Key Points
Question
What role does minimal residual disease (MRD) status have in acute lymphoblastic leukemia (ALL)?
Findings
We used prospective inclusion criteria to identify 39 studies with 13 637 patients. For both pediatric and adult patients with ALL, MRD negativity was associated with much better long-term outcome. For example, 10-year event-free survival for MRD negativity vs MRD was 77% vs 32% for pediatrics and 64% vs 21% for adults.
Meaning
In patients with ALL, MRD status is a useful indicator of therapeutic benefit in clinical practice and has potential for making drug development more efficient by providing early evidence of treatment benefit.
This meta-analysis examines the association of minimal residual disease status with acute lymphoblastic leukemia in children and adults.
Abstract
Importance
Minimal residual disease (MRD) refers to the presence of disease in cases deemed to be in complete remission by conventional pathologic analysis. Assessing the association of MRD status following induction therapy in patients with acute lymphoblastic leukemia (ALL) with relapse and mortality may improve the efficiency of clinical trials and accelerate drug development.
Objective
To quantify the relationships between event-free survival (EFS) and overall survival (OS) with MRD status in pediatric and adult ALL using publications of clinical trials and other databases.
Data Sources
Clinical studies in ALL identified via searches of PubMed, MEDLINE, and clinicaltrials.gov.
Study Selection
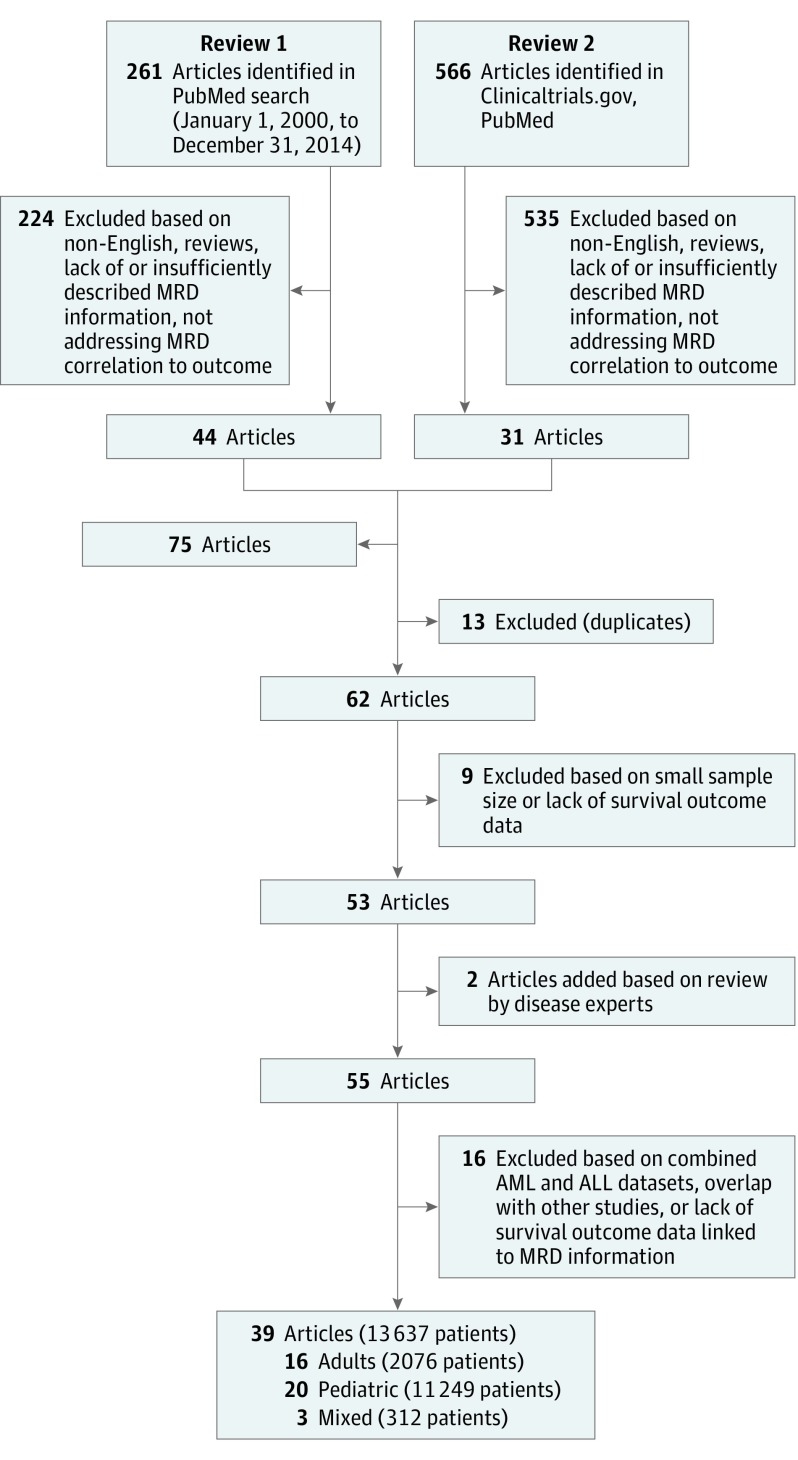
Our search and study screening process adhered to the PRISMA Guidelines. Studies that addressed EFS or OS by MRD status in patients with ALL were included; reviews, abstracts, and studies with fewer than 30 patients or insufficient MRD description were excluded.
Data Extraction and Synthesis
Study sample size, patient age, follow-up time, timing of MRD assessment (postinduction or consolidation), MRD detection method, phenotype/genotype (B cell, T cell, Philadelphia chromosome), and EFS and OS. Searches of PubMed and MEDLINE identified 566 articles. A parallel search on clinicaltrials.gov found 67 closed trials and 62 open trials as of 2014. Merging results of 2 independent searches and applying exclusions gave 39 publications in 3 arms of patient populations (adult, pediatric, and mixed). We performed separate meta-analyses for each of these 3 subpopulations.
