Key Points
Question
Can breast cancer of the chest wall be treated with chemoimmunotherapy?
Findings
In a single-arm phase 2 clinical trial of 14 patients, 5 achieved a compete response and 5 were partial responders for an overall response rate of 72%. Elevated pretreatment levels of peripheral blood programmed death-1–positive T cells and monocytic myeloid derived suppressor cells were associated with suboptimal response.
Meaning
Even in treatment-refractory disease, chemoimmunotherapy can induce remission. Peripheral blood biomarkers associated with response can be identified.
This phase 2, single-arm clinical trial examines the safety and objective response rate of topical imiquimod in combination with systemic chemotherapy for treatment of breast cancer cutaneous metastases.
Abstract
Importance
Salvage chemotherapy for recurrent chest wall lesions in breast cancer results in response rates of 20% to 30%. Preclinical studies showed significant disease regression could be induced in murine chest wall mammary cancers with a topical toll-like receptor (TLR)-7 agonist, imiquimod.
Objective
To evaluate the safety and objective response rate (ORR) of imiquimod in combination with systemic albumin bound paclitaxel in treatment-refractory breast cancer of the chest wall.
Design, Setting, and Particpants
A single arm phase 2 clinical trial of 15 patients with breast cancer previously treated in an academic medical center setting between 2009 and 2012 for chest wall disease that had recurred.
Interventions
Imiquimod cream, 5%, was applied topically to a designated target lesion once per day for 4 consecutive days on days 1 through 4, 8 through 11, 15 through 18, and 22 through 25 of a 28-day cycle, for 12 weeks. Albumin bound paclitaxel, 100 mg/m2, was given intravenously on days 1, 8, and 15, and repeated every 28 days over the 12-week period.
Main Outcomes and Measures
The primary endpoint was safety and ORR. Secondary endpoints included the generation of tumor-infiltrating lymphocytes and modulation of immune cell populations.
Results
The median age at baseline of the 15 study participants was 54 years (range, 46-92 years). Fourteen patients were evaluable. Combination therapy was associated with low-grade toxic effects. Of 358 adverse events 330 (92%) were grades 1 and 2. Five (36%) patients achieved a compete response and another 5 (36%) were partial responders for an overall response rate of 72% (10 of 14). The response duration was limited. Pretreatment levels of programmed death-1 (PD-1)+ peripheral blood T cells (PD-1+ cluster of differentiation [CD]4+; 95% CI, 2.68-6.63; P < .001 and PD-1+CD8+; 95% CI, 1.13-8.35; P = .01) and monocytic myeloid derived suppressor cells (mMDSC) (95% CI, 3.62-12.74; P = .001) greater than controls predicted suboptimal clinical response.
Conclusions and Relevance
Chemoimmunomodulation with a TLR-7 agonist and albumin bound paclitaxel is effective in inducing disease regression in treatment-refractory breast cancer chest wall metastases but responses are short-lived. Preexisting levels of cells indicating either T-cell exhaustion or systemic immunosuppression may be markers of selection for responsive patients.
Trial Registration
clinicaltrials.gov Identifier: NCT00821964
Introduction
Therapies for recurrent breast cancer chest wall disease are not curative and have low response rates. Studies in mammary tumors of transgenic mice demonstrate that topical toll-like receptor (TLR)-7 agonist, imiquimod stimulates type 1 cytokine secretion, up-regulates immune costimulatory molecules in the tumor, and results in inhibition of cancer growth. Taxanes also have immunomodulatory effects, increased interferon-γ production by antigen presenting cells, and enhanced natural killer (NK)-cell activity. Patients with breast cancer often demonstrate NK dysfunction and tumor antigen presenting cells promote type 2 cytokine secretion, dampening the development of cytotoxic T cells. Combination therapies that enhance interferon (IFN)-γ secretion may reverse established immune suppression. We examined whether the combination of topical imiquimod and weekly intravenous albumin-bound paclitaxel (nab-paclitaxel) could induce clinical responses in patients with treatment-refractory chest wall disease.
