This randomized clinical trial examines whether zoledronic acid every 12 weeks was noninferior to zoledronic acid every 4 weeks in patients with metastatic breast cancer involving bone who had previously received a standard dosing regimen of zoledronic acid and/or pamidronate.
Key Points
Question
Is zoledronic acid every 12 weeks noninferior to zoledronic acid every 4 weeks?
Findings
In this randomized clinical trial of 416 women, the efficacy of continuing zoledronic acid for an additional year every 12 weeks was noninferior to zoledronic acid every 4 weeks in women receiving monthly intravenous zoledronic acid and/or pamidronate disodium for 1 year or longer.
Meaning
These results may have a substantial influence on current clinical practice for treatment of patients with bone metastasis from breast cancer.
Abstract
Importance
Zoledronic acid, a potent bisphosphonate, is commonly administered to patients with bone metastases to reduce the risk of skeletal-related events (SREs). However, there have been concerns regarding its long-term monthly administration.
Objective
To examine whether zoledronic acid every 12 weeks was noninferior to zoledronic acid every 4 weeks in patients with metastatic breast cancer that involved the bone who had previously received a standard dosing regimen of zoledronic acid and/or pamidronate disodium.
Design, Setting, and Participants
OPTIMIZE-2 was a prospective, randomized, double-blind, multicenter phase 3 trial of intention-to-treat (full analysis set), evaluable (per protocol), and safety populations. Patients were randomized (1:1) to receive 4.0 mg of intravenous zoledronic acid every 4 or every 12 weeks with placebo for interim infusions for 1 year.
The study was conducted at 102 clinical trial centers in the United States from March 3, 2006, to July 25, 2013. Data analysis was performed from October 7, 2013, to March 24, 2014. The study randomized 416 women (≥18 years old) with bone metastases from breast cancer who previously received 9 or more doses of zoledronic acid and/or pamidronate during the first 10 to 15 months of therapy.
Main Outcomes and Measures
The primary end point was the proportion of patients with 1 or more SRE on study (SRE rate). The key secondary end points included time to first SRE and skeletal morbidity rate (SMR).
Results
A total of 416 women were randomized: 200 patients received zoledronic acid every 4 weeks (mean [SD] age, 59.2 [11.1] years; 173 were white [86.5%]), 203 patients received zoledronic acid every 12 weeks (mean [SD] age, 58.6 [11.2] years; 178 were white [87.7%]), and 13 patients received placebo (mean [SD] age, 60.8 [12.2] years; 13 were white [100%]). Baseline characteristics were similar in both zoledronic acid treatment arms. After 1 year of follow-up, SREs occurred in 44 patients (22.0%) in the zoledronic acid every 4 weeks group and 47 patients (23.2%) in the zoledronic acid every 12 weeks group (proportional difference of −1.2%; 1-sided 97.5% CI bound of the difference in SRE rate between arms, −9.8%; noninferiority P = .02). The time to first SRE between treatment groups was not statistically significantly different (hazard ratio [HR], 1.06; 95% CI, 0.70-1.60; P = .79). The mean (SD) SMR was 0.46 (1.06) vs 0.50 (1.50) events per year in the every 4 weeks vs every 12 weeks groups (P = .85). The safety profiles of the every 4 weeks and every 12 weeks groups were comparable, with 189 patients (95.5%) in the every 4 weeks group having at least 1 adverse event compared with 189 (93.5%) in the every 12 weeks group.
Conclusions and Relevance
The every 12 weeks regimen of zoledronic acid was noninferior to the every 4 weeks regimen for the proportion of patients experiencing 1 or more SRE. These results may have a substantial influence on current clinical practice for treatment of patients with bone metastasis from breast cancer.
Trial Registration
clinicaltrials.gov Identifier: NCT00320710
Introduction
Bone metastasis is a common cause of morbidity in patients with advanced solid tumors, such as breast cancer and prostate cancer. It is associated with various debilitating skeletal-related events (SREs), which include bone fractures, hypercalcemia, nerve compression, and severe pain. These skeletal complications, in turn, increase the need for palliative radiation or surgery to bone, limit functional independence, adversely affect the quality of life, and continue to cause morbidity of the affected patients.