Results
The 39 publications comprised 13 637 patients: 16 adult studies (2076 patients), 20 pediatric (11 249 patients), and 3 mixed (312 patients). The EFS hazard ratio (HR) for achieving MRD negativity is 0.23 (95% Bayesian credible interval [BCI] 0.18-0.28) for pediatric patients and 0.28 (95% BCI, 0.24-0.33) for adults. The respective HRs in OS are 0.28 (95% BCI, 0.19-0.41) and 0.28 (95% BCI, 0.20-0.39). The effect was similar across all subgroups and covariates.
Conclusions and Relevance
The value of having achieved MRD negativity is substantial in both pediatric and adult patients with ALL. These results are consistent across therapies, methods of and times of MRD assessment, cutoff levels, and disease subtypes. Minimal residual disease status warrants consideration as an early measure of disease response for evaluating new therapies, improving the efficiency of clinical trials, accelerating drug development, and for regulatory approval. A caveat is that an accelerated approval of a particular new drug using an intermediate end point, such as MRD, would require confirmation using traditional efficacy end points.
Introduction
Minimal residual disease (MRD) refers to the presence of disease in cases deemed to be in complete remission by conventional pathologic analysis. Detecting MRD in various hematological malignant diseases has been associated with higher relapse rates. These include chronic myeloid leukemia (CML), acute myeloid leukemia (AML), acute and chronic lymphoblastic leukemia (ALL and CLL), and multiple myeloma. In CML, the detection of chimeric BCR-ABL messenger RNA (mRNA) in peripheral blood has become the standard of care for assessing disease response, and has been used as a marker of disease response in registration studies.
In individual studies of ALL, the detection of MRD has been associated with poorer event-free survival (EFS) and overall survival (OS). US and European pediatric studies use risk stratification based on postinduction or postconsolidation MRD status, increasing therapy in cases with substantial MRD and/or decreasing therapy in patients who achieve MRD negativity.
Using MRD status to stratify risk in adult patients with ALL may play a role similar to that in pediatric patients with ALL. For example, a 2-stage risk-adapted study in adults found that 72% of patients who achieved MRD negativity were disease-free after 5 years compared with only 14% of the MRD-positive patients, regardless of clinical risk category.
Using MRD assessment to guide clinical treatment may depend on the strength of association, its robustness across studies, its dependence on disease subtype and the patient’s clinical and demographic characteristics. A critical consideration is the extent to which the ability to predict clinical outcome from a patient’s MRD status depends on treatment.
Minimal residual disease in patients with ALL can be measured in several ways, including by multiparametric flow cytometry (MFC), by polymerase chain reaction (PCR) of the IgH VDJ and/or TCR gene rearrangements, and by leukemia-specific fusion transcripts (eg, BCR-ABL). Although MRD levels assessed using molecular and immunophenotypic approaches are highly correlated, differences in sensitivity and the potential for analytic variability has clouded their interpretation.
Minimal residual disease is a measure of disease burden following a specific therapeutic intervention, and thus of therapeutic response. Minimal residual disease status has a potential impact on clinical management, trial design, and drug development. However, the wide adoption of MRD as a meaningful end point has been limited by the difficult interpretation of the data across heterogeneous studies, treatments, and individual patients. To understand the association of MRD with clinical outcomes EFS and OS, we performed a literature-based meta-analysis of ALL studies, distinguishing by patient age. We addressed the extent to which absence of MRD correlated with better long-term clinical outcome in the context of therapies considered in literature reports.
Methods
Data Sources
Two investigators (S.Z. and H.H.) conducted independent searches of PubMed, MEDLINE, and clinicaltrials.gov using various ranges of publication dates and key words, including “MRD,” “ALL,” “minimal residual,” and “acute leukemia.” We surveyed disease experts to augment our candidate list of published studies.
Study Selection
We excluded reviews and abstracts, studies with fewer than 30 patients, studies with insufficient description of MRD assessment, and studies that provided no information regarding survival end points (either EFS or OS) by MRD status. We performed separate analyses for adult, pediatric, and mixed adult/pediatric populations where the last category refers to studies that include at least 20% of both age groups (Figure 1).
Figure 1. Literature Search Diagram .
Data Extraction
We extracted from publications the following information when it was available: study sample size, median age and age range, median follow-up time, MRD detection method (PCR vs MFC using at least 3 colors), MRD cutoff level, MRD determination time point(s), ALL phenotype (B cell, T cell), cytogenetics including Philadelphia chromosome positivity (Ph+), and survival outcomes. Two researchers (S.Z. and L.M.) extracted the data independently.
We used a sequential approach when extracting hazard ratios (HRs), comparing patients who achieved MRD negativity with those who did not: (1) When available we used observed HRs and their CIs. (2) If Kaplan-Meier (KM) curves for both MRD-negative and MRD-positive groups were provided we used the commercial graph digitizer software DigitizeIt (version 2.1, Bormisoft) to extract coordinates of points on the curves and applied a numerical algorithm to reconstruct survival results. We then calculated HRs and CIs. When sample sizes over time were not available we adjusted the results by estimating the censoring over time based on reported follow-up time distribution and sample sizes. (3) For articles that provided survival proportions at fixed time points (eg, 3 years or 5 years), their standard deviations, and numbers of patients, we estimated the study’s HR and its CI assuming that the time-to-event distribution was exponential. We excluded a study if none of these 3 possibilities were available for either EFS or OS.
Data Synthesis
Searches of PubMed and MEDLINE found 268 to 566 articles depending on keywords and date range (January 1, 2000 to December 31, 2014, vs no restriction). A parallel search on clinicaltrials.gov using key words “acute lymphocytic leukemia,” “acute lymphoblastic leukemia,” “minimal residual disease,” and “MRD” found 67 closed trials and 62 open trials as of 2014. Publications related to these studies were identified and merged. We excluded reviews, abstracts, nonEnglish language articles, studies with MRD method obsolete or insufficiently described, and those with no survival end points. This left 62 publications with 30 285 patients. Applying additional exclusions of studies with fewer than 30 patients and insufficient outcome follow-up information reduced the total to 39 publications (16 adult, 20 pediatric, and 3 mixed) of distinct studies with 13 637 patients. These studies formed the basis of our statistical analyses (eTable 1 in the Supplement).