Methods
Participants
A targeted accrual of 15 patients with stage IV disease were enrolled from 2009-2012 after written informed consent was obtained. Institutional review board approval was granted by the University of Washington Cancer Consortium. Eligibility included treatment refractory progressive disease and measurable chest wall or cutaneous metastasis. Concurrent bisphosphonate, HER2-targeted, and endocrine therapies were allowed. Fourteen age-matched donor control blood samples were also analyzed. Participants were not compensated for their participation. The trial protocol is included in Supplement 1.
Study Design
We report a phase 2 single-arm study (eFigure 1 in Supplement 2). The objectives were safety and objective response rate (ORR). An ORR of 50% or higher or complete response (CR) rate of 10% or higher were benchmarks based on historical ORR with salvage chemotherapy of 30% and CRof less than 2%. Fifteen patients was statistically sufficient for 80% confidence that the ORR was within 0.16 of the true response rate. Toxic effects were evaluated by Common Terminology Criteria for Adverse Events, version 3.0. Target lesion(s) were assessed by modified World Health Organization criteria with response defined as the change in target lesion size over 13 weeks.
Tumor Analysis
Skin biopsy samples were obtained at baseline and week 13 and stained for CD3, CD4, CD8, CD25 and Forkhead box protein (FOXP)3. For each case, 3 high-powered fields (hpf) (original magnification × 40), representative of prominent areas of lymphocytic infiltrates, were scored. The total number of lymphocytes and other cells in the 3 hpfs were counted and reported as mean number of cells per hpf.
Flow Cytometry
The data are expressed as percentage PD-1+ among CD3+CD4+ and CD3+CD8+ cells, and CD14+ cells percentage of HLA-DR- cells (monocytic MDSC) and CD33+ cells percentage of Lin-HLA-DR- cells (granulocytic MDSC), and FOXP3+CD4+ cells percentage of CD3+ cells.
Statistical Analysis
Statistical analysis included a 2-tailed t test using GraphPad statistical software (version 7.01, GraphPad Software Inc). A P value less than .05 was considered statistically significant.
Results
Combination Chemoimmunotherapy
The median age at baseline of study participants was 54 years (range, 46-92 years). All patients had a chest wall malignant abnormality documented by skin biopsy. Eighty percent of patients were hormone receptor negative and had received 4 or more previous chemotherapies (eTable 1 in Supplement 2). Nine patients (60%) completed all treatment cycles, 3 (20%) completed 2 cycles, and 2 (13%) completed 1 cycle.
Of 358 toxic effects 330 (92%) were grades 1 or 2, and 27 (8%) were grade 3 (eTable 2 in Supplement 2). Three patients required nab-paclitaxel dose reduction of 20% for grade 3 neutropenia or neuropathy. There were no treatment discontinuations owing to toxic effects.
Patient Response to Combination Chemoimmunotherapy
Fourteen patients were evaluable. Five (36%) demonstrated CR with 4 of 5 confirmed pathologic CR (Figure 1). Five (36%) achieved a partial response (PR) with 1 pathologic CR demonstrating no evidence of tumor but increased inflammatory infiltrate. Three patients had stable disease (SD) and 1 had progressive disease. The ORR was 10 of 14 (72%). Responses were not associated with tumor size (eTable 3 and eFigure 3 in Supplement 2). Intratumoral T-cell infiltrates were present in all at baseline and varied from a sparse infiltrate (<7 CD3+ cells per hpf) to strong infiltration (66 CD3+ cells per hpf), but failed to show consistent trends pretreatment to posttreatment. The median duration of response after therapy was 12 weeks (range, 4-25 weeks) in patients with CR, 16 weeks (range, 4-28 weeks) in PR patients, and 3 weeks (range, 1-4 weeks) in SD patients.