Zoledronic acid is a potent bisphosphonate that inhibits osteoclast-mediated bone resorption. It has been approved for treatment of patients with bone metastases from solid tumors or multiple myeloma and for the management of tumor-induced hypercalcemia. Because SREs can occur repeatedly during metastatic disease that involves the bone, American Society of Clinical Oncology clinical guidelines recommend zoledronic acid be taken indefinitely as intravenous infusion every 3 to 4 weeks until there is deterioration of the general health of the patients.
Data regarding efficacy and safety of zoledronic acid beyond 1 year of treatment are limited. This lack of data is particularly significant in patients with breast cancer in whom survival with bone metastases usually exceeds 1 year. Additional concern regarding long-term administration of zoledronic acid pertains to its preferential binding and accumulation in bone, thus prolonging its pharmacologic activity after discontinuation of long-term treatment. OPTIMIZE-2 investigated the hypothesis that reduction in the dosing frequency of zoledronic acid from every 4 weeks to every 12 weeks, after 1 year of treatment with the standard (every 4 weeks) dosing regimen, would have continued efficacy.
Methods
Study Design and Patients
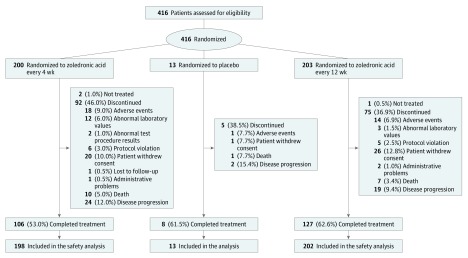
OPTIMIZE-2 was a prospective, randomized, double-blind, multicenter clinical study conducted at 102 study centers in the United States from March 3, 2006, to July 25, 2013. The trial protocol can be found in Supplement 1. Data analysis was performed from October 7, 2013, to March 24, 2014. Female patients 18 years or older with histologically confirmed breast cancer, 1 or more radiologically confirmed bone metastasis, Eastern Cooperative Oncology Group status of 2 or less, and life expectancy of 1 year or more were included. Patients were eligible if they had received zoledronic acid, pamidronate disodium, or a sequence of both for 9 doses or more during the first 10 to 15 months of treatment and were taking zoledronic acid or pamidronate at study entry. Patients who had a serum creatinine level higher than 3.0 mg/dL (to convert to micromoles per liter, multiply by 88.4) or a calculated creatinine clearance less than 30 mL/min/1.73 m2 (to convert to milliliters per second per square meter, multiply by 0.0167), active dental problems, or known hypersensitivity to zoledronic acid; who had previously received bisphosphonates other than zoledronic acid or pamidronate within the 12 months before study entry; or who had any changes in antineoplastic therapy within 30 days before randomization were excluded. Eligible patients were randomly assigned to receive 4.0 mg of intravenous zoledronic acid every 4 or 12 weeks. In the initial design, the OPTIMIZE-2 trial included a placebo arm (Figure 1); however, this hampered the recruitment of patients because bisphosphonates were already approved for this indication. Hence, the placebo arm was subsequently eliminated (eMethods in Supplement 2).
Figure 1. Patient Disposition (Full Analysis Set).
The study was conducted in adherence with the Good Clinical Practice guidelines, applicable local regulations, and the Declaration of Helsinki. The study protocol with all amendments and other patient-related materials were reviewed and approved by independent ethics committees or institutional review boards for each center. A full list of ethics committees and institutional review boards is given in the eMaterial in Supplement 2. All patients included in the analysis provided written informed consent.
Randomization and Blinding
Patients were randomized on a 1:1 ratio and stratified by duration of prior intravenous bisphosphonate therapy (10-15 months or >15 months) and a urinary N-telopeptide to creatinine (uNTX:Cr) ratio of 100 nmol of bone collagen equivalent per millimoles of creatinine or less or more than 100 nmol of bone collagen equivalent per millimoles of creatinine at screening. Randomization was performed by an interactive voice response system vendor using a validated system that automated the random assignment of patient numbers to randomization numbers. These randomization numbers were linked to the different treatment arms, which in turn were linked to medication numbers. Randomization data were kept strictly confidential, accessible only to authorized persons, until the time of unblinding.