Our search and study screening process adhered to the PRISMA Guidelines. Characteristics of the individual studies are presented in eTable 1 in the Supplement. In all the studies MRD was assessed in bone marrow. Some studies assessed MRD in blood as well.
Statistical Analyses
The primary end points were EFS and OS. We included disease-free, recurrence-free, relapse-free, and event-free survival in the definition of EFS.
For each study we obtained hazard ratios (MRD-negative vs MRD-positive) and CIs in 1 of the 3 ways described in Data Extraction above. We modeled log-HRs separately for pediatrics, adults, and mixed. We assumed that hazards were constant within each 6-month period of follow-up. We truncated results of all studies at 15 years. Each 6-month segment has its own study-specific hazard rate, and therefore also its own HR of MRD-negative vs MRD-positive.
We used 2 different approaches for statistical analysis, both of which allowed for the possibility that MRD status has different effects in different studies. The primary analysis used a Bayesian hierarchical model. The other approach was a traditional frequentist random-effects model. For the Bayesian analysis we assumed the prior distribution of the mean log-HR across studies to be normal with mean m and standard deviation s. We assumed noninformative prior distributions for m and s and for the time-segment-specific baseline hazard rates.
Unless indicated otherwise, all results reported are based on Bayesian hierarchical analysis. In particular, estimated HRs are posterior means and their variability is indicated by 95% Bayesian credible intervals (BCIs).
Studies that reported the observed HRs depending on MRD status and the associated CIs—case 1—contribute directly to the probability distributions of the log-HRs of the studies considered and in particular to inferences about the mean of the studies. For studies that reported KM curves for MRD-positive and MRD-negative groups—case 2—we estimated hazard rates within each time segment by reconstructing the time-to-event data. The contribution of studies that provided survival proportions only at particular time points—case 3—we assumed constant hazards over the time periods that were provided by the publications.
For all Bayesian analyses we found the joint posterior distributions of model parameters using Markov chain Monte Carlo (MCMC) methods. Because closed forms of the “full-conditional distributions” are not available, we generated these distributions using Gibbs sampling and a Metropolis-Hastings computational algorithm. We used statistical software R (version 3.1.2, R project; with packages survival_v2.38-1, rjags_v3-14, coda_v0.16-1, lattice_v0.20-29, and ggplot2_v1.0.1) and JAGS statistical software (version 3.4.0, http://mcmc-jags.sourceforge.net) for data analysis.
For the Bayesian analyses we plotted the means of EFS and OS distributions for patients who achieve MRD negativity vs those who did not. We used shading to show the 95% BCIs about these mean curves. Similarly we present the mean HR for MRD-negative vs MRD-positive and the 95% BCI of the HR.
In reporting probability distributions of HRs for subgroups of patients we included only those studies for which relevant published evidence was available. We excluded studies from a subgroup analysis if the publication did not include information regarding survival by MRD status for the subgroup in question. An extreme example that contained little evidence for an important subgroup is Philadelphia chromosome status in pediatric studies. Only 2 pediatric studies restricted eligibility by Ph-status and both studies consider only Ph-negative disease. As a consequence, for pediatric Ph-negative ALL the CI will be relatively wide and for pediatric Ph-positive ALL it does not exist.
This project was approved by the MD Anderson institutional review board.
Results
Studies Available for Analysis
A total of 39 studies (16 adult, 20 pediatric, and 3 mixed) were available for analysis, including 13 637 patients. These studies are described in eTable 1 in the Supplement. They used MRD status in various ways. Some were compilations of several clinical trials merged into a single study of MRD. For example, Borowitz et al assessed MRD status retrospectively on archival samples from 2143 pediatric patients enrolled in several Children’s Oncology Group trials. In another type of study, Vora et al used MRD status prospectively as a randomization criterion. The goal was to address the possibility of delaying and reducing treatment intensity. Patients with undetectable or low MRD (<0.01%) were randomized to 1 or 2 courses of delayed intensification therapy. In a third study type, Bassan et al used MRD status in adults as an indicator of recurrence risk, with MRD-negative patients receiving conventional therapy while those with MRD received high-dose therapy, including allogeneic transplantation.
MRD and Outcomes
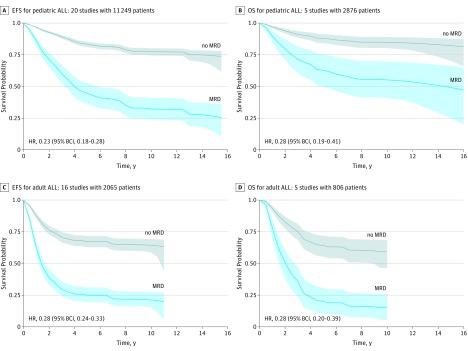
The overall EFS results for pediatric and adult studies are shown in Figure 2, A and C. For pediatric patients, EFS was better for those who achieved MRD negativity—estimated EFS of 77% at 10 years—compared with those with positive MRD—approximately 32% at 10 years. Although EFS for adult patients was inferior to that for pediatric patients in respective MRD groups, there was a similarly strong association between MRD and outcome. Adults who were MRD-negative had EFS of approximately 64% at 10 years vs only 21% for those who were MRD-positive. The relative benefit in EFS of having achieved MRD negativity was comparable in both age groups (HR, 0.23; 95% BCI, 0.18-0.28 for pediatric cases and HR, 0.28; 95% BCI, 0.24-0.33 for adults).
Figure 2. Estimated Survival Curves for Patient Groups With ALL.