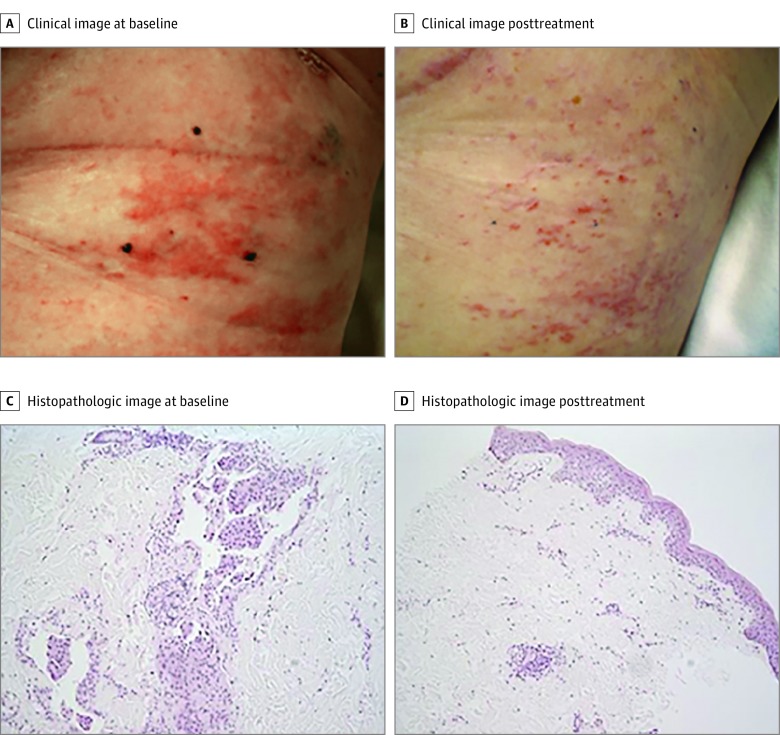
Figure 1. Patient Response to Combination Chemoimmunotherapy.
A patient with a complete response. A, Target lesion marked with 3 tattoos; B, complete resolution of target lesion; C, pathologic confirmation of carcinoma prior to therapy; and D, pathologic complete response and associated perivascular lymphocytic infiltration after the end of treatment. Hematoxylin-eosin stain (original magnification ×40).
Factors Associated With Suboptimal Clinical Response
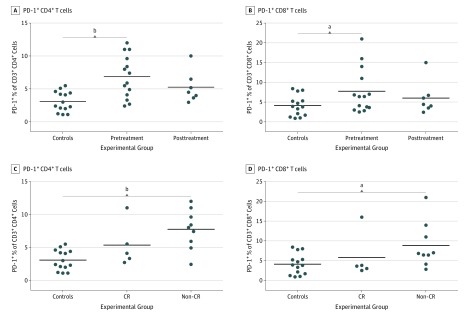
Patients had significantly elevated levels of PD-1+CD4+ peripheral blood T cells (mean, 6.6%; range, 2.4%-12.0%) compared with controls (mean, 3.1%; range, 1.1%-5.5%; 95% CI, 1.8-5.8; P < .001) (Figure 2A). Similarly, PD-1+CD8+ T cells were significantly elevated (mean, 7.1%; range, 2.5%-21.0%) compared with controls (mean, 4.1%; range, 0.9%-8.4%; 95% CI, 0.2%-7.1%; P = .04) (Figure 2B). The percent of PD-1 expressing CD4 or CD8 T cells was not modulated during therapy (pretreatment to posttreatment P values >.05). When pretreatment levels were assessed by response type, patients who achieved a CR had similar levels of PD-1+ T cells as volunteer donors (mean, 5.3%; range,2.7%-11.0%; P = .06; and mean, 5.8%; range, 2.5%-16.0%; P = .38) for CD4 and CD8 T-cells, respectively. PD-1+CD4+ and CD8+ T cells were significantly elevated in the pretreatment blood of patients developing suboptimal or no responses after therapy compared with controls (PD-1+CD4+ mean, 7.7%; range, 2.4%-12%; 95% CI, 2.7%-6.6%; P < .001 (Figure 2C) and PD-1+CD8+ mean, 8.8% (range, 2.8%-21%; 95% CI, 1.8%-8.4%; P = .01) (Figure 2D). There was no difference in NK or B cells between groups. Although breast cancer patients had elevated monocytes compared with controls (95% CI, 12.1-34.0; P = .002), these were not predictive of outcome.