Procedures
Patients received 4.0 mg of zoledronic acid intravenously (or renally dosed if baseline creatinine clearance was less than 60 mL/min) for at least 15 minutes. If baseline creatinine clearance was mild to moderately impaired at baseline (30-60 mL/min), dosage was reduced to 3.0 to 3.5 mg based on an algorithm provided in the product label. The study included a total of 15 scheduled visits (1 screening visit, 13 treatment visits, and 1 end-of-study visit). Patients in both groups had the same visit and infusion schedules. Patients in the every 12 weeks group received zoledronic acid every 3 visits followed by 2 placebo infusions. All patients received study-supplied, open-label, daily supplements of calcium (1000-2000 mg) and vitamin D (400-800 IU).
Outcomes
The primary objective of the study was to determine the efficacy, as measured by the SRE rate (the proportion of patients with ≥1 SRE during the study), of continued treatment with zoledronic acid every 4 weeks vs reduced zoledronic acid dosing frequency (every 12 weeks). The SREs were defined as pathologic bone fracture, radiation therapy or surgery to bone, and/or spinal cord compression. Initially, the primary comparison was for superiority compared with placebo; however, when the placebo arm was eliminated, the primary comparison was amended to noninferiority between the every 4 weeks and the every 12 weeks treatment regimens of zoledronic acid (eMethods in Supplement 2).
The secondary objectives were to determine the efficacy of continued treatment with zoledronic acid every 4 weeks vs reduced zoledronic acid dosing frequency (every 12 weeks) with regard to the time to first SRE, bone pain assessed by the Brief Pain Inventory (BPI) and analgesic consumption, the metabolic bone markers in association with dosing interval and SREs, and the skeletal morbidity rate (SMR) and to determine the safety of continued treatment with zoledronic acid every 4 weeks vs reduced zoledronic acid dosing frequency (every 12 weeks). The SMR was defined as the number of occurrences of any SRE, allowing for only 1 event in any 3-week interval, divided by the time at risk in years. The metabolic bone markers, including uNTX:Cr ratio and serum bone-specific alkaline phosphatase (BSAP) level, were assessed before infusion at 0, 12, 24, 36, and 48 weeks (eMethods in Supplement 2). The safety assessments consisted of monitoring and recording of all adverse events (AEs), including serious AEs with their severity and association with the study drug, regular monitoring of hematologic test results, blood chemical analyses, and careful monitoring for the possible occurrence of osteonecrosis of the jaw (panoramic radiography of the jaw at baseline and thereafter as clinically indicated) and atypical femoral fracture.
Statistical Analysis
The efficacy analysis was performed using the full analysis set (intention-to-treat population), which included all randomized patients. The primary efficacy variable was also analyzed using the per-protocol set (evaluable population), which included all randomized patients who met the entry criteria and had an evaluation at 3 months, did not take protocol-prohibited medications, and had not missed more than 50% of the scheduled study drug. The safety analysis was performed using the safety set, which included all randomized patients who received at least 1 dose of the study drug and who had at least 1 valid postbaseline safety assessment.
The hypothesis tested was that zoledronic acid every 12 weeks was noninferior to zoledronic acid every 4 weeks for SRE rate if the upper limit of the 2-sided 95% CI (equivalent to a 1-sided 97.5% CI) for the rate difference did not exceed 10%. The CI was calculated using the normal approximation to the binomial distribution with continuity correction. When this study was designed, no data were available from placebo-controlled randomized clinical trials in patients who had been pretreated for 1 year and continued to take zoledronic acid. The 10% noninferiority margin and the sample size were therefore determined based on SRE rate data from the first year of treatment. On the basis of assumed SRE rates of 48% in the every 4 weeks group, the initial sample size of OPTIMIZE-2 was calculated to be 705. Although OPTIMIZE-2 was ongoing, based on available data from the ZOOM study and a masked look at the pooled SRE data of OPTIMIZE-2 (which revealed a lower SRE rate of 21%), the sample size of OPTIMIZE-2 was recalculated to 423, including the 13 patients who had initially been randomized to placebo, for whom the results are not reported in this article (eMethods in Supplement 2). This new sample size had a power of 80% to detect the noninferiority with the noninferiority margin of 10%.