A, EFS: 3191 patients at time 0 with MRD and 8058 with no MRD. B, OS: 883 patients at time 0 with MRD and 1993 with no MRD. C, EFS: 711 patients at time 0 with MRD and 1354 with no MRD. D, OS: 242 patients at time 0 with MRD and 564 with no MRD. The plots show the means of the Bayesian hierarchical analyses. The shadings associated with each curve show the 95% BCIs for the mean survival proportion at the corresponding point in time of follow-up. All 20 pediatric studies and all 16 studies contributed to the EFS distributions (A and C) whereas only 5 of the studies in each age group contained information about OS (B and D). ALL indicates acute lymphoblastic leukemia; BCI, Bayesian credible intervals, EFS, event-free survival; MRD, minimal residual disease; OS, overall survival.
Figure 2, B and D show that the estimated benefit in OS of MRD negativity was identical in pediatric patients (HR, 0.28; 95% BCI, 0.19-0.41) and adults (HR, 0.28; 95% BCI, 0.20-0.39).
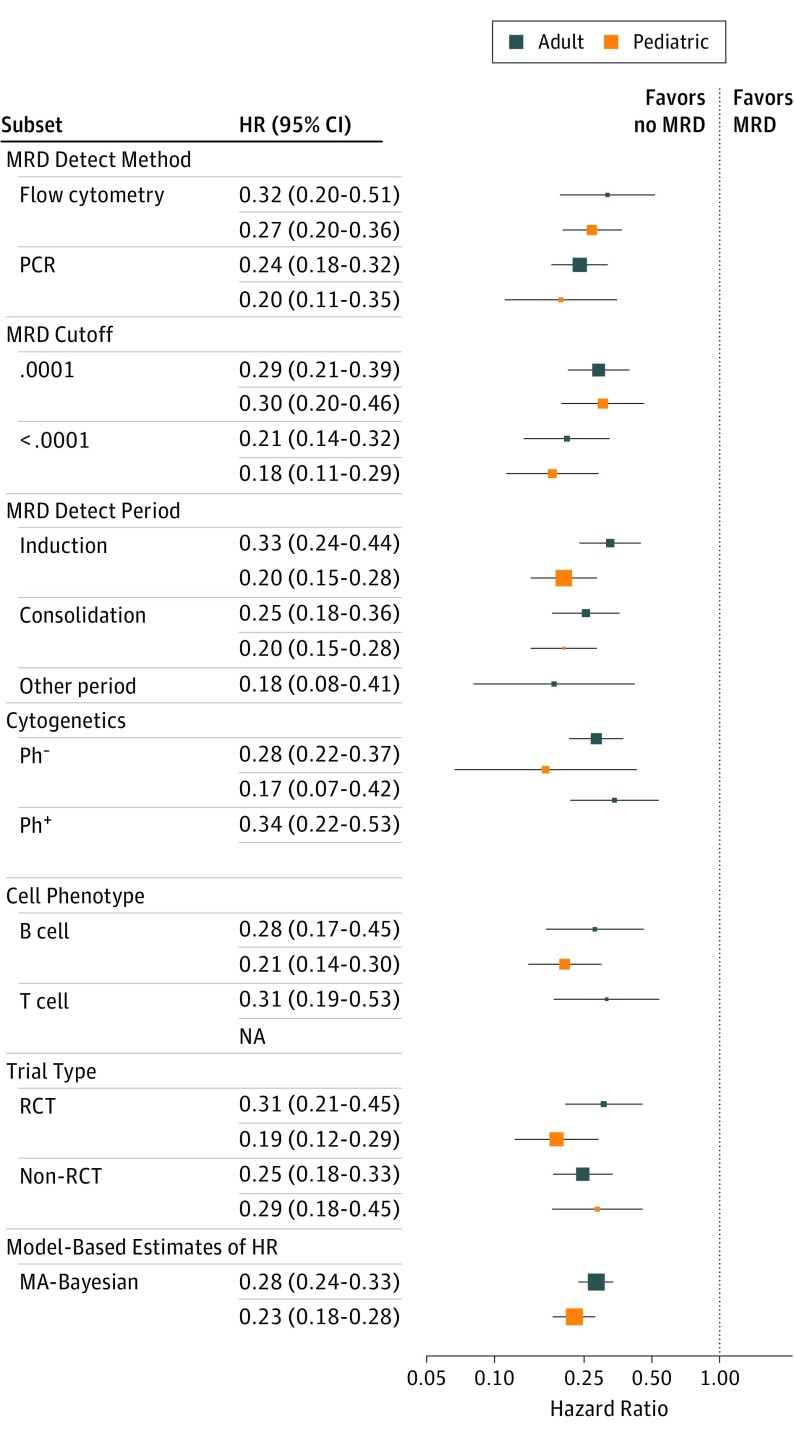
The HR for each individual study is shown in Figure 3, A and B.
Figure 3. Forest Plot of EFS HRs for Pediatric and Adult ALL Subtypes.
Numerical results are in eTable 2 in the Supplement. The dots represent the mean HR for the Bayesian hierarchical analysis. The horizontal lines show the 95% BCIs for the subgroup’s EFS HR. ALL indicates acute lymphoblastic leukemia; EFS, event-free survival; OS, overall survival.
There were only 3 studies in the mixed-age group. The results in these studies were very similar to those for the other 2 groups.