Figure 2. Pretreatment Elevated T-Cell PD1 Expression Associated With Suboptimal or No Clinical Response.
A, PD1+ percent of CD3+CD4+ cells; B, PD1+ percent of CD3+CD8+ cells in controls and patients before and after treatment. C, PD1+percent of CD3+CD4+ cells; D, PD1+ percent of CD3+CD8+ cells in controls, complete response (CR), and non-CR patients before treatment. Lines represent the means of the group.
aP < .05.
bP < .01.
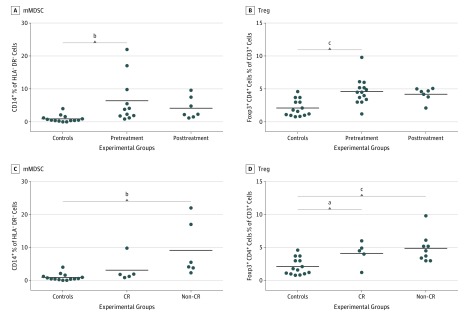
The mean pretreatment mMDSC level was 6.4% (range 0.9%-22.0%) in patients and 0.9% (range 0.0%-4.0%) in controls, (95% CI, 1.5%-9.4%; P < .01) (Figure 3A). The mean level of regulatory T (Treg) cells pretreatment was 4.6% (range, 1.2%-9.8%) compared with 2.1% (0.8%-4.6%) in controls, (95% CI, 1.2%-3.8%; P < .001) (Figure 3B). mMDSC levels were predictive of suboptimal response with patients not achieving a CR having elevated levels; mean, 9.1% (range, 2.3%-22.0%) significantly higher than controls (95% CI, 3.6%-12.7%; P = .001); whereas mMDSC levels in patients with CR were similar to donors; mean 3.1% (range, 0.9%-9.8%; P = .056) (Figure 3C). The Treg level was elevated in both complete (95% CI, 0.5-3.6; P = .01) and suboptimal responders (95% CI, 1.3-4.2; P < .001) compared with controls (Figure 3D).
Figure 3. Pretreatment Elevated Monocytic Myeloid-Derived Suppressor Cells Associated With Suboptimal or No Clinical Response.
mMDSC indicates monocytic myeloid derived suppressor cells; Treg, regulatory T cells. A, CD14+ cells percent human leukocyte antigen (HLA) DR- cells; B, FOXP3+CD4+ cells percent of CD3+ cells in controls and patients before and after treatment. C, CD14+ cells percent HLA DR- cells; D, FOXP3+CD4+ cells percent of CD3+ cells in controls, complete response (CR), and non-CR patients before treatment. Lines represent the means of the group.
aP < .05.
bP < .01.
cP < .001.
Discussion
In breast cancer, robust T-cell infiltrates have been associated with superior response rates to chemotherapy and increased progression free and overall survival. Most patients with breast cancer, however, have no to low levels of tumor infiltrating T cells (TIL). TLR-7 ligation activates dendritic cells in vivo, increasing antigen processing and presenting capabilities via type 1 interferon secretion. In the transgenic mouse mammary tumor virus neu transgenic model of mammary chest wall malignant abnormality, topical imiquimod induced type 1 cytokines and TIL. Inflammation resulting from imiquimod use elicited a self-regulatory response and secretion of IL-10 from CD4+ T cells limiting response duration in mice. Although imiquimod with nab-paclitaxel could induce pathologic CR in more than a third of patients, similar to mice, responses were of limited duration.