The secondary efficacy variables of time to first SRE and SRE-free survival were compared among treatment groups using stratified log-rank test. The SMR change from baseline and analgesic consumption was compared between groups using the stratified Cochran-Mantel-Haenszel test with modified ridit score. The Cochran-Mantel-Haenszel and log-rank test were stratified by the randomization stratification factors. Change from baseline in BPI composite pain score, uNTX:Cr ratio, and serum BSAP level between treatment groups were compared using analysis of covariance with baseline value as covariate and treatment and stratum as factors. P values were calculated by the analysis of covariance model. A 2-sided P < .05 was considered significant.
Results
A total of 416 women were randomized: 200 patients received zoledronic acid every 4 weeks (mean [SD] age, 59.2 [11.1] years; 173 were white [86.5%]), 203 patients received zoledronic acid every 12 weeks (mean [SD] age, 58.6 [11.2] years; 178 were white [87.7%]), and 13 patients received placebo (mean [SD] age, 60.8 [12.2] years; 13 were white [100%]) (Figure 1). The most common reasons for study discontinuation included consent withdrawal (20 [10.0%] vs 26 [12.8%] in the every 4 weeks group vs every 12 weeks group) and disease progression (24 [12.0%] vs 19 [9.4%] in the every 4 weeks group vs every 12 weeks group).
The baseline demographic and clinical characteristics were comparable between the 2 treatment groups (Table 1 and eResults and eTable 1 in Supplement 2). The median number of zoledronic acid infusions received during the study was 12 in the every 4 weeks group and 5 in the every 12 weeks group.
Table 1. Baseline and Demographic Characteristicsa.
| Characteristic | Zoledronic Acid Every 4 wk (n = 200) |
Zoledronic Acid Every 12 wk (n = 203) |
Placebo (n = 13) |
Total (N = 416) |
|---|---|---|---|---|
| Age, mean (SD), y | 59.2 (11.1) | 58.6 (11.2) | 60.8 (12.2) | 58.9 (11.1) |
| Age group, y | ||||
| <65 | 134 (67.0) | 148 (72.9) | 8 (61.5) | 290 (69.7) |
| ≥65 | 66 (33) | 55 (27.1) | 5 (38.5) | 126 (30.3) |
| Females | 200 (100) | 203 (100) | 13 (100) | 416 (100) |
| White | 173 (86.5) | 178 (87.7) | 13 (100) | 364 (87.5) |
| ECOG performance status | ||||
| 0-1 | 191 (95.5) | 195 (96.1) | 12 (92.3) | 398 (95.7) |
| ≥2 | 6 (3.0) | 6 (3.0) | 1 (7.7) | 13 (3.1) |
| Body mass index, mean (SD)b | 29.5 (6.2) (n = 177) |
29.7 (6.3) (n = 184) |
33.7 (8.6) (n = 12) |
29.7 (6.3) (n = 373) |
| BPI composite pain score, mean (SD) | 2.0 (1.9) (n = 176) |
2.2 (2.1) (n = 176) |
2.5 (1.7) (n = 12) |
2.14 (2.9) (n = 364) |
| Baseline serum creatinine | ||||
| Normal (<1.4 mg/dL) | 196 (98.0) | 192 (94.6) | 12 (92.3) | 400 (96.2) |
| Abnormal (≥1.4 mg/dL) | 2 (1.0) | 10 (4.9) | 1 (7.7) | 13 (3.1) |
| Duration of IV bisphosphonates prior to study, No. (%), mo | ||||
| >15 | 119 (59.5) | 106 (52.2) | 1 (7.7) | 226 (54.3) |
| ≤15 | 81 (40.5) | 97 (47.8) | 12 (92.3) | 190 (45.7) |
| uNTX:Cr ratio before studyc | ||||
| >100 | 9 (4.5) | 2 (1.0) | 11 (2.6) | |
| ≤100 | 191 (95.5) | 201 (99.0) | 13 (100) | 405 (97.4) |
| Time from initial diagnosis of cancer to randomization, wk | ||||
| Mean (SD) | 430 (334) (n = 199) |
369 (307) (n = 202) |
261 (291) (n = 13) |
395 (322) (n = 414) |
| Median (range) | 352 (48-1463) (n = 199) |
284 (44-1765) (n = 202) |
142 (62-885) (n = 13) |
296 (44-1765) (n = 414) |
Abbreviations: BPI, Brief Pain Inventory; ECOG, Eastern Cooperative Oncology Group; uNTX:Cr, urinary N-telopeptide to urinary creatinine.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4.