Relationships Between MRD Status and Outcome by Subgroup
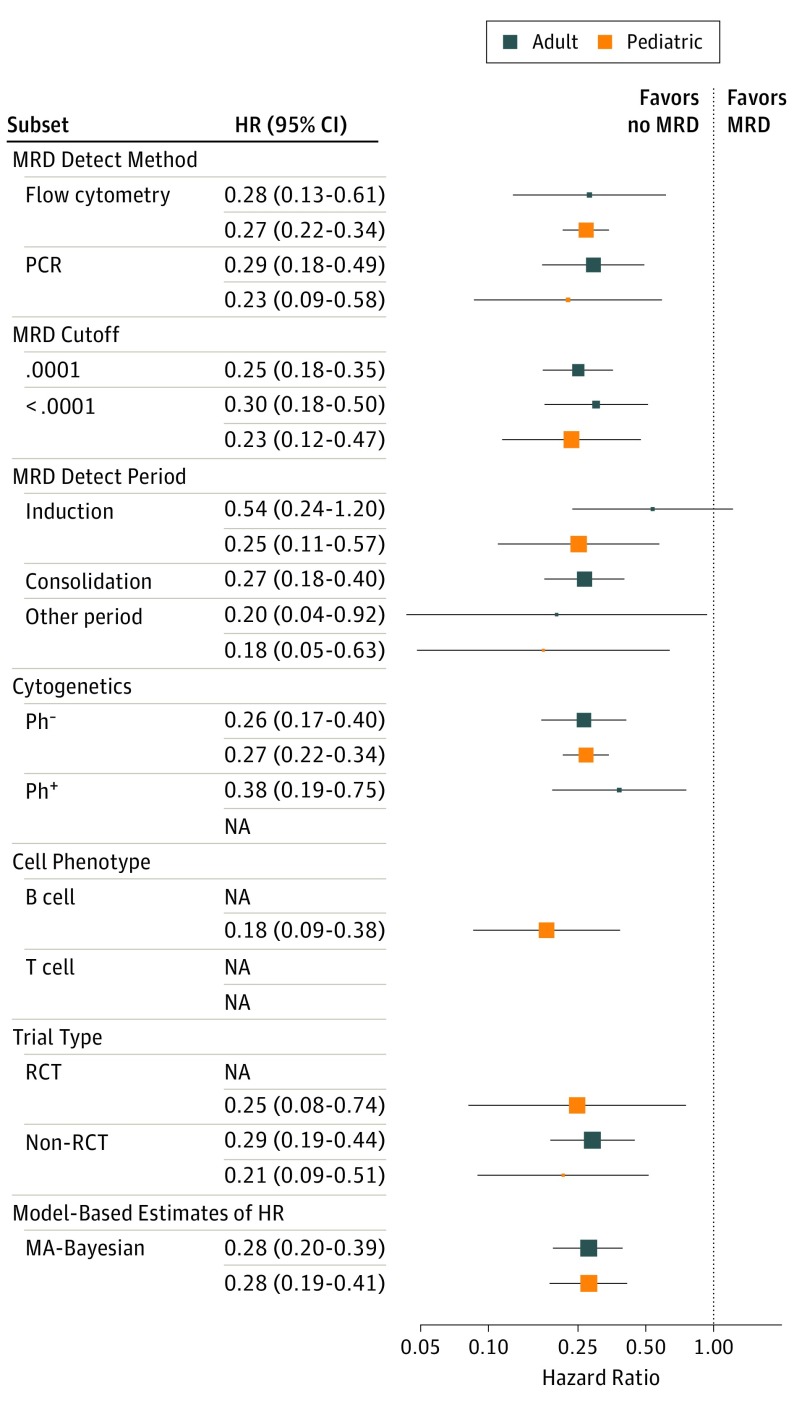
The HRs of EFS and OS depending on MRD status are shown in Figure 3 and Figure 4; eTable 2 in the Supplement for various subgroups: type of MRD detection (flow cytometry vs PCR), reported “cutoff” value of MRD measurement (≤0.01% vs 0.01%-1%), whether MRD determination was made at the end of induction or consolidation, whether including only Ph-negative or Ph-positive patients, B-cell or T-cell phenotype, and whether the study was a randomized clinical trial. None of the subgroupings suggest a differential effect of MRD on the HR of either EFS or OS.
Figure 4. Forest Plot of OS HRs for Pediatric and Adult ALL Subtypes.
Numerical results are in eTable 2 in the Supplement. The dots represent the mean HR for the Bayesian hierarchical analysis. The horizontal lines show the 95% BCIs for the subgroup’s EFS HR. ALL indicates acute lymphoblastic leukemia; EFS, event-free survival; OS, overall survival.
Discussion
Our meta-analysis demonstrates a consistent and strong association in ALL between MRD and clinical outcomes. The effect is substantial and robust in all subgroups of ALL that we were able to address, both for pediatric and adult patients. In particular, achieving MRD negativity had a consistent and beneficial effect in studies that used different therapies, different assessment methods and cutoff levels, time points, and disease and study subtypes.
Cutoff level showed an unexpected effect (Figure 3; eTable 2 in the Supplement). Namely, a larger cutoff level (>0.01% vs ≤0.01%) shows numerically smaller HRs for EFS, 0.18 vs 0.30 in pediatric ALL and 0.21 vs 0.29 in adult ALL. However, the numbers of studies involved in this comparison and the differences in hazard ratios is small. So this difference may be owing to unknown factors that differ by study and may not imply greater discrimination for the higher cutoff levels.
For the studies we considered, pediatric MRD-negative patients were much more likely to be disease-free after 10 years than those who were MRD-positive, 77% vs 32%, and alive, 84% vs 55%. For adult MRD-negative patients, 64% were disease-free at 10 years vs 21% for MRD-positive, and alive, 60% vs 15%. The HRs for pediatric patients were 0.23 for EFS and 0.28 for OS and for adult patients, 0.28 for both EFS and OS. Although the relative effects are similar, the hazards over time are very different for pediatric vs adult patients. Conditionally on MRD status, hazards are much higher for adult patients than for pediatric patients in the first 3 years. Hazards for adults were comparable or possibly even lower than for pediatric patients in subsequent years. This observation suggests greater heterogeneity in adult ALL, a heterogeneity beyond that which is distinguishable by MRD status.
Our study confirms MRD as a measure of disease burden that is an early response indicator for use in the design and conduct of clinical trials. As such, achieving MRD negativity may qualify as an end point for drug registration.
A precedent is neoadjuvant breast cancer in biomarker-defined subtypes. Accelerated regulatory approval requires showing substantial improvement in the rate of pathological complete response (pCR). The analogy in ALL would be demonstrating a substantial improvement in the rate of MRD negativity.
