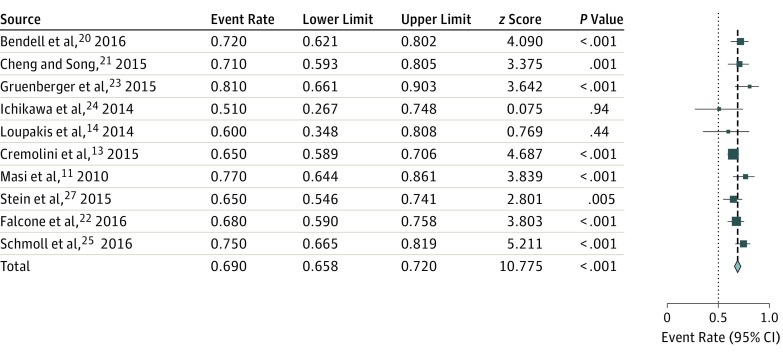
The rate of R0 resections in only published studies was 22.8% (95% CI, 14.1%-34.7%; I2 = 83.7%). In randomized studies, the rate of R0 resections was 23.3% (95% CI, 10.1%-45.3%; I2 = 91.6%), and in nonrandomized studies, the rate was 32.1% (95% CI, 17.9%-50.8%; I2 = 81.9%). Overall, 10 authors presented ORR data (complete and partial responses), with a pooled ORR of 69% (95% CI, 65%-72%; I2 = 25%; Figure 3).
Figure 3. Pooled Overall Response Rate With Fluorouracil, Oxaliplatin, and Irinotecan Plus Bevacizumab.
The sizes of the data markers vary according to the number of patients in the study.
The pooled median OS was 30.2 months (95% CI, 26.5-33.7 months) in 6 trials with data available. The pooled median PFS was 12.4 months (95% CI, 10.0-14.3 months) in 9 studies with data available. Efficacy results are presented in the Table.
Table. Summary Statistics of Pooled Analysis of FOLFOXIRI-Bev Efficacy.
| Characteristic | Range of Raw Values Between the Studies | Weighted Pooled Mean |
|---|---|---|
| Median OS, mo | 24.1-32.2 | 30.2 |
| Median PFS, mo | 9.2-18.6 | 12.4 |
| Response rate, % | 51-82 | 69 |
| Resection rate, % | 15.0-64.2 | 39.1 |
| R0 resection rate, % | 15.0-64.2 | 28.1 |
Abbreviations: FOLFOXIRI-Bev, fluorouracil, oxaliplatin, and irinotecan plus bevacizumab; OS, overall survival; PFS, progression-free survival.
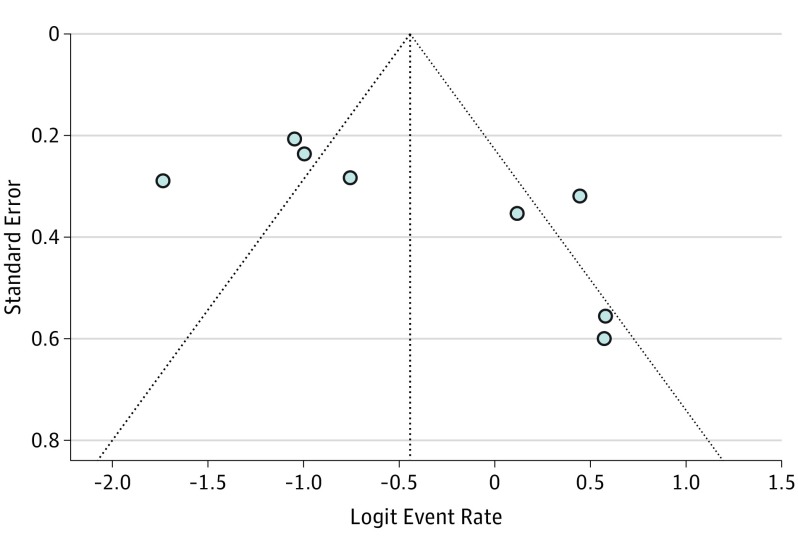
According to the Begg test, there was no obvious publication bias (Figure 4). However, the results of the Egger test were significant. The 1-study–removed procedure showed that the resection rate could be 35.4% when the study by Gruenberger et al was removed and could be 43.5% when the study by Bendell et al was removed.
Figure 4. Funnel Plot of Publication Bias for Resection Rate.
Discussion
The treatment of metastatic CRC has rapidly evolved during the last few years, and the outcomes of patients have significantly improved thanks to the availability of novel biologic targeted agents combined with traditional chemotherapy and to the refining of surgical indications. However, the resection of metastatic disease still represents the only strategy potentially leading to a definitive cure.
The results of this systematic review and pooled analysis confirm the significant clinical value of the intensification of chemotherapy in combination with the inhibition of angiogenesis in downsizing CRC metastases and facilitating secondary resection. Findings indicate that FOLFOXIRI-Bev is associated with an ORR of 70%, which translates to a 40% probability to undergo surgical conversion in a population initially deemed to have unresectable metastatic disease. The rates of R0 resections were also high, and these interventions were possible for more than 25% of all patients.