Data are presented as number (percentage) of patients unless otherwise indicated. The study was designed to include a placebo arm, with a randomization ratio of 2:2:1 for zoledronic acid every 4 weeks, zoledronic acid every 12 weeks, and placebo. Consequently, 13 patients were randomly assigned to the placebo arm. Subsequently, with the approval of bisphosphonates, the placebo arm was eliminated. All patients who were randomly assigned to the placebo arm were switched to zoledronic acid every 4 weeks; their efficacy data were analyzed separately and were not reported in this article.
Body mass index was calculated as weight in kilograms divided by height in meters squared.
uNTX:Cr ratio is reported as nanomoles of bone collagen equivalent per millimoles of creatinine.
Overall, 44 patients (22.0%) in the every 4 weeks group vs 47 patients (23.2%) in the every 12 weeks group experienced 1 or more SRE. The every 12 weeks group was determined to be noninferior to the every 4 weeks group for the proportion of patients experiencing 1 or more SRE because the proportional difference between groups of −1.2% had a 1-sided 97.5% CI bound (−9.8%) that was less than 10% (noninferiority P = .02). The per-protocol set analysis had similar results as the full analysis set analysis.
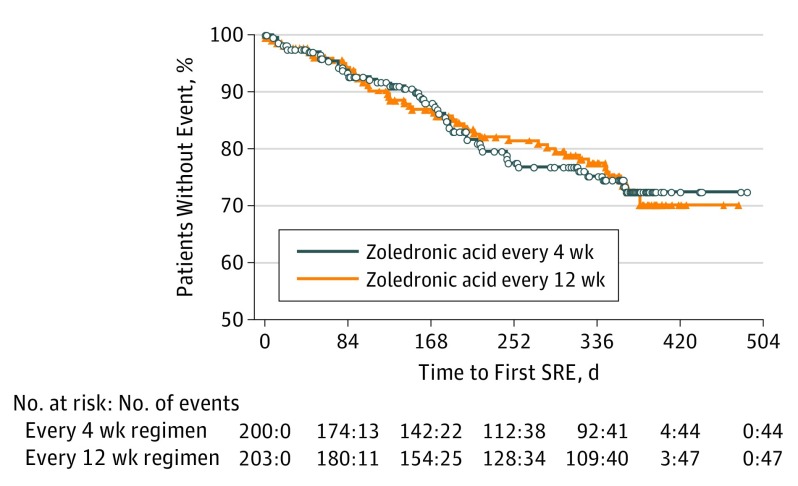
The time to first SRE between treatment groups was not statistically significantly different (hazard ratio [HR], 1.06; 95% CI, 0.70-1.60; P = .79) (Figure 2). A similar trend was estimated for time to multiple SRE events using the Andersen-Gill approach (HR, 0.97; 95% CI, 0.64-1.46; P = .88). The SRE-free survival between groups was not statistically significantly different (HR, 1.04; 95% CI, 0.70-1.54; log-rank test, P = .84). The most frequently recorded individual type of SRE in both treatment groups was radiation to bone (29 [14.5%] vs 23 [11.3%] in the every 4 weeks vs every 12 weeks group), followed by nonvertebral pathologic fractures (15 [7.5%] vs 18 [8.9%] in the every 4 weeks vs every 12 weeks group). The mean (SD) SMR was 0.46 (1.06) vs 0.50 (1.50) events per year in the every 4 weeks vs every 12 weeks group (P = .85).
Figure 2. Kaplan-Meier Curve for Time From Randomization to First Skeletal-Related Event (SRE).
Mean (SD) change from baseline in composite BPI score and analgesic consumption was not statistically different between groups. Mean (SD) change from baseline in uNTX:Cr ratio was statistically significantly higher in the every 12 weeks group than in the every 4 weeks group at week 36 only (P = .01) (eFigure, A in Supplement 2). No statistically significant mean changes from baseline in serum BSAP between the treatment groups were observed (eFigure, B in Supplement 2).