Minimal residual disease negativity leads to better clinical outcomes in the studies we considered. But this same relationship may not apply for therapies we did not consider. A new therapy might decrease the rate of MRD but not affect clinical outcome. Or it might have no effect on rate of MRD but still improve outcome. Using MRD as a primary end point for accelerated approval of a new drug will require a plan for confirming benefit on EFS or OS.
This issue is important in drug registration trials. For neoadjuvant therapy in breast cancer, achieving a pCR is associated with longer EFS and OS, especially when restricting to particular biomarker subtypes. The US Food and Drug Administration guidance describes routes to accelerated approval based on demonstrating substantial improvement in pCR rates. The critical requirement is evidence that improving pCR rate improves EFS. One route to registration is a single trial demonstrating improvement in both pCR rate (accelerated approval) and EFS (full approval). If applied to ALL, a prospectively randomized trial would have to show improvement in both rate of MRD and EFS.
The results shown in Figure 2 establish a hypothesis regarding the relationship between MRD and EFS regardless of therapy. If the control therapy has a 30% rate of MRD negativity then, under this hypothesis, 30% of the patients will be on the EFS curve for no MRD and 70% will be on the EFS curve for MRD. The resulting control EFS is the respective weighted average of the 2 curves. Make the analogous calculation for experimental therapy with its assumed rate of MRD negativity, perhaps derived from phase 2 trials. Comparing the 2 weighted averages establishes a hypothesis for the HR in a clinical trial with MRD negativity and EFS as coprimary end points.
This approach will likely give a more accurate estimate of EFS HR than traditional approaches. However, there are at least 3 sources of error. One is evident from Figure 2. The curves are estimates with their uncertainty indicated by the shading. This uncertainty should be considered when finding the predictive probability of trial success under the above hypothesis. The second source of error is the uncertainty in the assumed rates for MRD negativity for the 2 therapies. These rates in a future clinical trial may differ from historical rates. This source of error can also be incorporated into the predictive probability of trial success.
The third source of error is the most important: the hypothesis provided by Figure 2 may be wrong for 1 or both therapies. In particular, eliminating residual disease may not translate into the same effect on EFS or OS for a new therapy. A solution is using an adaptive phase 3 trial with frequent interim analyses to reestimate the trial’s sample size based on accumulating evidence about rate of MRD and its relationship with EFS. The hypothesis presented in Figure 2 can be updated for both therapies. Such designs will also confirm the association between achieving MRD negativity and reaching a clinical outcome. Sample size reestimation is best carried out based on frequent calculations of the predictive probability of trial success. Such calculations can also be used to stop the trial for futility.
The effects shown in Figure 2 are dramatic, but they may be misleading. Figure 2 compares 100% of the patients vs 0% of the patients who are MRD-negative. No therapy would qualify for such an extreme, especially since control therapy will have some benefit on MRD. Consider the EFS curves in Figure 2, A. The HR of 0.23 is a reflection of the distance between the MRD and no-MRD curves. The hazard of recurrence with MRD is reduced by 77% by achieving MRD negativity. A therapy that improves the rate of MRD negativity by 20 percentage points, say, from 30% for control to 50%, occupies only one-fifth of the distance between these 2 curves. The control arm EFS curve lies 30% of the way from the MRD curve to the no-MRD curve. The experimental arm lies halfway between the 2 curves. The EFS HR implied by Figure 2, A for those 2 derived curves is about 0.80 (calculation not shown). For a clinical trial to show an EFS improvement of this magnitude would require a sample size greater than 1000 patients.
The clear relationship between MRD and EFS that we quantified has applications besides clinical trial design: (1) in clinical practice, assigning patients who have MRD to alternative therapy, perhaps an allograft or a clinical trial; (2) as a research tool for better defining patients at high risk for recurrence and eligibility for clinical trials; (3) assigning highest priority for definitive evaluation in phase 3 trials for therapies that achieve the lowest rates of MRD; (4) providing supportive data in regulatory decisions based primarily on complete response rates with incomplete hematological recovery (as in the approval of blinatumomab); (5) extrapolating from disease types where a therapy has a known effect on other hematological malignant diseases where it shows a benefit on MRD.
Limitations
There are important caveats when using MRD. Cases in which EFS is long despite having MRD may reflect the genotypic and phenotypic heterogeneity of tumors or their hosts. The MRD-negative cases that relapse early may reflect the limits of assay sensitivity and a very actively growing tumor. Moreover, technical difficulties could give misleading results. Sensitive and specific standardized methods for MRD determination are not widely available outside of specialized centers. Standardization of PCR-based tests has been widely implemented in Europe. Most studies in the United States use flow cytometry for which standardization is more challenging. However, current MRD methods will likely be superseded by next-generation sequencing, which appears to be more sensitive than flow cytometry or PCR. Next-generation sequencing may also be easier to standardize. In addition, although we supplemented internet searches with surveys of disease experts, the bases of all our analyses are publications. So our study is subject to publication bias. Similarly, it is subject to any biases or errors of the original investigators. Finally, we did not have by-patient data and so could not draw strong conclusions about the roles of MRD in subsets of patients.
Minimal residual disease is a “biomarker” of disease in the powerful sense that MRD is the disease. However, no assay is perfect. Although MRD is a direct measure of disease burden and treatment response in ALL, there may be sanctuary sites in the body that contribute to relapse but are not measurable by conventional methods. The next major advance may be a more sensitive and standardized assessment of level of MRD as a continuous measure of disease burden. It would become as standard in clinical care as getting a hematocrit and a white blood count.