A multidisciplinary approach is mandatory to ensure that patients with upfront unresectable metastases may be properly managed. In fact, criteria for resectability have expanded in recent years, and an increasing number of patients can be converted to surgery after an initial systemic treatment approach.
Systemic conversion therapy may include combinations of chemotherapeutic agents (fluorouracil, irinotecan, or oxaliplatin) alone or in combination with monoclonal antibodies targeting angiogenesis (ie, bevacizumab) or agents that act on the epidermal growth factor receptor pathway (ie, cetuximab and panitumumab). Since probability to undergo liver surgery increases with response rate, the choice of the upfront chemotherapy regimen is of the utmost importance, especially for patients with only liver involvement. A recent meta-analysis showed that the association of monoclonal antibodies with standard chemotherapy has the potential to significantly enhance the efficiency of surgical conversion in patients with potentially resectable CRC liver metastases. Compared with anti–vascular endothelial growth factor agents, anti–epidermal growth factor receptor agents may be more effective, particularly in the subgroup of patients with wild-type RAS (KRAS, OMIM 190070; NRAS, OMIM 164790) biomarkers. No significant benefit was provided by the addition of either targeted agent for patients with mutant RAS.
Our findings are particularly interesting if we consider that only 3 studies among all examined included patients with LLD. As seen in the results of our secondary analyses, in this specific subgroup, overall and R0 resection rates are expected to be even higher (62.2% overall and 54.7% for R0 resection). In addition to LLD, receiving a high number of chemotherapy cycles (close to 12) seemed to significantly enhance the probability of undergoing secondary liver surgery. Consistent with these data, the results of the randomized phase 2 OLIVIA trial, conducted for 80 patients with upfront unresectable LLD, clearly demonstrated that FOLFOXIRI-Bev was better than fluorouracil and oxaliplatin plus bevacizumab in terms of ORR (81% vs 62%), overall resection rate (61% vs 49%), R0 resection rate (49% vs 23%), and PFS (18.6 vs 11.5 months). Although coming from indirect comparisons, our results seem to compare favorably with those reported in trials of doublet chemotherapy plus cetuximab, where R0 resection rates vary from 11.8% to 38.0%.
Results from studies evaluating chemotherapy plus anti–epidermal growth factor receptor agents refer to patients molecularly selected for not presenting with RAS mutations. Conversely, data from our analysis are derived from a series of biologically unselected patients in which more than 50% of tumors were RAS- or BRAF (OMIM 164757)–mutated overall. Since the adverse prognostic role of RAS and BRAF mutations in metastatic CRC is now well established, the inclusion of patient populations with such a poor prognosis was likely to result in a more conservative estimation of the treatment effect on patients with wild-type RAS, where the prognosis is expected to be reasonably better.
Finally, overall OS and PFS results (OS, 30.2 months; PFS, 12.4 months), although not striking, are highly consistent with those reported in the phase 3 TRIBE trial, probably reflecting the poor prognostic baseline characteristics of a population of patients with extensive metastatic involvement who were unselected for secondary resection intent.
Limitations
Some limitations of this meta-analysis must be pointed out. Some studies were small prospective single-institution phase 2 trials that could have overemphasized the final results. Also, the rate of resections could have been reported differently according to the primary site of disease (colon cancer with LLD) and the ability of the surgeons of the enrolling centers. Finally, the pooled analysis is not able to compare FOLFOXIRI-Bev with other active regimens but only describe the efficacy of this combination in an unselected population of patients with very advanced CRC. However, this systematic review includes all published series enrolling patients treated with FOLFOXIRI-Bev in phase 2 to 3 trials, including but not limited to the TRIBE study, and identifies a combination extremely useful for conversion purposes.
Conclusions
Our findings show that FOLFOXIRI-Bev represents a very effective therapeutic combination associated with a significant ORR. For patients with molecularly unselected and surgically unresectable metastatic CRC, triplet chemotherapy plus bevacizumab is able to not only control disease dissemination but induce rapid and deep cytoreduction that may translate into consistent probabilities to undergo secondary resection. Therefore, a multidisciplinary approach to advanced CRC is crucial to guarantee an optimal integration of highly active upfront treatments with surgical procedures to ultimately improve patients’ long-term outcome.