The safety profiles of the every 12 weeks and every 4 weeks groups were comparable. The most frequent AEs are given in Table 2. Ninety-four patients (47.5%) in the every 4 weeks group vs 86 patients (42.6%) in the every 12 weeks group reported grade 3 or 4 AEs. Fifty patients (25.3%) in the every 4 weeks group vs 51 patients (25.2%) in the every 12 weeks group experienced 1 or more treatment-emergent serious AEs. Overall, 34 patients (17.2%) in the every 4 weeks group and 17 patients (8.4%) in the every 12 weeks group experienced suspected study treatment-emergent AEs; 23 patients (11.6%) in the every 4 weeks group vs 18 patients (8.9%) in the every 12 weeks group discontinued study treatment because of treatment-emergent AEs. An increase in blood creatinine level was the most common treatment-emergent AE that led to discontinued use of the study drug, occurring in 6 patients in the every 4 weeks group vs 1 in the every 12 weeks group. Although overall more renal AEs occurred in the every 4 weeks group, renal failure occurred more frequently in the every 12 weeks group (eTable 2 in Supplement 2). Renal adverse events were comparable between the 2 treatment arms (eTable 2 in Supplement 2). Two adjudicated cases of osteonecrosis of the jaw were reported (both in the every 4 weeks group). No cases of adjudicated atypical femur fracture were reported in either treatment group. Numerically more cardiac events had occurred in the every 12 weeks group (n = 16) vs the every 4 weeks group (n = 15) (eResults in Supplement 2). A total of 18 patients died during the study: 10 patients (5.0%) in the every 4 weeks group, 7 patients (3.4%) in the every 12 weeks group, and 1 patient (7.7%) in the initial placebo group (eTable 3 in Supplement 2).
Table 2. Adverse Events Regardless of Study Drug Relationshipa .
| Adverse Event | No. (%) of Patients With Adverse Events | |||
|---|---|---|---|---|
| Zoledronic Acid Every 4 wk (n = 198) |
Zoledronic Acid Every 12 wk (n = 202) |
Placebo (n = 13) |
Total (N = 413) |
|
| Patients with ≥1 adverse event | 189 (95.5) | 189 (93.6) | 12 (92.3) | 390 (94.4) |
| Fatigue | 60 (30.3) | 68 (33.7) | 3 (23.1) | 131 (31.7) |
| Arthralgia | 65 (32.8) | 56 (27.7) | 6 (46.2) | 127 (30.8) |
| Nausea | 59 (29.8) | 53 (26.2) | 3 (23.1) | 115 (27.8) |
| Pain in extremity | 49 (24.7) | 48 (23.8) | 3 (23.1) | 100 (24.2) |
| Back pain | 53 (26.8) | 39 (19.3) | 2 (15.4) | 94 (22.8) |
| Constipation | 43 (21.7) | 35 (17.3) | 1 (7.7) | 79 (19.1) |
| Diarrhea | 40 (20.2) | 36 (17.8) | 2 (15.4) | 78 (18.9) |
| Vomiting | 32 (16.2) | 34 (16.8) | 4 (30.8) | 70 (16.9) |
| Headache | 33 (16.7) | 34 (16.8) | 1 (7.7) | 68 (16.5) |
| Musculoskeletal pain | 32 (16.2) | 30 (14.9) | 2 (15.4) | 64 (15.5) |
| Dyspnea | 32 (16.2) | 28 (13.9) | 2 (15.4) | 62 (15.0) |
| Anemia | 26 (13.1) | 34 (16.8) | 1 (7.7) | 61 (14.8) |
| Decreased appetite | 31 (15.7) | 27 (13.4) | 0 | 58 (14.0) |
| Musculoskeletal chest pain | 30 (15.2) | 25 (12.4) | 2 (15.4) | 57 (13.8) |
| Edema peripheral | 26 (13.1) | 27 (13.4) | 3 (23.1) | 56 (13.6) |
| Cough | 22 (11.1) | 28 (13.9) | 1 (7.7) | 51 (12.3) |
| Neuropathy peripheral | 20 (10.1) | 21 (10.4) | 3 (23.1) | 44 (10.7) |
| Dizziness | 24 (12.1) | 18 (8.9) | 1 (7.7) | 43 (10.4) |
| Upper respiratory tract infection | 18 (9.1) | 23 (11.4) | 1 (7.7) | 42 (10.2) |
The study was designed to include a placebo arm, with a randomization ratio of 2:2:1 for zoledronic acid every 4 weeks, zoledronic acid every 12 weeks, and placebo arms. Consequently, 13 patients were randomly assigned to the placebo arm. Subsequently, with the approval of bisphosphonates, the placebo arm was eliminated. All patients who were randomly assigned to the placebo arm were switched to zoledronic acid every 4 weeks; their efficacy data were analyzed separately and are not reported in this article.