Conclusions
Minimal residual disease status in patients with ALL is an indicator of therapeutic benefit in clinical practice. It has great potential for making drug development more efficient by providing early evidence of treatment benefit. Using MRD status is useful when designing efficient drug registration trials in ALL.
eTable 1. Characteristics of the studies included in this meta-analysis
eTable 2. Hazard ratios of EFS and OS and their respective Bayesian 95% credible intervals for each subgroup of patients in pediatric and adult ALL
eFigure 1. Hazard ratios of EFS and their 95% confidence intervals for the individual studies used in our pediatric ALL meta-analysis
eFigure 2. Hazard ratios of EFS and their 95% confidence intervals for the individual studies used in our pediatric ALL meta-analysis
References
- 1.Kantarjian H, Shah NP, Hochhaus A, et al. . Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260-2270. [DOI] [PubMed] [Google Scholar]
- 2.Saglio G, Kim DW, Issaragrisil S, et al. ; ENESTnd Investigators . Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251-2259. [DOI] [PubMed] [Google Scholar]
- 3.Buccisano F, Maurillo L, Gattei V, et al. . The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia. 2006;20(10):1783-1789. [DOI] [PubMed] [Google Scholar]
- 4.Walter RB, Buckley SA, Pagel JM, et al. . Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borowitz MJ, Wood BL, Devidas M, et al. . Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood. 2015;126(8):964-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gökbuget N, Kneba M, Raff T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia . Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868-1876. [DOI] [PubMed] [Google Scholar]
- 7.Böttcher S, Ritgen M, Fischer K, et al. . Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30(9):980-988. [DOI] [PubMed] [Google Scholar]
- 8.Korthals M, Sehnke N, Kronenwett R, et al. . The level of minimal residual disease in the bone marrow of patients with multiple myeloma before high-dose therapy and autologous blood stem cell transplantation is an independent predictive parameter. Biol Blood Marrow Transplant. 2012;18(3):423-431.e3. [DOI] [PubMed] [Google Scholar]
- 9.Borowitz MJ, Devidas M, Hunger SP, et al. ; Children’s Oncology Group . Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vora A, Goulden N, Wade R, et al. . Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199-209. [DOI] [PubMed] [Google Scholar]
- 11.Brüggemann M, Raff T, Flohr T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia . Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116-1123. [DOI] [PubMed] [Google Scholar]
- 12.Bassan R, Spinelli O, Oldani E, et al. . Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153-4162. [DOI] [PubMed] [Google Scholar]
- 13.Patel B, Rai L, Buck G, et al. . Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010;148(1):80-89. [DOI] [PubMed] [Google Scholar]
- 14.Gaipa G, Cazzaniga G, Valsecchi MG, et al. . Time point-dependent concordance of flow cytometry and real-time quantitative polymerase chain reaction for minimal residual disease detection in childhood acute lymphoblastic leukemia. Haematologica. 2012;97(10):1582-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irving J, Jesson J, Virgo P, et al. ; UKALL Flow MRD Group; UK MRD steering Group . Establishment and validation of a standard protocol for the detection of minimal residual disease in B lineage childhood acute lymphoblastic leukemia by flow cytometry in a multi-center setting. Haematologica. 2009;94(6):870-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malec M, van der Velden VH, Björklund E, et al. . Analysis of minimal residual disease in childhood acute lymphoblastic leukemia: comparison between RQ-PCR analysis of Ig/TcR gene rearrangements and multicolor flow cytometric immunophenotyping. Leukemia. 2004;18(10):1630-1636. [DOI] [PubMed] [Google Scholar]
- 17.Ryan J, Quinn F, Meunier A, et al. . Minimal residual disease detection in childhood acute lymphoblastic leukaemia patients at multiple time-points reveals high levels of concordance between molecular and immunophenotypic approaches. Br J Haematol. 2009;144(1):107-115. [DOI] [PubMed] [Google Scholar]
- 18.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broglio KR, Quintana M, Foster M, et al. . Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: A meta-analysis. JAMA Oncol. 2016;2(6):751-760. [DOI] [PubMed] [Google Scholar]
- 20.Bachanova V, Burke MJ, Yohe S, et al. . Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant. 2012;18(6):963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beldjord K, Chevret S, Asnafi V, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) . Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739-3749. [DOI] [PubMed] [Google Scholar]
- 22.Bowman WP, Larsen EL, Devidas M, et al. . Augmented therapy improves outcome for pediatric high risk acute lymphocytic leukemia: results of Children’s Oncology Group trial P9906. Pediatr Blood Cancer. 2011;57(4):569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen IM, Harvey RC, Mullighan CG, et al. . Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119(15):3512-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conter V, Bartram CR, Valsecchi MG, et al. . Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206-3214. [DOI] [PubMed] [Google Scholar]
- 25.Eckert C, Henze G, Seeger K, et al. . Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol. 2013;31(21):2736-2742. [DOI] [PubMed] [Google Scholar]
- 26.Eckert C, von Stackelberg A, Seeger K, et al. . Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia - long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013;49(6):1346-1355. [DOI] [PubMed] [Google Scholar]
- 27.Flohr T, Schrauder A, Cazzaniga G, et al. ; International BFM Study Group (I-BFM-SG) . Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22(4):771-782. [DOI] [PubMed] [Google Scholar]
- 28.Foster JH, Hawkins DS, Loken MR, Wells DA, Thomson B. Minimal residual disease detected prior to hematopoietic cell transplantation. Pediatr Blood Cancer. 2011;57(1):163-165. [DOI] [PubMed] [Google Scholar]
- 29.Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. . Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD Study. Br J Haematol. 2008;142(2):227-237. [DOI] [PubMed] [Google Scholar]
- 30.Imashuku S, Terui K, Matsuyama T, et al. ; multi-institutional collaborative study in Japan . Lack of clinical utility of minimal residual disease detection in allogeneic stem cell recipients with childhood acute lymphoblastic leukemia: multi-institutional collaborative study in Japan. Bone Marrow Transplant. 2003;31(12):1127-1135. [DOI] [PubMed] [Google Scholar]