eTable. Characteristics of Included Studies and Efficacy of FOLFOXIRI-Bevacizumab
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [DOI] [PubMed] [Google Scholar]
- 2.Garden OJ, Rees M, Poston GJ, et al. . Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(suppl 3):iii1-iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choti MA, Sitzmann JV, Tiburi MF, et al. . Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JJ, D’Angelica MI. Surgical management of hepatic metastases of colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):61-84. [DOI] [PubMed] [Google Scholar]
- 5.Maeda Y, Shinohara T, Nagatsu A, Futakawa N, Hamada T. Long-term outcomes of conversion hepatectomy for initially unresectable colorectal liver metastases. Ann Surg Oncol. 2016;23(suppl 2):S242-S248. [DOI] [PubMed] [Google Scholar]
- 6.Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16(8):1311-1319. [DOI] [PubMed] [Google Scholar]
- 7.Bokemeyer C, Bondarenko I, Makhson A, et al. . Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663-671. [DOI] [PubMed] [Google Scholar]
- 8.Douillard JY, Zemelka T, Fountzilas G, et al. . FOLFOX4 with cetuximab vs. UFOX with cetuximab as first-line therapy in metastatic colorectal cancer: The randomized phase II FUTURE study. Clin Colorectal Cancer. 2014;13(1):14-26.e1. [DOI] [PubMed] [Google Scholar]
- 9.Folprecht G, Gruenberger T, Bechstein WO, et al. . Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38-47. [DOI] [PubMed] [Google Scholar]
- 10.Okines A, Puerto OD, Cunningham D, et al. . Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer first BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101(7):1033-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masi G, Loupakis F, Salvatore L, et al. . Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(9):845-852. [DOI] [PubMed] [Google Scholar]
- 12.Cremolini C, Loupakis F, Antoniotti C, et al. . Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188-1194. [DOI] [PubMed] [Google Scholar]
- 13.Cremolini C, Loupakis F, Antoniotti C, et al. . FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306-1315. [DOI] [PubMed] [Google Scholar]
- 14.Loupakis F, Cremolini C, Masi G, et al. . Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609-1618. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. . Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 29, 2015.
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendell JC, Tan BR, Reeves JA, et al. Overall response rate (ORR) in STEAM, a randomized, open-label, phase 2 trial of sequential and concurrent FOLFOXIRI-bevacizumab (BEV) vs FOLFOX-BEV for the first-line (1L) treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC). In: 2016 Gastrointestinal Cancers Symposium; January 21, 2016; San Francisco, CA. Abstract 492. [Google Scholar]
- 21.Cheng Y, Song W. Efficacy of FOLFOXIRI versus XELOXIRI plus bevacizumab in the treatment of metastatic colorectal cancer. Int J Clin Exp Med. 2015;8(10):18713-18720. [PMC free article] [PubMed] [Google Scholar]
- 22.Falcone A, Cremolini C, Loupakis F, et al. FOLFOXIRI plus bevacizumab (bev) followed by maintenance with bev alone or bev plus metronomic chemotherapy (metroCT) in metastatic colorectal cancer (mCRC): the phase II randomized MOMA trial. In: 2016 ESMO Congress; October 10, 2016; Copenhagen, Denmark. Abstract LBA21. [Google Scholar]
- 23.Gruenberger T, Bridgewater J, Chau I, et al. . Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702-708. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa Y, Goto A, Kobayashi N, et al. FOLFOXIRI+B-mab showed powerful effect as preoperative chemotherapy for multiple liver metastases of colorectal cancer. In: 2014 AACR Annual Meeting; April 5, 2014; San Diego, CA. Abstract CT318. [Google Scholar]
- 25.Schmoll HJ, Garlipp B, Junghanb C, et al. FOLFOX/bevacizumab (beva) +/− irinotecan in advanced colorectal cancer (CRC): a randomized phase II trial (AIO KRK 0209, CHARTA). In: 2016 ESMO Congress; October 10, 2016; Copenhagen, Denmark. Abstract LBA22. [Google Scholar]
- 26.Shimada M, Morine Y, Imura S, et al. Impact of FOLFOXIRI regimen on early “conversion” and long-term outcome in patients with initially unresectable colorectal liver metastases. In: 2015 Gastrointestinal Cancers Symposium; January 15, 2015; San Francisco, CA. Abstract 416. [Google Scholar]
- 27.Stein A, Atanackovic D, Hildebrandt B, et al. . Upfront FOLFOXIRI+bevacizumab followed by fluoropyrimidin and bevacizumab maintenance in patients with molecularly unselected metastatic colorectal cancer. Br J Cancer. 2015;113(6):872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masi G, Allegrini G, Cupini S, et al. . First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of a phase II study with a simplified biweekly schedule. Ann Oncol. 2004;15(12):1766-1772. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Sun Y, Zhao B, Zhang H, Yu Q, Yuan X. Chemotherapy plus targeted drugs in conversion therapy for potentially resectable colorectal liver metastases: a meta-analysis. Oncotarget. 2016;7(34):55732-55740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douillard JY, Siena S, Peeters M, Koukakis R, Terwey JH, Tabernero J. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2015;51(10):1231-1242. [DOI] [PubMed] [Google Scholar]
- 31.Ye LC, Liu TS, Ren L, et al. . Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31(16):1931-1938. [DOI] [PubMed] [Google Scholar]
- 32.Kohne CH, Bokemeyer C, Folprecht G, et al. Chemotherapy plus cetuximab in patients with liver-limited or non-liver-limited KRAS wild-type colorectal metastases: a pooled analysis of the CRYSTAL and OPUS studies. In: 2012 ASCO Annual Meeting; June 1, 2012; Chicago, IL. Abstract 96. [Google Scholar]
- 33.Modest DP, Ricard I, Heinemann V, et al. . Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27(9):1746-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of Included Studies and Efficacy of FOLFOXIRI-Bevacizumab