Discussion
OPTIMIZE-2 met its primary end point, using a predefined noninferiority margin of 10%. Among patients who had received monthly intravenous zoledronic acid and/or pamidronate for 1 year or longer, the efficacy of continuing zoledronic acid treatment for an additional year at every 12 weeks was noninferior to zoledronic acid every 4 weeks. Of note, the upper limit of the 2-sided 95% CI (9.8%) was very close to the noninferiority boundary (10%). Similar observations were made in the ZOOM study. Safety profiles and bone marker profiles were similar between the 2 treatment arms.
In the previously published ZOOM study conducted in patients who had received 1 year of prior standard (every 4 weeks) zoledronic acid therapy, dosing with zoledronic acid every 12 weeks was noninferior to dosing every 4 weeks for efficacy. However, the study had limitations inherent to open-label study designs. In addition, the observed differences in bone turnover marker data between the 2 treatments in favor of the every 4 weeks treatment arm required further validation.
The difference in SMR in OPTIMIZE-2 was not statistically significant between the 2 zoledronic acid treatment groups. Similar to the ZOOM study, the most frequently recorded individual type of SRE in both treatment groups was radiation to the bone (14.5% in the every 4 weeks group and 11.3% in the every 12 weeks group). Similar results were obtained in the randomized Cancer and Leukemia Group B 70604 trial of 1822 bisphosphonate-naive patients with breast cancer, prostate cancer, and myeloma in which reduction in the dosing frequency of zoledronic acid from monthly to every 3 months was found to be noninferior.
The mean change from baseline profile of the bone turnover biomarkers (uNTX:Cr ratio and serum BSAP level) was comparable between the 2 treatment groups, except at one time point (36 weeks), wherein the every 12 weeks group had a greater change in uNTX:Cr ratio compared with the every 4 weeks group (P = .01). The clinical relevance of this single time point difference is not clear. Our results differ from the findings in the ZOOM study, which found separation in N-terminal telopeptide between the 2 treatment arms, in favor of the every 4 weeks treatment group, which, however, was not reproduced by our double-blind design. A feasibility study (REFORM) of women with metastatic breast cancer involving the bone and prior exposure to intravenous bisphosphonates randomized study participants to receive pamidronate every 3 to 4 weeks or to a deescalated interval of 12 weeks. The study reported a significant increase in the levels of 2 biomarkers (C-terminal telopeptide, P = .03, and BSAP, P = .01) in the 13 patients in the every 12 weeks group, in the absence of an association with SRE frequency. Because of the small sample size and the fact that the REFORM study was restricted to patients with low-risk bone metastasis, the data from this study may not allow extrapolation of the results to a larger generalized patient population.
In OPTIMIZE-2, the safety profiles of the 2 treatment arms were comparable, and there were no new or unexpected safety findings with zoledronic acid in the present study compared with the ZOOM study. The proportion of patients reporting serious AEs (grade 3 and 4 AEs) or the proportion of patients who experienced AEs that led to dose adjustment or interruption or study drug discontinuation was not obviously different between the 2 treatment groups. Regarding the AEs of special interest, the number of osteonecrosis of the jaw cases, cardiac ischemic AEs, and atrial fibrillation AEs were very low; 2 patients in the every 4 weeks group had an adjudicated osteonecrosis of the jaw event, whereas there were none in the every 12 weeks group. In the ZOOM study, 3 osteonecrosis of the jaw cases were reported in the every 4 weeks group and 4 osteonecrosis of the jaw cases in the every 12 weeks group. In the current study, no patient in any treatment group experienced an adjudicated atypical femur fracture event, with all femur fractures adjudicated. The proportion of patients who experienced renal treatment-emergent AEs was not obviously different between the 2 treatment groups.