- 31.Kang H, Chen IM, Wilson CS, et al. . Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia. Blood. 2010;115(7):1394-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krampera M, Vitale A, Vincenzi C, et al. . Outcome prediction by immunophenotypic minimal residual disease detection in adult T-cell acute lymphoblastic leukaemia. Br J Haematol. 2003;120(1):74-79. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Kim DW, Cho BS, et al. . Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26(11):2367-2374. [DOI] [PubMed] [Google Scholar]
- 34.Meleshko AN, Savva NN, Fedasenka UU, et al. . Prognostic value of MRD-dynamics in childhood acute lymphoblastic leukemia treated according to the MB-2002/2008 protocols. Leuk Res. 2011;35(10):1312-1320. [DOI] [PubMed] [Google Scholar]
- 35.Mortuza FY, Papaioannou M, Moreira IM, et al. . Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20(4):1094-1104. [DOI] [PubMed] [Google Scholar]
- 36.Pane F, Cimino G, Izzo B, et al. ; GIMEMA group . Significant reduction of the hybrid BCR/ABL transcripts after induction and consolidation therapy is a powerful predictor of treatment response in adult Philadelphia-positive acute lymphoblastic leukemia. Leukemia. 2005;19(4):628-635. [DOI] [PubMed] [Google Scholar]
- 37.Pulsipher MA, Langholz B, Wall DA, et al. . The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014;123(13):2017-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raff T, Gökbuget N, Lüschen S, et al. ; GMALL Study Group . Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910-915. [DOI] [PubMed] [Google Scholar]
- 39.Ravandi F, Jorgensen JL, Thomas DA, et al. . Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribera JM, Oriol A, Morgades M, et al. . Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32(15):1595-1604. [DOI] [PubMed] [Google Scholar]
- 41.Salah-Eldin M, Abousamra NK, Azzam H. Clinical significance of minimal residual disease in young adults with standard-risk/Ph-negative precursor B-acute lymphoblastic leukemia: results of prospective study. Med Oncol. 2014;31(5):938. [DOI] [PubMed] [Google Scholar]
- 42.Spinelli O, Peruta B, Tosi M, et al. . Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92(5):612-618. [DOI] [PubMed] [Google Scholar]
- 43.Stirewalt DL, Guthrie KA, Beppu L, et al. . Predictors of relapse and overall survival in Philadelphia chromosome-positive acute lymphoblastic leukemia after transplantation. Biol Blood Marrow Transplant. 2003;9(3):206-212. [DOI] [PubMed] [Google Scholar]
- 44.Stow P, Key L, Chen X, et al. . Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115(23):4657-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutton R, Shaw PJ, Venn NC, et al. . Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol. 2015;168(3):395-404. [DOI] [PubMed] [Google Scholar]
- 46.Tucunduva L, Ruggeri A, Sanz G, et al. . Impact of minimal residual disease on outcomes after umbilical cord blood transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia: an analysis on behalf of Eurocord, Cord Blood Committee and the Acute Leukaemia working party of the European group for Blood and Marrow Transplantation. Br J Haematol. 2014;166(5):749-757. [DOI] [PubMed] [Google Scholar]
- 47.Van der Velden VH, Corral L, Valsecchi MG, et al. ; Interfant-99 Study Group . Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia. 2009;23(6):1073-1079. [DOI] [PubMed] [Google Scholar]
- 48.Vidriales MB, Pérez JJ, López-Berges MC, et al. . Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value. Blood. 2003;101(12):4695-4700. [DOI] [PubMed] [Google Scholar]
- 49.Vilmer E, Suciu S, Ferster A, et al. ; Children Leukemia Cooperative Group . Long-term results of three randomized trials (58831, 58832, 58881) in childhood acute lymphoblastic leukemia: a CLCG-EORTC report. Leukemia. 2000;14(12):2257-2266. [DOI] [PubMed] [Google Scholar]
- 50.Zhao XS, Liu YR, Zhu HH, et al. . Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2012;91(2):183-192. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Goldwasser MA, Li A, et al. ; Dana-Farber Cancer Institute ALL Consortium . Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood. 2007;110(5):1607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berry DA. Bayesian clinical trials. Nat Rev Drug Discov. 2006;5(1):27-36. [DOI] [PubMed] [Google Scholar]
- 54.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis. 3rd ed Boca Raton, Florida: Chapman and Hall–CRC Press; 2013. [Google Scholar]
- 55.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration, 2011. http://handbook.cochrane.org. Accessed July 24, 2016.
- 56.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, West Sussex, United Kingdom: John Wiley and Sons, Ltd; 2009. [Google Scholar]
- 57.US Food and Drug Administration Guidance for industry: pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an end point to support accelerated approval. October 2014. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf. Accessed July 24, 2016.
- 58.Cortazar P, Zhang L, Untch M, et al. . Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. [DOI] [PubMed] [Google Scholar]
- 59.Berry DA, Hudis CA. Neoadjuvant therapy in breast cancer as a basis for drug approval. JAMA Oncol. 2015;1(7):875-876. [DOI] [PubMed] [Google Scholar]
- 60.Berry DA. Right-sizing adjuvant and neoadjuvant clinical trials in breast cancer. Clin Cancer Res. 2016;22(1):3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berry DA. Emerging innovations in clinical trial design. Clin Pharmacol Ther. 2016;99(1):82-91. [DOI] [PubMed] [Google Scholar]
- 62.Topp MS, Kufer P, Gökbuget N, et al. . Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493-2498. [DOI] [PubMed] [Google Scholar]
- 63.Wu D, Sherwood A, Fromm JR, et al. . High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4(134):134ra63. [DOI] [PubMed] [Google Scholar]
- 64.Larimore K, McCormick MW, Robins HS, Greenberg PD. Shaping of human germline IgH repertoires revealed by deep sequencing. J Immunol. 2012;189(6):3221-3230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the studies included in this meta-analysis
eTable 2. Hazard ratios of EFS and OS and their respective Bayesian 95% credible intervals for each subgroup of patients in pediatric and adult ALL
eFigure 1. Hazard ratios of EFS and their 95% confidence intervals for the individual studies used in our pediatric ALL meta-analysis
eFigure 2. Hazard ratios of EFS and their 95% confidence intervals for the individual studies used in our pediatric ALL meta-analysis