We found patients with suboptimal responses had higher levels of PD-1+ T cells in their blood indicating T-cell exhaustion and functional defects. Recent studies have demonstrated that PD1+CD4+ T-cell levels predict response to ipilimumab in prostate cancer. The lower the level of PD-1+ T cells prior to therapy, the more likely the clinical benefit. Myeloid cells, such as MDSC, inhibit CD4 T-cell activation via the PD-1/PDL-1 axis. T-cell activation could be restored in vivo in the presence of these immunosuppressive cells through the use of PD-1 blockade.
Combination immunotherapy for treatment of refractory chest wall lesions demonstrates a response rate higher than that reported for conventional chemotherapy. The addition of a PD-1 or PD-L1 directed monoclonal antibody to this combination regimen and a longer course of treatment could significantly improve response duration and potentially affect concurrent systemic disease.
Conclusions
Chemoimmunotherapy is an active regimen with low toxic effects. Inhibition of specific mechanisms of immune suppression, PD-1 up-regulation and mMDSC, could enhance efficacy and provide a new treatment approach to patients with refractory breast cancer in the chest wall.
Trial Protocol
eTable 1. Baseline patient characteristics
eFigure 1. Dosing schedule
eFigure 2. Flow diagram of patient progress through the trial
eTable 2. Detailed adverse events
eTable 3. Clinical data summary
eFigure 3. Response in larger lesion
References
- 1.Lu H, Wagner WM, Gad E, et al. Treatment failure of a TLR-7 agonist occurs due to self-regulation of acute inflammation and can be overcome by IL-10 blockade. J Immunol. 2010;184(9):5360-5367. [DOI] [PubMed] [Google Scholar]
- 2.Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009;38(4):283-290. [DOI] [PubMed] [Google Scholar]
- 3.Krneta T, Gillgrass A, Poznanski S, et al. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J Leukoc Biol. 2016;jlb.3A1215-552R. [DOI] [PubMed] [Google Scholar]
- 4.Olkhanud PB, Rochman Y, Bodogai M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 2011;186(10):5656-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim NK, Samuels B, Page R, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23(25):6019-6026. [DOI] [PubMed] [Google Scholar]
- 6.Martín M, Ruiz A, Muñoz M, et al. ; Spanish Breast Cancer Research Group (GEICAM) trial . Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: final results of the phase III Spanish Breast Cancer Research Group (GEICAM) trial. Lancet Oncol. 2007;8(3):219-225. [DOI] [PubMed] [Google Scholar]
- 7.Saunders Y, Stebbing J, Broadley K, Johnston SR. Recurrent locally advanced breast cancer: the treatment of chest wall disease with further chemotherapy. Clin Oncol (R Coll Radiol). 2001;13(3):195-199. [DOI] [PubMed] [Google Scholar]
- 8.Vassilomanolakis M, Koumakis G, Demiri M, Missitzis J, Barbounis V, Efremidis AP. Vinorelbine and cisplatin for metastatic breast cancer: a salvage regimen in patients progressing after docetaxel and anthracycline treatment. Cancer Invest. 2003;21(4):497-504. [DOI] [PubMed] [Google Scholar]
- 9.Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36(7):980-986. [DOI] [PubMed] [Google Scholar]
- 10.Disis ML, Dang Y, Coveler AL, et al. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol Immunother. 2014;63(2):101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354-1360. [DOI] [PubMed] [Google Scholar]
- 13.Le Mercier I, Poujol D, Sanlaville A, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73(15):4629-4640. [DOI] [PubMed] [Google Scholar]
- 14.Kwek SS, Lewis J, Zhang L, et al. Preexisting levels of CD4 T cells expressing PD-1 are related to overall survival in prostate cancer patients treated with ipilimumab. Cancer Immunol Res. 2015;3(9):1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding ZC, Lu X, Yu M, et al. Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T-cell responses through the PD-1-PD-L1 axis. Cancer Res. 2014;74(13):3441-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline patient characteristics
eFigure 1. Dosing schedule
eFigure 2. Flow diagram of patient progress through the trial
eTable 2. Detailed adverse events
eTable 3. Clinical data summary
eFigure 3. Response in larger lesion