Limitations
As discussed earlier, in the absence of SRE data from the second year of treatment with zoledronic acid vs placebo available at the time this trial was designed, the sample size and noninferiority margin of OPTIMIZE-2 were based on SRE data from the first year of treatment instead. However, the availability of data from the ZOOM study while OPTIMIZE-2 was ongoing led to an interim analysis of the masked pooled SRE rate data of available OPTIMIZE-2, and the sample size of OPTIMIZE-2 was reestimated. In addition, the deletion of the placebo arm made it difficult to assess the efficacy of continued treatment with zoledronic acid after the first year. Thus, the results should be interpreted with caution. After discussions with the US Food and Drug Administration, no changes are anticipated in the zoledronic acid label. Nevertheless, the efficacy and safety results of OPTIMIZE-2 are consistent with those reported from the ZOOM study.
Conclusions
OPTIMIZE-2 revealed that continuing zoledronic acid treatment for an additional year at a regimen of every 12 weeks was noninferior to an every 4 weeks regimen for efficacy and had a comparable safety profile in patients with bone metastasis from breast cancer who had completed 9 or more prior doses of intravenous bisphosphonate treatment, using a noninferiority margin of 10%. Further studies are warranted to investigate the effect of long-term treatment with zoledronic acid on bone saturation and bone retention rate as well as clinically relevant outcomes.
Trial Protocol
eMethods. Additional Details of the Study Methods
eMaterial. List of Independent Ethics Committees (IECs) or Institutional Review Boards (IRBs) by Study Center
eResults. Additional Details of the Study Results
eTable 1. Number of Infusions Before the Study
eTable 2. Renal Adverse Events
eTable 3. On-treatment Deaths
eFigure. Change From Baseline in Bone Biomarker Levels
References
- 1.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655-1664. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176. [DOI] [PubMed] [Google Scholar]
- 3.Clément-Demange L, Clézardin P. Emerging therapies in bone metastasis. Curr Opin Pharmacol. 2015;22:79-86. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8)(suppl):1588-1594. [DOI] [PubMed] [Google Scholar]
- 5.Weinfurt KP, Castel LD, Li Y, Timbie JW, Glendenning GA, Schulman KA. Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care. 2004;42(2):164-175. [DOI] [PubMed] [Google Scholar]
- 6.Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol. 2009;6(3):163-174. [DOI] [PubMed] [Google Scholar]
- 7.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(8):1860-1867. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RE, Major P, Lipton A, et al. . Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23(22):4925-4935. [DOI] [PubMed] [Google Scholar]
- 9.Green JR, Müller K, Jaeggi KA. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9(5):745-751. [DOI] [PubMed] [Google Scholar]
- 10.Zometa product characteristics. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/Zometa.pdf. Accessed March 6, 2016.
- 11.Van Poznak CH, Temin S, Yee GC, et al. ; American Society of Clinical Oncology . American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29(9):1221-1227. [DOI] [PubMed] [Google Scholar]
- 12.Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004;100(1):44-52. [DOI] [PubMed] [Google Scholar]
- 13.Yavas O, Hayran M, Ozisik Y. Factors affecting survival in breast cancer patients following bone metastasis. Tumori. 2007;93(6):580-586. [DOI] [PubMed] [Google Scholar]
- 14.Cremers SC, Papapoulos SE, Gelderblom H, et al. . Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res. 2005;20(9):1543-1547. [DOI] [PubMed] [Google Scholar]
- 15.Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag. 2008;4(1):261-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nancollas GH, Tang R, Phipps RJ, et al. . Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38(5):617-627. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Amadori D, Aglietta M, Alessi B, et al. . Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14(7):663-670. [DOI] [PubMed] [Google Scholar]
- 19.Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials. 1982;3(4):345-353. [DOI] [PubMed] [Google Scholar]
- 20.Himelstein AL, Rui Q, Novotny PJ, et al. . CALGB 70604 (Alliance): a randomized phase III study of standard dosing vs longer interval dosing of zoledronic acid in metastatic cancer [abstract 9501]. J Clin Oncol. 2015;33(33)(suppl). [Google Scholar]
- 21.Addison CL, Pond GR, Zhao H, et al. . Effects of de-escalated bisphosphonate therapy on bone turnover biomarkers in breast cancer patients with bone metastases. Springerplus. 2014;3(1):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Additional Details of the Study Methods
eMaterial. List of Independent Ethics Committees (IECs) or Institutional Review Boards (IRBs) by Study Center
eResults. Additional Details of the Study Results
eTable 1. Number of Infusions Before the Study
eTable 2. Renal Adverse Events
eTable 3. On-treatment Deaths
eFigure. Change From Baseline in Bone Biomarker Levels